Abstract
Background
Donor-specific antibodies (DSA), directed against human leucocyte antigens (HLA), are associated with increased risk for graft rejection in kidney transplantation. Anti-HLA antibodies detection by Luminex™ present high sensitivity and accuracy, but its interpretation after transplantation is not completely clear.
The aim of this study was to evaluate the impact of anti-HLA antibodies, preformed or de novo, on renal function, graft survival, and incidence of antibody-mediated acute rejection (AMR).
Material/Methods
A retrospective cohort of 86 kidney transplant recipients was divided into 3 groups according to the presence of anti-HLA antibodies before transplantation: donor-specific antibodies (DSA+, n=15), non-DSA (non-DSA, n=39), and negative pre-transplant panel reactive antibodies (PRA) that became positive after transplantation (PRA−, n=22). Forty-nine recipients with negative PRA pre- and post-transplantation were excluded. Antibody specificity and intensity of fluorescence (MFI) and their relationship with renal function, proteinuria, AMR, and graft failure were evaluated.
Results
Among patients who completed 1 year of follow-up, there was no significant difference in serum creatinine, estimated glomerular filtration rate, or proteinuria. AMR incidence was 9.5% in the DSA group, 2.3% in the non-DSA group, and 9.1% in the PRA− group. There was no correlation between fluorescence intensity and/or antibodies class (I or II) with increased risk of AMR. Thirteen grafts failed within 1 year post-transplant, there were 9 deaths due to infection, and only 1 due to AMR (PRA− group, DSA de novo at 3 months).
Conclusions
In contrast to previous reports, we did not find a correlation between incidence of AMR and MFI intensity in this series.
MeSH Keywords: Graft Rejection, Histocompatibility Antigens, Kidney Transplantation, Proteinuria
Background
Histocompatibility genes are involved in the immune response and rejection of transplanted tissues. In humans, histocompatibility molecules are called human leukocyte antigens (HLA), encoded by genes located in the short arm of chromosome 6. They are codominant genes, with expression of those of maternal and paternal origin. HLA is divided into 3 classes: I, whose genes encode HLA-A, B, and C molecules; II, which encode HLA-DR, DQ, and DP molecules; and III, which although classified within the HLA system, do not encode histocompatibility molecules but instead encode others with different functions in the immune system, such as complement components, cytokines, and enzymes. Class I antigens are constitutively expressed on the surface of all nucleated cells, while class II molecules are present only on the surface of antigen-presenting cells. Histocompatibility antigens can be identified by the complement-dependent antibody-mediated cellular cytotoxicity or by molecular biology techniques, as well as by deoxyribonucleic acid (DNA) extraction from nucleated cells and its subsequent amplification by polymerase chain reaction (PCR) [1].
Exposure to HLA antigens before or after transplantation can stimulate the production of antibodies against HLA antigens, both donor-specific (DSA) or non-DSA. In renal transplantation, the presence of DSA can cause acute or chronic rejection, even if pre-transplant complement-mediated cytotoxicity or flow cytometry cross-match tests are negative [2,3]. Anti-HLA antibodies subtypes have different pathogenicity [4] depending on their class, subclass, fluorescence intensity, and complement activation ability [5]. However, the cut-off level of antibody associated with a worse prognosis remains controversial [6]. The antibodies subclass phenotyping tests and their measurement of fluorescence intensity (MFI) with microspheres (Luminex™) [7] have high sensitivity and accuracy, although the clinical interpretation of their results and its relevance to the graft prognosis remain unknown [8]. Data from the Organ Procurement and Transplantation Network (OPTN) in patients on transplant waiting lists and transplant recipients in the United States shows an increase in the number of HLA mismatches between donors and recipients over the years [9]. Previous sensitization to HLA components, present in about 20% to 30% of the candidates enrolled in renal transplant waiting lists [9,10], is associated with an increase in waiting time on a transplant list and a higher risk for rejection after transplantation [10]. In the last decade, tracking the presence and intensity of anti-HLA antibodies after transplantation became a routine practice, although the correct interpretation of these results remains under debate.
The primary objective of this study was to evaluate the correlation between intensity and specificity of class I and II anti-HLA antibodies before and/or after renal transplantation, and the occurrence of rejection in kidney transplant recipients. Secondary objectives were to evaluate the effects of anti-HLA antibodies, and their specificity and intensity, on renal function and graft survival within 1 year after transplantation.
Material and Methods
Patients
We enrolled a retrospective cohort including renal transplant recipients from the Clinics Hospital – UNICAMP Renal Transplant Program. Inclusion criteria were: renal transplant recipients of living or deceased donors; older than 18 years at the time of transplantation; who had anti-HLA antibodies (donor-specific antibodies – DSA or non-DSA, classes I and II) detected before transplant and/or within 1 year after transplantation. Exclusion criteria were: patients younger than 18 years at the time of transplantation and persistence of negative panel reactive antibody (PRA) before and after transplantation.
All selected renal transplant recipients had previously been HLA typed and had been screened for anti-HLA antibodies on the waiting list for transplantation. All recipients had negative cross-match complement-cytotoxicity (CDC) prior to transplantation. Cross-matching by flow cytometry was not performed. Recipients from standard and expanded criteria donors were included according to the criteria proposed by the United Network for Organ Sharing (UNOS) in 2003 [11].
