Abstract
Background
ABO-incompatible (ABOi) living donor liver transplantation (LDLT) was accepted as a feasible therapy for end-stage liver disease after the introduction of rituximab. The present study investigated the association between ABO incompatibility and graft regeneration in patients who underwent LDLT.
Material/Methods
A total of 335 adult patients who underwent elective LDLT were divided into ABO-compatible (ABOc) and ABOi LDLT groups using propensity score (PS) matching of graft regeneration-related factors. Postoperative serial changes in graft volumes were compared between the groups. The factors associated with graft volume on postoperative day (POD) 21 were investigated in patients who underwent ABOi LDLT.
Results
In total, 300 (89.6%) patients underwent ABOc LDLT and 35 (10.4%) patients underwent ABOi LDLT. After PS matching, the ABOc and ABOi groups each included 32 paired patients. The absolute liver graft volumes on POD 21 were significantly lower in the ABOi group than those in the ABOc group in the PS-matched patients (1098.4 [964.0–1,162.0] vs. 1202.0 [1107.8–1455.2] mL; p=0.007). Major complications, including overall patient mortality during the follow-up period, did not differ between the groups. In patients who underwent ABOi LDLT, the preoperative graft volume/standard liver volume ratio and CD4+ cell level on POD 14 were independent factors related to liver graft volume on POD 21.
Conclusions
These results suggest that ABO incompatibility could affect postoperative liver graft regeneration. Therefore, graft regeneration must be investigated using a volumetric assessment in patients who have undergone ABOi LDLT.
MeSH Keywords: ABO Blood-Group System, Liver Regeneration, Liver Transplantation, Living Donors
Background
Liver transplantation (LT) is widely accepted as a definitive therapy for end-stage liver disease (ESLD). However, due to the donor liver graft shortage, patients suffering from ESLD are not transplanted in a timely manner [1]. Various types of LT, such as non-heart beating donor LT, split LT, and living donor liver transplantation (LDLT), have been performed to expand the donor pool, but the shortage of liver grafts persists [2,3]. Reaching beyond the ABO blood type barrier is considered less feasible for organ transplantation due to the strong immunological antibody-antigen reaction [4]. In addition, incompatible living liver donors have been emergently applied to rescue patients who present with rapid hepatic decompensation but remain on the waiting list; however, long-term outcomes are not good after ABO-incompatible (ABOi) LT [5]. The introduction of the anti-CD20 monoclonal antibody (rituximab) made ABOi LT a clinical reality. Thereafter, patient and graft outcomes improved after ABOi LDLT [6].
Partial liver grafts are required to regenerate rapidly due to the recipient demands of LDLT. A study by Kawasaki et al. [7] reported that transplanted grafts with insufficient mass against recipient body size did not undergo functional failure due to vigorous graft regeneration after LDLT. Previous studies have reported associations between liver graft regeneration and multiple factors, including recipient clinical status, graft ischemia-reperfusion injury, hepatic vascular hemodynamics, and donor condition in LDLT [8–11]. Immunosuppressive drugs also have negative or positive effects on graft regeneration after LDLT [12–14]. A complex immunoreaction develops between the donor graft and the host body in patients who undergo ABOi LDLT [4].
The present study investigated whether ABO incompatibility affects postoperative liver graft regeneration using a propensity score (PS) matching analysis in the LDLT setting. The incidence of major complications was compared between patients who underwent ABO-compatible (ABOc) and ABOi LDLTs. Finally, we analyzed the associations between perioperative factors and postoperative liver graft regeneration in patients who underwent ABOi LDLT.
Material and Methods
Study population
A total of 335 adult patients (age ≥18 years) undergoing elective ABOc or ABOi LDLT from March 2009 to February 2016 at St. Mary’s Hospital (Seoul, South Korea) were analyzed. Retrospective reviews of perioperative recipient and donor data were performed using the hospital electronic medical records system. Defective or inadequate data in the recipient’s or donor’s records were excluded from the present study. The Institutional Review Board of Seoul St. Mary’s Hospital Ethics Committee granted approval for this study (KC17RIS0001). Informed consent was waived due to the retrospective study design.
Surgery and anesthesia
The surgical procedure and anesthetic care of recipients undergoing LDLT were explained previously in detail [15,16]. Briefly, the piggyback method was applied using the right hepatic lobe of the donor, and by reconstructing the middle hepatic vein so that the segmental hepatic veins (from liver segments V and VIII) of the recipient anastomosed to the middle hepatic vein of the donor using a Dacron conduit with an inner diameter of 10 mm (Gelweave; Vascutek, Inchinnan, UK). Hepatic vessels, including the hepatic vein, portal vein, and hepatic artery, were serially anastomosed followed by biliary ductal reconstruction. After the anastomoses with the hepatic vessels, patency of hepatic blood flow was evaluated using spectral Doppler ultrasonography.
