Abstract
Background
Prospective evidence is lacking regarding the association between renal dysfunction and cardiovascular events after liver transplantation.
Material/Methods
Data were analyzed post hoc regarding renal function and major adverse cardiac events in a two-year prospective trial of de novo liver transplant recipients randomized at 30 days post-transplant to (i) everolimus [EVR]/reduced tacrolimus [EVR/rTAC] (ii) EVR with tacrolimus discontinued [TAC Elimination] or (iii) standard tacrolimus [TAC Control].
Results
By month 24 post-transplant, 32/716 patients had experienced a first major cardiac event (4.5%): 4.1% (10/245), 2.2% (5/229) and 7.0% (17/242) of patients in the EVR/rTAC, TAC Elimination and TAC Control groups, respectively (p=0.043). The cumulative eGFR area under the curve (AUC) from randomization to month 24 was 119 706, 123 082, and 105 946 mL in the EVR/rTAC, TAC Elimination, and TAC Control groups, respectively, corresponding to a mean eGFR AUC of 82.4, 83.0, and 71.9 mL/min/1.73 m2. Cox regression modeling showed that mean eGFR AUC was inversely associated with time to first major cardiac event: the hazard ratio per mL/min/1.73 m2 was −0.0000015 [95% CI −0.00000078; −0.0000024] (p<0.001).
Conclusions
These findings confirm retrospective evidence that the risk of major cardiac events increases with deteriorating renal function after liver transplantation and demonstrate the need for careful cardiovascular risk management in patients with renal impairment. Immunosuppression based on everolimus with tacrolimus withdrawal, or to a lesser extent tacrolimus reduction, improves both renal function and the risk of major cardiac events compared to standard tacrolimus therapy in liver transplant recipients.
MeSH Keywords: Death, Sudden, Cardiac; Glomerular Filtration Rate; Heart Failure; Immunosuppressive Agents; Liver Transplantation; Renal Insufficiency
Background
Improving long-term survival after liver transplantation remains a challenge, with 25% of patients dying within 5 years [1]. Reducing the toll of cardiovascular disease represents a key opportunity to increase survival rates. Cardiac events occur in 7–28% of liver transplant patients [2–6], depending on the definition applied, and increase mortality risk by more than 4-fold [3]. Approximately 5% of all deaths after the early post-transplant phase are accounted for by cardiovascular disease [2]. Older age [2,5,6], male sex [2,3,5], pre-transplant diabetes [2,5,7], pre-transplant hypertension [5], and nonalcoholic fatty liver disease as the indication for liver transplant [8] are established risk factors for cardiovascular mortality after liver transplantation.
Chronic renal dysfunction has also been shown to have a significant association with cardiovascular events in retrospective analyses [2,4,6,9]. One study of data from the Organ Procurement and Transplant Network (OPTN) assessed risk factors for cardiovascular mortality in 5057 patients undergoing liver transplant for nonalcoholic steatohepatitis and found moderate or severe kidney disease (estimated GFR [eGFR] <60 mL/min/1.73 m2) to incur a 1.8-fold increase in risk (p<0.001) [2]. A single-center retrospective analysis of 202 primary liver transplants has reported a doubling of the risk for both cardiovascular events and cardiovascular mortality in patients with eGFR <60 mL/min/1.73 m2 [6]. With 25–50% of patients showing moderate or severe renal dysfunction after liver transplantation [6,10,11], the impact of renal function on cardiovascular events is highly relevant. However, prospective studies of the association between renal function and cardiovascular events in liver transplant patients have hitherto been lacking.
Here, we examine the association between the evolution of renal function and the incidence of major adverse cardiac events over the first 2 years post-transplant in a post hoc analysis of data from an international, prospective, randomized trial of de novo liver transplant recipients [12,13].
Material and Methods
This was a post hoc analysis of the international, 24-month prospective, randomized, multicenter, 3-arm, open-label H2304 study in adult de novo primary liver transplant recipients (NCT00622869). The study has been described in detail elsewhere [12,13]. In brief, patients were randomized at 30 days post-transplant to receive: (i) everolimus [EVR] and reduced tacrolimus [EVR/rTAC], (ii) EVR with tacrolimus discontinued [TAC Elimination], or (iii) standard tacrolimus [TAC Control]. Corticosteroids were administered according to local practice (minimum 5 mg prednisolone/day until at least month 6 post-transplant). Patients were required to have eGFR ≥30 mL/min/1.73 m2 at study entry and at the point of randomization, with urine protein excretion <1.0 g/day.