End-points were renal function, proteinuria and panel of anti-HLA antibodies at 12 months. Secondary end-points were graft loss, death with functioning graft, or loss to follow-up.
The study population was divided into 3 groups, according to the presence of anti-HLA antibodies before transplantation: DSA+, with donor-specific antibodies; non-DSA, with non-donor-specific antibodies; and PRA−, with negative PRA pre-transplant and positive PRA post-transplant (DSA and/or non-DSA).
The study protocol was approved by University of Campinas Ethics Committee.
HLA typing and detection of HLA antibodies
Receptor and donor HLA were typed by deoxyribonucleic acid (DNA) amplification by PCR with molecular primers sequences (LABType™ SSO and Micro SSP™, One Lambda Inc, California, USA). DNA was obtained from a peripheral blood samples. Established volumes of amplification primers, phosphatized deoxyribonucleotides (dNTPs), and Taq DNA polymerase were mixed with the pre-aliquoted DNA samples. After denaturation and neutralization, the material was subsequently homogenized with hybridization buffer and micro-pearls, and then labelled with Streptavidin, R-Phycoerythrin conjugate (SAPE). The reaction was read on the LABScan™ 100, which identified the fluorescence intensity of phycoerythrin in each microsphere. The generated file was imported into the HLA fusion software (One Lambda Inc, California, USA) for analysis. HLA-A and B and DRB1 were routinely identified and, in cases where recipients also had anti-DQ antibodies, donor HLA-DQ antigens were also identified.
For detection of anti-HLA antibodies, recipient’s peripheral blood samples collected before and after transplant were incubated with microspheres labelled with class I and II HLA antigens (LABScreen™ Single Antigen HLA Class I LS1A04 and LABScreen™ Single Antigen HLA Class II LS2A01). Tests for antibody detection consisted of a panel of pearls encoded by color, which are coated with purified HLA antigens. Up to 100 different beads can be combined into 1 suspension for a single test. The HLA antibodies present in the serum bound to the antigens and were labelled with R-Phycoerythrin (PE)-goat anti-human conjugate. The flow analyzer LABScan™ 100 detected the fluorescent emission of PE of each pearl, and the result was analyzed by HLA fusion software (One Lambda Inc, California, USA). PRA was calculated based on the prevalence of HLA alleles of organ donors from São Paulo, Brazil, which is usually updated every 6 months and had about 2750 records by the time of the study.
Anti-human globulin-enhanced complement-dependent cytotoxicity-negative T cell cross-matches and NIH complement-dependent cytotoxicity B cell cross-matches were required for all kidney transplant recipients at the time of transplant, performed with the most recently collected recipient’s serum and donor’s cells obtained from lymph nodes or spleen. In case of recent recipient vaccination or transfusion, an additional CDC test was performed with another serum sample collected at the time of convocation for transplant. Only patients with negative T and B cell CDC were transplanted. All donor-recipient pairs were ABO-compatible.
According to service guidelines and regional and national laws, all kidney transplant recipients were HLA typed and screened to anti-HLA antibodies at the time of registry in the transplant waiting list. The panel reactive antibody (PRA) was calculated every 6 months and transplant candidate serum was collected every 3 months for possible cross-match testing. Information obtained by Luminex™ was used to guide post-transplantation immunosuppressive strategies and to define the need for monitoring of anti-HLA antibodies during post-transplant follow-up. There were no desensitization protocols in the center.
Immunosuppressive therapy and diagnosis of rejection
Induction immunosuppressive therapy in case of standard kidney donor and recipient with low immunological risk consisted of monoclonal anti-IL-2 receptor antibodies basiliximab 20 mg, IV, on the day of transplantation and on the 4th day post-transplant. In recipients of kidneys from expanded-criteria donors, non-identical HLA living donors or recipients considered as high immunological risk (DSA or panel reactive antibody higher than 50%), induction therapy was of IV anti-thymocyte globulin 3 to 7 mg/kg, dose-adjusted by lymphocytes count. All transplant patients received methylprednisolone 500 mg IV at the time of transplantation and remained on steroid therapy during the follow-up. Maintenance immunosuppression consisted of a combination of calcineurin inhibitor (tacrolimus 0.1 mg/kg bid, dose-adjusted according to blood levels) and sodium mycophenolate 720 mg bid, adjusted according to body surface, gastrointestinal tolerance, and white and red cell count in peripheral blood. None of the included patients received desensitization protocol before transplantation.
Rejection was suspected by increase in serum creatinine or new-onset proteinuria and confirmed by allograft biopsy graded according to the Banff 2013 classification, revised 2015 [12]. Paraffin-fixed biopsies were stained with monoclonal anti-C4d antibody, as previously described [13]. Positivity of C4d in peritubular capillaries was scored from 0 to 3+. Diagnosis of antibody-mediated acute rejection (AMR) was based on histologic criteria and presence of donor-specific antibodies, according to Banff classification [12].
Data collection and outcomes
Clinical and laboratory data were retrospectively collected from medical records and Renal Transplant Program databases, at the time of transplantation and at 1, 3, 6, and 12 months after transplantation. Data were transcribed and organized into a Microsoft™ Excel worksheet.