The patients were intraoperatively managed with balanced anesthesia. Hemodynamic homeostasis of the patients was adjusted with appropriate circulatory supplementation and a vasopressor was administered under invasive hemodynamic monitoring.
Grouping for the propensity score matching analysis
The study population was classified into the ABOc and ABOi LDLT groups. The perioperative recipient factors and donor-graft factors were applied in the PS matching analysis between the ABOc and ABOi groups. Preoperative recipient factors included age, sex, body mass index (BMI), comorbidities, model for end-stage liver disease (MELD) score, hepatic decompensation complications, and inflammatory markers. Intraoperative recipient factors included duration of surgery, use of a strong vasopressor (epinephrine or norepinephrine), severe post-reperfusion syndrome (PRS), average vital signs, total amount of blood products transfused, hourly fluid infusion, hourly urine output, average lactate, and the neutrophil-to-lymphocyte ratio (NLR). Donor-graft factors were age, sex, BMI, graft fatty percentage and type, total ischemic time, preoperative liver graft/standard liver volume (SLV) ratio, and average hepatic vascular hemodynamics on postoperative days (PODs) 1, 3, and 5.
Desensitization and immunosuppression protocol
All recipients scheduled for ABOi LDLT were treated with a single dose of intravenous rituximab (375 mg/m2 body surface area), which attenuates B cell immunity [17], 2 weeks before the surgery. Recipients’ blood samples were collected to measure the anti-ABO isohemagglutinin titers (anti-A IgM, anti-B IgM, anti-A IgG, and anti-B IgG) at hospital admission, at each point of plasmapheresis, and during the follow-up period. Fresh frozen plasma (FFP) in AB+ blood type was used for plasmapheresis. Plasmapheresis was continued to achieve the desired isohemagglutinin titer (≤1: 8) before surgery, and postoperative isoagglutinin titers were monitored and treated if the titers increased >1: 8 to 16.
The immunosuppression regimen (tacrolimus, methylprednisolone, and mycophenolate mofetil [MMF]) was administered postoperatively. The trough level of tacrolimus was preserved between 7 and 10 ng/mL for the first month after surgery and tapered between 5 and 7 ng/mL thereafter. Methylprednisolone was administered immediately before graft reperfusion and then gradually tapered. MMF was withdrawn 3–6 months after surgery. Basiliximab (interleukin-2 receptor blocker) was administered on the day of LDLT prior to the surgery and on POD 4.
Perioperative cluster of differentiation marker measurement
The cluster of differentiation (CD) marker levels (%), including CD 4+, 8+, 19+, 20+, and 25+ were estimated 1 day before surgery and on POD 14 in patients undergoing ABOi LDLT. Blood samples were obtained in test tubes (BD Vacutainer, K2 EDTA; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and sent to the laboratory for analysis of the CD markers using Navios flow cytometry (Beckman Coulter, Indianapolis, IN, USA).
Postoperative graft volume measurement
Liver graft volumes were measured using abdominal computed tomography (CT) images in the recipients and donors. Total liver volume and right lobe volume of donors were determined on preoperative volumetric CT images, and liver graft volumes of recipients were identified by postoperative volumetric CT images on PODs 7 and 21. Experienced radiologists measured absolute liver volume (ALV; mL) using volumetry software (AW VolumeShare 4; General Electric Healthcare, Chicago, IL, USA). Relative liver volume (RLV;%) was calculated as the ratio of the liver graft volume to the SLV. SLV was defined as follows: 1072.8×body surface area (BSA)−345.7 (BSA=weight [kg]0.425×height [cm]0.725×0.007184) [18].
Clinical outcomes
Postoperative clinical outcomes were liver graft volumes on PODs 7 and 21, hospital and intensive care unit (ICU) stays, early allograft dysfunction (EAD) [19], re-operation, infection, duration of mechanical ventilation, re-intubation, and major graft complications, including acute cellular rejection defined as clinically suspected findings and a Rejection Activity Index ≥3 [20], and acute antibody-mediated rejection (AMR) based on histopathological findings such as monocytic, eosinophilic, or neutrophilic microvascular/capillary inflammation [21], biliary complications, hepatic vessel thrombosis, and de novo cancer occurrence. Overall patient survival was assessed on the last out-patient visit during the follow-up period.
