Abstract
Background
Successful graft regeneration is important in living-donor liver transplantation (LDLT) because partial liver grafts are used. Early allograft dysfunction (EAD) is an intermediate outcome that affects the long-term postoperative course in liver transplantation. The aim of the present study was to investigate liver graft regeneration under EAD development in LDLT.
Material/Methods
The data of 226 patients who underwent LDLT from September 2010 to July 2014 were retrospectively analyzed. The patients were classified into 2 groups: one with and one without EAD. Graft regeneration, functional recovery, and long-term patient survival were compared between the 2 groups.
Results
The grafts grew more vigorously in the EAD group than in the non-EAD group, as evidenced by the larger absolute (ALV) and relative liver volumes (RLV) of the former on postoperative days (POD) 7 and 21. The median (interquartile range) RLVs of the non-EAD group versus the EAD group were as follows: 55.2 (47.9–65.8) vs. 53.7 (46.6–64.5)% preoperatively, p>0.05; 76.1 (66.9–85.7) vs. 86.7 (73.9–96.8)% on POD 7, p<0.01; 79.6 (69.3–91.2) vs. 93.7 (79.6–101.6)%, p<0.01 on POD 21. In the early postoperative period, hepatic function, measured as total bilirubin and international normalized ratio, was higher in the EAD group; however, after EAD development, graft function recovered in these patients. In the follow-up period, overall patient survival was comparable between the 2 groups.
Conclusions
The liver grafts of EAD patients steadily regenerated, such that the development of EAD did not affect long-term patient survival after LDLT.
MeSH Keywords: Liver Regeneration, Liver Transplantation, Living Donors, Primary Graft Dysfunction
Background
Successful graft regeneration is important in living-donor liver transplantation (LDLT) because partial liver grafts are used. The partial liver graft is required to meet the metabolic demands of the recipient; various factors, including graft volume, graft quality, portal hemodynamics, and immunology, affect the degree of postoperative graft regeneration in LDLT [1]. Insufficient graft mass is associated with graft dysfunction known as ‘small-for-size syndrome’ and leads to poor graft or recipient survival in LDLT [2].
Early allograft dysfunction (EAD) results from various factors, including a low graft-to-recipient weight ratio (GRWR), poor preoperative recipient condition, intraoperative graft injury, and poor donor-graft characteristics in liver transplantation (LT) [3–5]. In particular, previous studies have suggested that inadequate graft size was associated with EAD development because of the disparity between liver cell growth and function in LT [2,6,7]. However, postoperative graft regeneration under EAD development after LDLT has yet to be examined.
In the present study, we investigated postoperative graft regeneration between patients with and without EAD. Graft function recovery after EAD development was also evaluated and the survival of patients with and without EAD after LDLT was compared.
Material and Methods
Study population
In total, 257 adult patients (age ≥19 years) underwent LDLT for end-stage liver disease (ESLD) from September 2010 to July 2014 at St. Mary’s Hospital (Seoul, South Korea). Perioperative data of the recipients and donors were reviewed retrospectively in the hospital electronic medical records system after LDLT. Incomplete or missing data of the recipients and donors were not included in the analyses. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital Ethics Committee (KC17RISI0001). Informed consent was waived due to the retrospective study design.
Surgery and intraoperative management
Details of the surgical technique and anesthetic management in the recipients undergoing LDLT were previously described [8]. In summary, LDLT was performed according to the piggyback technique using the right hepatic lobe of the donor with middle hepatic vein reconstruction. The portal vein, hepatic artery, and bile duct were anastomosed consecutively, and hepatic vessel patency was evaluated by Doppler ultrasonography. The liver allograft was preserved using a histidine-tryptophan-ketoglutarate solution (Custodiol; Dr. Franz Köhler Chemie GmbH, Bensheim, Germany). Gross findings of the liver graft were examined and liver graft structures were adjusted by experienced transplant surgeons on a back table in the operating room. Balanced anesthesia was applied during the surgery. Strong vasopressors, such as epinephrine or norepinephrine, were administered if hemodynamic instability was not corrected using adequate fluid resuscitation under invasive hemodynamic monitoring. Diuretics were administered when oliguria continued despite supplementing the deficient circulatory volume. Sodium bicarbonate was infused if an arterial pH <7.1 or absolute value of base excess ≥10 was present.
Immunosuppression
The immunosuppression regimen consisted of 3 drugs, including a calcineurin inhibitor (tacrolimus or cyclosporine), mycophenolate mofetil (MMF), and steroid (prednisolone), in accordance with our hospital’s LDLT protocol [9]. The trough level of tacrolimus was maintained within 7 and 10 ng/mL for the first month after LDLT, and within 5 and 7 ng/mL thereafter. The trough level of cyclosporine was sustained within 100 and 150 ng/mL for the first month after LDLT, and within 50 and 100 ng/mL thereafter. Steroid was gradually tapered within the first month after LDLT. MMF was also tapered between 3 and 6 months after LDLT. An IL-2 receptor blocker, such as basiliximab, was administered on the day of LDLT before the surgery and on the fourth day after LDLT [10].