Primary outcomes were: allograft function, estimated by the study equation of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (14); proteinuria, evaluated by determining the urinary protein-to-creatinine ratio; specificity and intensity of anti-HLA antibodies at 12 months after transplantation. Secondary outcomes included a composite graft survival, considering graft failure, return to dialysis, death with a functioning graft, and loss to follow-up.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or as median and range and/or percentages. Continuous variables among the groups were compared using the Kruskal-Wallis test, whereas categorical variables were compared using Pearson chi-square tests. Graft survival was assessed using Kaplan-Meier analysis. Values of p<0.05 were considered statistically significant. Data were analyzed with the GraphPad Prism 7.0c™ for Mac (La Jolla CA, USA).
Results
General characteristics according to anti-HLA antibodies before transplantation
From a transplant group of 548 renal transplant recipients from 2012 to 2016, 91 fulfilled the inclusion criteria. Five were excluded because of incomplete donor HLA typing information. Forty-nine recipients without pre-transplant anti-HLA antibodies remained with undetectable antibodies during the first-year follow-up and were excluded from the general analysis. The remaining 86 recipients were distributed into 3 groups according to the presence and the specificity of anti-HLA antibodies before transplantation (Figure 1).
Figure 1.
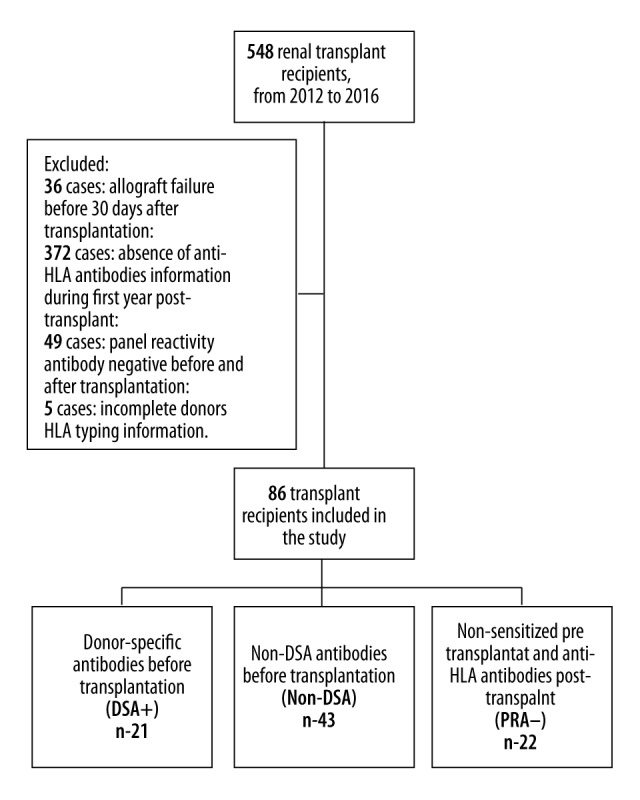
Study population and analyzed groups.
The majority of included patients were male recipients, and groups were similar in age, etiology of chronic kidney disease, length of pre-transplant dialysis, number of previous sensitizing events (transfusion, previous transplantation or pregnancies), donor serum creatinine, cold ischemia time, and need for and number of dialysis sessions before hospital discharge post-transplant (Table 1). As expected, according to our protocol, induction therapy with polyclonal anti-thymocyte globulin was more frequent in previously sensitized recipients (93.3% DSA+ group and 76.9% non-DSA group).
Table 1.
General characteristics of the groups according to the presence of anti-HLA antibodies before transplantation.
| DSA+ (n=21) | Non-DSA (n=43) | PRA− (n=22) | p | |
|---|---|---|---|---|
| Transplant recipients | ||||
| Age (years) | 44.2±10.6 | 45.7±11.2 | 42.5±13.15 | 0.81 |
| Male, n (%) | 12 (57.1) | 17 (39.5) | 16 (72.7) | 0.35 |
| Etiology of CKD (%) | 0.99 | |||
| Unknown | 5 (23.80) | 12 (27.9) | 5 (22.7) | |
| Systemic arterial hypertension | 4 (19.05) | 7 (16.3) | 3 (13.6) | |
| Chronic glomerulonephritis | 5 (23.8) | 5 (11.6) | 4 (18.2) | |
| Diabetes mellitus | 0 (0) | 5 (11.6) | 3 (13.6) | |
| Other | 7 (33.35) | 14 (32.6) | 7 (31.8) | |
| Months on dialysis | 39.5±28.3 | 50.6±42.3 | 34.5±23.0 | 0.23 |
| Transfusions pre-transplant, n (%) | 14 (66.7) | 21 (48.8) | 9 (40.9) | 0.55 |
| Previous transplant, n (%) | 5 (23.8) | 3 (6.9) | 0 (0) | 0.25 |
| Pregnancies before transplant, n (%) | 6 (66.7) | 21 (80.8) | 5 (83.3) | 0.89 |
| HLA-A, B and DR Mismatches | 3.6±0.8 | 3.2±1.2 | 3.9±1.1 | 0.06 |
| Class I PRA pre-transplant (%) | 36.5±32.5 | 38.6±29.2 | 0 | <0.01 |
| Class II PRA pre-transplant (%) | 41.1±40.1 | 17.1±25.9 | 0 | <0.01 |
| Donors | ||||
| Deceased donors, n (%) | 20 (95.2) | 39 (90.7) | 17 (77.3) | 0.70 |
| Age (years) | 43.4±11.3 | 42.3±15.6 | 39.9±12.3 | 0.69 |
| Male, n (%) | 11 (53.4) | 28 (65.1) | 9 (40.9) | 0.73 |
| Expanded-criteria donors (%) | 6 (28.6) | 20 (46.5) | 3 (13.6) | 0.29 |
| Serum creatinine (mg/dl) | 1.25±0.8 | 1.3±1.0 | 1.3±1.2 | 0.76 |
| Transplantation | ||||
| Initial immunosuppressive therapy | ||||
| Thymoglobulin (%) | 20 (95.2) | 34 (79.1) | 5 (22.7) | <0.01 |
| Thymoglobulin (mg/kg) | 5.95±1.7 | 4.22±2.4 | 1.06±2.1 | <0.01 |
| Basiliximab (%) | 1 (4.8) | 9 (20.9) | 14 (6.6) | <0.01 |
| Tacrolimus (%) | 19 (90.5) | 40 (93.0) | 21 (95.4) | 0.10 |
| Mycophenolate (%) | 20 (95.2) | 40 (93.0) | 22 (100) | 0.95 |
| Cold ischemia (hours) | 21.0±4.6 | 21.4±5.5 | 18.6±4.6 | 0.16 |
| DGF, n (%) | 13 (61.9) | 23 (53.5) | 13 (59.1) | 0.10 |
| HD before hospital discharge, (n) | 2.6±3.4 | 1.9±2.7 | 3.2±4.1 | 0.40 |
| Urine output higher than 1 L (days) | 4.8±3.9 | 5.9±6.6 | 7.0±9.7 | 0.95 |
CKD – chronic kidney disease; PRA – panel reactive antibody; DGF – delayed graft function, HD – hemodialysis.
