Abstract
Background
Inflammatory activity of the artery can be assessed by measuring 18F-fluorodeoxyglucose (18F-FDG) uptake with positron emission tomography computed tomography (PET/CT). Improvement in vascular function after renal transplantation has been reported, but no studies have used 18F-FDG PET/CT to examine the changes in vascular inflammation. This study investigated the changes in the inflammatory activity in the carotid artery after renal transplantation in patients with chronic kidney disease (CKD).
Material/Methods
18F-FDG PET/CT was performed before and at 4 months after transplantation. We quantified 18F-FDG uptake as the target-to-background ratio (TBR) in the carotid artery in 10 CKD patients. TBR was evaluated in the whole carotid artery (WH) and most-diseased segment (MDS), and the mean and maximum values were analyzed. The concentrations of inflammatory cytokines, including tumor necrosis factor-alpha, interleukin-6, plasminogen activator inhibitor-1, and endothelin-1, were measured.
Results
Eight patients showed a decrease in mean or maximum TBR. The average mean or maximum TBRs in the WH and MDS of the right and left arteries were all reduced after transplantation. The average mean TBR for the right WH decreased significantly (% reduction [95% CI]) by −5.74% [−15.37, −0.02] (p=0.047). TBRs did not correlate significantly with cytokine concentrations. The changes in cytokine concentrations after transplantation varied.
Conclusions
18F-FDG uptake by the WH and MDS tended to reduce after renal transplantation. Therefore, renal transplantation may confer an anti-inflammatory effect on carotid atherosclerosis in patients with CKD; however, this effect is not large enough to be demonstrated in this study with small sample size.
MeSH Keywords: Atherosclerosis; Carotid Artery Diseases; Fluorodeoxyglucose F18; Kidney Transplantation; Positron-Emission Tomography; Renal Insufficiency, Chronic
Background
Patients with chronic kidney disease (CKD) have an extremely high risk of developing cardiovascular disease (CVD), which is the leading cause of death in this population [1,2]. Traditional risk factors for CVD in CKD patients are hypertension, diabetes, dyslipidemia, and smoking [3]. However, traditional risk factors underestimate the CVD risk in CKD patients [4]. Renal transplantation has a survival advantage over dialysis and reduces the CVD risk even with the persistence of traditional risk factors [5–7]. These findings imply a role of CKD-related, nontraditional risk factors in the development of CVD. Inflammation is one of the main contributing factors to the increased CVD risk in CKD patients. C-reactive protein (CRP), a marker of inflammation, predicts progression of atherosclerosis [8] and increased CVD risk in CKD patients [9]. CRP levels have been shown to decrease [10,11], and carotid intima-media thickness (IMT) [11], endothelial function [12–14], and aortic stiffness [15] to improve after renal transplantation. These changes may be associated with the reduced CVD risk in CKD patients.
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography computed tomography (PET/CT) is an imaging technique frequently used for cancer surveillance. Atherosclerotic plaque inflammation can be imaged with 18F-FDG PET/CT by assessing the increased 18F-FDG uptake by the arterial wall [16,17]. The arterial 18F-FDG uptake is an independent predictor for future CVD beyond that predicted by the Framingham risk score [18]. Increased arterial inflammation measured by 18F-FDG uptake using 18F-FDG PET/CT was reported recently in CKD patients without overt atherosclerotic disease [19]. However, it is unknown whether the inflammatory activity of the carotid artery decreases after renal transplantation. In this study, we evaluated the changes in arterial inflammatory activity after renal transplantation by measuring 18F-FDG uptake by carotid arteries using 18F-FDG PET/CT.
Material and Methods
Patients and study design
This study was a prospective, single-center trial to evaluate the changes in inflammatory activity of the carotid artery after renal transplantation. Ten patients with stage 5 CKD who were planning to receive a living-donor renal transplant were enrolled between January 2014 and June 2016. The exclusion criteria were prior stroke or acute myocardial infarction, previous percutaneous coronary intervention or coronary artery bypass graft surgery, use of statin treatment within the previous 4 months, age less than 18 years, or pregnancy. After enrollment, patients underwent a baseline 18F-FDG PET/CT scan before renal transplantation and any immunosuppressive or desensitization therapy and were followed by a second 18F-FDG PET/CT scan at 4 months after renal transplantation. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board of Incheon St. Mary’s Hospital (OC13OISI0113). All patients provided written informed consent. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism”.