Definition of early allograft dysfunction
EAD was indicated by the presence of 1 of the following after LDLT without surgical complications: serum total bilirubin level ≥10 mg/dL and international normalized ratio (INR) ≥1.6 on postoperative day (POD) 7, and an alanine (ALT) or aspartate (AST) aminotransferase level >2,000 U/L within the first POD 7 [5]. According to the EAD definition, the study population was classified into 2 groups: non-EAD vs. EAD.
Liver volume measurement
Abdominal computed tomography (CT) images of the donors and recipients were used to measure liver graft regeneration. The total liver volume and right lobe volume of the donors were measured preoperatively using volumetric CT scans. Postoperative volumetric CT scans of recipients were used to determine the liver graft volume at PODs 7 and 21. Volumetry software (AW VolumeShare 4; General Electric Healthcare, Chicago, IL, USA) was used by expert radiologists to calculate the absolute liver volume (ALV). The ratio of the estimated graft volume to the standard liver volume (SLV) was used to calculate the relative liver volume (RLV) (SLV=1072.8×body surface area [BSA]–345.7; BSA=weight [kg]·0425×height [cm]·0725×0.007184) [11].
Perioperative recipient and donor-graft findings
Preoperative variables of the recipients included age, sex, body mass index (BMI), etiology of LDLT, comorbidity, history of abdominal surgery, hemodialysis, emergency operation, model for end-stage liver disease (MELD) score, complications of hepatic decompensation, and transthoracic echocardiography findings. The laboratory parameters included hematocrit, sodium, C-reactive protein (CRP), total bilirubin, INR, creatinine, AST, ALT, albumin, ammonia, and glucose.
Intraoperative variables of the recipients included duration of surgery, continuous renal replacement therapy (CRRT), strong vasopressor (epinephrine or norepinephrine) use, incidence of severe post-reperfusion syndrome (PRS), indicated by severe hemodynamic instability, fatal arrhythmia, requirement for strong vasopressors, and prolonged or recurrent fibrinolysis [12], average values of vital signs during the whole surgery, blood product transfusions, hourly fluid infusions and urine output, and doses of the drugs administered. The average values of the levels of lactate, brain natriuretic peptide (BNP), and glucose, all measured at the pre-anhepatic, anhepatic and neo-hepatic phases, are presented.
Donor-graft factors included age, sex, BMI, steatosis percentage and type, and total ischemic time. Hepatic vascular hemodynamics, including hepatic artery resistive index (HARI) and portal venous flow (PVF), were measured on PODs 1, 3, and 5, and these values were averaged.
During postoperative graft function recovery, serum levels of total bilirubin, INR, AST, and ALT were measured on PODs 1, 7, 14, 21, and 28. Recipient survival was evaluated on the last outpatient visit during the follow-up period.
Statistical analysis
Continuous data are expressed as median (interquartile range, IQR) and compared using the Mann-Whitney U test. The normality of the continuous data was determined using the Shapiro-Wilk test. Categorical data are presented as number (proportion) and were evaluated using the χ2 test or Fisher’s exact test, as appropriate. Postoperative changes in liver graft volumes and liver function markers (total bilirubin, INR, AST, and ALT) were evaluated by repeated-measures ANOVA (RM-ANOVA) with the Bonferroni post hoc test. The associations between perioperative recipient and donor-graft factors and EAD development were evaluated by forward and backward univariate logistic regression analyses. Potentially significant factors (p≤0.1) in univariate analyses were included in multivariate logistic regression analyses. The values are expressed as the odds ratio [95% confidence interval (95% CI)]. Correlations of the perioperative changes in RLV with the postoperative changes in markers of hepatic function were examined using the Spearman correlation method. Recipient survival was evaluated in the non-EAD and EAD groups using the Kaplan-Meier method and the results compared to the log-rank test. All tests were two-sided and a p-value <0.05 was considered significant. Statistical analyses were performed using SPSS (ver. 19.0 for Windows; SPSS Inc., Chicago, IL, USA) and MEDCALC (ver. 11.0 for Windows; MedCalc Software, Mariakerke, Belgium).
