Abstract
Background
Prolonged QT interval is an integral part of the definition of cirrhotic cardiomyopathy. The aim of this study was to analyze the relationship between QT corrected (QTc) and the etiology and the severity of liver disease in relation to the complications of cirrhosis in candidates for orthotropic liver transplantation (OLTx).
Material/Methods
From 360 consecutive patients with end-stage liver disease (ESLD) consulted by a designated cardiologist, 160 patients underwent OLTx. The QTc was calculated according to 3 formulas in 151 ECG tracings with good quality. The severity of liver disease was assessed according to Child-Pugh classification and model for end-stage liver disease (MELD). This was a single-center study with register-based follow-up design.
Results
Prolonged QTc over 440 ms was found in 51 subjects (33.8%), but none had prolonged QTc >500 ms. QTc corrected by Fridericia (F) formula was more suitable for patients with ESLD. We found no correlation between QTc interval and severity of liver disease. The QTc interval was higher in patients with alcoholic cirrhosis when compared to patients with viral hepatitis and ESLD of other etiologies. We observed a higher QTc interval in patients with gastroesophageal varices and encephalopathy. We did not notice any significant difference in the effect of the QTc interval on survival.
Conclusions
QTc interval might be associated with etiology and complication of ESLD. The prolonged QT interval is not associated with higher all-cause mortality after OLTx.
MeSH Keywords: Cardiomyopathies, Electrocardiography, End Stage Liver Disease, Liver Transplantation, Mortality
Background
The QT interval seems to be an electrocardiographic marker of autonomous nervous system function, which approximates the duration of the repolarization of the ventricular myocardium [1,2].
It is proven that QT interval prolongation is associated with a higher risk of severe arrhythmias and may provoke torsades des pointes and thus lead to ventricular fibrillation [3]. Population studies have shown a relationship between the QTc interval and all-cause mortality, cardiovascular mortality, and sudden cardiac death [4,5].
Prolongation of the QT interval is a very common electrophysiological abnormality in patients with cirrhosis [6]. However, sudden cardiac death and arrhythmias are not as common. Reasons for QT interval prolongation still remain unclear and it must be corrected for heart rate (QTc). The literature, however, presents some discrepancies in the methodology for the correction of QT interval in patients with liver disease. The most suitable formula for QT interval correction in this cohort is the Fridericia formula.
Prolonged QT interval is an integral part of the definition of cirrhotic cardiomyopathy (CCM), which is defined as a chronic cardiac dysfunction characterized by blunted systolic function in response to stress or exercise, and/or impaired diastolic function, and electrophysiological abnormalities, especially with QT interval prolongation, in the absence of other known cardiac disease.
There are some controversies about the relationship of the QT interval with the etiology and severity of liver disease. Moreover, the prognostic value of a prolonged QT interval in cirrhotic patients, especially those who undergo OLTx, is unclear.
The aim of this study was to analyze the relationship between QTc corrected with Fridericia formula and the etiology and the severity of liver disease in relation to the complications of cirrhosis in candidates for liver transplantation. Further, we evaluated the effect of the QT interval on mortality after liver transplantation.
Material and Methods
Group of patients
We studied 360 consecutive patients with end-stage liver disease (ESLD) who were consulted by a designated cardiologist from the 1st Department of Cardiology at the Medical University of Warsaw between April 1st, 2011 and April 30th, 2013. All patients were hospitalized and operated on in the Department of General, Transplant, and Liver Surgery of the Medical University of Warsaw.
Of the patients included in the study, 160 underwent liver transplantation at our university hospital; however, due to the unsatisfactory quality of 9 ECG tracings, we included only 151 ECG tracings for further analysis. Derivation of the study population is presented in Figure 1.
Figure 1.
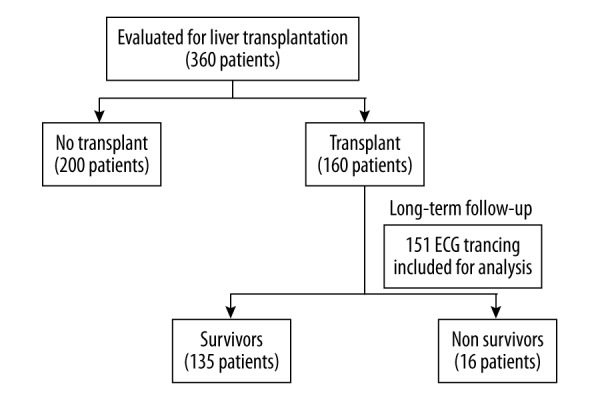
Derivation of the study population.