18F-FDG PET/CT and image analysis
18F-FDG PET/CT scans were performed as previously published (Discovery Ste, GE Healthcare) [18,20]. 18F-FDG PET/CT images were analyzed as previously described [21]. Arterial 18F-FDG uptake was measured in the right and left carotid arteries every 3 mm starting 2 cm below the carotid artery bifurcation and continuing superiorly to 2 cm into the internal carotid artery. The intensity of 18F-FDG uptake was quantified by measuring the maximum and mean standardized uptake value (SUV) corrected for body weight. The SUV score of the artery was corrected for background venous activity by dividing the average blood SUV estimated from the internal jugular veins and was defined as the target-to-background ratio (TBR). TBR was evaluated in the whole carotid artery (WH) and the most-diseased segment (MDS). The MDS, in turn, was defined as the 1.5 cm arterial segment, centered on the slice of artery, demonstrating the highest 18F-FDG uptake at baseline. TBR was assessed using 2 different approaches. The first approach was to define the average maximum TBR activity. WH-TBRmax was calculated as the average maximum TBR of the WH segments. MDS-TBRmax was calculated as the average maximum TBR derived from 3 contiguous axial segments of the MDS. The second approach was to define the average mean TBR activity. WH-TBRmean was calculated as the average mean TBRs for the WH segments. MDS-TBRmean was calculated as the average mean TBRs of the MDS. Among the 2 carotid arteries, the artery with the highest 18F-FDG uptake at baseline was identified as the index vessel, as previously described [21,22]. The index vessel TBR was calculated using 2 different approaches; calculating WH-TBRmax and MDS-TBRmax of the index vessel. Two nuclear medicine physicians who were uninformed of the study protocol made the 18F-FDG uptake measurements, and then the 2 measurements were averaged.
Outcome definitions
The primary objective of this study was to evaluate whether renal transplantation reduced the TBRs of the WH and MDS from baseline. The absolute and percent changes in the average maximum TBRs within the WH (WH-TBRmax) and the MDS (MDS-TBRmax) were assessed. The absolute and percent changes in the average mean TBRs within the WH (WH-TBRmean) and the MDS (MDS-TBRmean) were also assessed. The changes in TBRs were analyzed separately in the right and left carotid arteries, and the changes in the WH-TBRmax and MDS-TBRmax of the index vessel were analyzed.
Laboratory examinations
Venous blood samples were obtained from all patients after a 12-hour overnight fast at the same time points used for the 18F-FDG PET/CT scans. Enzyme immunoassay assays were used to measure the concentrations of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), plasminogen activator inhibitor-1 (PAI-1), and endothelin-1 (all from R&D Systems, Minneapolis, MN, USA). Serum CRP concentration was measured using a high-sensitivity immunoturbidimetric method. Renal function was calculated using the Modification of Diet in Renal Disease formula for estimated glomerular filtration rate (eGFR) [23]. Changes in the concentrations of TNF-α, IL-6, PAI-1, endothelin-1, eGFR, CRP, total cholesterol (TC), triglyceride, low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) were also analyzed.
Statistical analyses
The data are expressed as median and interquartile range (IQR). The Wilcoxon signed-rank test was used to assess differences in the TBRs and other parameters before and after renal transplantation. Associations between the TBR of the MDS-TBRmax of the index vessel and other variables were tested using Spearman correlation analysis. Statistical analyses were performed using the Statistical Analysis Software package (SAS version 9.1, SAS Institute, Cary, North Carolina, USA). A p value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the 10 patients. The median age (IQR) was 48 years (36.75, 58.25). Four patients (40%) were men, and 5 patients (50%) had diabetes mellitus. None of the patients had previous CVD, and 6 patients (60%) had visible plaque on carotid ultrasound. One patient (10%) had a panel-reactive antibody level of more than 50%, none had donor-specific antibody, and 4 patients (40%) received an ABO-incompatible renal transplantation. For desensitization therapy, 1 patient (10%) received rituximab only, and 4 patients (40%) received rituximab, plasmapheresis, and intravenous immunoglobulin. Corticosteroids were tittered to a dose of 5 or 10 mg/day by the time of the second 18F-FDG PET/CT scan in all patients.
Table 1.
Baseline characteristics.
