Abstract
Background
The aim of this study was to investigate serum concentrations of visfatin, irisin, and omentin in patients with end-stage lung diseases (ESLD) before and after lung transplantation (LTx) and to find relationship between adipokines levels and clinical outcomes.
Material/Methods
Fourteen consecutive lung transplant recipients (six males and seven females; age 32.0±14.2 years; body mass index (BMI) 21.8±5.3 kg/m2) who underwent lung transplantation with initial diagnosis of respiratory failure due to cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF) were included. Visfatin, irisin, and omentin serum levels were assayed using commercially available ELISA kits at four time points: the day of LTx (day 0), 72 hours (day 3), one month (day 30) and three months (day 90) after LTx.
Results
Omentin serum concentration decreased significantly within three days after LTx (350.5±302.0 to 200.0±0.90 ng/mL; p<0.05), while visfatin serum levels decreased later, 30 days after Ltx (4.81±3.78 to 0.78±0.35 [0.4–1.1] pg/mL; p<0.05). Downregulated serum levels of both adipokines remained stable for the next two months (256.0 [201.7–642.9] ng/mL and 0.77±0.76 pg/mL, respectively; p<0.05). Serum levels of irisin were unchanged before and after Ltx. Immunosuppressive regimen did not affect serum levels of the analyzed adipokines.
Conclusions
The study showed for the first time serum omentin and visfatin levels to be decreased after LTx in ESLD patients. Successful LTx contributes to the improvement of impaired lung function parameters and attenuation of ongoing inflammatory process, resulting in altered visfatin and omentin serum levels. Additional influence of immunosuppressive treatment on omentin and visfatin serum concentration cannot be excluded.
MeSH Keywords: Adipokines, Immunosuppressive Agents, Lung Transplantation
Background
Lung transplantation is a therapeutic, medical-surgical procedure indicated for end-stage-lung diseases (ESLDs), which are terminal and irreversible with available current medical treatment. Obesity or overweight, insulin resistance, or diabetes may contribute to chronic inflammation and exacerbate ESLDs progression.
Adipokines are the bioactive proteins that manifest hormone-like attributes with properties similar to some chemokines, cytokines and growth factors. Adipokines also regulate fibrogenesis, angiogenesis, and autoimmune processes [1,2]. Therefore, changes in the circulatory levels of some adipokines appear to have a proatherogenic, pro-inflammatory, and anti-insulin effects [3].
Visfatin is an adipokine mainly expressed in visceral fatty tissue but also is produced in the myocardium, skeletal muscle, lungs, immune cells, bone marrow, and liver [4]. Visfatin undergoes self-phosphorylation which enhances its catalytic function in nicotinate and nicotinamide metabolism [4]. Increased visfatin serum levels were found in some diseases associated with obesity, atherogenesis, insulin-resistance, and inflammatory process [5–11].
It has been considered as a pro-inflammatory and immunomodulating factor, which is also associated with vascular smooth muscle cell maturation and plaque destabilization [4,6]. Visfatin, known as pre-B-cell colony-enhancing factor-1 (PBEF) was originally cloned as a putative cytokine shown to enhance the maturation of B-cell precursors in the presence of interleukin-7 (IL-7) and stem cell factor [12]. It enhances activation of leukocytes, synthesis of adhesion molecules, production of proinflammatory cytokines, inhibits neutrophil apoptosis, stimulates proangiogenic activity and affects cancer cells proliferation and viability [7,8,12–16].
Irisin is a novel myokine, proteolytically processed from the product of the FNDC5 gene prior to being released into the circulation [17]. Irisin is regulated by peroxisome proliferator-activated receptor-coactivator-1 (PGC1) and it has been proposed to mediate the beneficial effects of exercise on metabolism, inducing the browning of subcutaneous adipocytes and thermogenesis by increasing uncoupling protein-1 (UCP1) levels [17]. In humans, contradictory effects of physical exercise on irisin production have been reported [18–20]. The circulating irisin concentration levels were inversely correlated with adiponectin and positively with body mass index (BMI), fasting glucose and total cholesterol [21]. Irisin is an exercise-induced myokine that is secreted into the circulation following proteolytic cleavage from its cellular form, fibronectin-type III domain-containing 5 (FNDC5) [22]. It reverses diet-induced obesity and diabetes by stimulating thermogenesis in rodents through increasing brown adipocyte-like cell abundance [22,23] within white fat. As it appears paradoxical that exercise should increase secretion of a thermogenic hormone, it has been hypothesized that the mechanism evolved from shivering-related muscle contraction to augment nonshivering thermogenesis (NST) through brown adipose tissue expansion [17,22].