Electrocardiogram
As part of the routine cardiac workup, ECG was performed on every patient. During retrospective analysis, we analyzed 151 ECG tracings of patients with end-stage liver disease who underwent OLTx.
ECG tracings were recorded during elective hospitalization as part of the qualification for OLTx. All 12-lead ECGs were recorded at rest and all tracings were performed at a paper speed of 25 mm/s and an amplification of 10 mm/mV. Measurements were taken from lead II from 1 ECG and were calculated by the cardiologist.
QT intervals were measured according to the standard criteria from the start of the QRS complex to the end of the T wave. The end of the wave was defined as the point of return to the isoelectric line. In ECGs where the return of the T wave to base line was not easily determined, the end of the T wave was defined as the nadir of the curve between the T and U waves (drawn along the steepest part of the descending portion of the T wave).
The corrected QT interval (QTc) was calculated according to:
– Bazett [7] (B) formula: QT/RR1/2
– Fridericia [8] (F) formula: QT/RR1/3
– Hodges [9] (H) formula: QT + 1.75 (HR – 60)
The normal QTc interval (B) was established as below 440 ms.
Severity of liver disease
Data regarding demographic characteristics, laboratory results, cardiovascular risk factors, etiology of liver disease (alcoholic, viral, non-alcoholic steatohepatitis, cryptogenic, and other) and severity of liver disease (Child-Pugh classification and model for end-stage liver disease [MELD]), presence of ascites, gastroesophageal varices, and history of overt encephalopathy were recorded retrospectively.
Compensated disease was defined as Child-Pugh class A and decompensated disease included Child-Pugh class B or C patients.
Follow-up
This was a single-center study with a register-based follow-up design. We used the Polish Civil Personal Registration Number unique to each Polish citizen to link information from the national registry of deaths at the Ministry of the Interior and Administration and clinical data obtained for this study.
Mortality rates were calculated based on death irrespective of cause as a single end-point. Observations were censored at the date of last available follow-up (18 July 2014).
Statistical methods
Continuous variables of baseline characteristics were summarized with means ±SD, whereas frequencies and percentages were used for categorical variables. Electrocardiographic parameters such as QT intervals are presented as medians with the first quartile (Q1) and the third quartile (Q3). Comparisons were made using Fisher’s exact test for nominal variables. Unpaired t tests were used for comparison of continuous variables. Differences between living and deceased patients at the end of the follow-up were analyzed by the independent-samples t test for normally distributed data and the Mann-Whitney U test for non-normally distributed data.
Univariate analyses and multivariate analyses (by stepwise multivariate model building) were assessed with a Cox proportional hazards model. Hazard ratios (HRs) are presented with 95% confidence intervals (95% CI). The corresponding Kaplan-Meier curves for stroke occurrences were also plotted and compared using the log-rank test. Statistical significance for all analyses was determined as p<0.05. All analyses were undertaken using STATISTICA version 12 software.
Ethics aspect
Although ethics approval is not required for registry-based and retrospective studies in Poland, our study was approved by the Ethics Committee at the Medical University of Warsaw.
Results
Baseline characteristics
The baseline characteristics of patients and clinical aspects of ESLD are shown in Table 1.
Table 1.
Baseline characteristics of patients.
| Characteristic/variable | Value |
|---|---|
| Demographics | |
| Age (years), mean ±SD | 49±12.3 |
| Gender (male), n (%) | 95 (62.9%) |
| Etiology of liver disease | |
| Alcoholic, n (%) | 27 (17.9%) |
| Viral hepatitis (Hepatitis B or C), n (%) | 68 (45%) |
| Autoimmune cirrhosis, n (%) | 36 (23.8%) |
| Other | 20 (13.2%) |
| Severity of ESLD at the time of cardiac consultation | |
| Child-Pugh score (units), mean ±SD | 7.8±2.1 |
| Child-Pugh class A, n (%) | 50 (33.1%) |
| Child-Pugh class B, n (%) | 73 (48.3%) |
| Child-Pugh class C, n (%) | 28 (18.5%) |
| MELD score (units), mean ±SD | 11.8±4.6 |
| Complications of end-stage liver disease | |
| Hepatocellular carcinoma (HCC), n (%) | 28 (18.5%) |
| Ascites, n (%) | 41 (27.2%) |
| Gastroesophageal varices grade III–IV, n (%) | 48 (31.8%) |
| History of bleeding from gastroesophageal varices | 29 (19.2%) |
| History of overt encephalopathy, n (%) | 32 (21.2%) |
MELD – model for end-stage liver disease.