Statistical analysis
Comparisons of perioperative recipient factors and donor-graft factors were evaluated using the Mann-Whitney U-test, and χ2 test or Fisher’s exact test, as appropriate, before PS matching. The normality of the continuous data was tested using the Shapiro–Wilk test. A PS matching analysis was used to minimize the influence of potential confounding factors on intergroup differences based on ABO compatibility. The PS scores were elicited from matched patients at a 1: 1 ratio using greedy matching algorithms without replacement. Standardized mean differences <0.25 were considered to indicate well-balanced matching between the groups [22,23]. The Wilcoxon signed-rank sum test and McNemar’s test were applied to the pair-matched data. Continuous data are expressed as median and interquartile range (IQR), and categorical data are shown as numbers and proportions. The perioperative determinants that affected liver graft volume on POD 21 were analyzed using univariate and multivariate linear regression in patients undergoing ABOi LDLT. Potentially significant factors (p<0.1) in the univariate analysis were included in a multivariate analysis. When various perioperative factors were correlated with each other, the most clinically relevant factors were selected. The values in univariate and multivariate linear analyses are presented as β (regression coefficient) and 95% confidence intervals (CIs). All tests were two-sided, and a p-value <0.05 was considered significant. Overall survival of patients was evaluated using the Kaplan-Meier analysis and compared between the groups using the log-rank test. We used stratified Cox regression to account for the PS matching. The stratified Cox regression analysis was applied to evaluate the hazard ratio (HR) and 95% CI of ABO compatibility on overall survival of patients. All statistical analyses were performed using the SAS 9.4 software package (SAS Institute, Cary, NC, USA).
Results
Study population
Ninety-five patients were excluded due to deficient or missing donor-related variables (10 patients), radiological images of liver grafts (11 patients), intraoperative hemodynamic variables of recipients (10 patients), and emergency LDLT (64 patients). Eventually, 335 patients were enrolled in our study. The study populations were featured as follows: median age was 54.0 (IQR: 49.0–59.0) years; mainly male sex (70.7%); median BMI was 23.8 (IQR: 22.0–26.4) kg/m2. The median follow-up period was 3.3 (IQR: 1.3–5.4) years after LDLT. Hepatocellular carcinoma was diagnosed in 177 (52.8%) patients, and the most common indication for LDLT was hepatitis B virus (60.3%), followed by alcohol abuse (20.9%), hepatitis C virus (7.5%), toxin and drug-related hepatitis (3.8%), autoimmune hepatitis (2.7%), and cryptogenic hepatitis (4.8%). The median MELD score was 13.7 (IQR: 9.2–23.4) points, and hepatic decompensation, including severe encephalopathy (West-Haven criteria III or IV), variceal hemorrhage, and ascites (>1 L), was seen in 77 (22.9%), 89 (26.5%), and 143 (42.6%) patients, respectively.
A total of 300 (89.6%) patients underwent ABOc LDLT. Identical LDLTs were performed in 201 (60.0%) patients, and compatible LDLTs were conducted in 99 (29.5%) patients. Thirty-five (10.4%) patients underwent ABOi LDLT. The preoperative frequency of plasmapheresis was 5.0 (IQR: 4.0–6.0) to reduce the isohemagglutinin titer to <1: 8 in patients who underwent ABOi LDLT.
Comparison of perioperative factors before and after propensity score matching
Before PS matching, preoperative NLR and sodium levels were higher in the ABOi group than in the ABOc group, but AST and ALT levels were lower in the ABOi group than in the ABOc group. The mean intraoperative NLR was higher in the ABOi group than in the ABOc group, and more bicarbonate was administered to the ABOi group than to the ABOc LDLT group. Donor-graft findings, such as age, sex, BMI, preoperative liver graft volume/SLV ratio, graft fatty percentage and type, total ischemic time, and hepatic vascular hemodynamic, were not different between the ABOc and ABOi groups (Table 1). After PS matching, no significant differences in perioperative factors were found in the recipients or donors between the ABOc and ABOi groups (Table 2).
Table 1.
Perioperative characteristics between the ABO-Compatible and ABO-Incompatible living donor liver transplantation groups before propensity score-matching.