Omentin is a novel adipokine that is shown to be decreased in obesity and negatively associated with BMI, insulin resistance, and metabolic syndrome in adults [24–29]. The biological functions of omentin are not fully known, however, there are some reports about its role in energy homeostasis. It is suggested that it enhances insulin sensitivity, transmembrane transport of glucose stimulated by insulin in isolated human adipocytes, and regulates fat distribution [25,30]. Omentin inhibits C-reactive protein (CRP), tumor necrosis factor (TNF) α and nuclear factor (NF) κB signaling pathways, reduces adhesion molecules and has anti-inflammatory effect on smooth muscles and endothelium and it may be protective in lung injury [31–33].
The primary aim of the study was to assess the variability of adipokine levels in patients with ESLDs before and after lung transplantation. The overarching goal was to find the relationship between adipokines and clinical outcomes. Assessment of adipokine levels will facilitate a better understanding of their role in ESLDs before and after transplantation, and help to determine their potential usefulness as prognostic factors.
Material and Methods
The study population included six men and eight women (mean age 32.0±14.2 years) who underwent LTx at Department of Cardiac Surgery and Transplantation at Silesian Centre for Heart Diseases in Zabrze is characterized in Table 1. Three study patients had received single lung transplantation (SLT; one left lung and two right lungs), and 11 patients had received double lung transplantation (DLT). The referring diagnosis before LTx was cystic fibrosis (n=8), chronic obstructive pulmonary disease (COPD) (n=3), idiopathic pulmonary fibrosis (IPF) (n=2), and arteriovenous fistulas (n=1). Patient’s mean waist circumstance (WC) was 75.0±4.1 cm and mean hip circumference (HC) 88.4±6.5 cm. The mean body mass index (BMI) was 18.0±1.5 kg/m2. All patients have received intravenous anti-thymocyte globulin (ATG) as induction agent for a minimum of three days at average dose of 75 mg, but different maintenance immunosuppressive regimen, i.e., cyclosporine (CSA, n=11), mycophenolate mofetil (MM, n=7), tacrolimus (FL-506, n=3), sirolimus (n=4) and prednisolone (n=14). Maintenance immunosuppression was started post-transplant day 4–7, target level of cyclosporine is (250–300 ng/mL), tacrolimus (15–17 μg/mL), and sirolimus (8–12 ng/mL) and these were reached 10–14 days after LTx.
Table 1.
Characteristic of the study group.
| Parameters | LTx patients | Normal range |
|---|---|---|
| N (male/female) | 14 (6/8) | – |
| Age [years] | 32.0±14.2 | – |
| WC [cm] | 75.0±4.1 [70.5–78.8]* | – |
| HC [cm] | 88.1±6.4 | – |
| BMI [kg/m2] | 18.0±1.5 [17.0–20.1]* | 18.5–24.99 |
| RBC [×106/ul] | 4.6±0.86 | 4.5–5.9 |
| WBC [×103/ul] | 15.7±8.08 | 4.3–10 |
| HGB [mmol/l] | 8.1±1.35 | 8.7–11.2 |
| PLT [×103/ul] | 176.5±84.75 [127.3–296.8]* | 150–350 |
| INR | 1.2±0.23 | 0.85–1.3 |
| AST [U/l] | 23.0±5.38 [17.5–28.3]* | 10–34 |
| ALT [U/l] | 15.5±5.75 [12.0–23.5]* | 6–44 |
| GGTP [U/l] | 16.0±12.13 [10.0–34.3]* | 5–61 |
| Cr [μmol/l] | 62.2±24.48 | 62–106 |
| HDL [mmol/l] | 0.3±0.13 [0.2–0.5]* | 0.9–1.7 |
| LDL [mmol/l] | 1.8±0.87 | 1.3–3.36 |
| TG [mmol/l] | 2.5±1.37 | 0.4–1.82 |
| Protein [g/l] | 75.3±15.09 | 60–80 |
| Albumin [g/l] | 36.9±8.52 | 35–50 |
| CRP [mg/l] | 12.6±14.58 | <5 |
| Distance in 6MWT [m] | 332.0±128.8 | – |
| FEV1%N | 25.3±17.51 [20.0–55.0]* | 80–120 |
| FVC%N | 53.4±23.5 | 80–120 |
Median ±quartile deviation [lower-upper quartile];
WC – waist circumstance; HC – hip circumference; FEV1 – Forced Expiratory Volume; FVC – Forced Vital Capacity; %N – the percentage of a due value.