GFR was estimated using the 4-variable Modification of Diet in Renal Disease [MDRD4] formula [14]:
To evaluate trends in renal function from randomization to month 24, eGFR values documented at each study visit (months 1, 2, 3, 4, 5, 6, 9, 12, 18, and 24) were used to calculate the area under the curve (AUC), using the standard trapezoidal rule [15].
Information on major cardiac events was collected as part of the standard adverse event reporting procedures at each study visit as reported based on the investigators’ judgement. Information on any major cardiac event considered to be a serious adverse event by the investigator was captured up to 30 days after the last dose of study drug. Major cardiac events were defined in accordance with MedDRA Standardized MedDRA Queries (SMQs) as ischemic heart diseases, cardiac failure, ischemic stroke, and sudden death. Thus, the following MedDRA preferred terms were included as major cardiac events: acute myocardial infarction, myocardial infarction, angina pectoris, unstable angina pectoris, cardiac failure, congestive cardiac failure, coronary artery arteriosclerosis, coronary artery disease, coronary artery insufficiency, ischemic stroke, myocardial ischemia, pulmonary edema, and sudden death.
The association between treatment group and time to occurrence of first major cardiac event was assessed by the Kaplan-Meier method. The association between eGFR AUC as a continuous variable and the occurrence of first major cardiac event was assessed using a standard Cox regression model.
All analyses were based on patients in the intent-to-treat (ITT) population, consisting of all randomized patients. Statistical analysis was performed using SAS software (version 9.3) SAS Institute Inc., Cary, NC, USA.
Results
In total, 719 patients were randomized and formed the ITT population (EVR/rTAC 245, TAC Elimination 231, TAC Controls 243). The mean age was 54 years and the majority of patients were male (72.7%) (Table 1). The most frequent indications for liver transplantation were alcoholic cirrhosis (23.7%) and hepatitis C infection (24.3%). At randomization, 38.8% of patients had diabetes and 22.3% had a history of cardiac disorders, with a similar distribution of both parameters between treatment groups (Table 1). The MELD score was approximately 19 in all these treatment arms.
Table 1.
Population characteristics at randomization by treatment group (ITT population).
| EVR/rTAC N=245 | TAC Elimination N=231 | TAC Control N=243 | |
|---|---|---|---|
| Age (years) | 53.6 (9.2) | 53.2 (10.8) | 54.5 (8.7) |
| Male sex, n (%) | 180 (73.5) | 164 (71.0) | 179 (73.7) |
| Body mass index (kg/m2) | 25.1 (4.2) | 25.3 (4.3) | 24.5 (4.2) |
| Diabetes, n (%) | 95 (38.8) | 83 (35.9) | 101 (41.6) |
| Primary disease leading to liver transplantation, n (%) | |||
| Alcoholic cirrhosis | 71 (29.0) | 49 (21.2) | 51 (21.0) |
| Hepatitis C | 62 (25.3) | 56 (24.2) | 57 (23.5) |
| Hepatocellular carcinoma | 42 (17.1) | 31 (13.4) | 35 (14.4) |
| Hepatitis B | 17 (6.9) | 17 (7.4) | 15 (6.2) |
| Other | 53 (21.6) | 78 (33.8) | 85 (35.0) |
| MELD score* | 19.2 (9.0) | 19.6 (7.5) | 19.0 (7.6) |
| eGFR, mL/min/1.73 m2 | 81.1 (32.6) | 82.6 (37.2) | 78.0 (27.5) |
| eGFR <60mL/min/1.73 m2 (%) | 71 (29.0) | 61 (26.4) | 61 (25.1) |
| Medical history of cardiac disorders, n (%) | 58 (23.7) | 49 (21.2) | 53 (21.8) |
| Mitral valve incompetence | 7 (2.9) | 9 (3.9) | 9 (3.7) |
| Tricuspid valve incompetence | 7 (2.9) | 4 (1.7) | 4 (1.6) |
| Left ventricular hypertrophy | 8 (3.3) | 8 (3.5) | 0 |
| Coronary artery disease | 6 (2.4) | 5 (2.2) | 10 (4.1) |
| Atrial fibrillation | 5 (2.0) | 4 (1.7) | 5 (2.1) |
Based on laboratory values only.