The most common indications for liver transplantation were viral cirrhosis (45%), autoimmunogenic cirrhosis (23.8%), and alcoholic cirrhosis (17.9%). The average age was 49 years and 62.9% of the patients were males.
Of the study population, 50 (33.1%) patients were in Child-Pugh A class, 73 (48.3%) were in Child-Pugh B class, and 28 (18.5%) were in Child-Pugh C class. The mean MELD score was 11.8±4.6. Hepatocellular carcinoma (HCC) was diagnosed in 28 (18.5%) patients. Gastroesophageal varices grades III–IV were present in 48 (31.8%), and 29 (19.2%) patients had a history of bleeding from gastroesophageal varices.
Treatment with beta-blockers was administered to 69 (45.7%) patients who did not take any other drugs affecting the QT interval, including: antiarrhythmic drugs, macrolides, antihistamines, anxiolytic drugs, or tricyclic antidepressants. No patients from this cohort had history of previous MI or family history of long QT syndrome. QT interval could be altered due to acute ischemia, left bundle branch block (LBBB), or right bundle branch block, but we did not observe these conditions on ECG recordings.
Electrocardiogram
With regard to ECG variable values in candidates for OLTx, mean heart rate was 84±18 beats per minute (bpm) and median QT interval was 360 milliseconds (ms), (Q1, Q3: 320, 400 ms). The QTc interval was different depending on the formula used: median QTc (B) was 425 ms (Q1, Q3: 400, 454 ms), median QTc (F) was 379 ms (Q1, Q3: 359, 409 ms), and median QTc (H) was 406 ms (Q1, Q3: 386, 430 ms), p<0.001.
Prolonged QTc (B) >440 ms was found in 51 subjects (33.8%), but none had prolonged QTc >500 ms. Among those 51 subjects there were 4 women (only one women had corrected QT interval greater than 470 ms). We found no correlation between QTc interval calculated with 3 formulas and severity of liver disease assessed by Child-Pugh score and MELD score (Table 2). The median QTc (F) did not differ in Child-Pugh classes (Figure 2).
Table 2.
ECG parameters regarding severity of liver disease according to Child-Pugh classification and MELD score.
| ECG parameter | Child-Pugh class A | Child-Pugh class B | Child-Pugh class C | p |
|---|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | Median (Q1–Q3) | ||
| HR | 83 (74–95) | 79 (71–96) | 78 (69–92) | 0.43 |
| QT | 360 (320–380) | 360 (320–400) | 380 (350–415) | 0.10 |
| QTc (B) | 419 (400–450) | 425 (393–454) | 431 (419–462) | 0.28 |
| QTc (F) | 374 (359–398) | 377 (353–403) | 397 (373–425) | 0.07 |
| QT (H) | 398 (386–424) | 405 (383–429) | 423 (401–435) | 0.10 |
| ECG parameter | MELD <11 (median) | MELD ≥11 (median) | p | |
| Median (Q1–Q3) | Median (Q1–Q3) | |||
| HR | 82 (72–96) | 79 (71–94) | 0.39 | |
| QT | 360 (320–400) | 360 (340–400) | 0.77 | |
| QTc (B) | 427 (404–451) | 424 (393–457) | 0.43 | |
| QTc (F) | 377 (360–402) | 380 (354–409) | 0.81 | |
| QT (H) | 411 (390–426) | 405 (380–430) | 0.57 | |
Q1 – the first quartile; Q3 – the third quartile.
Figure 2.
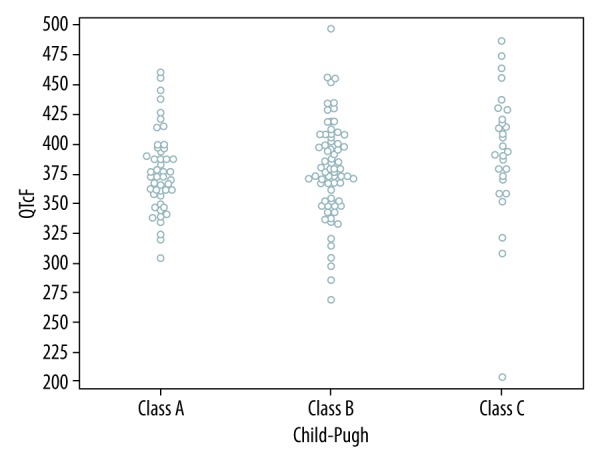
Relationship between QTc (F) interval and Child-Pugh score.