Patients were classified into three groups of immunosuppressive drugs (CSA n=7; FK-506, n=3 and CSA+sirolimus, n=4).
Donor characteristics, including donor age, BMI, cold ischemia time, and partial pressure of oxygen (on 100% fraction of inspired oxygen just before lung harvest), are presented in Table 2.
Table 2.
Characteristic of donors.
| Parameters | Donors |
|---|---|
| N (Male/Female) | 14 (9/5) |
| Age [years] | 33.9±12.8 |
| BMI [kg/m2] | 22.5±2.0 |
| Cold ischemia [min] | 370.9±87.1 |
| PaO2 [mmHg] | 460.2±79.0 |
PaO2 – partial pressure of oxygen.
No episodes of acute rejection were observed in the study group, and during 3-month observation period no episodes of chronic rejection were observed.
Blood samples were collected upon admission to the hospital before transplantation (day 0) and three days (day 3), one month (day 30), and three months (day 90) after LTx.
Blood samples were collected from an antecubital vein and then placed into a BD Vacutainer.
For adipokines analysis, blood samples were immediately centrifuged at 2,500× g for 20 minutes to obtain serum and stored at −80°C, thawing only once immediately before analysis.
Adipokines and myokine concentration levels were determined enzymatically using commercial ELISA Kits (Human Visfatin (Nampt) ELISA, Catalogue no. RAG004R; IRISIN ELISA, Catalogue no. RAG018R; HUMAN OMENTIN-1 ELISA Catalogue no. RD191100200R; by BioVendor, Czech Republic). The sensitivity of the sets was adequately 30 pg/mL for visfatin, 1 ng/mL for irisin and 0.5 ng/mL for omentin. The test precision within an assay for visfatin is 5.63%, for irisin it is 6.909% and for omentin it is 3.65%. The test precision for an assay for visfatin is 5.93%, for irisin it is 9.066%, and for omentin it is 4.6%. The visfatin levels range from healthy donors in serum is from 0.2 to >1.5 ng/mL. The irisin level range in human plasma and serum is from 0.2 to 2 μg/mL. The omentin range depends on age and sex and is from 200 to 1,399 ng/mL.
Medical assessment included spirometry using Jaeger-Masterlab (Erich Jaeger GmbH, Wurtzburg, Germany) and six-minute walking test according to Guyatt and ATS Statement [34,35].
After lung transplantation no one of the study group represent respiratory failure.
Informed consent was obtained in each case. The study was approved by the Ethics Committee of the Medical University of Silesia in Katowice (approval number KNW-1-161/N/4/0 and KNW-1-195/K/5/0) and was in compliance with the ethical guidelines of the Declaration of Helsinki.
Statistical analysis
The results of continuous variables are expressed as mean ±SD for data with normal distribution and median and quartile deviation for the other. The normality of the distribution was assessed using Shapiro-Wilk W-test.
Comparisons between analyzed parameters were made using the Student t-test, Mann-Whitney U test, Wilcoxon matched-pairs test and Kruskal-Wallis H test. Correlations between parameters were calculated using the Spearman’s rank correlation test. Statistical significance was set at the p level <0.05. All statistical analysis was performed using Statistica 10.0 (StatSoft, Poland).
Results
Visfatin concentration decreased significantly 30 days after LTx (4.81±3.78 before versus 0.77±0.76 pg/mL 30 days after; p<0.05). Decreased visfatin levels have remained unchanged for the next two months (Figure 1). There was no change in irisin serum levels before and after transplantation (Figure 2). Omentin level decreased significantly within three days after transplantation (from 350.5±302.0 ng/mL to 200.0±0.90 ng/mL; p<0.05) (Figure 3), in further observation significantly changes were also observed when matched-pairs test was calculated (Table 3.).
Figure 1.