Continuous variables are shown as mean (SD). eGFR – estimated GFR (calculated by abbreviated Modification of Diet in Renal Disease [MDRD4] equation; EVR – everolimus; MELD – Model for End-stage Liver Disease; TAC – tacrolimus.
Treatment with statin therapy during the study was more frequent in the EVR/rTAC and TAC Elimination groups than in TAC Controls (22.0%, 21.4%, and 12.0%, respectively), and hypercholesterolemia (11.0%, 9.2%, and 3.7%) and hyperlipidemia (8.6%, 10.5% and 2.1%) were reported more frequently as adverse events (Table 1).
Data on major cardiac events were available in 716 patients (EVR/rTAC 245, TAC Elimination 229, TAC Controls 242). By month 24 post-transplant, 32 patients had experienced a first major cardiac event (32/716, 4.5%), representing an incidence of 26 events per 1000 patient years. The most frequent events were angina pectoris (n=6), cardiac failure (n=6), coronary artery disease (n=5), and cardiac failure (n=4) (Supplementary Table 1). Major cardiac events occurred in 4.1% (10/245), 2.2% (5/229), and 7.0% (17/242) of patients in the EVR/rTAC, TAC Elimination, and TAC Control groups, respectively. Figure 1 illustrates a Kaplan-Meier analysis of the time to first occurrence of major cardiac events according to treatment group. Over the 2-year study, the lowest probability of an event was seen in the TAC Elimination group, followed by the EVR/rTAC group, with the highest probability in the TAC Control arm (Figure 1). The difference in risk across all 3 groups was found to be significantly different (log rank p=0.043) (Figure 1).
Figure 1.
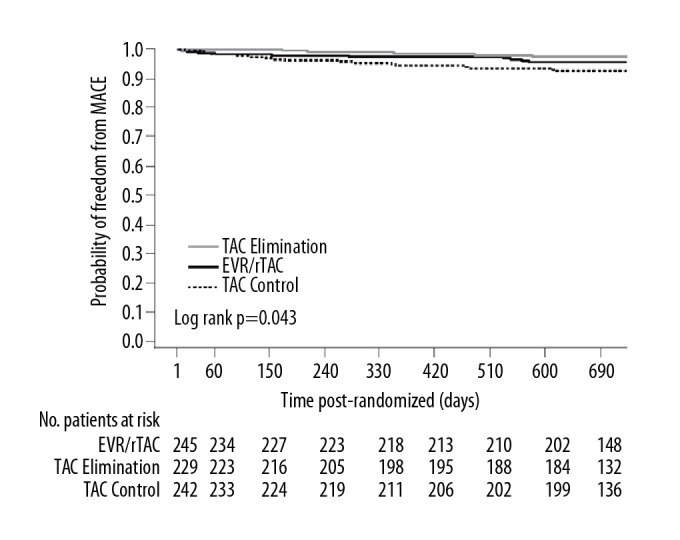
Kaplan-Meier (KM) plot of major cardiac events (MACE) to month 24 according to treatment group (ITT population). EVR – everolimus; rTAC – reduced tacrolimus; TAC – tacrolimus.
Renal function (eGFR) at randomization was comparable between groups (mean 81.1, 82.6, and 78.0 mL/min/1.73 m2 in the EVR/rTAC, TAC Elimination, and TAC Control groups, respectively; p=0.553) (Table 1). Mean eGFR at baseline was thus within 60–89 mL/min/1.73 m2 in both groups, representing kidney damage with mildly reduced eGFR (chronic kidney disease [CKD] stage 2) [16]. The proportion of patients with eGFR <60 mL/min/1.73 m2 (moderate or severe CKD [16]) was 26.8%, with no marked differences between groups (Table 1). At the month 24 study visit, mean (SD) eGFR was 74.7 (26.1), 67.8 (21.0), and 77.5 (26.2) mL/min/1.73 m2 in the EVR/rTAC, TAC Elimination, and TAC Control groups, respectively (p=0.007).