Graft function and proteinuria outcomes according to the groups
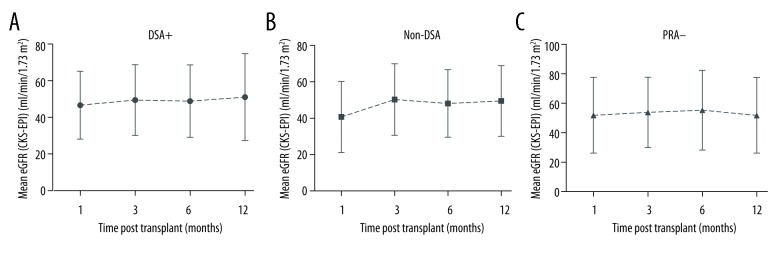
There was no significant difference in renal function among the groups in the analyzed period. The mean eGFR (CKD-EPI, ml/min/1.73 m2) at 1 month was 46.6±18.5 in the DSA+ group, 41.6±19.9 in the non-DSA group and 52.6±26.1 in the PRA− group (p=0.24). At 12 months, eGFRs in the 3 groups were 51.0±23.7, 50.66±19.9, and 52.5±26.0 (p=0.98), respectively (Figure 2). Proteinuria was also similar in all 3 groups within the first year of follow-up.
Figure 2.
Mean estimated glomerular filtration rate (eGFR) (ml/min/1.73 m2), according to the groups, over time after renal transplantation.
Post-transplant anti-HLA antibodies characteristics and rejection episodes
The characteristics of the anti-HLA antibodies in the post-transplant follow-up, according to the groups, are shown in Table 2. In the DSA+ group, 33.3% of patients persisted with specific antibodies detectable at the end of the first year post-transplant, but with lower fluorescence intensity compared to baseline (p<0.05). In the non-DSA group, 6.97% developed de novo DSA within the first 3 months post-transplant, with subsequent reduction of its fluorescence intensity during the follow-up, becoming undetectable 12 months after transplantation. In the PRA− group, the detection of DSA was later and most were class II DSA.
Table 2.
Characteristics of anti-HLA antibodies, according to groups, at 3 and 12 months after transplantation.
| DSA+ | Non-DSA | PRA− | ||||
|---|---|---|---|---|---|---|
| 3rd month n=20 |
12th month n=15 |
3rd month n=43 |
12th month n=39 |
3rd month n=22 |
12th month n=20 |
|
| DSA | ||||||
| Class I, n (%) | 4 (40.0) | 2 (40.0) | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| Class II, n (%) | 4 (40.0) | 3 (60.0) | 1 | 0 (0.0) | 0 (0.0) | 3 (60.0) |
| Class I + II, n (%) | 2 (20.0) | 0 (0.0) | 1 | 0 (0.0) | 0 (0.0) | 2 (40.0) |
| Higher DSA (MFI), medium (min–max) | 2591.5 (483–15810) | 980.0 (712–4400) | 800 (600–837) | - | 680 | 15890 (6200–21434) |
| Sum DSA (MFI) | 5302.6±5541.9 | 1933.6±1493.7 | 922.3±372.4 | - | 680±0.0 | 27451.6±20332.7 |
| Higher DSA >3000MFI, n (%) | 5 (50.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (100.0) |
| Nonspecific anti-HLA | ||||||
| Class I, n (%) | 2 (20.0) | 5 (55.5) | 11 (50.0) | 7 (58.4) | 4 (66.6) | 2 (25.0) |
| Class II, n (%) | 3 (30.0) | 4 (44.5) | 5 (41.6) | 2 (16.6) | 0 (0.0) | 3 (37.5) |
| Class I + II, n (%) | 5 (50.0) | 0 (0.0) | 6 (27.4) | 3 (25.0) | 2 (33.3) | 3 (37.5) |
DSA – donor-specific antibody; MFI – maximum fluorescence intensity; sd – standard deviation; min – minimum; max – maximum; n – number.