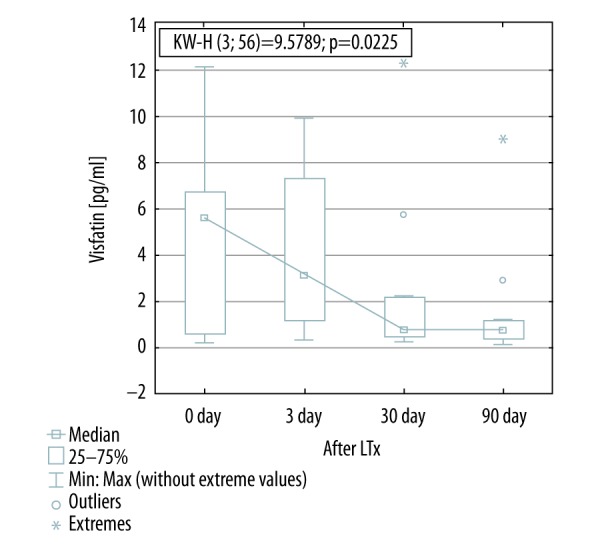
Visfatin concentration before and after lung transplantation. KW-H, Kruskal-Wallis H test.
Figure 2.
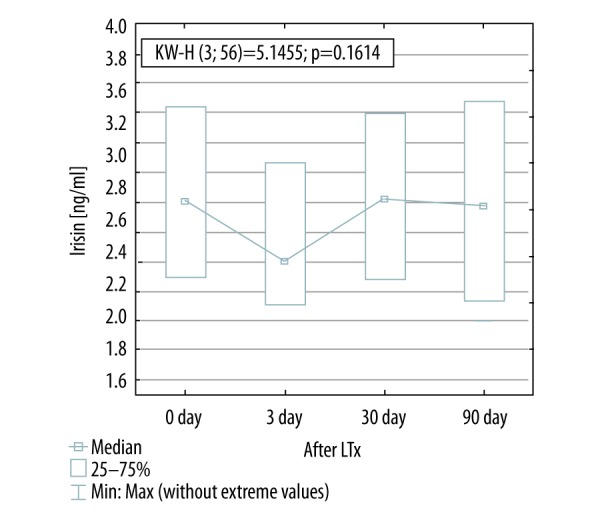
Irisin concentration before and after lung transplantation. KW-H, Kruskal-Wallis H test.
Figure 3.
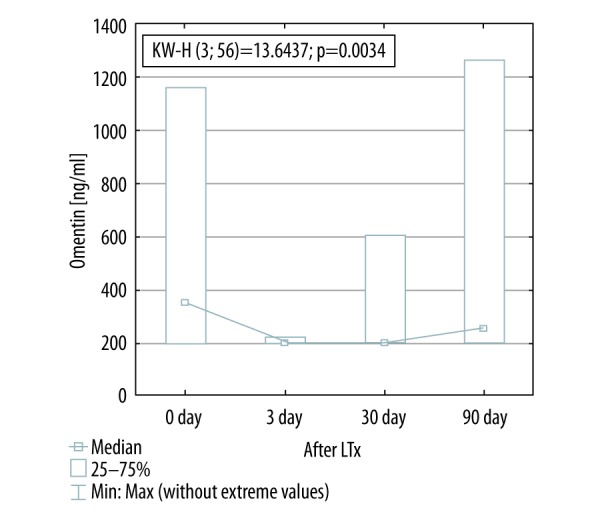
Omentin concentration before and after lung transplantation. KW-H, Kruskal-Wallis H test.
Table 3.
Distribution of adipokines concentration and laboratory parameters before and after lung transplantation.