The QTc (F) interval was higher in patients with alcoholic etiology of liver disease when compared to patients with viral hepatitis and hepatitis of other etiologies (394 ms, 380 ms, and 370 ms, retrospectively, p=0.017) (Table 3; Figure 3).
Table 3.
ECG parameters regarding etiology of liver disease.
| ECG parameter | Viral hepatitis | Alcoholic cirrhosis | Another etiology | p |
|---|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | Median (Q1–Q3) | ||
| HR | 79 (72–89) | 75 (70–90) | 89 (71–107) | 0.040 |
| QT | 360 (340–400) | 360 (360–400) | 345 (320–380) | 0.014 |
| QTc (B) | 424 (400–454) | 436 (402–459) | 425 (394–453) | 0.53 |
| QTc (F) | 380 (363–409) | 394 (374–419) | 370 (349–392) | 0.017 |
| QT (H) | 402 (390–426) | 420 (386–433) | 409 (380–430) | 0.33 |
Q1 – the first quartile; Q3 – the third quartile.
Figure 3.
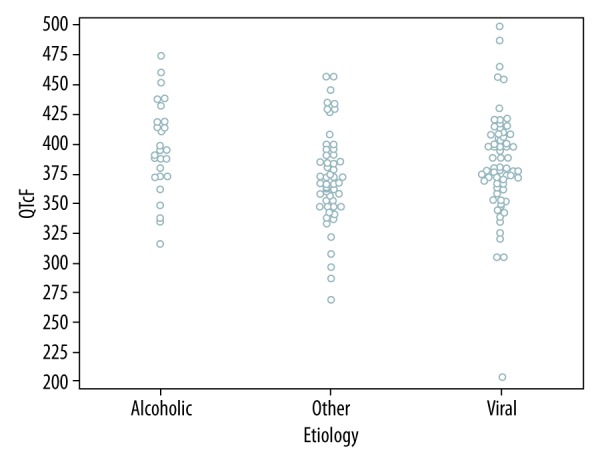
Relationship between QTc (F) interval and etiology of liver disease.
Patients with complications of liver disease such as ascites and encephalopathy had a higher QTc (F) interval (394 vs. 374 ms, p=0.015; 390 vs. 375 ms, p=0.05, retrospectively). There were no differences in QTc (F) interval among patients with and without HCC and gastroesophageal varices (Table 4).
Table 4.
ECG parameters regarding complication of ESLD.
| Complication of ESLD (+) | Complication of ESLD (−) | p | |
|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | ||
| ECG parameter | HCC (+) | HCC (−) | |
| HR | 86 (76–96) | 79 (71–94) | 0.22 |
| QT | 355 (320–380) | 360 (340–400) | 0.08 |
| QTc (B) | 413 (394–444) | 426 (400–459) | 0.22 |
| QTc (F) | 373 (348–394) | 382 (362–409) | 0.12 |
| QT (H) | 386 (380–421) | 413 (386–431) | 0.10 |
| ECG parameter | Ascites (+) | Ascites (−) | |
| HR | 78 (71–95) | 81 (71–96) | 0.48 |
| QT | 360 (360–400) | 360 (320–400) | 0.036 |
| QTc (B) | 436 (415–464) | 424 (398–450) | 0.055 |
| QTc (F) | 394 (372–415) | 374 (353–400) | 0.015 |
| QT (H) | 419 (400–433) | 400 (383–424) | 0.02 |
| ECG parameter | Gastroesophageal varices (+) | Gastroesophageal varices (−) | |
| HR | 78 (69–88) | 82 (72–97) | 0.042 |
| QT | 360 (340–400) | 360 (320–400) | 0.25 |
| QTc (B) | 428 (399–456) | 424 (400–454) | 0.97 |
| QTc (F) | 385 (369–413) | 377 (353–403) | 0.29 |
| QT (H) | 409 (385–432) | 406 (388–426) | 0.86 |
| ECG parameter | Encefalopathy (+) | Encefalopathy (−) | |
| HR | 77 (72–90) | 81 (71–96) | 0.37 |
| QT | 360 (360–400) | 360 (320–400) | 0.081 |
| QTc (B) | 443 (406–465) | 424 (400–450) | 0.14 |
| QTc (F) | 390 (370–420) | 375 (357–400) | 0.0500 |
| QT (H) | 419 (393–435) | 401 (386–425) | 0.086 |
ESLD – end-stage liver disease; Q1 – the first quartile; Q3 – the third quartile.
