Abstract
Background
The persisting organ shortage in the field of transplantation recommends the use of marginal kidneys which poorly tolerate ischemic damage. Adenosine triphosphate (ATP) depletion during cold ischemia time (CIT) is considered crucial for graft function. We tested different strategies of kidney perfusion before transplantation in the attempt to improve the technique.
Material/Methods
Twenty human discarded kidneys from donors after brain death and with at least 20 hours of CIT were randomized to the following experimental groups (treatment time three-hours at 4°C): a) static cold storage (CS); b) static cold hyperbaric oxygenation (Hyp); c) hypothermic perfusion (PE); d) hypothermic perfusion in hyperbaric oxygenation (PE-Hyp); and e) hypothermic oxygenated perfusion (PE-O2).
Results
Histological results showed that perfusion with or without oxygen did not produce any endothelial damage. A depletion of ATP content following the preservation procedure was observed in CS, PE, and Hyp, while PE-Hyp and PE-O2 were associated with a net increase of ATP content with respect to baseline level. In addition, PE-Hyp was associated with a significant downregulation of endothelial isoform of nitric oxide synthase (eNOS) gene expression and of hypoxia inducible factor-1α (HIF-1α).
Conclusions
Hyperbaric or normobaric oxygenation with perfusion improves organ metabolic preservation compared to other methods. This approach may prevent the onset of delayed graft function, but clinical trials are needed to confirm this.
MeSH Keywords: Adenosine Triphosphate, Delayed Graft Function, Organ Preservation, Organs at Risk, Pulsatile Flow
Background
One of the major challenges in the field of transplantation is the shortage of donor organs. Many patients waiting for transplant die during the waiting time or wait many years for kidney transplantation, with detrimental effects on their quality of life and increasing morbidity and related costs [1–4]. Effective strategies, which safely extend the donor pool, are therefore advocated.
During the last 20 years, the two main policies attempting to achieve this purpose were living donation and expanded criteria donors [5–8]. These donors are presumed to provide a worse outcome than conventional donors and many research protocols have been developed to reduce preservation injury (PI) and PI-related complications [9]. Static cold storage (SCS) has been the standard technique in clinical practice for liver and kidney preservation using specific solutions (Belzer UW, Custodiol, Celsior) able to prevent cellular swelling [10,11].
Hypothermic machine perfusion (HMP) of the kidney and the liver in the preimplantation phase has been widely explored and shown that high tissue adenosine triphosphate (ATP) levels at reperfusion time and the continuous perfusion of the microcirculation improve graft dysfunction and survival [9,12–18]. Oxygen during perfusion seems to add further improvements to marginal grafts, including donation after circulatory death [19–22].
Furthermore, organ preservation by normothermic machine perfusion (NMP) has proven to be superior to SCS in animal and clinical studies [23–27]. NMP mimics the physiological environment by maintaining a normal temperature and providing substrates for cellular metabolism, oxygen and nutrition [23,26–27].
Another possible strategy for reducing PI could be treatment with hyperbaric oxygen that has been shown to improve lipid peroxidation and mitochondrial oxidative phosphorylation capacity in rat livers [28,29] and protects against the ischemia-reperfusion injury in rat kidneys [30].
The present study was designed in order to assess the impact of normobaric and hyperbaric oxygenation combined with hypothermia and pulsatile perfusion on the structure and metabolic activity of discarded human kidneys.
Material and Methods
Our research group developed an innovative machine able to perfuse organs at hypothermic temperature (4°C), also providing oxygen to the graft in normobaric or hyperbaric condition.
We report the experimental results of 20 kidneys discarded for kidney transplantation due to clinical reasons and with at least 20 hours of static cold ischemia time (CIT) which underwent different organ preservation strategies.
Study design
From May 2014 to June 2015, kidneys from donors after brain death not suitable for transplantation and offered for research after informed consent from the relatives were randomized in the following experimental groups: a) Static cold storage (CS); b) Static cold hyperbaric oxygenation (Hyp); c) Hypothermic perfusion (PE); d) Hypothermic perfusion in hyperbaric oxygenation (PE-Hyp); and e) Hypothermic oxygenated perfusion (PE-O2).
Ethical approval was granted by the Ethics Committee of University of Bologna Sant’Orsola- Malpighi Hospital and the experimental phases reported were conducted in accordance with institutional guidelines. Organ retrieval was performed by our surgical team according to the current protocols of the National Transplant Center. All kidneys initially were stored in Celsior (Waters Medical Systems of Institute Georges Lopez) at 4°C until organ preparation following standard back-table procedures. For kidneys of group c, d, and e, the renal artery was dissected and cannulated by means of an 8 Fr, 10 Fr, or 12 Fr vascular cannula (Medtronic-UK), based on renal artery size. Each treatment was accomplished under hypothermic conditions (4°C) for three hours and using 1 L of Celsior.
Machine perfusion
The perfusion device was developed by Medica S.P.A and Centro Iperbarico S.R.L. under the scientific management of the author MR. It provides pulsatile perfusion through three peristaltic pumps, each driven by an individual motor with the dual purpose of perfusing the organ with the preservation solution and, at the same time, maintaining constant liquid circulation around the organ within the hyperbaric chamber. The first pump, used only in hyperbaric treatments, breaks the gas-liquid interface facilitating the diffusion of the compressed gaseous mixture into the preservation solution. The second and third peristaltic pumps are used to perfuse the organ through suction and discharge pipes connected to the organ’s blood vessels.
The organ is connected through the cannulated vascular access with sterile tubing (PVC and silicon) to the machine. Flow and pressure values during perfusion are assessed by means of specific sensors, auto-regulated and displayed on the device’s screen in real time. To achieve oxygenation of the organs during perfusion, it is possible to use a membrane oxygenator integrated in the perfusion circuit that provides oxygen to the preservation fluid (normobaric oxygenation) or a pressurizable chamber where the graft is placed during the treatment (hyperbaric oxygenation).