| Parameters | Before | 72 h after | 1 month after | 3 months after |
|---|---|---|---|---|
| Visfatin [pg/ml] | 4.81 ±3.78***,**** | 4.07 ±3.23**** | 0.77±0.76 [0.5–2.0]* | 0.78 ±0.35 [0.4–1.1]* |
| Irisin [ng/ml] | 2.77 ±0.24** | 2.53 ±0.32 | 2.78 ±0.30 | 2.77 ±0.36 |
| Omentin [ng/ml] | 350.5 ±302.0**,*** [202.3–806.4]* | 200.1 ±0.9***,**** [199.7–201.5]* | 202.3±33.0**** [199.9–266.0]* | 256.0 ±220.6 [201.7–642.9]* |
| Glucose [mmol/l] | 5.36 ±0.76 | 5.69 ±1.09 | 5.87±1.34**** | 5.38 ±0.83 |
| Insulin [mU/l] | 7.04 ±1.27 [6.6–9.2]* | 7.59 ±2.81*** [6.9–12.5]* | 6.92±0.71 [6.6–8.0]* | 7.46 ±1.77 [6.9–10.5]* |
| HOMA-IR | 1.78 ±0.28 [1.4–2.0]* | 1.90 ±0.67 [1.7–3.1]* | 2.12 ±0.63 | 1.87 ±0.58 [1.5–2.7]* |
| HOMA-IR ≥2.5 number of patients | 3 | 4 | 3 | 4 |
| Total cholesterol [mmol/l] | 3.26 ±1.23***,**** | 3.40 ±1.21***,**** | 4.82 ±1.88 | 4.76 ±1.67 |
| HbA1C [%] | 5.64 ±0.65*** | 5.78 ±0.50***,**** | 5.16 ±0.77 | 5.36 ±0.57 |
Median ±quartile deviation [lower-upper quartile]; statistically significant changes with p<0.05:
vs. 72 hours after transplantation;
vs. 1 month after transplantation;
vs. 3 months after transplantation.
The detailed analysis of adipokine levels before and after lung transplantation is shown in Table 3.
There was no association between serum levels of analyzed adipokines and BMI, WC, HC, and age before transplantation.
However, serum concentrations of analyzed adipokines were correlated with fasting glucose, insulin, HOMA-IR, total cholesterol, and HbA1C levels. The positive correlation between irisin and total cholesterol before transplantation (r=0.66; p<0.05), omentin and total cholesterol one month after transplantation (r=0.69; p<0.05), omentin and total cholesterol three months after transplantation (r=0.77; p<0.05) was found. Omentin was negatively associated with HbA1C three months after LTx (r=−0.54; p<0.05). HOMA-IR was negatively related to irisin one month after (r=−56; p<0.05), and omentin three months after LTx (r=−0.58; p<0.05).
Relationship between immunosuppressive regimens and adipokine serum levels
Patients were classified into three groups with respect to immunosuppressive drug regimen (CSA, n=7; FK-506, n=3 and CSA+sirolimus, n=4). There was no difference in visfatin and omentin serum levels between patients administered CSA, FK-506, and CSA+sirolimus. However, significant difference were found in serum irisin levels between FK-506 and CSA+sirulimus subgroups one month after LTx (2.45±0.05 ng/mL versus 3.10±0.24 ng/mL; p=0.01) and three months after LTx (2.45±0.33 ng/mL versus. 3.20±0.25 ng/mL; p=0.03).
Relationship between spirometric parameters, distance in 6MWT and adipokine serum levels
There was no statistically significant correlation between adipokines levels and 6MWT, pO2, FEV1, and FVC in patients before lung transplantation, regardless of underlying lung disease.
FVC (percentage of a due value) measured three months after LTx correlated positively with serum irisin at the same time point (r=0.72; p<0.05). The distance in 6MWT reached in examination three months after LTx was positively associated with serum irisin at the same time point (r=0.64; p<0.05). To assess adipokines plasma levels and pulmonary function it was calculated correlation between delta (difference before and after transplantation) distance in 6MWT. Significant correlation was found between visfatin levels one month after transplantation and delta6MWT (r=0.62; p<0.05).
Relationship between donor parameters and adipokine serum levels
Negative statistically significant correlation was found between age of donors and visfatin serum levels one months after transplantation (r=0.5818, p=0.047). No correlation was observed between BMI, paO2, and cold ischemia of donors and adipokine levels of recipients.
Discussion
ESLD patients represent very low physical activity and an increased chronic inflammatory process. Every LTx patient receives a strong immunosuppression that significantly modulates the inflammatory response.
As far as we know, this is the first study aimed at assessment of novel adipokines and myokine serum levels in patients undergoing LTx and evaluation of relationship between these adipokines and myokine and clinical outcomes.