Results
In total, 31 patients were excluded because of deficient or incorrect data, including liver graft volumes preoperatively (n=6), or on PODs 7 (n=5) and 21 (n=4), vital sign parameters (n=8), and laboratory variables (n=8). Eventually, data for 226 patients were analyzed, and EAD development occurred in 28 (12.4%) patients. The study population was predominantly male (70.4%), the average age was 52.7±8.3 years, and the average BMI was 24.5±3.8 kg/m2. The average follow-up period was 3.7±1.7 years after LDLT. The most frequent etiology of LDLT was hepatitis B virus (61.1%), followed by alcohol abuse (19.5%), drug- or toxin-related hepatitis (6.6%), hepatitis C virus (5.3%), autoimmune hepatitis (2.2%), and cryptogenic findings (5.3%). The mean MELD score was 17±9 points, and 164 patients (72.6%) suffered from ESLD-related complications before the surgery.
In the preoperative findings of the recipients (Table 1), patients with EAD underwent emergency LDLT more frequently and had a higher incidence of hepatorenal syndrome than those without EAD. CRP levels were higher in the EAD group than in the non-EAD group. Among the intraoperative findings, the frequency of CRRT, incidence of severe postreperfusion syndrome, total transfusion of fresh frozen plasma (FFP), and average BNP level were higher in the EAD group than in the non-EAD group. Donor-graft factors, including donor age, steatosis, and total ischemic time, did not differ between the 2 groups, nor did average postoperative hepatic vascular flows (HARI and PVF). The HARI values of each group were within the reference range (0.55–0.70) [13].
Table 1.
Comparison of demographic recipient and donor-graft findings between the non-EAD and EAD groups in living donor liver transplantation.
| n=226 | Non-EAD group | EAD group | p |
|---|---|---|---|
| 198 | 28 | ||
| Preoperative recipient finding | |||
| Age (years) | 53 (48–59) | 54 (49–57) | 0.92 |
| Gender (male) | 143 (72.2%) | 16 (57.1%) | 0.10 |
| Body mass index (kg/m2) | 24.2 (22.1–26.1) | 24.1 (21.6–27.1) | 0.88 |
| Etiology | 0.43 | ||
| Alcohol | 39 (19.7%) | 5 (17.9%) | |
| Hepatitis B | 119 (60.1%) | 19 (67.9%) | |
| Hepatitis C | 12 (6.1%) | 0 (0.0%) | |
| Autoimmune | 4 (2.0%) | 1 (3.6%) | |
| Drug & toxin | 12 (6.1%) | 3 (10.7%) | |
| Cryptogenic findings | 12 (6.1%) | 0 (0.0%) | |
| Emergency operation | 29 (14.6%) | 13 (46.4%) | <0.001 |
| MELD score (points) | 15 (9–23) | 15 (9–29) | 0.68 |
| Complications of hepatic decompensation | |||
| Severe encephalopathy* | 11 (5.6%) | 3 (10.7%) | 0.39 |
| Varix | 51 (25.8%) | 6 (21.4%) | 0.61 |
| Hepatorenal syndrome | 15 (7.6%) | 6 (21.4%) | 0.03 |
| Ascites (>1L) | 82 (41.4%) | 11 (39.3%) | 0.83 |
| Laboratory value | |||
| Hematocrit (%) | 29.4 (25.3–35.3) | 31.7 (24.0–36.2) | 0.85 |
| Sodium (mEq/L) | 139.0 (135.0–142.0) | 139.5 (137.3–142.8) | 0.27 |
| C-reactive protein (mg/dL) | 0.3 (0.1–0.9) | 1.7 (0.2–2.5) | <0.01 |
| Platelet count (x109/L) | 63.0 (46.0–112.0) | 64.0 (38.0–97.3) | 0.35 |
| Intraoperative recipient finding | |||
| Total operation time (min) | 510 (459–570) | 518 (460–603) | 0.43 |
| Continuous renal replacement therapy | 9 (4.5%) | 5 (17.9%) | 0.02 |
| Severe postreperfusion syndrome | 37 (18.7%) | 10 (35.7%) | 0.04 |
| Blood products transfusion (unit) | |||
| Packed red blood cell | 7 (3–11) | 68 (6–11) | 0.22 |
| Fresh frozen plasma | 7 (4–10) | 10 (5–15) | 0.04 |
| Platelet concentrate | 0 (0–6) | 3 (0–9) | 0.47 |
| Cryoprecipitate | 0 (0–0) | 0 (0–0) | 0.82 |
| Hourly fluid administration (mL/kg/h) | 9.8 (7.5–12.6) | 10.3 (7.1–13.0) | 0.69 |
| Hourly urine output (mL/kg/h) | 1.4 (0.7–2.0) | 1.3 (0.7–2.3) | 0.98 |
| Mean lactate (mmol/L) | 4.0 (3.2–5.4) | 4.6 (3.6–5.4) | 0.40 |
| Mean brain natriuretic peptide (pg/mL) | 145 (107–154) | 219 (151–341) | 0.02 |
| Donor-graft finding | |||
| Age (years) | 32 (24–42) | 37 (29–47) | 0.07 |
| Gender (male) | 117 (59.1%) | 12 (42.9%) | 0.15 |
| Body mass index (kg/m2) | 23.0 (21.1–25.5) | 22.9 (20.3–25.0) | 0.38 |
| Steatosis percentage (%) | 3.0 (0.0–5.0) | 5.0 (0.0–5.0) | 0.58 |
| Steatosis type | 0.25 | ||
| None | 51 (25.8%) | 10 (35.7%) | |
| Microvesicular | 6 (3.0%) | 2 (7.1%) | |
| Macrovesicular | 120 (60.6%) | 15 (53.6%) | |
| Mixed | 21 (10.6%) | 1 (3.6%) | |
| Total ischemic time (min) | 102 (72–130) | 81 (66–118 | 0.19 |
| Average of hepatic circulation postoperative days 1, 3 and 5 | |||
| Hepatic artery resistive index | 0.7 (0.6–0.7) | 0.6 (0.6–0.7) | 0.44 |
| Portal venous flow (mL/min) | 2360 (1635–2764) | 2440 (1389–3482) | 0.94 |
EAD – early allograft dysfunction; MELD – model for end-stage liver disease
Severe encephalopathy: West-Haven criteria III or IV. Values are expressed as numbers (portions) and median (interquartile).