Normobaric oxygenation
The oxygenator is a component of the tubing system that provides oxygen to the organ preservation fluid. It is a microporous hollow fiber membrane oxygenator consisting of a gas exchange module with an integrated heat exchanger. The device, supplied as single-use and ethylene oxide sterile, is intended to be used in an extracorporeal perfusion circuit to oxygenate and remove carbon dioxide from the fluid during routine perfusion procedures for up to six hours in duration.
Hyperbaric oxygenation
The device consists of a pressurizable (“hyperbaric”) chamber designed to accommodate the organ fully immersed in the preservation solution. The chamber is compressed with a mixture of medical oxygen (95%) and carbon dioxide (5%) until a higher pressure than the atmospheric one is obtained. The minimum partial pressure of oxygen (pO2) was inversely proportional to the normalized flow (mL/min/g of the organ); the device allows pressurization up to 200 kilopascals by a certified medical gas cylinder. An appropriate reduction gear, certified for use with medical oxygen, reduces the gas pressure. The mixture is dissolved in the preservation solution in a concentration directly proportional to the pressure exerted by the gas on the gas-liquid interface (Henry’s law). The hyperbaric chamber is placed inside a thermal conditioning apparatus, which can be powered from 12 to up to 220 volts. The device has the ability to maintain the hypothermic (or, where appropriate, hyper-thermic) condition within the range of –5°C/40°C (23°F/104°F), for all the time necessary. To guarantee effective organ preservation during transport, there is a retro-actuated control of the refrigerator temperature and of the storage chamber for the organ.
Kidney perfusion and oxygenation parameters
The renal perfusion rate was set in order to reach an arterial pressure of 25–30 mmHg. To provide an adequate oxygen supply with pO2 level of the preservation solution close to 750 mmHg, the oxygenation setting was between 4 L and 2 L of O2 in normobaric condition and at 1.5 atm in hyperbaric condition. All the parameters were chosen based on preliminary experiments performed during machine tuning.
Histological analysis
Kidney biopsies were obtained prior to (T0) and after treatment (T1). All core-needle biopsies were fixed in formalin and embedded in a paraffin block by standard procedures (see supplemental file). Sections underwent histochemical staining (Hematoxylin-Eosin, Trichrome stain, Periodic-acid Schiff) and examination to reveal any tissue change possibly related to preservation damage.
In T0 and T1 biopsies, all the components of kidney parenchyma were histologically evaluated for the purposes of the study: glomeruli: signs of glomerular ischemia, occurrence and diffusion of glomerular sclerosis; interstitium: occurrence of fibrosis and/or inflammatory infiltrate; tubules: occurrence of tubulocyte atrophy, tubulocyte vacuolization, luminal cylinders, acute tubular necrosis; and arteries: occurrence of myointimal thickening, detachment of endothelial cells and other signs of arterial/arteriolar damage.
Immunohistochemistry
Immunohistochemistry (IHC) for CD34 (mouse monoclonal antibody directed against human CD34, clone QBEnd/10, Roche Diagnostics) and CD31 (mouse monoclonal antibody directed against human CD31, clone JC70, Roche Diagnostics) was automatically performed with BenchMark XT® Immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA) following the manufacturer’s instructions. IHC was carried out on formalin-fixed paraffin embedded 2-μm-thick sections. Slides were first dewaxed in xylol (30 minutes) and rehydrated through grade washes of ethanol: 100% (five minutes), 95% (three minutes) and 70% (one minute). Nuclei were counterstained with Gills hematoxylin (Sigma Chemicals). Negative controls were obtained by omitting the primary antibody.
Semi-quantitative immunohistochemistry
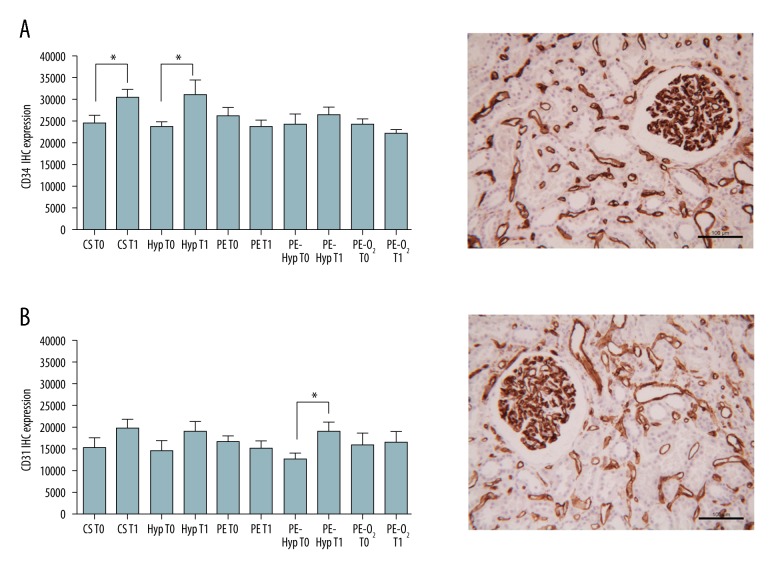
Immunohistochemical images were acquired using a Leitz Diaplan light microscope (Wetzlar, Germany) connected to a JVC 3CCD video camera (KY-F55B, Yokohama, Japan). Semi-quantitative analysis was performed with Image-Pro Plus® 6 software (Media Cybernetics, Silver Spring, MD, USA) using digital images taken at 10× of magnification. For each experimental condition, the positive area intensely stained with endothelial cell markers (CD31, CD34) was quantified; at least three randomly selected tissue areas corresponding to an average area of 303622.56 μm2 were recorded; to make the selection comparable we decided to select images that exclusively contained a single glomerulus (as seen in Figure 1, right column); the values were scored separately.
Figure 1.
Representative immunohistochemical images (right column) and semi-quantitative analysis (left column) of kidney tissue expressing CD34 (A) and CD31 (B) endothelial cell markers. T0 and T1 values are reported separately. (A, B) Scale bars=100 μm. (A) * p-value=0.0187 for CS group, * p-value=0.0466 for Hyp. (B) *p-value=0.0109 for Hyp-PE group; unpaired Student’s t-test.