The first intriguing finding refers to visfatin, a pro-inflammatory, immunomudulating and proapoptotic adipokine. Visfatin concentration decreased significantly one month after LTx, and has remained unchanged for the next two months. Visfatin potentiates TNFα and IL-6 production in human peripheral blood mononuclear cells (PBMCs) [36]. Visfatin also may induce vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 synthesis directly in endothelial cells and leukocytes by activation of nuclear factor (NF-κβ) [36,37]. These findings suggest that visfatin directly, together with TNFα, or through the induction of TNFα, may enhance adhesion molecules production and therefore plays a pivotal role in the regulation of necro-inflammatory process in the lungs and facilitates migration of immune cells to the site of inflammation. In the lungs, visfatin was associated with acute lung injury (ALI) [38]. The inhibition of visfatin synthesis has been shown to attenuate inflammation and apoptosis associated with severe virus infection in lung endothelium [36]. Visfatin levels correlate positively with IL-6 in patients with COPD but not in healthy controls [39]. Additionally, serum visfatin was positively associated with TNFα and IL-8. Visfatin was associated with parenchymal impairment in patients with emphysematous COPD. This suggests that visfatin may have a pivotal role in the inflammatory processes in COPD. A negative correlation was found between visfatin and pulmonary diffusion capacity indicating visfatin to be associated with parenchymal impairment [39]. Patients referred for LTx presented significantly impaired lung function parameters (FEV1, VC) and poor physical activity (6MWT), which evidently improved after successful LTx. In our study, a significant decrease in serum visfatin levels was found one month after LTx, and was stable at the same level in two months. It correlated with improvement in lung function tests and 6MWT. Moreover, no study group patient represented respiratory failure after LTx. This finding is in accordance with data published by Liu et al. who described that normoxemia may contribute to decreased visfatin concentration [40].
Our study results suggest that with regard to the proinflammatory action of visfatin, which promotes proinflammatory cytokine synthesis, the inflammation associated with ESLD of the newly transplanted organ significantly decreases after LTx.
Another explanation of downregulation of visfatin accompanied by decreased inflammation may be related to introduction of immunosuppression. There have not been any data published on relationship between immunosuppression and visfatin levels so far.
According to the International Society for Heart and Lung Transplantation (ISHLT) consensus document for the selection of lung transplant candidate’s, the level of physical activity of these patients is very low.
Irisin is secreted by skeletal myocytes and might potentially be used as a biomarker of physical inactivity in ESLDs. In a study of Ijiri et al., a non-significant correlation between 6MWD, PFTs and serum level of irisin in COPD patients. They showed that irisin concentration in these patients was lower than in a control group. In this study, exercise training has resulted in upregulation of irisin levels. At the cellular level, exercise training may improve bioenergetic functions of skeletal muscle, including the irisin release in patients with COPD [41]. Our research does not support these conclusions despite the fact that the level of mobility of patient improved. Despite daily rehabilitation, improved mobility, lack of respiratory failure, the concentration of irisin remained at the same level throughout the course our study.
No study so far has been conducted on omentin in ESLD patients qualified for LTx. Omentin is an adipokine with anti-inflammatory and insulin-sensitizing effect. It has been shown to activate AMP-activated protein kinase (AMPK) and eNOS, inhibiting protein kinase B(PKB/Akt) pathways at the same time. Moreover, it inhibits CRP, TNFα, and NF κB signaling pathways, reduces adhesion molecules activity and synthesis, and thus has anti-inflammatory effect on smooth muscle cells and endothelium [33,42,43]. Administration with recombinant human omentin inhibits TNFα, decreases inflammation, and dilates vascular vessels, suggesting its potential therapeutic role in inflammation-related conditions [44]. Furthermore, omentin is needed for monocyte chemotactic protein (MCP)-1 production in lung epithelium and causes airway inflammation in mice with asthma. Additionally, given the fact that omentin blocks proinflammatory cytokines, TNFα, and signaling pathway of NF κB, it may be protective in lung injury. Furthermore, considering the similarity of omentin and adiponectin, we hypothesize that omentin exerts anti-inflammatory effect in lung injury. However, the potential proinflammatory effect of omentin may not be ignored as well.
Omentin level sharply decreased significantly within 72 hours after lung transplantation, without changes in further observation. Many other studies have shown increase in omentin levels as compensatory mechanism of insulin-resistance. Our study did not show any difference in HOMA-IR before and after LTx. This finding suggests that omentin level changes are not related to glucose metabolism or increased tissue sensitivity to insulin. We have also not found any correlation between omentin levels and lung function tests.