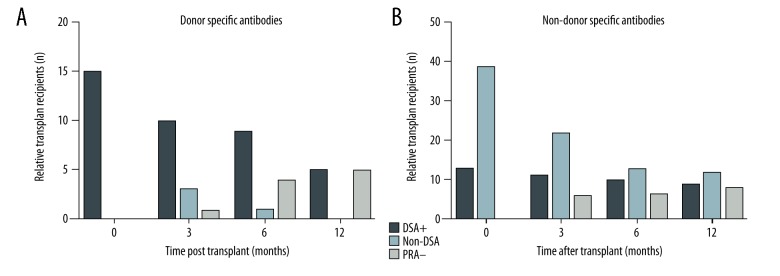
There was a reduction in the number of cases with donor specific antibodies in the DSA+ group and Non-DSA group, while the number of cases with donor specific antibodies increased in the PRA− group over the period (Figure 3A). In the non-DSA group, there was a reduction by more than 50% in the number of recipients with detectable nonspecific anti-HLA antibodies during the follow-up (Figure 3B). However, in the DSA+ group, the prevalence of recipients with detectable nonspecific anti-HLA antibodies remained stable over time.
Figure 3.
Distribution of renal transplant recipients with donor-specific anti-HLA antibodies (A) and non-donor-specific antibodies (B), according to the groups, after renal transplantation.
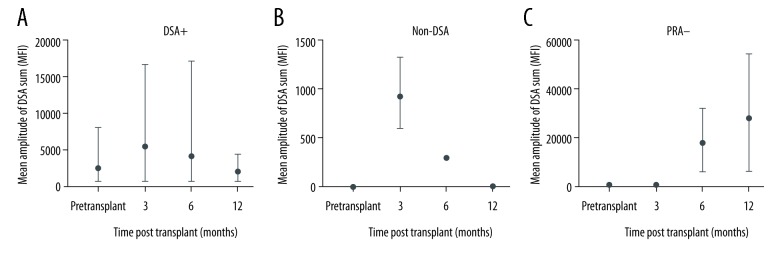
In the DSA+ group, intensity of fluorescence of specific anti-HLA antibodies before transplant ranged from 620 to 8000 MFI, with a mean of 2392 MFI. At 3 months, the fluorescence intensity was 5302 MFI, with subsequent reduction, reaching 1933.6 MFI at 12 months (Figure 4A). In the non-DSA group, mean fluorescence intensity of de novo DSA remained below 1000 MFI throughout the assessed period, reaching the maximum value of 922 MFI in the third month after transplantation (Figure 4B). In the PRA− group, all de novo DSA showed fluorescence intensity above 5000 MFI after 6 months of follow-up, reaching a mean of 27 451 MFI at 12 months (Figure 4C).
Figure 4.
(A–C) Mean and amplitude of sum of DSA values (MFI), according to the groups, after renal transplantation.
The mean overall allograft biopsy time was 2.4±3.1 months, occurring later in the DSA+ group, without significant differences compared to other groups. There was no statistical difference among the groups in the occurrence of biopsy-proven antibody-mediated acute rejection (AMR) or acute cell-mediated rejection (ACR) (Table 3). Graft biopsies of patients from groups with preformed anti-HLA antibodies (DSA+ and non-DSA+) had more interstitial fibrosis and tubular atrophy (IF/TA) compared to the group without preformed antibodies (PRA−), but the difference was not statistically significant.
Table 3.
Characteristics of graft biopsies according to the groups.
| Total | DSA+ | Non-DSA | PRA− | p | |
|---|---|---|---|---|---|
| Biopsy (%) | 36 (41.9) | 6 (28.6) | 19 (44.2) | 11 (50.0) | 0.68 |
| Biopsy time (months) | 2.4±3.1 | 3.0±3.8 | 2.55±3.15 | 1.65±2.4 | 0.70 |
| Glomerulus count | 18.9±8.8 | 16.8±8.5 | 18.53±9.52 | 21.9±7.5 | 0.23 |
| Glomerulosclerosis (%) | 1.4±1.6 | 1.2±1.2 | 1.75±1.77 | 1.0±1.6 | 0.42 |
| AMR, n (%) | 5 (5.8) | 2 (9.5) | 1 (2.32) | 2 (9.1) | 0.92 |
| ACR, n (%) | 14 (16.3) | 1 (4.8) | 7 (16.28) | 6 (27.3) | 0.67 |
| Borderline | 5 (5.8) | 0 (0) | 3 (6.97) | 2 (9.1) | 0.93 |
| Banff 1 | 1 (1.2) | 0 (0) | 1 (2.32) | 0 (0.0) | 0.98 |
| Banff 2 | 7 (8.1) | 1 (4.8) | 3 (6.97) | 3 (13.6) | 0.97 |
| Banff 3 | 1 (1.2) | 0 (0) | 0 (0.0) | 1 (4.5) | 0.81 |
| IF/TA (%) | 23 (26.7) | 10 (47.6) | 9 (20.93) | 4 (18.2) | 0.39 |
| IF/TA 1 | 16 (18.60) | 9 (42.85) | 5 (11.62) | 2 (13.6) | 0.09 |
| IF/TA 2 | 5 (5.81) | 1 (4.76) | 3 (6.97) | 1 (4.5) | 0.99 |
| IF/TA 3 | 2 (2.32) | 0 (0) | 1 (2.32) | 1 (4.5) | 0.98 |
AMR – antibody-mediated rejection; ACR – acute cell rejection; IF/TA – interstitial fibrosis and tubular atrophy (Banff 2013/15).