Transmission electron microscopy
Small tissue fragments were fixed in Karnowsky fixative (2% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer) and processed for transmission electron microscopy (TEM) analysis.
Each specimen was rinsed in phosphate buffer, post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer for one hour at room temperature, dehydrated with graded ethanol (from 30% to 100%) and embedded in Araldite resin. The ultrathin sections were counterstained with uranyl acetate and lead citrate before examination in a Philips CM10 (FEI Company, Milan, Italy) transmission electron microscope equipped with a Gatan camera; digital images were captured using the FEI proprietary software Olympus SIS Megaview SSD digital camera.
Assessment of metabolic parameters
The pH, lactate concentration, oxygen (pO2), and carbon dioxide (pCO2) partial pressure were assessed in the perfusate collected from the organ reservoir at T0 and T1 by means of hemo gas analyzer (Gem Premier 3500, Instrumentation Laboratory-Werfen S.P.A., Barcelona, Spain).
Tissue ATP level determination
Tissue samples were collected and immediately frozen in liquid nitrogen at T0 and T1. After protein extraction, ATP content was assessed through an ATP determination kit (Cat. N A22066, Thermo Fisher, Waltham, MA, USA).
Total proteins were extracted from each tissue sample using a buffer containing 150 mM NaCl, 20 mM Na2HPO4/NaH2PO4, 10% glycerol, 1% Triton X-100, 100 mM PMSF, 100 mM DTT, and a mix of protease inhibitors (Roche; Basel, Swiss). Total protein concentration was determined by the Lowry method; then each sample was diluted to a final concentration of 1 μg/μL. Then 5 μg of total protein were used for the determination of the tissue ATP content on a Glomax 20/20 single tube luminometer (Promega, Fitchburg, WI, USA) following the manufacturer’s protocol. For each sample, the absolute ATP concentration was obtained by interpolating the luminescence value on a standard curve, then the ATP level was expressed as the ratio of T1 over T0 (Figure 3).
Figure 3.
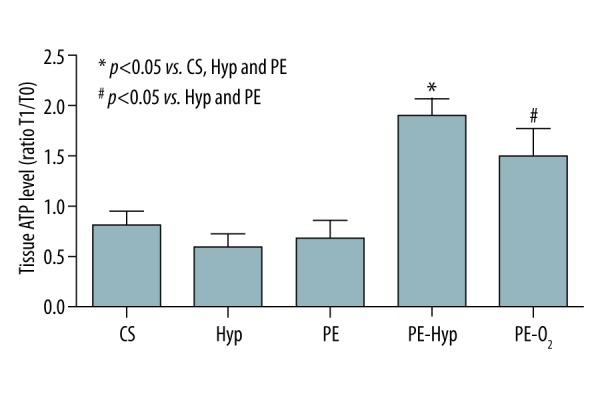
Tissue adenosine triphosphate (ATP) level. Results are expressed as the ratio between ATP content at T1 and T0. The results of post-hoc analysis are indicated by asterisk.
Gene expression analysis
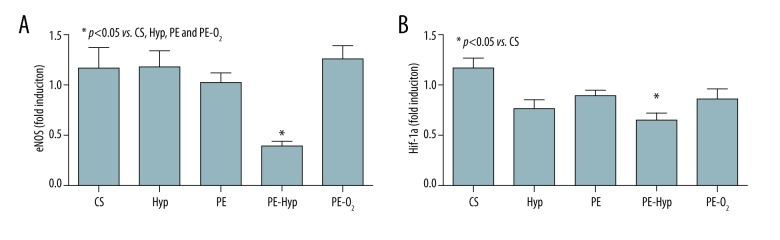
Total RNA was extracted from each tissue sample, collected as aforementioned, following the Trizol RNA isolation protocol and reverse transcribed by using a SuperScript Vilo Master Mix (Invitrogen; Waltham, MA, USA). The cDNA was amplified through a semi-quantitative real-time qPCR on an iCycler (Biorad, Hercules, CA, USA) using SYBR GreenER qPCR SuperMix (Invitrogen, Waltham, MA, USA). The primers used were: hypoxia inducible factor 1 alpha (HIF-1α) (FW AACATAAAGTCTGCAACATGGAAG; RV TTTGATGGGTGAGGAATGGG), endothelial nitric oxide synthase (eNOS) (FW GTGGCTGGTACATGAGCACT; RV GTCTTTCCACAGGGACGAGG), beta actin (FW CCTGGACTT CGAGCAAGAGATG; RV GGAAGGAAGGCTGGAAGAGTG) and beta-2microglobulin (FW CTTTCTGGCCTGGAGGCTATC; RV CTTTCTGGCCTGGAGGCTATC). Data were analyzed according to the 2−ΔΔct method in which the expression of each gene at T0 was used as the reference value. Gene expression level was therefore reported as fold induction of T1 over T0 (Figure 4A, 4B).
Figure 4.
Gene expression of endothelial nitric oxide synthase (eNOS) (A) and hypoxia inducible factor 1α (HIF-1α) (B). Data presented are fold induction of sample at T1 over T0. The results of post-hoc analysis are indicated by asterisk.
Data analysis
Data are expressed as the ratio between values measured at T0 and T1 or absolute value. For continuous variables, data are presented as mean and SD. For all variables, the Kolmogorov-Smirnov test was used to test the normality of the distribution while the homogeneity of variance was evaluated through the Levene test, then differences between groups were evaluated by one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test for intergroup comparisons. The correlation between ATP level and pCO2 was performed following Pearson’s method. Differences between sample categories following IHC semi-quantification were determined using an unpaired Student’s t-test. All tests used were two-sided and p values less than 0.05 were considered statistically significant. SPSS version 20.0 and GraphPad Prism 5.0 software were used for statistical analysis.