| N=10 | |
|---|---|
| Age, years, median (IQR) | 48.0 (36.75, 58.25) |
| Recipient male, n (%) | 4 (40) |
| Diabetes mellitus, n (%) | 5 (50) |
| Cause of ESRD, n (%) | |
| Diabetes | 5 (50) |
| Hypertension | 1 (10) |
| Chronic GN | 2 (20) |
| Others | 2 (20) |
| Previous CV disease, n (%) | 0 (0) |
| History of smoking, n (%) | 3 (30) |
| BMI, kg/m2, median (IQR) | 22.37 (20.13, 27.93) |
| Dialysis vintage, months, median (IQR) | 3.36 (1.45, 24.53) |
| eGFR, ml/min/1.73 m2, median (IQR) | 5.38 (4.29, 6.75) |
| Donor male, n (%) | 6 (60) |
| Donor age, years, median (IQR) | 41.50 (35.0, 52.75) |
| Systolic BP (mmHg), median (IQR) | 140.0 (120.0, 150.0) |
| Diastolic BP (mmHg) median (IQR) | 80.0 (77.50, 92.50) |
| LV ejection fraction (%), median (IQR) | 62.5 (58.5, 68.3) |
| Rt carotid IMT (mm), median (IQR) | 0.60 (0.55, 0.90) |
| Lt carotid IMT (mm), median (IQR) | 0.65 (0.58, 1.08) |
| Carotid plaque on US, n (%) | 6 (60) |
| TC, mg/dL | 167.0 (146.0, 239.0) |
| Triglyceride, mg/dL | 132.50 (95.25, 220.50) |
| HDL-cholesterol, mg/dL | 36.50 (27.0, 53.50) |
| LDL-cholesterol, mg/dL | 100.0 (88.50, 167.0) |
| HLA mismatch No, median (IQR) | 3.0 (1.75, 4.0) |
| Panel reactive antibody >50%, n (%) | 1 (10) |
| Presence of DSA, n (%) | 0 (0) |
| ABO incompatible, n (%) | 4 (40) |
| Induction therapy | |
| Basiliximab | 10 (100) |
| Anti-thymocyte globulin | 0 (0) |
| Maintenance immunosuppression | |
| Tacrolimus (%) | 10 (100) |
| Mycophenolate mofetil (%) | 10 (100) |
| Desensitization therapy | |
| Rituximab only (%) | 1 (10) |
| Rituximab/Plasmapheresis/IVIG (%) | 4 (40) |
| None | 5 (50) |
IQR – interquartile range; ESRD – end-stage renal disease; BP – blood pressure; CVD – cardiovascular disease; eGFR – estimated glomerular filtration rate; BP – blood pressure; LV – left ventricular; IMT – intima-media thickness; TC – total cholesterol; HDL-C – high-density lipoprotein-cholesterol; LDL-C – low-density lipoprotein-cholesterol; HLA – human leukocyte antigen; IVIG – intravenous immunoglobulin.
Changes in carotid arterial inflammatory activity after renal transplantation
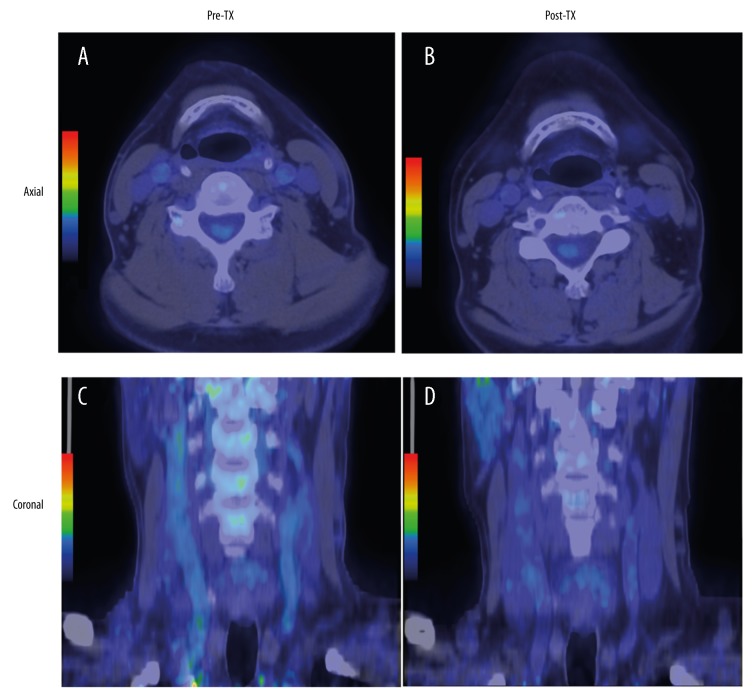
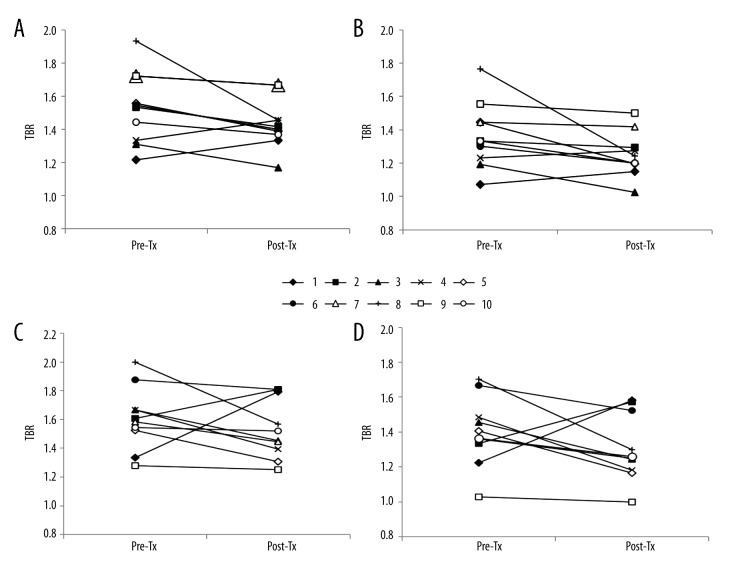
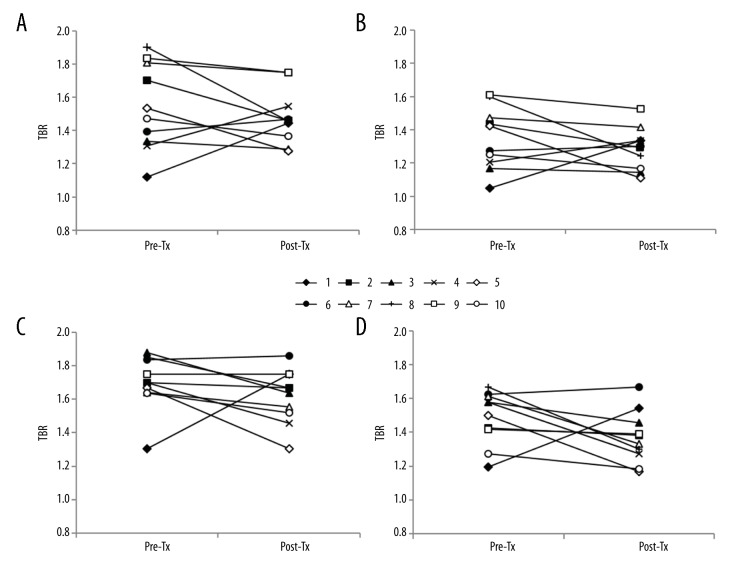
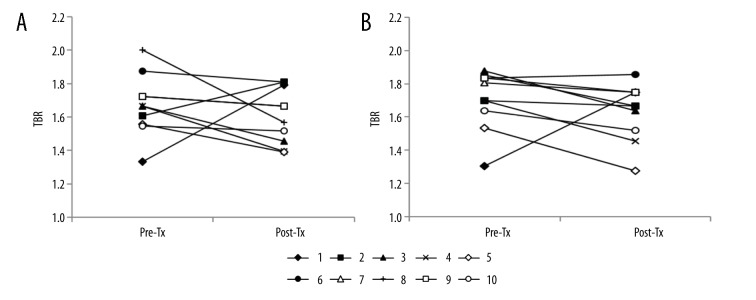