Patients who were taking beta-blockers had a lower heart rate (75 vs. 92, p<0.001) and a higher QTc(F) interval (391 vs. 374 ms, p=0.009).
Follow-up
Overall, there were 16 deaths (10.6%) after liver transplantation within the study population. The mean period of follow-up was over 2 years (736.5±260.6 days) and the mean time on the waiting list was approximately 3 months (110.2±101.3 day).
There was no significant difference between survivors and non-survivors in HR, QTc (B), or QTc (F) (Figure 4). In the Cox regression analysis, there were no differences in the QTc (F) interval between patients who were still alive at the end of follow-up and those who had died (hazard ratio [HR] 1.0039; 95% confidence interval [CI]: 1.001–1.007). We did not find any significant difference in the effect of QTc (F) interval on survival regarding severity of liver disease and etiology (in all subgroups, HR 1).
Figure 4.
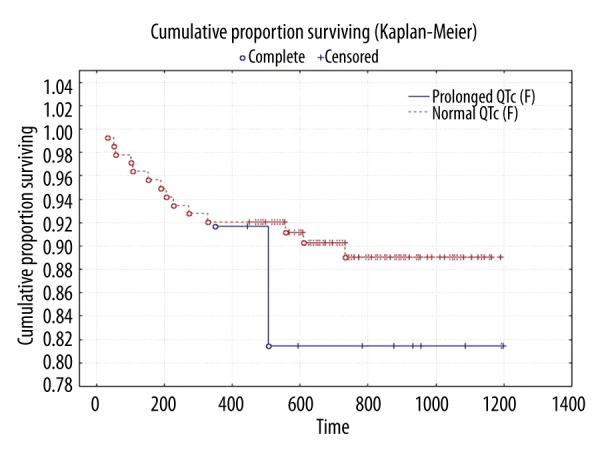
Kaplan-Meier curves of survival. QTc (F) <440 ms and ≥440 ms.
Discussion
Comparison of the formulas for QT interval correction
To ensure accurate interpretation, the QT interval should undergo adequate rate correction (QTc) in order to compare measurements taken at different times and at different heart rates [10].
Several formulas have been proposed to calculate the QTc, and multiple studies have compared these formulas in different patient groups and in a wide range of heart rates [11,12]. Although the Bazett formula is the most often used correction method in clinical settings, the Fridericia formula is recommended by the Food and Drug Administration (FDA) [13] for clinical trials on drug safety.
Every formula has its limitations [14]. The Bazett formula contains an overcorrection at high heart rates and an undercorrection at lower heart rates, but the Fridericia formula gives the opposite results of undercorrection at faster heart rates and overcorrection at slower heart rates [15].
QTc calculation in patients with liver disease
Because of these limitations and the tendency for tachycardia in patients with cirrhosis, many studies have been performed on this group of patients. Typically, these studies are done to identify new heart rate correction formulas or to validate already existing methods.
Thus, Zambruni [16] derived a new population-specific QT correction formula (QTc=QT/RR1/3,02), which is quite similar to the Fridericia formula (QTc=QT/RR1/3). Both are identified as the best rate correction in patients with cirrhosis and thus are suggested for use in patients with liver disease. However, online calculators for QT interval correction are only available for the Bazett and Fridericia formulas. The Fridericia formula seems to be more appropriate for clinical settings and should be used more widely in patients with liver disease.
We used different methods to calculate the QT correction but took into account that the Fridericia formula is the most suitable for candidates for OLTx and should be applied in the clinical setting. However, in most studies, the Bazett formula is used (Bernardi et al. [6], Bal et al. [17], Mohamed et a. [18]).
Prevalence of QT prolongation in patients with liver disease
A prolonged QTc interval (defined as QTc interval corrected with the Bazett formula >440 ms) has been reported in patients with liver disease with a prevalence assessed as 40% [17] (Bal et al.), 46.8% [6] (Bernardi et al.) and 55% [16] (Zambruni et al.).