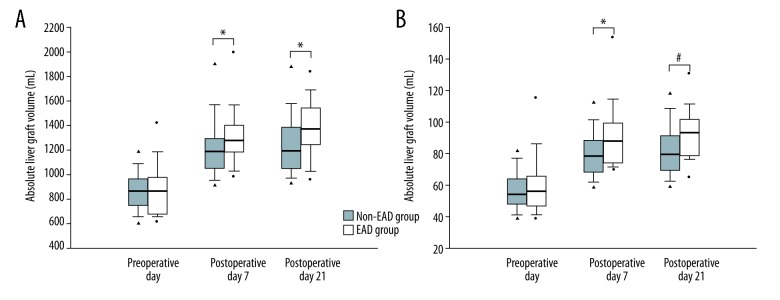
Although the absolute and relative graft volumes at the time of transplantation were comparable between the non-EAD and EAD patients, the graft volumes on PODs 7 and 21 were significantly greater in the former group (Figure 1). The median (IQR) of the ALVs of the non-EAD and EAD groups was as follows: 836.1 (736.3–975.2) vs. 866.8 (668.2–995.0) mL preoperatively, p>0.05; 1,170.0 (1,021.4–1,298.9) vs. 1,313.8 (1,179.5–1,471.0) mL on POD 7, p<0.01; and 1,185.4 (1,042.2–1,392.9) vs. 1,442.0 (1,160.4–1,580.0) mL on POD 21, p<0.01. The median (IQR) standard liver volumes of the non-EAD group and EAD group were 1,541.5 (1,395.5–1,644.4) and 1,501.6 (1,348.8–1,674.3) mL, respectively. The median (IQR) RLVs of the non-EAD and EAD groups were as follows: 55.2 (47.9–65.8) vs. 53.7 (46.6–64.5)% preoperatively, p>0.05; 76.1 (66.9–85.7) vs. 86.7 (73.9–96.8)% on POD 7, p<0.01; and 79.6 (69.3–91.2) vs. 93.7 (79.6–101.6)% on POD 21, p<0.01.
Figure 1.
Comparison of postoperative (A) absolute and (B) relative liver graft volumes of the non-EAD and EAD groups after living-donor liver transplantation. The box plots show the median (line in the middle of the box), interquartile range (box), 5th and 95th percentiles (whiskers), and outliers (dots). * p≤0.01, # p≤0.001.
In assessment of the association between perioperative factors and EAD development (Table 2), univariate analyses showed that preoperative recipient factors (heart disease, emergency operation, hepatorenal syndrome, and CRP level), intraoperative recipient factors (CRRT, severe postreperfusion syndrome, mean heart rate, FFP transfusion, and mean BNP level), and donor-graft factors (RLV on POD 7 and change in the RLV between the preoperative value and POD 7) were potentially related to EAD development. In multivariate analyses, emergency operation, preoperative CRP level, and intraoperative average BNP level were identified as independent factors favoring EAD (AUC 0.785; 95% CI [0.676–0.894]; p≤0.01). In the predictive model of EAD development, the RLV on POD 7, and the changes in RLV between the preoperative value and POD 7 were not independently involved.
Table 2.