The 18F-FDG uptake by both carotid arteries was compared between the baseline and after transplantation using the axial and coronal images of 18F-FDG PET/CT scans (Figure 1). The changes in TBRs in the WH and MDS of the right and left carotid arteries are shown in Figures 2 and 3, respectively. Eight of the 10 patients (80%) showed a reduction in right WH-TBRmax and WH-TBRmean, and left MDS-TBRmean, WH-TBRmax, and WH-TBRmean. Seven patients (70%) showed a reduction in right MDS-TBRmax and MDS-TBRmean, and left MDS-TBRmax. Figure 4 shows the changes in WH-TBRmax and MDS-TBRmax in the index vessel. Eight of the 10 patients (80%) showed a reduction in WH-TBRmax and MDS-TBRmax in the index vessel.
Figure 1.
Representative images of 18F-FDG PET/CT of carotid artery before and after renal transplantation. The upper row is axial images (A, B), and the lower row is coronal images (C, D). Note the reduction in 18F-FDG uptake in both carotid arteries after transplantation (B, D) compared to baseline (A, C). Pre-Tx – before renal transplantation; Post-Tx – after renal transplantation.
Figure 2.
Changes in TBR values of right and left WH in individual participants after renal transplantation. (A) WH-TBRmax of right carotid artery. (B)WH-TBRmean of right carotid artery. (C) WH-TBRmax of left carotid artery. (D) WH-TBRmean of left carotid artery. Pre-Tx – before renal transplantation; Post-Tx – after renal transplantation; TBR – target-to-background ratio.
Figure 3.
Changes in TBR values of right and left MDS in individual participants after renal transplantation. (A) MDS-TBRmax of right carotid artery. (B) MDS-TBRmean of right carotid artery. (C) MDS-TBRmax of left carotid artery (D) MDS-TBRmean of left carotid artery. Pre-Tx – before renal transplantation; Post-Tx – after renal transplantation; TBR – target-to-background ratio.
Figure 4.
Changes in TBR values of the index vessel in individual participants after renal transplantation. (A) WH-TBRmax of the index vessel. (B) MDS-TBRmax of the index vessel. Pre-Tx – before renal transplantation; Post-Tx – after renal transplantation; TBR – target-to-background ratio.
Table 2 shows the absolute and percent changes in TBRs. These tendencies were observed for reductions in right WH-TBRmax, MDS-TBRmax, and MDS-TBRmean (% reduction [95% CI]): −6.50% [−12.81, 1.54]; −4.06% [−13.42, 9.38]; and −4.47% [−13.64, 7.29], respectively. Tendencies were also observed for reductions in left WH-TBRmax, WH-TBRmean, MDS-TBRmax, and MDS-TBRmean (% reduction [95% CI]) by −6.13% [−15.17, 8.32]; −8.37% [−17.62, 6.56]; −6.14% [−14.53, 7.01]; and −7.29% [−17.91, 4.18], respectively. The right WH-TBRmean was significantly reduced from baseline (% reduction [95% CI] by −5.74% [−15.37, −0.02], p=0.047). In the index vessel, the WH-TBRmax and MDS-TBRmax tended to decrease from baseline (% reduction [95% CI]) by −3.36 [−14.11, 8.88] and −5.87 [−13.83, 6.80], respectively.