In a recent study by Carvahleiro et al., prevalence of prolonged QTc (according to the Fridericia formula) was established at the level of 19% [19].
Normal QTc values
Most reported criteria for normal and abnormal values for the QTc are derived from the Bazett formula because it has been the formula most frequently used in medical publications. The QTc (according to the Bazett formula) is considered as abnormal if it is over 440 ms. Based on a study with more than 10 000 healthy subjects, the upper limit for a normal QT interval calculated with the Fridericia formula is suggested to be 460 ms [20]. However, there are some articles suggesting greater values: the upper limit of the normal QTc values of approximately 460 ms for men and 470 ms for women.
A QTc interval of over 500 ms is associated with an increased risk of torsades de pointes. We did not observe a QTc interval of 500 ms in any ECG tracing. No cases of sudden cardiac death were observed in our cohort. However, the literature does report cases describing subjects with a prolonged QT interval and arrhythmias during surgery or pharmacotherapy.
Explanation of QT interval prolongation
Numerous investigations are underway to explain the pathomechanism of QT interval prolongation in patients with liver disease. There is some data suggesting correlations between the QT interval and increased plasma concentrations of endothelin-1 (ET-1), cytokines (for example interleukin-1 [IL-1], IL6, TNFα), norepinephrine, autonomic dysfunction, and structural myocytes abnormalities [16].
QTc interval and etiology of liver disease
Mohamed et al. did not find a significant correlation between the QTc and the etiology of liver disease [18]. However, Bal et al. found that prolonged QTc interval was more common in patients with alcoholic cirrhosis compared to those with nonalcoholic cirrhosis (60% vs. 35%, p=0.001) [17].
QT interval and severity of liver disease
Our study only found a trend for a higher QTc interval in patients with a Child class of B and C compared to A, but this difference did not reach statistical significance. However, Bernardi et al. observed a QT interval prolongation relative to the severity of the disease [6]. In accordance to that observation, Mohamed et al. found a significant correlation between the QTcmax and severity of the disease [18]. Bal et al. showed that the mean QTc interval was higher in Child C class compared to Child B [17].
Our findings are in agreement with a recent study that showed QTc (F) before OLTx did not vary significantly with MELD or Child-Pugh score [19]. There are some possible explanations of these observation. First of all, as previously mentioned, the Bazett formula is not suitable for cirrhotic patients because it may overcorrect the QT interval due to increased heart rate, which is common in this cohort. Another explanation may be the use of beta-blockers in a large portion of our patients (18.5%) with hepatocellular carcinoma; these patients were more severe and had higher priority on the waiting list for OLTx.
Beta-blockers
The wide use of beta-blockers in our population may be an explanation for these results, especially in subjects with more advanced liver disease. This is supported by the observation of higher QTc intervals in patients taking beta-blockers. Beta-blockers are used to shorten the QT interval. Recently, beta-blockers have become widely used in treatment of patients with advanced liver disease, especially if gastroesophageal varices are present, for primary or secondary prevention for bleeding from those varices. Because of the high prevalence of diagnosed gastroesophageal varices in our study population and a history of previous bleeding from those varices, patients taking beta-blockers were not excluded. However, in most studies on QT interval, patients taking beta-blockers were excluded.
QTc interval and complication of liver disease
The correlation of the QTc interval with complications in liver disease remains unclear and has not been widely studied. We observed a higher QTc (F) interval in patients with ascites and encephalopathy. In some studies, it was reported that acute gastrointestinal bleeding might further prolong the QTc interval in cirrhosis [21]. We only enrolled candidates for OLTx with high prevalence of complication of liver disease. The severity of liver disease was prominent with a high mean MELD score. The advantage of the present study is the analysis of the QTc interval in subjects with HCC, diagnosed in 28 (18.5%) of our patients. HCC is a reason for early qualification for OLTx, regardless of the MELD score. In a recent study, QTc (B) interval was found to be prolonged and longer in cirrhotic patients with ascites than in patients without ascites (472 vs. 531 ms, p<0.001) [22].
QTc interval after OLTx
There are only a few studies assessing changes of the QT interval after OLTx. The Bazett formula was used in the 2 most prominent studies, so there is a possibility of overcorrection of the QT interval and it could be an explanation for the high prevalence of patients with a QTc prolongation.