Association of perioperative recipient and donor-graft factors with early allograft dysfunction using univariate and multivariate logistic regression analyses.
| Univariate logistic regression | Multivariate logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|
| β | Odd ratio | 95% CI | p | β | Odd ratio | 95% CI | p | |
| Preoperative recipient factor | ||||||||
| MELD score (points) | 0.02 | 1.02 | 0.97–1.06 | 0.45 | ||||
| Heart disease | 1.61 | 5.00 | 0.80–31.34 | 0.09 | ||||
| Emergency operation | 1.62 | 5.05 | 2.18–11.71 | <0.001 | 1.56 | 4.76 | 1.72–13.21 | <0.01 |
| Hepatorenal syndrome | 1.24 | 3.47 | 1.21–9.90 | 0.02 | ||||
| C-reactive protein (mg/dL) | 0.30 | 1.35 | 1.12–1.63 | <0.01 | 0.23 | 1.26 | 1.04–1.53 | 0.02 |
| Intraoperative recipient factor | ||||||||
| CRRT | 1.51 | 4.54 | 1.40–14.7 | 0.01 | ||||
| Severe postreperfusion syndrome | 0.88 | 2.42 | 1.03–5.67 | 0.04 | ||||
| Mean heart rate (beats/min) | 0.03 | 1.03 | 1.00–1.06 | 0.04 | ||||
| Fresh frozen plasma (unit) | 0.07 | 1.07 | 1.01–1.13 | 0.02 | ||||
| Mean brain natriuretic peptide (pg/mL) | 0.01 | 1.01 | 1.00–1.01 | <0.01 | 0.01 | 1.01 | 1.00–1.01 | <0.001 |
| Donor-graft factor | ||||||||
| Relative liver volume (RLV)* | ||||||||
| On preoperative day | 0.01 | 1.01 | 0.99–1.04 | 0.28 | ||||
| On POD 7 | 0.02 | 1.02 | 1.00–1.04 | 0.02 | ||||
| The RLV change between preoperative day and POD 7 | 0.02 | 1.02 | 0.99–1.04 | 0.09 | ||||
MELD – model for end-stage liver disease; CRRT – continuous renal replacement therapy; POD – postoperative day.
Relative liver volume: graft volume to standard liver volume ratio (%).
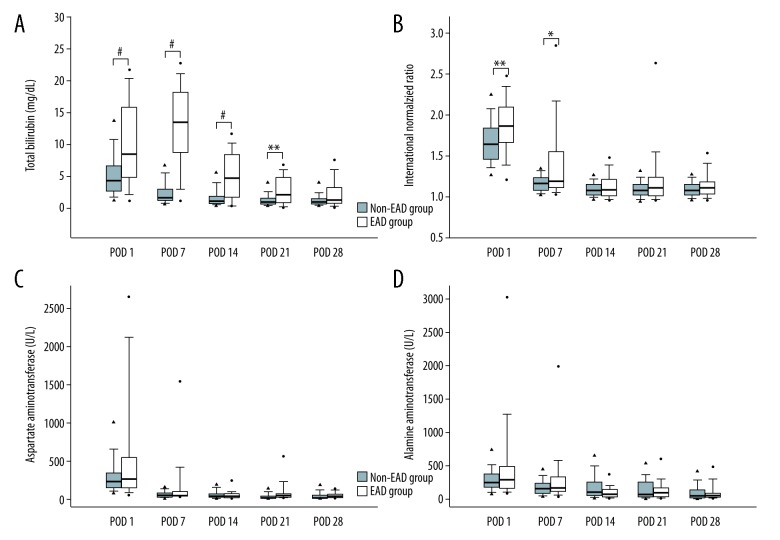
As shown in Figure 2, serial changes in liver function markers, including total bilirubin, INR, AST, and ALT, were compared between the groups after LDLT. The postoperative levels of total bilirubin were higher in the EAD group than in the non-EAD group between PODs 1 and 21, but the patients with EAD showed distinct recovery of cholestasis from POD 7 to 28. The levels of INR on PODs 1 and 7 were higher in the EAD group than in the non-EAD group but did not differ significantly thereafter. The AST and ALT levels of the 2 groups were comparable.
Figure 2.
Comparison of hepatic functional markers, including (A) total bilirubin, (B) international normalized ratio, (C) aspartate aminotransferase, and (D) alanine aminotransferase in the non-EAD and EAD groups on postoperative days (POD) 1, 7, 14, 21, and 28 after living-donor liver transplantation. The box plots show the median (line in the middle of the box), interquartile range (box), 5th and 95th percentiles (whiskers), and outliers (dots). * p<0.05, ** p≤0.01, # p≤0.001.
As shown in Figure 3, there were no significant differences in overall patient survival between patients with and without EAD development after LDLT.
Figure 3.