The trajectory of eGFR over the 24-month study was assessed by calculating eGFR AUC from randomization. As shown in Figure 2, the cumulative eGFR AUC to month 24 was highest in the TAC Elimination group (123 082 mL), slightly lower in the EVR/rTAC group (119 706 mL) and distinctly lower in the TAC Control group (105 946 mL). These values corresponded to a mean eGFR AUC of 82.4, 83.0, and 71.9 mL/min/1.73 m2 over the 24-month study period.
Figure 2.
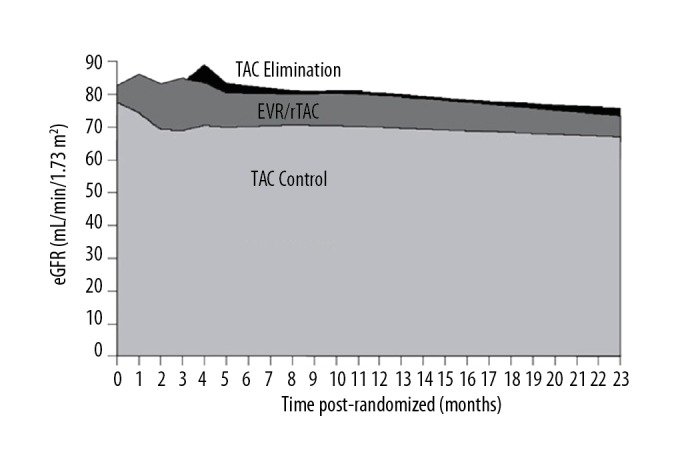
Area under the curve (AUC) of estimated GFR (eGFR) from baseline to month 24 according to treatment group (ITT population). EVR – everolimus; rTAC – reduced tacrolimus; TAC – tacrolimus.
Cox regression modeling showed that that the mean eGFR AUC from randomization to month 24 post-transplant was inversely associated with the time to first occurrence of a major cardiac event: the hazard ratio per mL/min/1.73 m2 was −0.0000015 [95% CI–0.00000078; −0.0000024] (p<0.001).
Discussion
Analysis of this large dataset from a prospective, randomized trial with protocol-defined monitoring of renal function and adverse events confirms retrospective observations [2,4,6,9] that the risk of major cardiac events is inversely associated with renal function, as assessed by eGFR AUC, over the first 2 years after liver transplantation.
There is convincing evidence from large studies in non-transplant populations that renal dysfunction (eGFR <60 mL/min/1.73 m2) is significantly associated with a higher risk for major cardiac events [17] and cardiovascular mortality [18]. One study observed a 32% increased risk for myocardial infarction with each 10 mL/min/1.73 m2 decrease in eGFR [19]. A number of uremic-specific cardiovascular risk factors have been identified in the general population, including anemia, hyperparathyroidism, hyperhomocysteinemia, high lipoprotein(a) levels, and low vitamin C [20], which would be expected to apply equally in liver transplant recipients with poor renal function. Additionally, transplant recipients are subject to chronic renal insults associated with long-term immunosuppression, most prominently dose-dependent nephrotoxicity associated with calcineurin inhibitors (CNIs) [21]. In the present 2-year study, patients receiving everolimus with reduced or discontinued tacrolimus had better renal function than the TAC Control group, as demonstrated by higher eGFR AUC values. This is consistent with the findings of 2 previous randomized trials in liver transplantation [22,23], including the PROTECT study [24], in which early introduction of everolimus with CNI withdrawal resulted in higher mean eGFR. Notably, both the EVR/rTAC and TAC Elimination groups were associated with a significant reduction in risk for major cardiac events versus standard CNI therapy based on Kaplan-Meier analysis, but with a more marked effect in the TAC Elimination group. This observation supports the premise that cardiac events become more likely as renal function deteriorates. Other randomized studies of everolimus with CNI withdrawal in liver transplantation, including the PROTECT study [22,24], have not described the incidence of major cardiac events [22–24], but in renal transplantation the recent ELEVATE study reported that early conversion from CNI therapy to everolimus was associated with a significant reduction in such events at 12 months post-transplant [25].