Results
Features of the discarded kidneys
The kidneys were discarded for various reasons: high Remuzzi score [31], donor age, or too long CIT. The mean donor age was 70±4 years while the minimum CIT before the start of the planned treatment was 20 hours. Data were comparable among all experimental groups (Table 1).
Table 1.
Features of the discharged kidney enrolled in the 5 study groups.
| CS (4) | Hyp (4) | PE (4) | PE-Hyp (4) | PE-O2 (4) | p | |
|---|---|---|---|---|---|---|
| Mean donor age | 74±6 | 74±6 | 69±5 | 69±5 | 70±4 | n.s. |
| Mean cold ischemia time | 22.3±2.2 | 22.3±2.2 | 26.8±3.3 | 22.8±1.6 | 26.5±1.4 | n.s. |
| Mean Remuzzi score | 5 | 5 | 6 | 5.5 | 4.5 | n.s. |
| Mean pO2 at T0 | 67±4 | 65±7 | 75±5 | 72±3 | 68±7 | n.s. |
| Mean pO2 at T1 | 176±48 | 282±29* | 106±37 | 366±83* | 710±39* | <0.001* |
| Mean pCO2 at T0 | 7±2.5 | 6.3±2.5 | 6.5±4.4 | 5.3±1.3 | 4.3±0.5 | n.s. |
| Mean pCO2 at T1 | 8.3±3.3 | 12±3.5* | 7±2.5 | 40±19.6* | 4.8±1 | <0.001* |
| Mean lactic acid at T0 | 0.3±0.2 | 0.2±0 | 4.3±5.9 | 0.7±0.8 | 2.2±0.6 | n.s. |
| Mean lactic acid at T1 | 0.5±0.4 | 0.3±0.2 | 12.7±9.9* | 2.1±2.6 | 9.5±5.5* | <0.001* |
| Mean pH at T0 | 6.99±0.05 | 7.03±0.04 | 6.96±0.08 | 6.98±0.01 | 7.04±0.03 | n.s. |
| Mean pH at T1 | 6.96±0.07 | 6.85±0.02 | 6.85±0.08 | 6.80±0.01 | 7±0.21 | n.s. |
CS – static cold storage; Hyp – static cold hyperbaric oxygenation with 1.5 atm; PE – hypothermic perfusion with arterial pressure of 25–30 mmHg; PE-Hyp – hypothermic perfusion in hyperbaric oxygenation with arterial pressure of 25–30 mmHg and with 1.5 atm; PE-O2 – hypothermic oxygenated perfusion with arterial pressure of 25–30 mmHg and with 2–4 L of O2 level maintaining pO2 close to 700 mmHg; T0 – before starting the treatment; T1 – at the end of three hours of treatment. All preservation treatments were performed for three hours at 4°C with 1 L Celsior solution.
Histological analysis and immunohistochemistry
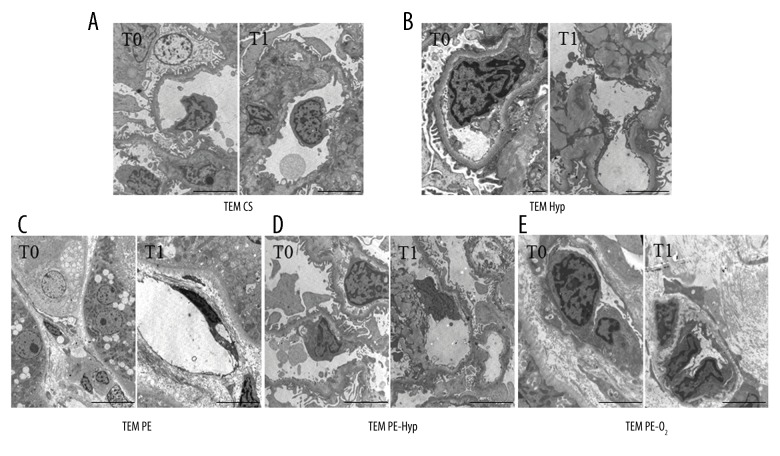
Each experimental group included organs (minimum one and maximum three) at T0 with pathological evidences of glomerular sclerosis, tubular injury, mild interstitial fibrosis, interstitial chronic fibrosis, or arterial myointimal thickening. After T1, endothelial damage appeared in one or two cases for all groups without any correlation to perfusion or oxygen. An increased expression of CD31 and CD34, indicating a well-preserved tissue, was observed in the CS, Hyp, and PE-Hyp groups; instead, expression was not significant for PE and PE-O2 (Figure 1, left column). TEM showed endothelial injury only for the PE-group (Figure 2C) respect to the CS, Hyp, PE-Hyp and PE-O2 groups (Figure 2A, 2B, 2D, 2E, respectively).
Figure 2.
Representative ultrastructural images of endothelial cells seen in glomerular loop and interstitium. (A) Scale bars=5 μm; (B) T0 scale bar=2 μm, T1 scale bar=5 μm; (C) T0 scale bar=5 μm, T1 scale bar=5 μm; (D, E) scale bars=5 μm.
Metabolic evaluation of the preservation solution
As expected, pO2 was significantly higher in the Hyp, PE-Hyp, and PE-O2 groups compared to the CS and PE groups. At T1, the lactate concentration was significantly higher in all perfusion groups except for PE-Hyp. The pCO2 was significantly higher in the Hyp and PE-Hyp groups with respect to all the other groups. Of note, pCO2 levels were not evaluated in the PE-O2 group since the oxygenator was equipped with a CO2 scavenger. Data expressed as mean and SD are reported in Table 1.
Tissue ATP content
The amount of ATP content for each preservation group is shown in Figure 3 and presented as the ratio of T1 over T0 samples. In the CS, Hyp, and PE groups a net depletion of ATP content following the preservation procedure was observed. On the contrary PE-Hyp as well as PE-O2 were associated with a net increase of ATP content with respect to baseline levels. As a result, at the end of the preservation the amount of ATP was significantly higher in the PE-Hyp group with respect to the CS, Hyp, and PE groups, while ATP level in PE-O2 was significantly increased only with respect to the PE and Hyp groups. Additionally, grouping all samples with the exception of PE-O2 for the CO2 of the oxygenator device scavenger showed a significant correlation between ATP content and pCO2 (Pearson correlation 0.759, p=0.001).