Table 2.
Change in TBR in the WH and MDS.
| Baseline, median (IQR) | Post-transplantation, median (IQR) | % change from baseline, median (95% CI) | P | |
|---|---|---|---|---|
| WH | ||||
| Right TBRmax | 1.54 (1.33, 1.72) | 1.41 (1.36, 1.51) | −6.50 (−12.81, 1.54) | 0.09 |
| Right TBRmean | 1.33 (1.22, 1.47) | 1.22 (1.18, 1.32) | −5.74 (−15.37, −0.02) | 0.047 |
| Left TBRmax | 1.59 (1.48, 1.72) | 1.49 (1.37, 1.80) | −6.13 (−15.17, 8.32) | 0.20 |
| Left TBRmean | 1.38 (1.31, 1.53) | 1.25 (1.18, 1.54) | −8.37 (−17.62, 6.56) | 0.22 |
| MDS | ||||
| Right TBRmax | 1.50 (1.33, 1.81) | 1.46 (1.35, 1.60) | −4.06 (−13.42, 9.38) | 0.33 |
| Right TBRmean | 1.35 (1.20, 1.50) | 1.30 (1.16, 1.35) | −4.47 (−13.64, 7.29) | 0.24 |
| Left TBRmax | 1.70 (1.64, 1.84) | 1.65 (1.50, 1.75) | −6.14 (−14.53, 7.01) | 0.14 |
| Left TBRmean | 1.54 (1.38, 1.61) | 1.36 (1.25, 1.48) | −7.29 (−17.91, 4.18) | 0.09 |
| Index vessel | ||||
| WH-TBRmax | 1.67 (1.55, 1.76) | 1.62 (1.44, 1.80) | −3.36 (−14.11, 8.88) | 0.24 |
| MDS-TBRmax | 1.75 (1.61, 1.84) | 1.67 (1.50, 1.75) | −5.87 (−13.83, 6.80) | 0.09 |
IQR – interquartile range; CI – confidence interval; WH – whole carotid artery; TBRmax – maximum target-to-background ratio; TBRmean – mean target-to-background ratio; MDS – most-diseased segment.
Change in eGFR and concentrations of CRP, lipids, and cytokines
Table 3 shows the changes in eGFR, laboratory results, and cytokine levels. The eGFR and total cholesterol and HDL-C concentrations increased significantly from baseline. The TNF-α and endothelin-1 concentrations did not change after renal transplantation, but the IL-6 and PAI-1 concentrations increased significantly after renal transplantation. The changes in cytokine concentrations were analyzed further according the administration of rituximab. TNF-α concentration did not change significantly in rituximab-treated patients but decreased significantly in rituximab-untreated patients. IL-6 concentration increased significantly in rituximab-treated patients but did not change significantly in rituximab-untreated patients. PAI-1 concentration increased significantly in both rituximab-treated and untreated patients. Endothelin-1 concentration did not change significantly in rituximab-treated or untreated patients.
Table 3.
Change in eGFR, CRP, lipids, and cytokine levels.
| Baseline, median (IQR) | Post-transplantation, median (IQR) | P | |
|---|---|---|---|
| eGFR, ml/min/1.73 m2 | 5.38 (4.29, 6.75) | 71.97 (55.08, 78.04) | 0.005 |
| CRP, mg/L | 0.42 (0.34, 0.50) | 0.55 (0.37, 1.32) | 0.24 |
| TC, mg/dL | 167.0 (146.0, 239.0) | 237.0 (217.25, 293.0) | 0.02 |
| Triglyceride, mg/dL | 132.50 (95.25, 220.50) | 162.0 (129.0, 204.75) | 0.72 |
| HDL-cholesterol, mg/dL | 36.50 (27.0, 53.50) | 55.0 (41.50, 75.50) | 0.047 |
| LDL-cholesterol, mg/dL | 100.0 (88.50, 167.0) | 142.50 (133.75, 168.25) | 0.074 |
| TNF-α, pg/mL | 14.95 (13.87, 18.25) | 13.42 (11.62, 16.38) | 0.45 |
| Rituximab-untreated group (n=5) | 15.13 (13.60, 18.19) | 12.07 (11.16, 13.87) | 0.04 |
| Rituximab-treated group (n=5) | 14.76 (13.62, 18.55) | 15.41 (12.86, 25.00) | 0.50 |
| IL-6, pg/mL | 3.50 (2.67, 6.04) | 15.49 (4.12, 320.09) | 0.03 |
| Rituximab-untreated group (n=5) | 3.60 (2.88, 6.18) | 5.55 (3.50, 143.01) | 0.50 |
| Rituximab-treated group (n=5) | 3.16 (2.63, 4.95) | 23.33 (5.94, 655.10) | 0.04 |
| PAI-1, ng/mL | 1.25 (0.94, 1.47) | 3.57 (2.59, 4.56) | 0.005 |
| Rituximab-untreated group (n=5) | 1.20 (0.91, 1.41) | 4.13 (2.44, 4.65) | 0.04 |
| Rituximab-treated group (n=5) | 1.29 (0.68, 1.72) | 3.10 (2.43, 4.43) | 0.04 |
| Endothelin-1, pg/mL | 297.25 (253.35, 395.23) | 334.85 (280.88, 423.25) | 0.51 |
| Rituximab-untreated group (n=5) | 302.20 (214.90, 377.30) | 371.30 (257.85, 441.60) | 0.23 |
| Rituximab-treated group (n=5) | 292.30 (271.35, 410.05) | 311.40 (259.55, 411.10) | 0.69 |
IQR – interquartile range; eGFR – estimated glomerular filtration rate; CRP – C-reactive protein; TC – total cholesterol; HDL-C – high-density lipoprotein-cholesterol; LDL-C – low-density lipoprotein-cholesterol; TNF-α – tumor necrosis factor-alpha; IL-6 – interleukin-6; PAI-I – plasminogen activator inhibitor-1.