Graft loss within the first year after transplantation and its association with anti-HLA antibodies
Graft failure included the cases of chronic kidney disease with need for renal replacement therapy and death with functional graft, caused by infectious or cardiovascular diseases. The main cause of graft failure in this series was death from infection, with 5 cases (23.8%) in the DSA+ group, 4 (9.3%) in non-DSA group and 1 (4.5%) in the PRA− group. There was 1 case of AMR caused by de novo DSA DQ-type in the PRA− group, with graft loss and return to dialysis. One-year death-censored graft survival was similar among the 3 groups (Figure 5).
Figure 5.
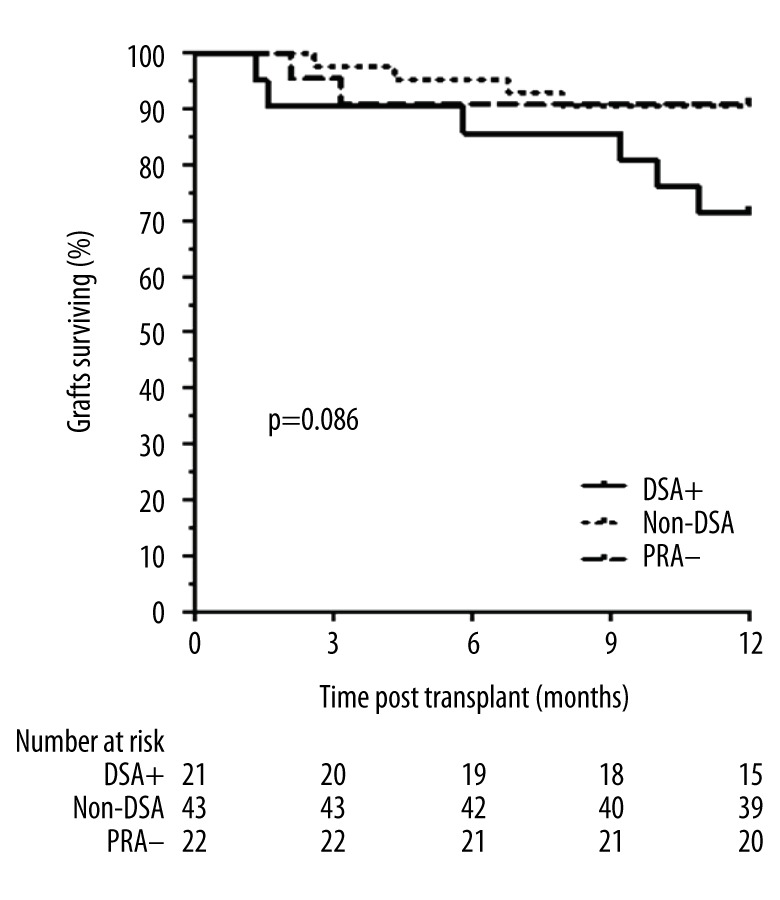
Death-censored kidney graft survival of patients according anti-HLA antibodies before transplantation (donor-specific antibodies: DSA+, non-DSA, without anti-HLA antibodies: PRA−).
In the analyzed period, 7 cases of cytomegalovirus infection were diagnosed – 1 in the DSA+ group, 5 in the non-DSA group, and 1 in the PRA− group – without a significant difference. Presumed infection by polyomavirus, detected by the presence of decoy cells on urine cytology, occurred in 3 cases in the DSA+ group, 12 in the non-DSA group, and 2 in the PRA− group, but without statistical difference.
Discussion
Several studies have shown the relationship between presence of anti-HLA antibodies and the reduction of graft survival in renal transplant recipients [5] and with an increased risk of acute antibody-mediated rejection [2]. AMR has been described as an ongoing process that, in addition to acute injury, can be responsible for the development of proteinuria, chronic dysfunction, and graft failure [5]. Recent studies reported differences between potentially pathogenic characteristics of these antibodies in relation to their class, subclass, fluorescence intensity, and complement activation capacity [5]. The prevalence of pre-transplant DSA in our global population during the study period was of 3.8%. However, in the present analysis, as detection of DSA pre-transplant was an inclusion criterion, the DSA+ group corresponds to one-quarter of followed patients, similar to the de novo DSA group. Half of studied patients maintained unspecific anti-HLA antibodies pre- or post-transplant. In our series, there was no statistical difference in the incidence of AMR among the groups. Aubert et al. [15] studied 113 renal transplant recipients, of which 9.7% had DSA, and also did not find an association between pre-transplant DSA and development of AMR in the follow-up. These results differ from other studies that revealed a higher incidence of AMR after transplantation in recipients with pre-transplant DSA [15,16]. In our series, the incidence of AMR in the DSA+ group was of 9.52% within the first year post-transplant, which is lower than in other previous reports where AMR ranged from 32.3% to 55% [17,18]. Malheiro et al. [2] studied 462 renal transplant recipients and found an incidence of 37.5% of AMR in the group with preformed DSA (n=40). AMR in these patients also occurred earlier than in patients without DSA, especially in the presence of fluorescence intensity higher than 3000 MFI. In our cohort, there was no association between antibodies fluorescence intensity and development of AMR, since in half of rejection cases the intensity of DSA fluorescence was lower than 1000 MFI. Class II antibodies, either alone or in combination with class I antibodies, are reported to be related to an increased risk of graft immune damage than class I antibodies [15,16,19]. In this series, however, such an association was not demonstrated, since half of AMR cases occurred in the presence of class I DSA Ab. This result is similar to that obtained by Aubert et al. [15], who found an association between class I antibodies and AMR in their cohort. The differences between our results and other studies may be due to the virtual cross-match screening pre-transplant, routinely done in our unit, where renal transplant recipients with single DSA fluorescence intensity higher than 2000 MFI or combination of more than 1 DSA antibody are considered as high risk or ineligible for renal transplantation with a selected donor. Another possible cause for the noted differences is that protocol biopsies are not performed, so cases of subclinical AMR may not have been detected in our cohort.