Our interesting finding of the study was the downregulation of two adipokines with different properties because visfatin is a proinflammatory factor and omentin has an anti-inflammatory effect. Visfatin is mainly expressed in visceral fatty tissue, but also is produced in lungs, immune cells, bone marrow, and liver. It is a proinflammatory and immunomodulating factor. In our study group because of low BMI values and thus a paucity of adipose tissue, the remaining tissues were mainly responsible for the secretion of this adipokine. Reducing the inflammatory disease associated with primary disease and the effect of immunosuppression inhibiting immune cells appears to be a major factor in the decline of this level of adipokine. The level of omentin correlates negatively to BMI, which may be higher in patients with low BMI. Omentin also inhibits C-reactive protein and has anti-inflammatory effect on smooth muscle and protects against lung damage. After a successful lung transplant, these effects may be less important and cause downregulation of this adipokine. Particularly, the decrease in serum level of omentin is already seen after 72 hours post-transplantation.
There were some limitations of our study. The group of patients was relatively small and not homogenous. Lung transplantation and postoperative treatment is a complex medical procedure, therefore, many other factors may be associated with adipokine level changes.
Most importantly, our study was not designed to address potential mechanisms behind the observed associations. Results of our study were not compared between two groups of sick patients with ESLD and non-advanced lung disease and healthy volunteers. Patients’ records lacked information on certain variables, including diffusing capacity (DLCO), serum levels of TNF-alpha and IL-6, and body plethysmography. Measurements of these parameters were not performed in all patients; therefore, they were not included in further analyses.
Conclusions
The present results introduce the adipocytokines visfatin, irisin, and omentin in patients before and after lung transplantation. The procedure of LTx influenced decreasing of plasma concentration of omentin and visfatin. There were no changes of serum concentration of irisin in patients who underwent lung transplantation at our institution. These findings suggest that visfatin and omentin might have a proinflammatory role in ESLD. Immunosuppressive drugs may affect irisin serum levels. Further studies are needed to evaluate if analyzed novel adipokines and myokine could be used as biomarkers in patients with ESLD.
Footnotes
Source of support: The financial support from Medical University of Silesia in Katowice was received by statutory work number KNW-1-161/N/4/0 and KNW-1-195/K/5/0
References
- 1.Kukla M, Mazur W, Bułdak RJ, Zwirska-Korczala K. Potential role of leptin, adiponectin and three novel adipokines-visfatin, chemerin and vaspin-in chronic hepatitis. Mol Med. 2011;17(11–12):1397–410. doi: 10.2119/molmed.2010.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14(11–12):741–51. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah A, Mehta N, Reilly MP. Adipose inflammation, insulinresistance and cardiovascular disease. J Parenter Enteral Nutr. 2008;32:38–44. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl TB, Yndestad A, Skjelland M, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possiblerole in inflammation and plaque destabilization. Circulation. 2007;115(8):972–80. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 5.Filippatos TD, Randeva HS, Derdemezis CS, et al. Visfatin/PBEF and atherosclerosis-related diseases. Curr Vasc Pharmacol. 2010;8(1):12–28. doi: 10.2174/157016110790226679. [DOI] [PubMed] [Google Scholar]
- 6.Liu SW, Qiao SB, Yuan JS, Liu DQ. Association of plasma visfatin levels with inflammation, atherosclerosis, and acute coronary syndromes in humans. Clin Endocrinol (Oxf) 2009;7(1):202–7. doi: 10.1111/j.1365-2265.2008.03453.x. [DOI] [PubMed] [Google Scholar]
- 7.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor(PBEF)/visfatin: Visfatin novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–16. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 8.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178(3):1748–58. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 9.Kukla M, Berdowska A, Gabriel A, et al. Association between hepatic angiogenesis and serum adipokine profile in non-obese chronic hepatitis C patients. Pol J Pathol. 2011;62(4):218–28. [PubMed] [Google Scholar]
- 10.Kukla M, Ciupińska-Kajor M, Kajor M, et al. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Pol J Pathol. 2010;61(3):147–53. [PubMed] [Google Scholar]
- 11.Kukla M, Zwirska-Korczala K, Gabriel A, et al. Visfatin serum levels in chronic hepatitis C patients. J Viral Hepat. 2010;17(4):254–60. doi: 10.1111/j.1365-2893.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 12.Samal B, Sun Y, Stearns G, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–37. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia SH, Li Y, Parodo J, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113(9):1318–27. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adya R, Tan BK, Punn A, et al. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: Novel insights into visfatininduced angiogenesis. Cardiovasc Res. 2008;78:356–65. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- 15.Bułdak RJ, Bułdak Ł, Polaniak R, et al. Visfatin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: An in vitro study. Oncol Rep. 2013;29(2):771–78. doi: 10.3892/or.2012.2175. [DOI] [PubMed] [Google Scholar]