In the study by Mohamed et al. [18], 52 patients underwent transplantation and there was significant improvement in the QTc interval after OLTx (p=0.001). Prolonged QTc (B) interval (>440 ms) improved within 3 months after OLTx in 32 of the 44 patients with prolonged QT interval before OLTx. In the study with the highest number of patients (162) who underwent OLTx, the authors examined the QTc for 6 months after OLTx. In 91 (56%) patients, a prolonged QTc interval (B) was observed. In this group, in 50 (55%) subjects the QTc normalized, in 3 the QTc (3.3%) remained unchanged and prolonged, in 26 (28%) improvement was observed but remained prolonged, and in 12 (13.3%) QTc was even prolonged further. However, 9 patients with a normal QTc before OLTx had a prolonged QTc (>440 ms) after OLTx. We enrolled a similar number of patients (160 patients who had OLTx) into our study, but for technical reasons we were only able to analyze 151 ECG tracings.
Prognostic value of the QTc interval in the cardiovascular disorders cohort
Several population-based studies have previously reported an association between the QTc and all-cause mortality. In the Framingham Heart Study population (n=6895; mean follow-up=27.5 years), a significant relation was observed between a prolonged QTc(B) (450 ms for males and 470 ms for females) with all-cause mortality (HR, 1.84; 95% CI, 1.36–2.49; P<0.0001) and coronary heart disease mortality (HR, 2.63; 95% CI, 1.36–5.11; P=0.004), but not with sudden cardiac death (HR, 1.83; 95% CI, 0.56–5.91; P=0.31). A similar relationship between a prolonged QTc (F) interval and all-cause mortality is not so well established [4].
Prognostic value of the QTc interval in the liver disease cohort
In the study by Mohamed et al., patients who died had a prolonged QTcmax (B) with a median of 495 ms (range, 455–522 ms) compared with a median of 470 ms (range, 410–533 ms) in survivors. However, no significant difference in pretransplant QT values between survivors and non-survivors was noticed (p=0.1). This finding is in agreement with our results. Among the 52 patients who underwent transplantation, 7 died (5 of them within 30 days of surgery and 1 after 147 days), but long-term follow-up among survivors was performed. However, an assessment of QT interval in survivors 3 months after OLTx was done.
Effect of QTc interval on long-term survival
There is no study assessing long-term mortality in patients who underwent OLTx regarding the QT interval. There is a lack of evidence on the effect of QT interval prolongation on long-term survival, except for an 8.9-year follow-up study in patients with liver disease who were not transplanted. This retrospective study looked at a cohort of 409 patients with compensated liver disease. Lower survival was observed in patients with a prolonged QTc interval (P=0.03), but when survival was adjusted for the Child-Pugh score, there were no differences in survival regarding the QT interval.
Both studies support our observation that a prolonged QT interval is not associated with higher mortality. To the best of our knowledge, the present study has the longest follow-up in patients who underwent OLTx.
QT interval and CCM
In many studies, the QT interval was not discussed regarding diagnosis of CCM. However, prolongation of the QT interval is listed as one of the key criteria for its diagnosis. The QT interval should be analyzed with other cardiovascular abnormalities present in patients with cirrhosis and/or portal vein hypertension.
Methodology
In our study, we measured the QT interval only in lead II, while other studies (Mohamed et al., Zambruni et al., Bernardi et al.) calculated an average maximal QT interval in any of the 12 leads. However, U waves are more prominent in the left chest leads and less prominent in lead II, with the end of the T wave in lead II corresponding to the end of significant repolarization in any other lead. Therefore, by convention, lead II has been chosen to measure the QT interval [2].
It is suggested to take QT interval measurement in leads II and V5 or V6 and the longest value should be noted. In our study, we worked on standard 12-lead ECG tracings at a speed of 25 mm/s with an amplitude of 10 mm/mV. This is generally considered as adequate for accurate measurement of the QT interval. In some studies, higher paper speeds (50 mm/s) were used, but a higher speed may increase the risk of distortion of low-amplitude U waves, making measurement more difficult [23].
Study limitations
There are several limitations of our analysis. First, this was the single-center study and it was conducted in a retrospective manner. We did not correlate the long-term mortality with the presence of any perioperative cardiovascular events. The cause of death could not be identified since only the date of death was available from the National Registry regarding patients who died. We did not compare the presence of QTc prolongation with the diagnosis of CCM. However, in other studies, this correlation was also not discussed.