Relationships between carotid arterial inflammatory activity and other variables
The relationships between the TBR of the index vessel and other variables were analyzed. In the index vessel, no significant correlations were observed between MDS-TBRmax and the eGFR (rho=−0.32, p=0.16) or with the concentrations of TC (rho=−0.07, p=0.76), TG (rho=−0.18, p=0.45), LDL-C (rho=−0.05, p=0.85), HDL-C (rho=−0.11, p=0.64), CRP (rho=−0.03, p=0.92), TNF-α (rho=−0.07, p=0.78), PAI-1 (rho=−0.31, p=0.18), or endothelin-1 (rho=−0.15, p=0.52). A significant correlation was observed between MDS-TBRmax and IL-6 concentration (rho=−0.46, p=0.044). The WH-TBRmax of the index vessel did not correlate significantly with the eGFR (rho=−0.20, p=0.41) or with the concentrations of TC (rho=−0.02, p=0.95), TG (rho=−0.12, p=0.62), LDL-C (rho=−0.16, p=0.50), HDL-C (rho=−0.04, p=0.86), CRP (rho=0.27, p=0.24), IL-6 (rho=−0.22, p=0.35), TNF-α (rho=0.15, p=0.53), PAI-1 (rho=−0.24, p=0.30), or endothelin-1 (rho=0.16, p=0.51).
Discussion
The results of the present study showed that the 18F-FDG uptake by the carotid arteries of CKD patients decreased after renal transplantation. Seven or 8 of the 10 patients showed a reduction in 18F-FDG uptake by the WH or MDS. The WH-TBRmean of the right carotid artery was significantly reduced from baseline. The MDS-TBRmax, MDS-TBRmean, and WH-TBRmax of both carotid arteries and the WH-TBRmean of the left carotid artery showed a tendency for a reduction from baseline. These findings suggest that renal transplantation may confer an anti-inflammatory effect on carotid atherosclerosis in CKD patients.
Only a few studies have used imaging techniques to examine the change in vascular disease after renal transplantation in CKD patients [24]. Carotid IMT is a surrogate marker for atherosclerosis [25] and is associated with inflammation in CKD patients [26,27]. One study found that carotid IMT improved after renal transplantation [11]. However, it was unclear whether the inflammatory activity in the carotid artery was reduced after renal transplantation. 18F-FDG PET/CT is approved as an imaging technique for the assessment of atherosclerotic plaque inflammation [16,17]. Arterial 18F-FDG uptake is associated with atherosclerosis progression [28] and independently predicts future CVD [18]. Studies have reported the use of 18F-FDG PET/CT to monitor inflammation in the vessel wall during certain pharmacological and nonpharmacological interventions [21,22,29,30].
It has recently been shown that CKD patients without overt atherosclerotic disease have increased arterial 18F-FDG uptake [19]. In this study, we included CKD patients without overt atherosclerotic disease and only those who had not received statin treatment within the previous 4 months because statins can attenuate atherosclerotic inflammation [21]. Previous studies using 18F-FDG PET/CT have evaluated the changes in WH-TBRmax and MDS-TBRmax in the index vessel [21,22,29,30]. The MDS represents the site of the most severe inflammation in the vessel, whereas the WH is a mixture of more or less diseased segments; therefore, the treatment effects may be larger in the MDS than in the WH [21]. Although not statistically significant, the percent change was larger in the MDS-TBRmax than in the WH-TBRmax in the index vessel. We also calculated the TBRmax and TBRmean of the WH and MDS in the right and left carotid arteries because our aim was to assess the various aspects of changes in carotid atherosclerosis. Although not all patients showed a reduction in carotid 18F-FDG uptake, 70–80% of patients showed a reduction. A trend for a reduced 18F-FDG uptake was observed, and the right WH-TBRmean was significantly reduced from baseline.