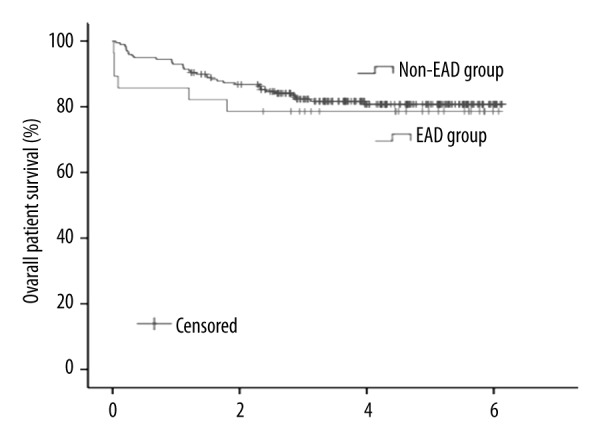
Comparison of overall patient survival after living-donor liver transplantation between the non-EAD and EAD groups. The 1-, 3-, and 5-year survival rates were 93%, 82%, and 81% in the non-EAD group, and 86%, 79%, and 79% in the EAD group, respectively (p=0.605).
In analyses of the correlation between the postoperative changes in RLV and hepatic functional markers (Table 3), the decrease in the total bilirubin level of the non-EAD group from POD 1 to POD 21 correlated with the increase in the RLV between the preoperative value and POD 21. In the whole study cohort, and in patients without EAD, the decreases in the INR level from POD 1 to POD 7 correlated with the increases in the RLV between the preoperative value and POD 7. In the whole study cohort and in patients with and without EAD, the decrease in INR level between POD 1 and POD 21 was correlated with increase in the RLV between the preoperative value and POD 21. Particularly, in EAD patients, there was a moderate correlation between change in the RLV and change in the INR level during the first 3 weeks after LDLT (correlation coefficient: −0.475; p<0.05).
Table 3.
Correlation between changes of relative liver graft volume and hepatic functional markers after living donor liver transplantation.
| Changes of hepatic functional markers between PODs 1 and 7 | ||||
|---|---|---|---|---|
| Relative liver graft volume change# | Total bilirubin | INR | AST | ALT |
| In whole study cohort | −0.01 | −0.25*** | 0.03 | 0.03 |
| In the non EAD group | −0.10 | −0.24*** | 0.02 | 0.03 |
| In the EAD group | −0.02 | −0.27 | 0.01 | 0.05 |
| Changes of hepatic functional markers between PODs 1 and 21 | ||||
| Relative liver graft volume change## | Total bilirubin | INR | AST | ALT |
| In whole study cohort | −0.13 | −0.24*** | 0.06 | 0.03 |
| In the non EAD group | −0.19** | −0.21** | −0.01 | −0.01 |
| In the EAD group | 0.30 | −0.48* | 0.15 | 0.11 |
POD – postoperative day; INR – international normalized ratio; AST – aspartate aminotransferase; ALT – alanine aminotransferase; EAD – early allograft dysfunction.
Changes of relative liver graft volume between preoperative day and postoperative day 7;
Changes of relative liver graft volume between preoperative day and postoperative day 21;
p<0.05,
p≤0.01,
p≤0.001.
Discussion
The main finding of this study was that liver graft regeneration ability was maintained despite EAD development after LDLT. In the early postoperative period, hepatic function, as measured using total bilirubin and INR levels, was higher in the EAD group than in the non-EAD group. However, graft function in patients with EAD recovered within 1 month after surgery. In the follow-up period, overall patient survival was comparable between patients with and without EAD after LDLT.
A previous study by Olthoff et al. [5] reported that the overall incidence of EAD after LT ranged from 21% to 25%, and that EAD development was significantly associated with the 6-month mortality of both the grafts and the patients. Other studies have suggested that EAD development was closely related to 1-year graft loss (retransplantation and primary non-function) [4], and 1-, 3-, and 5-year patient and graft survival rates were worse in the patients with EAD than in those without EAD in the LT setting [3]. Risk factors for EAD development include recipient MELD score, donor age, surgery duration, transfusion requirement, and graft steatosis [3,5]. Insufficient graft mass compared to recipient body size (GRWR <0.8% or RLV <40%) at the time of transplantation is related to a poor clinical outcome [14]. However, rapid graft regeneration in a patient with portal hyperperfusion is associated with a poor outcome for both grafts and patients [15]. In many reports, graft edema probably contributed to the observed increase in graft volume within the first 2 weeks after LT [16]. In the present study, the absolute and relative graft volumes of the 2 groups at the time of the surgery were comparable, although the grafts of EAD patients more vigorously regenerated than those of patients without EAD, as assessed during the first 3 weeks after LDLT. As the increase in graft size was determined over a sufficient period of time, an overestimate because of graft edema was unlikely. In addition, because the hepatic blood flow (HARI and PVF) supplying grafts with and without EAD is similar, effects of portal hyperperfusion on increasing graft sizes could essentially be ruled out.