Gene expression of eNOS and HIF1α
As shown in Figure 4A, the mRNA level of eNOS was reduced in the PE-Hyp group, while in the other groups, including PE-O2, the mRNA level was not decreased. As a result, PE-Hyp was associated with a significant downregulation of eNOS gene expression with respect to CS, PE, Hyp, and PE-O2 groups. The gene expression of HIF-1α was also evaluated. All preservation modalities were associated with a slight reduction in the expression of HIF1α with respect to CS, reaching statistical significance only in the PE-Hyp group (Figure 4B).
Expression of genes involved in the inflammatory response (interleukin-6) and cellular apoptosis (caspase-3) were also evaluated; however, no significant differences between groups were seen when the gene expression levels in T1 samples were compared to that of T0 samples (data not shown).
Discussion
The present experimental study indicated that three hours of hypothermic perfusion with hyperbaric or normobaric oxygenation restored ATP levels, a signal of organ functional activity. These improvements were possible even with extended criteria grafts declared not suitable for transplantation and after a prolonged CIT (more than 20 hours). An increased ATP content during organ preservation may lead to better outcomes and reduced risks of organ dysfunction after transplantation [32]. Furthermore, the reduced gene expression of both eNOS and HIF1α in the PE-Hyp group may suggest optimal delivery of both oxygen and nutritional support to the graft.
Metabolic evaluations showed that the high pO2 level detected at T1 in the Hyp, PE-O2, and PE-Hyp groups was related to a higher level of pCO2 for Hyp and, mostly, to the PE-Hyp group showing an increased metabolic activity of the graft. Therefore, the different pCO2 level between the Hyp and PE-Hyp groups suggests that only hyperbaric oxygenation without perfusion may lead renal cells to not efficiently exploit the O2 dissolved in the storage solution to maintain their basal metabolism. A high pCO2 level was not detected in the PE-O2 group, due to the CO2 scavenger of the oxygenator device. In the PE group pCO2 did not change at T1 with respect to T0; only perfusion was less effective in maintaining the renal basal metabolism. Like the perfusion groups, PE and PE-O2 had higher levels of lactic acid in the preservation solution because the organs were flushed, but PE-Hyp did not show this increased level. During perfusion, the hyperbaric oxygenation probably stimulated a better aerobic metabolism, yielding higher ATP and lower lactate concentrations, as previously reported by other authors in different experimental settings [33–35]. The reduced lactate production may contribute to the beneficial effect of the hyperbaric oxygenation in ischemia/reperfusion injury. Furthermore, hyperbaric perfusion showed a significantly different gene expression of HIF-1α and eNOS. These results again suggested a reduction of the preservation injury and a better delivery of oxygen to the tissue. HIF1α expression normally occurs during SCS as an adaptive response to sub-optimal oxygen tension, therefore its reduced expression in the PE-Hyp group indicates optimal oxygen delivery to the graft [36]. The expression of eNOS in the same group also supports this hypothesis. Indeed, it was reported that eNOS gene expression was upregulated during hypoxia, thus favoring vasodilation in order to ensure an adequate blood supply [37].
The principal mechanisms of action of hyperbaric oxygenation are based on antioxidant enzymes induction, inflammatory cytokine suppression, angiogenesis, vasculogenesis, and enhancement of stem cell mobilization [38–45]. An early event associated with post-ischemic tissue reperfusion is the adherence of circulating neutrophils to vascular endothelium by β2 integrin. Some authors have shown that in many tissues, including liver and kidney, hyperbaric oxygen, unlike normobaric oxygen, temporarily arrests the adherence/sequestration of neutrophils by inhibiting the β2 integrin function and induces the release of antioxidant enzymes and anti-inflammatory proteins [40].
Our study explored only some aspects of these beneficial effects, but the absence of damage at histological examination, the improved metabolic activity, and a different gene expression seem to establish a good basis for the novel application of PE-Hyp of grafts in clinical settings.
All experimental procedures in each group were conducted setting arterial pressure and the time of perfusion after preliminary validation. We selected 30 mmHg as arterial pressure for the perfusion because, as reported by other authors [46] and in our initial experimental phase (data not reported), higher arterial pressure values (50–60 mmHg) were associated with endothelial damage and altered parenchymal architecture. Confirming our hypothesis, IHC and ultrastructural analysis showed that the glomerular and interstitial endothelial cells were well preserved.
We set the timing of treatment at three hours in order to keep the CIT as short as possible, due to its relation to organ dysfunction and survival [47,48]. Additionally, in our preliminary experiments, longer treatment times were not associated with better features of kidneys in terms of metabolic activity and tissue damage (data not reported). Other groups have reported one hour as an effective time of treatment with oxygen for liver transplantation and in the setting of donation after circulatory death [20].
Many case series have reported CIT values between 10 and 18 hours [8,9] and >30 hours [7]. Often, CIT is secondary to recipient selection and dialysis before transplantation. In many circumstances, therefore, the kidneys are available for pre-transplant treatments during recipient preparation and three hours seemed a reasonable compromise between a short CIT and the opportunity of improving the quality of the preserved graft.
Other clinical strategies for improving graft function are currently under debate, such as reduced donor temperature, sub-normothermic perfusion, or use of a cell-free oxygen carrier [49–52]. Up until now, there has been no evident clinical data in favor of one strategy compared to the others, but in agreement with some authors’ suggestions [53], our group believes that oxygenation during hypothermia is effective and simple. In addition, we used hyperbaric oxygenation, which has been reported as kidney perfusion procedure since 1966 [54,55], and has been used by many researchers in the past on animal bloodless perfusion kidneys. Hyperbaric oxygen therapy (HBO) continues to be investigated in the field of renal disease. Heiachi et al. confirmed that the HBO after renal ischemia reperfusion injury of rats decreased apoptosis and increased cellular proliferation [56]. We have applied hyperbaric oxygen in kidney perfusion ex-situ, cited among oxygenation techniques [22], and have not yet applied it for clinical human kidney preservation. This represents a novel aspect of our research work on organ perfusion.