The question arises whether other effects of mTOR inhibition contributed to the reduced rate of major cardiac events in the everolimus-treated cohorts, in addition to improved renal function. A significant direct cardioprotective effect seems unlikely. Despite evidence that switching to a mammalian target of rapamycin (mTOR) inhibitor reduces left ventricular remodeling after heart transplantation [26,27], early conversion to everolimus has not been found to exert a clinically relevant effect on ventricular mass in kidney transplantation [25,28]. Studies have shown mixed results concerning improved blood pressure after switching to everolimus from CNI [25,28], while data suggesting a beneficial effect on arterial stiffness [29,30] was not borne out in the recent ELEVATE trial [25]. The immunomodulatory effects of everolimus may attenuate the process of atherosclerosis, based on studies in heart transplant recipients [31,32], which would contribute to a lower risk for cardiac events. Interestingly, the reduced risk for major cardiac events in the everolimus-treated cohorts was observed despite the known hyperlipidemic effect of mTOR inhibitors [33, 34] which was again apparent in the present cohort of patients, indicating that any adverse risk associated with this hyperlipidemic influence is outweighed by other effects.
This analysis was undertaken post hoc but benefitted from prospective collection of data on eGFR and adverse events over the first 2 years after liver transplantation in a large cohort of patients. The study population had similar mean baseline eGFR values to the general liver transplant population [35]. Patients with severe renal dysfunction (<30 mL/min/1.73 m2, i.e., CKD stage 4 or 5) were excluded, and the results are not necessarily applicable in that context, but such patients represent a relatively small proportion of recipients. It should be noted that although the randomized PROTECT study found no increase in biopsy-proven acute rejection when CNI elimination was performed more gradually and basiliximab induction was given [22], continuation of a reduced-exposure CNI regimen after introducing everolimus may overall be a more appropriate option than CNI withdrawal [12].
Conclusions
These findings confirm retrospective evidence that the risk of major cardiac events increases with deteriorating renal function after liver transplantation. In view of the risk of cirrhotic cardiomyopathy and prevalence of conventional cardiovascular risk factors in liver transplant candidates, the European Association for the Study of the Liver (EASL) recommends that all candidates should undergo cardiovascular screening [36]. EASL advises routine assessment with an electrocardiogram and transthoracic echocardiography, with stress echocardiograms reserved for high-risk cases (i.e., more than 2 risk factors or if the patient is >50 years old) [36]. The present results underscore the importance of such screening procedures and highlight that particularly careful cardiovascular risk management is advisable in liver transplant patients with renal impairment. Furthermore, the analysis demonstrates that an immunosuppressive regimen based on everolimus with tacrolimus withdrawal, or to a lesser extent tacrolimus reduction, improves both renal function and the risk of major cardiac events compared to standard treatment with tacrolimus. Selection of an everolimus-based immunosuppressive regimen may be advantageous in avoiding major cardiac events in liver transplant recipients with renal dysfunction.
Supplementary Table
Supplementary Table 1.
First major adverse cardiac events to month 24, by MedDRA preferred term, according to treatment group (ITT population).
| EVR/rTAC N=245 | TAC Elimination N=231 | TAC Control N=243 | |
|---|---|---|---|