Conclusions
The most suitable formula for QT interval correction (QTc) in patients with ESLD is the Fridericia formula (F), not the more-frequently used Bazett formula. There was no obvious correlation between QTc interval calculated with the 3 formulas and severity of liver disease assessed by Child-Pugh score and MELD score. However, QTc interval might be associated with complications of ESLD. The QTc (F) interval was higher in patients with alcoholic etiology of liver disease when compared to patients with viral hepatitis and hepatitis of other etiologies. The prolonged QT interval was not associated with higher all-cause mortality after OLTx.
Acknowledgments
Participating investigators who collected data and cared for the patients participating in the study.
Footnotes
Source of support: Self financing
Conflict of interest
None.
References
- 1.Browne KF, Zipes DP, Heger JJ, et al. Influence of the autonomic nervous system on the Q-T interval in man. Am J Cardiol. 1982;50:1099–e103. doi: 10.1016/0002-9149(82)90425-8. [DOI] [PubMed] [Google Scholar]
- 2.Garson A., Jr How to measure the QT interval – what is normal? Am J Cardiol. 1993;72:14B–16B. doi: 10.1016/0002-9149(93)90034-a. [DOI] [PubMed] [Google Scholar]
- 3.van Noord C, Eijgelsheim M, Stricker BH. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16–23. doi: 10.1111/j.1365-2125.2010.03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noseworthy PA, Peloso GM, Hwang SJ, et al. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol. 2012;17:340–48. doi: 10.1111/j.1542-474X.2012.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen JB, Graff C, Rasmussen PV, et al. Risk prediction of cardiovascular death based on the QTc interval: Evaluating age and gender differences in a large primary care population. Eur Heart J. 2014;35:1335–44. doi: 10.1093/eurheartj/ehu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardi M, Calandra S, Colantoni A, et al. Q-T interval prolongation in cirrhosis: Prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 7.Bazett HC. An analysis of the time-relations of the electrocardiograms. Heart. 1920;7:353–70. [Google Scholar]
- 8.Fridericia LS. Die systolendauer im elektrokardiogramm bei normalen menschen und bei herzkranken. Acta Med Scand. 1920;53:469–86. [in German] [Google Scholar]
- 9.Hodges MS, Salerno D, Erlinen D. Bazett’s QT correction reviewed: Evidence that a linear QT correction for heart rate is better. J Am Coll Cardiol. 1983;1:694. [Google Scholar]
- 10.Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–20. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 11.Aytemir K, Maarouf N, Gallagher MM, et al. Comparison of formulae for heart rate correction of QT interval in exercise electrocardiograms. Pacing Clin Electrophysiol. 1999;22(9):1397–401. doi: 10.1111/j.1540-8159.1999.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 12.Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70(7):797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs – Questions and Answers (R1) Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073161.pdf.
- 14.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Indik JH, Pearson EC, Fried K, Woosley RL. Bazett and Fridericia QT correction formulas interfere with measurement of drug-induced changes in QT interval. Heart Rhythm. 2006;3(9):1003–7. doi: 10.1016/j.hrthm.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Zambruni A, Di Micoli A, Lubisco A, et al. QT interval correction in patients with cirrhosis. J Cardiovasc Electrophysiol. 2007;18:77–82. doi: 10.1111/j.1540-8167.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 17.Bal JS, Thuluvath PJ. Prolongation of QTc interval: Relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23:243–48. doi: 10.1034/j.1600-0676.2003.00833.x. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23(5):1128–34. doi: 10.1002/hep.510230529. [DOI] [PubMed] [Google Scholar]
- 19.Carvalheiro F, Rodrigues C, Adrego T, et al. Diastolic dysfunction in liver cirrhosis: Prognostic predictor in liver transplantation? Transplant Proc. 2016;48(1):128–31. doi: 10.1016/j.transproceed.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: The effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Trevisani F, Di Micoli A, Zambruni A, et al. QT interval prolongation by acute gastrointestinal bleeding in patients with cirrhosis. Liver Int. 2012;32(10):1510–15. doi: 10.1111/j.1478-3231.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 22.Abbas WA, Ahmed SMK, Abdel Aal AM, et al. Galactin-3 and brain natriuretic peptide versus conventional echocardiography in the early detection of cirrhotic cardiomyopathy. Turk J Gastroenterol. 2016;27:367–74. doi: 10.5152/tjg.2016.16100. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg I, Moss AJ, Zareba W. QT interval: How to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17:333–36. doi: 10.1111/j.1540-8167.2006.00408.x. [DOI] [PubMed] [Google Scholar]