Our results suggest that emergency operation, preoperative CRP level, and the intraoperative average BNP level are independently associated with EAD development. Previous reports showed that these factors affected postoperative outcomes. In one study, patient and graft survival rates were worse in emergency versus non-emergency LT [17], and in another high study, serum CRP levels were closely related to poor overall survival in recipients with hepatocellular carcinoma after LT [18]. In addition, a high serum BNP level was shown to predict cirrhotic cardiomyopathy and was associated with 1-year patient mortality [19,20]. However, there was no significant relationship between the increase in graft volume during the first week after surgery and EAD development in this study.
Previous experimental studies have suggested that hepatocytes maintain their function during liver regeneration [21]. After partial hepatectomy, gluconeogenesis in remnant liver cells is induced as an adaptive response of the liver to prevent hypoglycemia via liver-specific transcription factors, including CCAAT-enhancer-binding protein [22,23]. Other transcription factors, including signal transducer and activator of transcription 3 and hepatic nuclear factor-1, are upregulated by liver injury and play important roles in maintaining homeostasis during liver repair [24]. In LDLT, because partial grafts are transplanted, the graft regenerating capacity during the early postoperative period is important to overcome hepatic decompensation in the recipient [2]. A previous study by Kawasaki et al. [25] reported that patients with small grafts did not suffer from postoperative graft failure and exhibited good survival rate 3 years after LDLT, because of vigorous allograft growth. In the present study, increased graft volume after surgery occurred in parallel with decreases in the levels of graft function markers (total bilirubin, INR, AST, and ALT). The correlation between the increase in graft size and the decreased levels of graft function markers, particularly total bilirubin and INR, was statistically significant. In patients with EAD, the correlation between graft volume and changes in the INR level on POD 21 was moderate. As for the overall patient survival rate, there were no differences between patients with and without EAD after LDLT. We speculate that the steady regenerative ability of the grafts under conditions of EAD is related to the subsequent recovery of hepatic function. This finding may be of interest in the development of measures to improve long-term survival in patients with EAD after LDLT.
Our study had several limitations. First, the exclusion of a large number of patients in the non-EAD group during the long-term follow-up may have compromised the accuracy of the reported survival rates. Second, in the EAD group, we could not assess the differences between patients who died early and those who survived. Further study is required to elucidate the differences in graft regeneration and recovery among recipients who develop EAD. Third, the factors associated with graft regeneration in EAD patients could not be investigated. Previous studies have indicated that many inflammatory mediators, including interleukin-6 and tumor necrosis factor-α, were related to liver regeneration [26–30]. Further research is required to establish the factors that improve graft regeneration under EAD in LDLT and the results may contribute to improving the currently poor survival among recipients with EAD. Finally, because only right lobe LDLT was performed in our hospital, we could not investigate the differences in graft regeneration according to type of graft (right lobe graft, left lobe graft, and left lateral segment graft) during the development of EAD. The types of liver grafts differ not only in graft volume but also in anatomical properties, and these differences may affect graft regeneration and functional recovery under conditions of EAD in LDLT.
Conclusions
Liver grafts of patients with EAD after LDLT steadily regenerate and achieve functional recovery within 1 month after surgery. However, further studies are needed to identify the factors involved in graft regeneration and EAD development in LDLT. Nonetheless, EAD development on POD 7 was an intermediate and modifiable outcome in terms of long-term patient survival.