| Acute myocardial infarction | 1 | 0 | 2 |
| Angina pectoris | 1 | 1 | 4 |
| Angina unstable | 1 | 0 | 0 |
| Cardiac failure | 2 | 0 | 4 |
| Cardiac failure congestive | 0 | 1 | 1 |
| Coronary artery arteriosclerosis | 1 | 0 | 0 |
| Coronary artery disease | 2 | 0 | 3 |
| Coronary artery insufficiency | 0 | 1 | 0 |
| Ischemic stroke | 0 | 0 | 1 |
| Myocardial infarction | 0 | 1 | 0 |
| Myocardial ischemia | 1 | 0 | 1 |
| Pulmonary edema | 1 | 0 | 0 |
| Sudden death | 0 | 1 | 1 |
| Total | 10 | 5 | 17 |
Acknowledgements
With grateful thanks to the H2304 Study Investigators. Argentina: A.C. Gadano, Buenos Aires; L. McCormack, Buenos Aires; L. Toselli, San Martin; S. Yantorno, Buenos Aires; F. Zingalie, Rosario. Australia: J. Chen, Bedford Park; G. Jeffrey, Nedlands; R. Jones, Heidelberg; G. McCaughan, Camperdown. Brazil: G. Cantisani, Porto Alegre; O. Castro, Ribeirao Preto; L.F. Moreira, Rio de Janerio; J.C. Wiederkehr, Blumenau. Belgium: O. Detry, Liege; F. Nevens, Leuven; X. Rogiers, Gent. Canada: N. Kneteman, Edmonton; P. Marotta, London; G. Therapondros, Toronto; E. Yoshida, Vancouver. Colombia: L. Caicedo, Cali; G. Mejía, Bogotá. Czech Republic: P. Trunecka, Praha. France: E. Boleslawski, Lille; F. Durand, Clichy; C. Duvoux, Creteil; J. Hardwigsen, Marseilles; M. Neau-Cransac, Bordeaux; G. Pageaux, Montpellier; F. Saliba, Villejuif. Germany: L. Fischer, Hamburg; D.B. Foltys, Mainz; S. Jonas, Leipzig; S. Beckebaum, Essen; P. Neuhaus, Berlin; J. Schmidt, Heidelberg; A. Schnitzbauer, Regensburg. Hungary: J. Jaray, Budapest. Ireland: A. McCormick, Dublin. Israel: R. Nakache, Tel-Aviv. Italy: L. De Carlis, Milan; F. Filopponi, Pisa; G.E. Gerunda, Modena; M. Rossi, Rome; M. Salizzoni, Turin. Netherlands: H.J. Metselaar, Rotterdam. Russia: S. Gautier, Moscow; A.V. Zhao, Moscow. Spain: B. Bilbao Aguirre, Barcelona; J. Fabregat, Llobregat; I. Herero, Pamplona; V. Cuervas Mons, Majadanonda; A. Moya, Valencia; M. Navasa, Barcelona; J. Ortiz de Urbina, Baracaldo; M. Salcedo, Madrid. Sweden: B-G Ericzon, Stockholm. United Kingdom: N. Heaton, London; A. MacGilchrist, Edinburgh. USA: A. Alsina, Tampa; R. Brown, New York; S.Gordon Burroughs, Houston; W.C. Chapman, St Louis; K. Chavin, Charleston; S.S. Cheng, Dallas; S.D. Coloquhoun, Los Angeles; C. Doria, Philadelphia; G. Everson, Aurora; S. Feng, San Francisco; J.J. Fung, Cleveland; R. Gedaly, Lexington; S. Geevarghese, Nashville; J. Goss, Houston; J. Hong, Los Angeles; M.A. Huang, Detroit; L. Johnson, Washington; M. Johnson, Atlanta; G.B. Klintmalm, Dallas; V. Kohli, Oklahoma City; B. Koneru, Newark; T. Kozlowski, Chapel Hill; H Merhav, Houston; S. Orloff, Portland; L. Sher, Los Angeles; D. Sudan, Durham; L.W. Teperman, New York; G. Testa, Chicago; K. Watt, Rochester.
Abbreviations
- AUC
area under the curve
- CKD
chronic kidney disease
- CNI
calcineurin inhibitor
- EASL
European Association for the Study of the Liver
- eGFR
estimated glomerular filtration rate
- EVR
everolimus
- ITT
intent-to-treat
- MACE
major adverse cardiac event
- MDRD4
four-variable Modification of Diet in Renal Disease
- mTOR
mammalian target of rapamycin
- OPTN
Organ Procurement and Transplant Network
- SMQs
Standardized MedDRA Queries
- TAC
tacrolimus
Footnotes
Source of support: The H2304 study and this analysis was funded by Novartis Pharma AG, Basel, Switzerland
Conflicts of interest
Faouzi Saliba has received speaker honoraria and/or research grants from Novartis, Astellas, Genzyme, Gilead, Abbvie, Merck Sharp & Dohme, Pfizer, Chiesi, Gambro and Baxter. Lutz Fischer is a member of a Data and Safety Monitoring Board for Novartis. Paolo de Simone is an advisory board member for Novartis, Astellas, and Chiesi. Peter Bernhardt and Giovanni Bader are employees of Novartis. John Fung is a member of a Data and Safety Monitoring Board for Novartis.