Most patients in the DSA+ group (95.24%) received anti-thymocyte globulin at the mean dose of 6 mg/kg as immunosuppressive induction therapy, associated with full-dose tacrolimus, which would be more effective in preventing short-term rejection in previously sensitized patients, as reported in other studies [20]. This could explain the low AMR rate found during first-year follow-up in this series. Maintenance therapy with tacrolimus and mycophenolate is also reported to be related to a lower prevalence of anti-HLA antibodies and with a lower AMR incidence and better outcomes at 2-year follow-up, increasing graft survival [21]. Moreover, the association of low doses of steroids in maintenance therapy, despite not finding direct evidence of this in transplant recipients, is widely used in situations of rapid and effective suppression of the immune response, such as in many autoimmune diseases [21]. All DSA+ patients had gradual reduction of steroids during the follow-up, with similar steroid and antiproliferative drugs doses in the immunosuppressive maintenance therapy at 12 months. There was no significant modification in immunosuppressive therapy in the recipients who presented reduction of fluorescence intensity. In this group, the blood level of tacrolimus was of 6.6±1.4 ng/mL at 1 month, whereas at 12 months it was 6.2±1.9 ng/mL, without statistical difference in trough level over time (p=0.57). Considering all DSA+ group recipients, we also did not observe a significant alteration in maintenance immunosuppression (p=0.30). In our study, therefore, the maintenance immunosuppression used does not explain the variation in fluorescence intensity among DSA+ group recipients.
The prevalence of de novo DSA in the non-DSA group (6.97%) was lower than in the PRA− group (27.27%). de Souza et al. [22], studying a cohort of 111 kidney transplant recipients, found that 90.9% of recipients with negative pre-transplant PRA remained with negative PRA in post-transplant follow-up. The incidence of anti-HLA antibodies after transplant in the non-previously sensitized group in our study was 31%, higher than previously described by de Sousa et al. [22]. This difference can be a consequence of study design, as in our series all cases were investigated for suspected rejection, while in the de Souza study there was prospective monitoring. Therefore, the exclusion of the persistently non-sensitized recipients did not impair the overall analysis, since most non-sensitized patients did not develop anti-HLA antibodies after transplantation, and the main mechanism responsible for the allograft dysfunction at the indication of antibodies screening test would be independent of the action of anti-HLA antibodies. In our study, the PRA− group received less potent initial immunosuppression, which may have influenced the post-transplant development of DSA in some recipients in this group. We also observed a trend of more mismatches in the PRA− group compared to the groups with preformed anti-HLA antibodies (DSA+ and non-DSA), which could also have contributed to a higher incidence of DSA in the PRA− group. However, it is not a routine practice in our service to monitor post-transplant anti-HLA antibodies in patients considered as being at low immunological risk and who are asymptomatic. In all cases of the PRA− group, antibody screening was performed for suspected rejection, which may have overestimated the incidence of DSA.
We observed 5 cases of AMR within the first year post-transplant, without a statistical difference among the groups. We were not able to find a correlation between fluorescence intensity or antibodies subtypes with a higher risk of AMR in this series. This could be a result of sample size or the short follow-up period.
The evolution of renal function of the DSA+ group (serum creatinine, eGFR, and proteinuria) did not show a statistically significant difference compared to non-DSA and PRA− groups during the first year post-transplant. The low fluorescence intensity of specific HLA antibodies and the short follow-up period could explain these findings, since lower levels of antibodies are most frequently associated with subclinical rejection and later graft dysfunction, with negative impact on long-term survival [23].
A higher incidence of death due to infection was observed in the groups with pre-transplant anti-HLA antibodies (DSA+ and non-DSA). In these cases, immunosuppressive induction with higher doses of thymoglobulin could justify the higher incidence of opportunistic infections, compared to patients who received lower doses of thymoglobulin or basiliximab as induction therapy [24]. However, we did not find any cases of graft failure due to rejection in these groups during the 1-year follow-up.
Considering the retrospective cohort, this study has some limitations, such as the absence of protocol biopsy, impairing the diagnosis of subclinical AMR cases and the short follow-up, which was possibly insufficient to detect long-term changes in renal function and proteinuria.
Conclusions
In our cohort, renal transplantation in recipients with preformed anti-HLA antibodies had a low incidence of AMR, and graft function was similar among patients with or without pre-transplant anti-HLA antibodies. Development of de novo DSA occurred earlier in pre-sensitized patients compared with those with zero PRA pre-transplant, but data could be masked by the lack of protocol post-transplant DSA monitoring for low-risk patients. Prospective studies with longer follow-up are needed to verify potential effects of anti-HLA antibodies on subclinical rejection, proteinuria, and long-term graft survival.