Some limitations of our study have to be acknowledged. We did not have a healthy control kidney to use as a reference; ethical issues do not allow us to use organs suitable for transplantation. Furthermore, study cases were not transplanted and so we have no data about graft function and survival. However, our results may be the basis for advanced clinical investigations into the beneficial effect of hypothermic oxygenated perfusion in normobaric or hyperbaric condition for marginal kidneys before transplantation.
Conclusions
Hyperbaric or normobaric oxygenation and dynamic hypothermic perfusion improve organ metabolic preservation compared to other treatments that were tested in our study. Thus, these advanced organ preservation techniques may avoid the risk of delayed graft function; however, clinical trials are needed to confirm our study findings (ClinicalTrials.gov ID: NCT03031067).
Supplementary file
Results of histological analysis and immunohistochemistry in detail
a) CS
The 4 grafts of the control group had mainly mild interstitial fibrosis, tubular atrophy and glomerular sclerosis at T0.
After CS (T1), 2/4 grafts showed a histologically visible tubular injury, characterized by simplification and focal vacuolization of tubulocytes, and appearance of ATN was observed in 2 cases. One graft showed endothelial damage. At T1, IHC showed an increased expression of the investigated proteins (Figure 1, left column); in particular, CD34 increased significantly. These results, indicating well preserved tissue, were confirmed by ultrastructural examination; indeed, in T1 samples, endothelial cells of glomeruli and interstitium showed excellent morphology including nuclei with dispersed chromatin and small nucleoli as well as a normal complement of cytoplasm organelles (Figure 2A).
b) Hyp
Among the 4 grafts of this group, 1 had some degree of tubular injury with ATN at T0.
At T1, 3 grafts showed the same morphological picture as to T0 and endothelial damage appeared in 1 graft. The expression of CD31 and CD34 increased (Figure 1, left column); a significant CD34 increase was seen. At ultrastructural examination, endothelial cells were normal without any feature of subtle cell damage (Figure 2B).
c) PE
Among the 4 grafts of this group, 2 had some degree of tubular injury with ATN at T0, and diffuse glomerular sclerosis was seen in one. After PE, 2/4 grafts showed the same morphology as T0, while 2 grafts had some degree of endothelial injury. Unlike the previous group, a decreased and non-significant expression of CD31 and CD34 was seen at IHC (Figure 1, left column). TEM showed endothelial cell injury; markedly electron dense shrunken cells with cytoplasm vacuolization (Figure 2C).
d) PE-Hyp
In this group only 1 case was characterized by diffuse glomerular sclerosis, tubular atrophy with interstitial chronic phlogosis and arterial myointimal thickening. At T1, 2 grafts showed the same morphology as at T0, while the other 2 showed a global worsening of histologic appearance, with glomerular simplification, tubular atrophy and endothelial loss.
The CD31 and CD34 expression increased in this group too, and CD31 was significantly expressed after the treatment (Figure 1, left column). Accordingly, TEM revealed unremarkable endothelial cells (Figure 2D).
e) PE-O2
Among the 4 grafts of this group, 3 showed some degree of tubular injury with ATN at T0.
After PE-O2, 3/4 grafts showed the same morphology as at T0, while 1 graft had some degree of endothelial injury. The CD31 and CD34 expression did not vary after the treatment; in particular, CD31 showed a slightly increased expression whereas CD34 decreased a little (Figure 1, left column). TEM of endothelial cells did not show any difference after the treatment (Figure 2E).
Formal-fixed paraffin-embedded tissues processing protocol
-
Preparation and cassetting
Fixed tissue is sent to the Histology facility.
If not done yet, grossing, slicing and cassetting are achieved by our technical staff.
-
Processing: wax infiltration step inside tissue sample
Dehydration: 3 alcohol baths with growing concentrations, 70–85–90%, preventing tissue damage (distortion, hardening). Water is finally removed by 3 final absolute alcohol baths.
Clearing: 3 toluene baths will enable to replace alcohol trapped inside tissues and to be a miscible solvent with wax.
Wax infiltration: hot wax baths (44–60°C) will solidify the tissue.
-
Tissue embedding
Tissue is orientated inside a mold filled with hot paraffin.
-
Tissue sectioning using a microtome.
Most of the time, histology sections are 4 μm thick. Wax ribbons are transferred onto a warm water bath (43–45°C) and spread on a microscope glass slide before drying at least for one hour at 45°C.
-
Routine stain
-
HE (Hematoxylin-Eosin) or HPS (Hematoxylin-Phloxin-Safran)
– Deparaffinize
– 2 baths of toluene (95–100%) 3 minutes each
– 3 baths of alcohol (80–95–100%) 3 minutes each
– Rince in tap water
– Gill Hematoxylin for 3 to 10 minutes
– Sodium Bicarbonate for 5 to 10 seconds
– Rince in tap water
– Eosin/Phloxin 1 minute
– Rince in tap water
– 3 baths of absolute alcohol 40 seconds each. (Safran. for 3 to 10 minutes)
– 3 baths of absolute alcohol 40 seconds each
– 3 baths of toluene
Special stains
-
Pathologist analysis.
Acknowledgments
The authors thank the following people for their important scientific contribution and assistance during the experimental phase and the development of the study: Emanuela Marcelli, Professor; Laura Cercenelli, MD; Ferdinando E. Giannone, PhD; Alberto Balduzzi, MD; Annalisa Amaduzzi, MD; Sergio Emiliani; Sergio Salani; Stefano Agnini; Piergiorgio Marotti, MD; Gabriella Sangiorgi, MD; Lorenza Ridolfi, MD; Elisa Casadio, MD; Caterina Cicognani, Dr; Juric Primoz, Dr; Gian Luca Grazi, MD; Gaetano La Manna, Professor.; Giorgia Comai, MD; Giovanni Liviano, MD; Olga Baraldi, MD; Vania Cuna, MD; Matteo Cescon, Professor.; Massimo Del Gaudio, MD; Alessandro Cucchetti, MD; Antonietta D’Errico, Professor; Marco Di Laudo, MD; Federica Odaldi, MD; Lorenzo Maroni, MD.