References
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR annual data report 2015: Liver. Am J Transplant. 2017;17(Suppl 1):174–251. doi: 10.1111/ajt.14126. [DOI] [PubMed] [Google Scholar]
- 2.VanWagner LB, Lapin B, Skaro AI, et al. Impact of renal impairment on cardiovascular disease mortality after liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Int. 2015;35:2575–83. doi: 10.1111/liv.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piazza NA, Singal AK. Frequency of cardiovascular events and effect on survival in liver transplant recipients for cirrhosis due to alcoholic or nonalcoholic steatohepatitis. Exp Clin Transplant. 2016;14:79–85. doi: 10.6002/ect.2015.0089. [DOI] [PubMed] [Google Scholar]
- 4.Di Maira T, Rubin A, Puchades L, et al. Framingham score, renal dysfunction, and cardiovascular risk in liver transplant patients. Liver Transpl. 2015;21:812–22. doi: 10.1002/lt.24128. [DOI] [PubMed] [Google Scholar]
- 5.Albeldawi M, Aggarwal A, Madhwal S, et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18:370–75. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 6.Josefsson A, Fu M, Björnsson E, et al. Pre-transplant renal impairment predicts posttransplant cardiac events in patients with liver cirrhosis. Transplantation. 2014;98:107–14. doi: 10.1097/01.TP.0000442781.31885.a2. [DOI] [PubMed] [Google Scholar]
- 7.Kuo HT, Lum E, Martin P, Bunnapradist S. Effect of diabetes and acute rejection on liver transplant outcomes: An analysis of the organ procurement and transplantation network/united network for organ sharing database. Liver Transpl. 2016;22:796–804. doi: 10.1002/lt.24414. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 9.Rubin A, Sánchez-Montes C, Aguilera V, et al. Long-term outcomes of ‘long-term liver transplant survivors’. Transplant Int. 2013;26:740–50. doi: 10.1111/tri.12118. [DOI] [PubMed] [Google Scholar]
- 10.Leithead JA, Ferguson JW, Bates CM, et al. Chronic kidney disease after liver transplantation for acute liver failure is not associated with perioperative renal dysfunction. Am J Transplant. 2011;11:1905–15. doi: 10.1111/j.1600-6143.2011.03649.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishi H, Shibagaki Y, Kido R, et al. Chronic renal outcome after living donor liver transplantation. Clin Transplant. 2013;27:90–97. doi: 10.1111/ctr.12013. [DOI] [PubMed] [Google Scholar]
- 12.De Simone P, Nevens F, De Carlis L, et al. H2304 Study Group. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am J Transplant. 2012;12:3008–20. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saliba F, De Simone P, Nevens F, et al. H2304 Study Group. Renal function at two years in liver transplant patients receiving everolimus: Results of a randomized, multicenter study. Am J Transplant. 2013;31:1734–45. doi: 10.1111/ajt.12280. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh ST. Using trapezoidal rule for the area under a curve calculation. Proceedings of SUGI-27; Orlando, Florida, USA. April 14–17; Paper 229–27 (Poster). Available at http://www2.sas.com/proceedings/sugi27/p229-27.pdf. [Google Scholar]
- 16.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: The debate should be about patient prognosis – a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–20. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Cea Soriano L, Johansson S, Stefansson B, Rodriguez LA. Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with type 2 diabetes: Associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol. 2015;14:38. doi: 10.1186/s12933-015-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira JP, Girerd N, Pellicori P, et al. Renal function estimate and Cockcroft-Gault formulas for predicting cardiovascular mortality in population-based, cardiovascular risk, heart failure and post-myocardial infarction cohorts: The Heart ‘Omics’ in AGEing (HOMAGE) and the high-risk myocardial infarction database initiatives. BMC Med. 2016;14:181. doi: 10.1186/s12916-016-0731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugts JJ, Knetsch AM, Mattace-Raso FU, et al. Renal function and risk of myocardial infarction in an elderly population: the Rotterdam Study. Arch Intern Med. 2005;165:2659–65. doi: 10.1001/archinte.165.22.2659. [DOI] [PubMed] [Google Scholar]
- 20.Wright J, Hutchison A. Cardiovascular disease in patients with chronic kidney disease. Vasc Health Risk Manag. 2009;5:713–22. doi: 10.2147/vhrm.s6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman JR. Chronic calcineurin inhibitor nephrotoxicity-lest we forget. Am J Transplant. 2011;11:693–97. doi: 10.1111/j.1600-6143.2011.03504.x. [DOI] [PubMed] [Google Scholar]