Footnotes
Conflict of interest
None.
Source of support: Marcos Vinicius de Sousa, e-mail: marcosnefro@gmail.com
References
- 1.Mackay IR, Rosen FS, Klein J, Sato A. The HLA system. N Engl J Med. 2000;343(10):702–9. doi: 10.1056/NEJM200009073431006. [DOI] [PubMed] [Google Scholar]
- 2.Malheiro J, Tafulo S, Dias L, et al. Analysis of preformed donor-specific anti-HLA antibodies characteristics for prediction of antibody-mediated rejection in kidney transplantation. Transpl Immunol. 2015;32(2):66–71. doi: 10.1016/j.trim.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Khovanova N, Daga S, Shaikhina T, et al. Subclass analysis of donor HLA-specific IgG in antibody-incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure. Transpl Int. 2015;28(12):1405–15. doi: 10.1111/tri.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan SC, Vo AA. Donor-specific antibodies in allograft recipients: Etiology, impact and therapeutic approaches. Curr Opin Organ Transplant. 2014;19(6):591–97. doi: 10.1097/MOT.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 5.Gosset C, Lefaucheur C, Glotz D. New insights in antibody-mediated rejection. Curr Opin Nephrol Hypertens. 2014;23(6):597–604. doi: 10.1097/MNH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 6.Higgins R, Lowe D, Daga S, et al. Pregnancy-induced HLA antibodies respond more vigorously after renal transplantation than antibodies induced by prior transplantation. Hum Immunol. 2015;76(8):546–52. doi: 10.1016/j.humimm.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Picascia A, Infante T, Napoli C. Luminex and antibody detection in kidney transplantation. Clin Exp Nephrol. 2012;16:373–81. doi: 10.1007/s10157-012-0635-1. [DOI] [PubMed] [Google Scholar]
- 8.Picascia A, Grimaldi V, Napoli C. From HLA typing to anti-HLA antibody detection and beyond: The road ahead. Transplant Rev. 2016;30:187–94. doi: 10.1016/j.trre.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 10.Gebel HM, Bray RA. Approaches for transplanting the sensitized patient: Biology versus pharmacology. Nephrol Dial Transplant. 2008;23:2454–57. doi: 10.1093/ndt/gfn108. [DOI] [PubMed] [Google Scholar]
- 11.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–25. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 12.Loupy A, Haas M, Solez K, et al. The Banff 2015 Kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17:28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampaio WLV, Mazzali M. C4d deposits in borderline rejection: An early marker for chronic renal dysfunction? Transplant Proc. 2014;46(6):1710–12. doi: 10.1016/j.transproceed.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubert V, Venetz JP, Pantaleo G, Pascual M. Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum Immunol. 2009;70(8):580–83. doi: 10.1016/j.humimm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Pollinger HS, Stegall MD, Gloor JM, et al. Kidney transplantation in patients with antibodies against donor HLA class II. Am J Transplant. 2007;7(4):857–63. doi: 10.1111/j.1600-6143.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- 17.Caro-Oleas JL, González-Escribano MF, González-Roncero FM, et al. Clinical relevance of HLA donor-specific antibodies detected by single antigen assay in kidney transplantation. Nephrol Dial Transplant. 2012;27(3):1231–38. doi: 10.1093/ndt/gfr429. [DOI] [PubMed] [Google Scholar]
- 18.Lefaucheur C, Suberbielle-Boissel C, Hill GS, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8(2):324–31. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 19.Visentin J, Marroc M, Guidicelli G, et al. Clinical impact of preformed donor-specific denatured class I HLA antibodies after kidney transplantation. Clin Transplant. 2015;29(5):393–402. doi: 10.1111/ctr.12529. [DOI] [PubMed] [Google Scholar]
- 20.Brokhof MM, Sollinger HW, Hager DR, et al. Antithymocyte globulin is associated with a lower incidence of de novo donor-specific antibodies in moderately sensitized renal transplant recipients. Transplantation. 2014;97(6):612–17. doi: 10.1097/TP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorling A, Rebollo-Mesa I, Hilton R, et al. Can a combined screening/treatment programme prevent premature failure of renal transplants due to chronic rejection in patients with HLA antibodies: Study protocol for the multicentre randomised controlled OuTSMART trial. Trials. 2014;15:30. doi: 10.1186/1745-6215-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza PS, David-Neto E, Panajotopolous N, et al. Dynamics of anti-human leukocyte antigen antibodies after renal transplantation and their impact on graft outcome. Clin Transplant. 2014;28(11):1234–43. doi: 10.1111/ctr.12451. [DOI] [PubMed] [Google Scholar]
- 23.Zachary AA, Leffell MS. HLA mismatching strategies for solid organ transplantation – a balancing act. Front Immunol. 2016;7:1–14. doi: 10.3389/fimmu.2016.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nafar M, Dalili N, Poor-Reza-Gholi F, Ahmadpoor P, et al. The appropriate dose of thymoglobulin induction therapy in kidney transplantation. Clin Transplant. 2017;31:1–8. doi: 10.1111/ctr.12977. [DOI] [PubMed] [Google Scholar]