Footnotes
Source of support: Department of General Surgery and Transplantation has received funding by Emilia-Romagna Region, known as “Fondo per la Modernizzazione”, and University of Bologna
Conflict of interest
None.
References
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant. 2017;17(Suppl 1):21–116. doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong G, Howard K, Chapman JR, et al. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PLoS One. 2012;7:e29591. doi: 10.1371/journal.pone.0029591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252–59. doi: 10.1001/jamasurg.2014.2038. [DOI] [PubMed] [Google Scholar]
- 4.Keller EJ, Kwo PY, Helft PR. Ethical considerations surrounding survival benefit-based liver allocation. Liver Transpl. 2014;20:140–46. doi: 10.1002/lt.23780. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigue JR, LaPointe Rudow D, Hays R American Society of Transplantation. Living donor kidney transplantation: Best practices in live kidney donation-recommendations from a consensus conference. Clin J Am Soc Nephrol. 2015;10:1656–57. doi: 10.2215/CJN.00800115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reese PP, Boudville N, Garg AX. Living kidney donation: outcomes, ethics, and uncertainty. Lancet. 2015;385:2003–13. doi: 10.1016/S0140-6736(14)62484-3. [DOI] [PubMed] [Google Scholar]
- 7.Khan MA, El-Hennawy H, Farney AC, et al. Analysis of local versus imported expanded criteria donor (ECD) kidneys: A single center experience with 497 ECD kidney transplants. Clin Transplant. 2017 doi: 10.1111/ctr.13029. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Głyda M, Włodarczyk Z, Czapiewski W. Results of renal transplantation from expanded criteria deceased donors-a single-center experience. Ann Transplant. 2012;17:35–42. doi: 10.12659/aot.882634. [DOI] [PubMed] [Google Scholar]
- 9.Jochmans I, Akhtar MZ, Nasralla D, et al. Past, present, and future of dynamic kidney and liver preservation and resuscitation. Am J Transplant. 2016;16:2545–55. doi: 10.1111/ajt.13778. [DOI] [PubMed] [Google Scholar]
- 10.O’Callaghan JM, Knight SR, Morgan RD, Morris PJ. Preservation solutions for static cold storage of kidney allografts: A systematic review and meta-analysis. Am J Transplant. 2012;12:896–906. doi: 10.1111/j.1600-6143.2011.03908.x. [DOI] [PubMed] [Google Scholar]
- 11.Dikdan GS, Mora-Esteves C, Koneru B. Review of randomized clinical trials of donor management and organ preservation in deceased donors: Opportunities and issues. Transplantation. 2012;94:425–41. doi: 10.1097/TP.0b013e3182547537. [DOI] [PubMed] [Google Scholar]
- 12.Tedesco-Silva H, Jr, Mello Offerni JC, Ayres Carneiro V, et al. Randomized trial of machine perfusion versus cold storage in recipients of deceased donor kidney transplants with high incidence of delayed graft function. Transplant Direct. 2017;3:e155. doi: 10.1097/TXD.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brat A, Pol RA, Leuvenink HG. Novel preservation methods to increase the quality of older kidneys. Curr Opin Organ Transplant. 2015;20:438–43. doi: 10.1097/MOT.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 14.Gill J, Dong J, Eng M, et al. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time. Transplantation. 2014;97:668–74. doi: 10.1097/01.TP.0000438637.29214.10. [DOI] [PubMed] [Google Scholar]
- 15.La Manna G, Conte D, Cappuccilli ML, et al. An in vivo autotransplant-model of renal preservation: Cold storage versus machine perfusion in the prevention of ischemia/reperfusion injury. Artif Organs. 2009;33:565–70. doi: 10.1111/j.1525-1594.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 16.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant. 2010;10:372–81. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 17.Jochmans I, O’Callaghan JM, Pirenne J, Ploeg RJ. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors. Transpl Int. 2015;28:665–76. doi: 10.1111/tri.12530. [DOI] [PubMed] [Google Scholar]
- 18.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–69. doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 19.Minor T, Paul A, Efferz P, et al. Kidney transplantation after oxygenated machine perfusion preservation with Custodiol-N solution. Transpl Int. 2015;28:1102–8. doi: 10.1111/tri.12593. [DOI] [PubMed] [Google Scholar]
- 20.Westerkamp AC, Karimian N, Matton AP, et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100:825–35. doi: 10.1097/TP.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 21.Kron P, Schlegel A, de Rougemont O, et al. Short, cool, and well oxygenated - HOPE for kidney transplantation in a rodent model. Ann Surg. 2016;264:815–22. doi: 10.1097/SLA.0000000000001766. [DOI] [PubMed] [Google Scholar]
- 22.Hosgood SA, Nicholson HF, Nicholson ML. Oxygenated kidney preservation techniques. Transplantation. 2012;93:455–59. doi: 10.1097/TP.0b013e3182412b34. [DOI] [PubMed] [Google Scholar]
- 23.Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. J Heart Lung Transplant. 2017;36(7):744–53. doi: 10.1016/j.healun.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16:3282–85. doi: 10.1111/ajt.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bral M, Gala-Lopez B, Bigam D, et al. Preliminary single-center canadian experience of human normothermic ex vivo liver perfusion: Results of a clinical trial. Am J Transplant. 2017;17:1071–80. doi: 10.1111/ajt.14049. [DOI] [PubMed] [Google Scholar]
- 26.Kaths JM, Paul A, Robinson LA, Selzner M. Ex vivo machine perfusion for renal graft preservation. Transplant Rev (Orlando) 2017 doi: 10.1016/j.trre.2017.04.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16:3235–45. doi: 10.1111/ajt.13875. [DOI] [PubMed] [Google Scholar]