- 16.Buldak RJ, Gowarzewski M, Buldak L, et al. Viability and oxidative response of human colorectal HCT-116 cancer cells treated with visfatin/eNampt in vitro. J Physiol Pharmacol. 2015;66(4):557–66. [PubMed] [Google Scholar]
- 17.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–68. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488:9–10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 19.Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61(12):1725–38. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecker SH, Zavin A, Cao P, et al. Expression of the Irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5(6):812–18. doi: 10.1161/CIRCHEARTFAILURE.112.969543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):769–78. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 22.Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrovic N, Walden TB, Shabalina IG, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oswiecimska J, Suwala A, Swietochowska E, et al. Serum omentin levels in adolescent girls with anorexia nervosa and obesity. Physiol Res. 2015;64:701–9. doi: 10.33549/physiolres.932841. [DOI] [PubMed] [Google Scholar]
- 25.De Sousa Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–61. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 26.Tan BK, Adya R, Farhatullah S, et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: Ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–8. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 27.Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 28.El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med. 2011;28:1194–200. doi: 10.1111/j.1464-5491.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 29.Kukla M, Waluga M, Adamek B, et al. Omentin serum concentration and hepatic expression in chronic hepatitis C patients – together or apart? Pol J Pathol. 2015;66(3):231–38. doi: 10.5114/pjp.2015.54956. [DOI] [PubMed] [Google Scholar]
- 30.Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:1253–61. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 31.Wang C. Obesity, inflammation, and lung injury (OILI): The good. Mediators Inflamm. 2014;2014:978463. doi: 10.1155/2014/978463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazama K, Usui T, Okada M, et al. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012;686(1–3):116–23. doi: 10.1016/j.ejphar.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Zhong X, Li X, Liu F, et al. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem Bioph Res Commun. 2012;425(2):401–6. doi: 10.1016/j.bbrc.2012.07.110. [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 35.ATS Statement. Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–17. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 36.Gao W, Mao Q, Feng AW, et al. Inhibition of pre-B cell colony-enhancing factor attenuates inflammation and apoptosisinduced by pandemic H1N1 2009 in lung endothelium. Resp Physiol Neurob. 2011;178(2):235–41. doi: 10.1016/j.resp.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Wijesekara N, Krishnamurthy M, Bhattacharjee A, et al. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285(44):33623–31. doi: 10.1074/jbc.M109.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye SB, Simon BA, Maloney JP, et al. Pre-B-cell colonyenhancing factor as a potential novel biomarker in acute lung injury. Am J Resp Crit Care. 2005;171(4):361–70. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 39.Leivo-Korpela S, Lehtimäki L, Hämälainen M, et al. Adipokines NUCB2/Nesfatin-1 and Visfatin as novel inflammatory factors in chronic obstructive pulmonary disease. Mediat Inflam. 2014;2014:232167. doi: 10.1155/2014/232167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Ji Y, Chen J, et al. Circulating visfatin in chronic obstructive pulmonary disease. Nutrition. 2009;25(4):373–78. doi: 10.1016/j.nut.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Ijiri N, Kanazawa H, Asai K, et al. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology. 2015;20(4):612–17. doi: 10.1111/resp.12513. [DOI] [PubMed] [Google Scholar]
- 42.Yamawaki H, Kuramoto J, Kameshima S, et al. Omentin, a novel adipocytokine inhibits TNF induced vascular inflammation in human endothelial cells. Biochem Bioph Res Commun. 2011;408(2):339–43. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 43.Kazama K, Usui T, Okada M, et al. Omentin plays an anti-inflammatory role through inhibitionof TNF-α-induced superoxide production in vascular smoothmuscle cells. Eur J Pharmacol. 2012;686(1–3):116–23. doi: 10.1016/j.ejphar.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 44.Yamawaki H. Vascular effects of novel adipocytokines: Focuson vascular contractility and inflammatory responses. Biol Pharm Bull. 2011;34(3):307–10. doi: 10.1248/bpb.34.307. [DOI] [PubMed] [Google Scholar]