The literature shows that endothelial function improves within 1 month after transplantation [12,13]. However, we did not observe a marked reduction in the arterial inflammatory activity. The reason for this discrepancy is unclear but may be because our patients were receiving various immunosuppressive agents. It has been reported that rituximab improves endothelial function and reduces inflammation in patients with rheumatoid arthritis [31]. Mycophenolate mofetil was shown to decrease atherosclerotic lesion size in an animal study [32] and to attenuate plaque inflammation in patients with carotid artery stenosis [33]. By contrast, tacrolimus is associated with vascular inflammation and endothelial dysfunction in animal models [34,35] and in endothelial and vascular smooth muscle cells [36]. These drugs may interfere with each other’s effects on the process of atherosclerosis. Additionally, the vascular response may be affected by several confounders such as diabetes mellitus or the use of antidiabetic or antihypertensive drugs.
We measured the concentrations of proinflammatory, prothrombotic, and vasoconstrictive cytokines to determine whether these cytokine changes correlate with the changes in arterial 18F-FDG uptake. IL-6 and PAI-1 concentrations increased after renal transplantation, whereas TNF-α and endothelin-1 concentrations did not change significantly. Rituximab can increase the production of the proinflammatory cytokines TNF-α and IL-6 [37–39], and the cytokine levels changed differently according to the administration of rituximab. The decrease in TNF-α concentration in rituximab-untreated patients was noteworthy. PAI-1 is a prothrombotic cytokine, and its concentration increased in both rituximab-treated and untreated patients. Renal transplantation does not reverse coagulopathy in CKD patients [40]. Endothelin-1, a vasoconstrictive cytokine, also did not change significantly in either rituximab-treated or untreated patients. Our results differ from those of a previous report that showed a decrease in endothelin-1 concentration at 3 months after transplantation [15]. However, it has also been reported that endothelin-1 concentration does not correlate with the IMT in peripheral arteries in CKD patients [41]. These findings may suggest that changes in these cytokine concentrations do not correlate with the early changes in arterial 18F-FDG uptake. Probably the time window for detection was too soon that cytokines levels are unlikely to stabilize four months after transplantation
Our study has several limitations. First, the number of patients was small and not all patients showed a consistent pattern of change in arterial 18F-FDG uptake. Second, the follow-up 18F-FDG PET/CT was taken at a relatively short interval after transplantation because previous studies reported that endothelial function improves within 1 month after transplantation [12,13], and because previous interventional studies using 18F-FDG PET/CT to monitor arterial inflammation have taken follow-up 18F-FDG PET/CT within 4 months from baseline [21,29]. Our results did not show the long-term effects of renal transplantation on carotid atherosclerotic inflammation. Third, an effect of immunosuppressive agents on carotid inflammation cannot be excluded.
Conclusions
Serial 18F-FDG-PET/CT of the carotid arteries showed that the 18F-FDG uptake by the WH and MDS was reduced in the early post-transplantation period. Renal transplantation may confer an anti-inflammatory effect on carotid atherosclerosis in CKD patients by improving renal function, and18F-FDG PET/CT can be an assessment tool for atherosclerosis. These findings need further investigation to determine the long-term effects of renal transplantation on atherosclerosis.
Footnotes
Source of support: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2014R1A1A3A04050919)
Conflict of interest
None.
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–95. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Pozzoni P, Tentori F, del Vecchio L. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant. 2003;18(Suppl 7):vii2–9. doi: 10.1093/ndt/gfg1072. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Elsayed EF, et al. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–24. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Hunsicker LG. A survival advantage for renal transplantation. N Engl J Med. 1999;341:1762–63. doi: 10.1056/NEJM199912023412310. [DOI] [PubMed] [Google Scholar]
- 6.van Dijk PC, Jager KJ, de Charro F, et al. Renal replacement therapy in Europe: The results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant. 2001;16:1120–29. doi: 10.1093/ndt/16.6.1120. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 8.Benedetto FA, Tripepi G, Mallamaci F, Zoccali C. Rate of atherosclerotic plaque formation predicts cardiovascular events in ESRD. J Am Soc Nephrol. 2008;19:757–63. doi: 10.1681/ASN.2007070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zebrack JS, Anderson JL, Beddhu S, et al. Do associations with C-reactive protein and extent of coronary artery disease account for the increased cardiovascular risk of renal insufficiency? J Am Coll Cardiol. 2003;42:57–63. doi: 10.1016/s0735-1097(03)00564-3. [DOI] [PubMed] [Google Scholar]