- 28.Ijichi H, Taketomi A, Soejima Y, et al. Effect of hyperbaric oxygen on cold storage of the liver in rats. Liver Int. 2006;26:248–53. doi: 10.1111/j.1478-3231.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- 29.Giannone FA, Treré D, Domenicali M, et al. An innovative hyperbaric hypothermic machine perfusion protects the liver from experimental preservation injury. ScientificWorldJournal. 2012;2012:573410. doi: 10.1100/2012/573410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao DS, Wu YK, Fu SJ, et al. Hyperbaric oxygenation protects against ischemia-reperfusion injury in transplanted rat kidneys by triggering autophagy and inhibiting inflammatory response. Ann Transplant. 2018;23:75–82. doi: 10.12659/aot.901102. [DOI] [PubMed] [Google Scholar]
- 31.Remuzzi G, Cravedi P, Perna A, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354:343–52. doi: 10.1056/NEJMoa052891. [DOI] [PubMed] [Google Scholar]
- 32.Wijermars LG, Schaapherder AF, de Vries DK, et al. Defective postreperfusion metabolic recovery directly associates with incident delayed graft function. Kidney Int. 2016;90:181–91. doi: 10.1016/j.kint.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 33.Nylander G, Nordstrom H, Lewis DH, Larsson J. Metabolic effects of hyperbaric oxygen in post ischemic muscle. Plast Reconstr Surg. 1987;79:91–97. doi: 10.1097/00006534-198701000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Bosco G, Yang ZJ, Nandi J, et al. Effects of hyperbaric oxygen on glucose, lactate, glycerol and anti-oxidant enzymes in the skeletal muscle of rats during ischemia and reperfusion. Clin Exp Pharmacol Physiol. 2007;34:70–76. doi: 10.1111/j.1440-1681.2007.04548.x. [DOI] [PubMed] [Google Scholar]
- 35.Nørlinger TS, Nielsen PM, Qi H, et al. Hyperbaric oxygen therapy reduces renal lactate production. Physiol Rep. 2017;5:e13217. doi: 10.14814/phy2.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 37.Coulet F, Nadaud S, Agrapart M, Soubrier F. Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J Biol Chem. 2003;278:46230–40. doi: 10.1074/jbc.M305420200. [DOI] [PubMed] [Google Scholar]
- 38.Thom SR. Hyperbaric oxygen therapy. J Intensive Care Med. 1989;4:58–74. [Google Scholar]
- 39.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Thom SR. Hyperbaric oxygen – its mechanisms and efficacy. Plast Reconstr Surg. 2011;127:131S–41S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, Chang Q, Cox RA, et al. Hyperbaric oxygen attenuates apoptosis and decreases inflammation in an ischemic wound model. J Invest Dermatol. 2008;128:2102–12. doi: 10.1038/jid.2008.53. [DOI] [PubMed] [Google Scholar]
- 42.Buras J, Holt D, Orlow D, et al. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit Care Med. 2006;34:2624–29. doi: 10.1097/01.CCM.0000239438.22758.E0. [DOI] [PubMed] [Google Scholar]
- 43.Yu S, Chiu J, Yang S, et al. Preconditioned hyperbaric oxygenation protects the liver against ischemia-reperfusion injury in rats. J Surg Res. 2005;128:28–36. doi: 10.1016/j.jss.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Zhou C, Calvert J, et al. Multiple effects of hyperbaric oxygen on the expression of HIF-1a and apoptotic genes in a global ischemia-hypotension rat model. Exp Neurol. 2005;191:198–210. doi: 10.1016/j.expneurol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 45.Benson RM, Minter LM, Osborne BA, Granowitz EV. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin Exp Immunol. 2003;134:57–62. doi: 10.1046/j.1365-2249.2003.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maathuis MH, Manekeller S, van der Plaats A, et al. Improved kidney graft function after preservation using a novel hypothermic machine perfusion device. Ann Surg. 2007;246:982–91. doi: 10.1097/SLA.0b013e31815c4019. [DOI] [PubMed] [Google Scholar]
- 47.Bahde R, Vowinkel T, Unser J, et al. Prognostic factors for kidney allograft survival in the Eurotransplant Senior Program. Ann Transplant. 2014;19:201–9. doi: 10.12659/AOT.890125. [DOI] [PubMed] [Google Scholar]
- 48.Butala NM, Reese PP, Doshi MD, Parikh CR. Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation. 2013;95:1008–14. doi: 10.1097/TP.0b013e3182855544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fontes P, Lopez R, van der Plaats A, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15:381–94. doi: 10.1111/ajt.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9:e110642. doi: 10.1371/journal.pone.0110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosgood SA, Barlow AD, Hunter JP, Nicholson ML. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg. 2015;102:1433–40. doi: 10.1002/bjs.9894. [DOI] [PubMed] [Google Scholar]
- 52.Niemann CU, Feiner J, Swain S, et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015;373:405–14. doi: 10.1056/NEJMoa1501969. [DOI] [PubMed] [Google Scholar]
- 53.Schlegel A, Kron P, Graf R, et al. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014;61:1267–75. doi: 10.1016/j.jhep.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JM. Hyperbaric bloodless perfusion of the kidney. Br J Surg. 1966;53:802–6. doi: 10.1002/bjs.1800530918. [DOI] [PubMed] [Google Scholar]
- 55.Van Zyl JJ, Van Zyl JA, Lochner A, et al. Renal function and metabolism of isolated baboon kidneys following prolonged bloodless hypothermic, hyperbaric storage with helium and oxygen. Ann Surg. 1968;168:95–109. doi: 10.1097/00000658-196807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migita H, Yoshitake S, Tange Y, et al. Hyperbaric oxygen therapy suppresses apoptosis and promotes renal tubular regeneration after renal ischemia/reperfusion injury in rats. Nephrourol Mon. 2016;8:e34421. doi: 10.5812/numonthly.34421. [DOI] [PMC free article] [PubMed] [Google Scholar]