- 22.Fischer L, Klempnauer J, Beckebaum S, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation – PROTECT. Am J Transplant. 2012;12:1855–65. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 23.Masetti M, Montalti R, Rompianesi G, et al. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10:2252–62. doi: 10.1111/j.1600-6143.2010.03128.x. [DOI] [PubMed] [Google Scholar]
- 24.Sterneck M, Kaiser GM, Heyne N, et al. Long-term follow-up of five yr shows superior renal function with everolimus plus early calcineurin inhibitor withdrawal in the PROTECT randomized liver transplantation study. Clin Transplant. 2016;30:741–48. doi: 10.1111/ctr.12744. [DOI] [PubMed] [Google Scholar]
- 25.Holdaas H, de Fijter JW, Cruzado JM, et al. ELEVATE Study Group. Cardiovascular parameters to 2 years after kidney transplantation following early switch to everolimus without calcineurin inhibitor therapy: An analysis of the randomized ELEVATE Study. Transplantation. 2017;101:2612–20. doi: 10.1097/TP.0000000000001739. [DOI] [PubMed] [Google Scholar]
- 26.Raichlin E, Chandrasekaran K, Kremers WK, et al. Sirolimus as primary immunosuppressant reduces left ventricular mass and improves diastolic function of the cardiac allograft. Transplantation. 2008;86:1395–400. doi: 10.1097/TP.0b013e318189049a. [DOI] [PubMed] [Google Scholar]
- 27.Kushwaha SS, Raichlin E, Sheinin Y, et al. Sirolimus affects cardiomyocytes to reduce left ventricular mass in heart transplant recipients. Eur Heart J. 2008;29:2742–50. doi: 10.1093/eurheartj/ehn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murbraech K, Massey R, Undset LH, et al. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients – a three-yr serial echocardiographic substudy of the randomized controlled CENTRAL trial. Clin Transplant. 2015;29:678–84. doi: 10.1111/ctr.12565. [DOI] [PubMed] [Google Scholar]
- 29.Seckinger J, Sommerer C, Hinkel UP, et al. Switch of immunosuppression from cyclosporine A to everolimus: Impact on pulse wave velocity in stable de-novo renal allograft recipients. J Hypertens. 2008;26:2213–19. doi: 10.1097/HJH.0b013e32830ef940. [DOI] [PubMed] [Google Scholar]
- 30.Joannidès R, Monteil C, de Ligny BH, et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant. 2011;11:2414–22. doi: 10.1111/j.1600-6143.2011.03697.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosing K, Fobker M, Kannenberg F, et al. Everolimus therapy is associated with reduced lipoprotein-associated phospholipase A2 (Lp-Pla2) activity and oxidative stress in heart transplant recipients. Atherosclerosis. 2013;230:164–70. doi: 10.1016/j.atherosclerosis.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Vitiello D, Neagoe PE, Sirois MG, White M. Effect of everolimus on the immunomodulation of the human neutrophil inflammatory response and activation. Cell Mol Immunol. 2015;12:40–52. doi: 10.1038/cmi.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster AC, Lee LW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: A systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–48. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- 34.Holdaas H, Potena L, Saliba F. mTOR inhibitors and dyslipidemia in transplant recipients: A cause for concern? Transplant Rev (Orlando) 2015;29:93–102. doi: 10.1016/j.trre.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Burra P, Senzolo M, Masier A, et al. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41:350–56. doi: 10.1016/j.dld.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 36.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–85. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
First major adverse cardiac events to month 24, by MedDRA preferred term, according to treatment group (ITT population).
| EVR/rTAC N=245 | TAC Elimination N=231 | TAC Control N=243 | |
|---|---|---|---|
| Acute myocardial infarction | 1 | 0 | 2 |
| Angina pectoris | 1 | 1 | 4 |
| Angina unstable | 1 | 0 | 0 |
| Cardiac failure | 2 | 0 | 4 |
| Cardiac failure congestive | 0 | 1 | 1 |
| Coronary artery arteriosclerosis | 1 | 0 | 0 |
| Coronary artery disease | 2 | 0 | 3 |
| Coronary artery insufficiency | 0 | 1 | 0 |
| Ischemic stroke | 0 | 0 | 1 |
| Myocardial infarction | 0 | 1 | 0 |
| Myocardial ischemia | 1 | 0 | 1 |
| Pulmonary edema | 1 | 0 | 0 |
| Sudden death | 0 | 1 | 1 |
| Total | 10 | 5 | 17 |