- 10.Molnar MZ, Naser MS, Rhee CM, et al. Bone and mineral disorders after kidney transplantation: Therapeutic strategies. Transplant Rev (Orlando) 2014;28:56–62. doi: 10.1016/j.trre.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz MI, Sonmez A, Saglam M, et al. A longitudinal study of inflammation, CKD-mineral bone disorder, and carotid atherosclerosis after renal transplantation. Clin J Am Soc Nephrol. 2015;10:471–79. doi: 10.2215/CJN.07860814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocak H, Ceken K, Yavuz A, et al. Effect of renal transplantation on endothelial function in haemodialysis patients. Nephrol Dial Transplant. 2006;21:203–7. doi: 10.1093/ndt/gfi119. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz MI, Saglam M, Caglar K, et al. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation. 2005;80:1660–66. doi: 10.1097/01.tp.0000183750.22675.be. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz MI, Sonmez A, Saglam M, et al. Longitudinal analysis of vascular function and biomarkers of metabolic bone disorders before and after renal transplantation. Am J Nephrol. 2013;37:126–34. doi: 10.1159/000346711. [DOI] [PubMed] [Google Scholar]
- 15.Ignace S, Utescu MS, De Serres SA, et al. Age-related and blood pressure-independent reduction in aortic stiffness after kidney transplantation. J Hypertens. 2011;29:130–36. doi: 10.1097/HJH.0b013e32833f5e68. [DOI] [PubMed] [Google Scholar]
- 16.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 17.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–24. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 18.Figueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–59. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Bernelot Moens SJ, Verweij SL, van der Valk FM, et al. Arterial and cellular inflammation in patients with CKD. J Am Soc Nephrol. 2017;28:1278–85. doi: 10.1681/ASN.2016030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudd JH, Myers KS, Bansilal S, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: Implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–96. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–17. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 22.Fayad ZA, Mani V, Woodward M, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): A randomised clinical trial. Lancet. 2011;378:1547–59. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 25.Tardif JC, Heinonen T, Orloff D, Libby P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation. 2006;113:2936–42. doi: 10.1161/CIRCULATIONAHA.105.598987. [DOI] [PubMed] [Google Scholar]
- 26.Lemos MM, Jancikic AD, Sanches FM, et al. Intima-media thickness is associated with inflammation and traditional cardiovascular risk factors in non-dialysis-dependent patients with chronic kidney disease. Nephron Clin Pract. 2010;115:c189–94. doi: 10.1159/000313033. [DOI] [PubMed] [Google Scholar]
- 27.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease – a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216:446–51. doi: 10.1016/j.atherosclerosis.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Abdelbaky A, Corsini E, Figueroa AL, et al. Focal arterial inflammation precedes subsequent calcification in the same location: A longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6:747–54. doi: 10.1161/CIRCIMAGING.113.000382. [DOI] [PubMed] [Google Scholar]
- 29.Tawakol A, Singh P, Rudd JH, et al. Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. J Am Coll Cardiol. 2014;63:86–88. doi: 10.1016/j.jacc.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 30.van Wijk DF, Sjouke B, Figueroa A, et al. Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J Am Coll Cardiol. 2014;64:1418–26. doi: 10.1016/j.jacc.2014.01.088. [DOI] [PubMed] [Google Scholar]
- 31.Hsue PY, Scherzer R, Grunfeld C, et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc. 2014;3:e001267. doi: 10.1161/JAHA.114.001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Vietinghoff S, Koltsova EK, Mestas J, et al. Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation. J Am Coll Cardiol. 2011;57:2194–204. doi: 10.1016/j.jacc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Leuven SI, van Wijk DF, Volger OL, et al. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis. 2010;211:231–36. doi: 10.1016/j.atherosclerosis.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 34.Shing CM, Fassett RG, Brown L, Coombes JS. The effects of immunosuppressants on vascular function, systemic oxidative stress and inflammation in rats. Transpl Int. 2012;25:337–46. doi: 10.1111/j.1432-2277.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 35.Takeda Y, Miyamori I, Furukawa K, et al. Mechanisms of FK 506-induced hypertension in the rat. Hypertension. 1999;33:130–36. doi: 10.1161/01.hyp.33.1.130. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues-Diez R, Gonzalez-Guerrero C, Ocana-Salceda C, et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep. 2016;6:27915. doi: 10.1038/srep27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones JD, Hamilton BJ, Skopelja S, Rigby WF. Induction of interleukin-6 production by rituximab in human B cells. Arthritis Rheumatol. 2014;66:2938–46. doi: 10.1002/art.38798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal A, Vieira CA, Book BK, et al. Rituximab, anti-CD20, induces in vivo cytokine release but does not impair ex vivo T-cell responses. Am J Transplant. 2004;4:1357–60. doi: 10.1111/j.1600-6143.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 39.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–64. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 40.Ballow A, Gader AM, Huraib S, et al. Successful kidney transplantation does not reverse the coagulopathy in patients with chronic renal failure on either hemo or peritoneal dialysis. Saudi J Kidney Dis Transpl. 2007;18:177–85. [PubMed] [Google Scholar]
- 41.Nezami N, Sepehrvand N, Mirchi M, et al. Serum and tissue endothelin-1 are independent from intima-media thickness of peripheral arteries in patients with chronic kidney disease. Vascular. 2015;23:382–90. doi: 10.1177/1708538114551195. [DOI] [PubMed] [Google Scholar]