Abbreviations
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- EAD
early allograft dysfunction
- INR
international normalized ratio
- LDLT
living-donor liver transplantation
- MELD
model for end-stage liver disease
- MMF
mycophenolate mofetil
- POD
postoperative day
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Olthoff KM, Emond JC, Shearon TH, et al. Liver regeneration after living donor transplantation: Adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015;21:79–88. doi: 10.1002/lt.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiuchi T, Tanaka K, Ito T, et al. Small-for-size graft in living donor liver transplantation: How far should we go? Liver Transpl. 2003;9:S29–35. doi: 10.1053/jlts.2003.50198. [DOI] [PubMed] [Google Scholar]
- 3.Lee DD, Croome KP, Shalev JA, et al. Early allograft dysfunction after liver transplantation: An intermediate outcome measure for targeted improvements. Ann Hepatol. 2016;15:53–60. doi: 10.5604/16652681.1184212. [DOI] [PubMed] [Google Scholar]
- 4.Salvalaggio PR, Felga GE, Afonso RC, Ferraz-Neto BH. Early allograft dysfunction and liver transplant outcomes: A single center retrospective study. Transplant Proc. 2012;44:2449–51. doi: 10.1016/j.transproceed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–49. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 6.Nishizaki T, Ikegami T, Hiroshige S, et al. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575–80. doi: 10.1097/00000658-200104000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaton N. Small-for-size liver syndrome after auxiliary and split liver transplantation: Donor selection. Liver Transpl. 2003;9:S26–28. doi: 10.1053/jlts.2003.50197. [DOI] [PubMed] [Google Scholar]
- 8.Chae MS, Koo JM, Park CS. Predictive role of intraoperative serum brain natriuretic peptide for early allograft dysfunction in living donor liver transplantation. Ann Transplant. 2016;21:538–49. doi: 10.12659/aot.899255. [DOI] [PubMed] [Google Scholar]
- 9.Choi HJ, Kim DG, Na GH, et al. The clinical outcomes of patients with portal vein tumor thrombi after living donor liver transplantation. Liver Transpl. 2017;23:1023–31. doi: 10.1002/lt.24782. [DOI] [PubMed] [Google Scholar]
- 10.Na GH, Hong TH, You YK, Kim DG. Clinical analysis of patients with hepatocellular carcinoma recurrence after living-donor liver transplantation. World J Gastroenterol. 2016;22:5790–99. doi: 10.3748/wjg.v22.i25.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinemann A, Wischhusen F, Puschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366–68. doi: 10.1002/lt.500050516. [DOI] [PubMed] [Google Scholar]
- 12.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14:504–8. doi: 10.1002/lt.21381. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Noguera A, Montserrat E, Torrubia S, Villalba J. Doppler in hepatic cirrhosis and chronic hepatitis. Semin Ultrasound CT MR. 2002;23:19–36. doi: 10.1016/s0887-2171(02)90027-2. [DOI] [PubMed] [Google Scholar]
- 14.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–27. doi: 10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 15.Yagi S, Iida T, Taniguchi K, et al. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005;11:68–75. doi: 10.1002/lt.20317. [DOI] [PubMed] [Google Scholar]
- 16.Bekheit M, Rajakannu M, Bucur P, et al. Serial volumetric assessment of large for size liver grafts after whole cadaveric liver transplant in adults: do large liver grafts shrink in size? HPB (Oxford) 2016;18:200–6. doi: 10.1016/j.hpb.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman RB, Jr, Steffick DE, Guidinger MK, et al. Liver and intestine transplantation in the United States, 1997–2006. Am J Transplant. 2008;8:958–76. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 18.An HJ, Jang JW, Bae SH, et al. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2012;18:1406–14. doi: 10.1002/lt.23512. [DOI] [PubMed] [Google Scholar]
- 19.Kim YK, Shin WJ, Song JG, et al. Evaluation of intraoperative brain natriuretic peptide as a predictor of 1-year mortality after liver transplantation. Transplant Proc. 2011;43:1684–90. doi: 10.1016/j.transproceed.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Saner FH, Neumann T, Canbay A, et al. High brain-natriuretic peptide level predicts cirrhotic cardiomyopathy in liver transplant patients. Transpl Int. 2011;24:425–32. doi: 10.1111/j.1432-2277.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- 21.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 22.Diehl AM, Yang SQ. Regenerative changes in C/EBP alpha and C/EBP beta expression modulate binding to the C/EBP site in the c-fos promoter. Hepatology. 1994;19:447–56. [PubMed] [Google Scholar]
- 23.Greenbaum LE, Cressman DE, Haber BA, Taub R. Coexistence of C/EBP alpha, beta, growth-induced proteins and DNA synthesis in hepatocytes during liver regeneration. Implications for maintenance of the differentiated state during liver growth. J Clin Invest. 1995;96:1351–65. doi: 10.1172/JCI118170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leu JI, Crissey MA, Leu JP, et al. Interleukin-6-induced STAT3 and AP-1 amplify hepatocyte nuclear factor 1-mediated transactivation of hepatic genes, an adaptive response to liver injury. Mol Cell Biol. 2001;21:414–24. doi: 10.1128/MCB.21.2.414-424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawasaki S, Makuuchi M, Matsunami H, et al. Living related liver transplantation in adults. Ann Surg. 1998;227:269–74. doi: 10.1097/00000658-199802000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: Deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–46. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47:309–12. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 29.Pachowka M, Zegarska J, Ciecierski R, Korczak-Kowalska G. The role of IL-6 during the late phase of liver regeneration. Ann Transplant. 2008;13:15–19. [PubMed] [Google Scholar]
- 30.Diehl AM, Yin M, Fleckenstein J, et al. Tumor necrosis factor-alpha induces c-jun during the regenerative response to liver injury. Am J Physiol. 1994;267:G552–61. doi: 10.1152/ajpgi.1994.267.4.G552. [DOI] [PubMed] [Google Scholar]