| Characteristics | Total subject (n=335) | ABOc (n=300) | ABOi (n=35) | p | |||
|---|---|---|---|---|---|---|---|
| Preoperative recipient findings | |||||||
| Age (years) | 54.0 | (49.0–59.0) | 53.0 | (49.0–59.0) | 55.0 | (50.0–58.0) | 0.566 |
| Gender (Male) | 237 | (70.7%) | 210 | (70.0%) | 27 | (77.1%) | 0.379 |
| Body mass index (kg/m2) | 23.8 | (22.0–26.4) | 23.9 | (21.8–26.4) | 23.4 | (22.2–25.2) | 0.546 |
| Diagnosis | 0.949 | ||||||
| Alcohol | 70 | (20.9%) | 64 | (21.3%) | 6 | (17.1%) | |
| Hepatitis B | 202 | (60.3%) | 179 | (59.7%) | 23 | (65.7%) | |
| Hepatitis C | 25 | (7.5%) | 22 | (7.3%) | 3 | (8.6%) | |
| Autoimmune | 9 | (2.7%) | 9 | (3.0%) | 0 | (0.0%) | |
| Toxin and drung | 13 | (3.8%) | 12 | (4.0%) | 1 | (2.9%) | |
| Cryptogenic | 16 | (4.8%) | 14 | (4.7%) | 2 | (5.7%) | |
| Comorbidity | |||||||
| Diabetes mellitus | 87 | (25.9%) | 78 | (26.0%) | 9 | (25.7%) | 0.971 |
| Hypertension | (18.2%) | 53 | (17.7%) | 8 | (22.9%) | 0.451 | |
| MELD score (points) | 13.7 | (9.2–23.4) | 14.1 | (9.4–23.6) | 13.4 | (8.4–20.2) | 0.385 |
| Hepatic decompensation | |||||||
| Severe encephalopathy | 77 | (22.9%) | 68 | (22.7%) | 9 | (25.7%) | 0.685 |
| Variceal hemorrahage | 89 | (26.5%) | 82 | (27.3%) | 7 | (20.0%) | 0.353 |
| Ascites (>1L) | 143 | (42.6%) | 131 | (43.7%) | 12 | (34.3%) | 0.288 |
| Laboratory parameters | |||||||
| Hematocrit (%) | 29.7 | (25.4–35.8) | 29.8 | (25.3–35.9) | 29.6 | (26.4–35.1) | 0.960 |
| NLR | 2.2 | (1.4–4.0) | 2.10 | (1.3–3.8) | 3.2 | (2.2–4.8) | 0.005 |
| PLR | 64.3 | (28.1–95.8) | 65.8 | (31.0–95.7) | 62.5 | (3.0–104.7) | 0.149 |
| Platelet count (x103/μL) | 62.0 | (46.0–101.0) | 62.0 | (46.0–101.7) | 59.0 | (38.0–99.0) | 0.547 |
| Total bilirubin (mg/dL) | 1.8 | (0.8–6.8) | 1.9 | (0.9–6.8) | 1.7 | (0.6–3.7) | 0.289 |
| Creatinine (mg/dL) | 0.8 | (0.6–1.0) | 0.7 | (0.6–1.1) | 0.8 | (0.6–0.9) | 0.707 |
| International normalized ratio | 1.4 | (1.2–1.8) | 1.4 | (1.2–1.8) | 1.4 | (1.2–1.7) | 0.798 |
| AST (unit/L) | 46.0 | (31.0–74.0) | 47.0 | (33.0–77.0) | 34.0 | (26.0–57.0) | 0.009 |
| ALT (unit/L) | 31.0 | (21.0–49.0) | 32.0 | (22.0–52.7) | 22.0 | (18.0–37.0) | 0.003 |
| Sodium (mEq/L) | 140.0 | (136.0–142.0) | 139.0 | (136.0–141.0) | 141.0 | (139.0–143.0) | 0.027 |
| Intraoperative recipient findings | |||||||
| Duration of surgery (min) | 515.0 | (465.0–585.0) | 520.0 | (465.0–589.0) | 505.0 | (455.0–530.0) | 0.182 |
| Strong vasopressor | 51 | (15.2%) | 47 | (15.7%) | 4 | (11.4%) | 0.509 |
| Severe PRS | 49 | (14.6%) | 46 | (15.3%) | 3 | (8.6%) | 0.284 |
| Average of vital signs | |||||||
| CVP (mmHg) | 9.6 | (8.0–11.5) | 9.6 | (8.0–11.5) | 10.3 | (7.7–11.5) | 0.918 |
| MPAP (mmHg) | 18.8 | (16.3–20.8) | 18.6 | (16.1–20.8) | 18.9 | (17.6–20.8) | 0.303 |
| Stroke volume variation (%) | 6.6 | (5.0–8.6) | 6.6 | (5.0–8.5) | 6.5 | (5.0–10.3) | 0.921 |
| Cardiac index (L/min/m2) | 4.1 | (3.6–4.9) | 4.1 | (3.6–4.8) | 4.0 | (3.2–5.0) | 0.984 |
| SVRI (dynes-sec/cm5/m2) | 1266.1 | (1011.5–1581.1) | 1284.4 | (1011.5–1591.5) | 1231.0 | (1007.1–1321.4) | 0.631 |
| Mean blood pressure (mmHg) | 77.0 | (70.9–83.5) | 77.0 | (70.9–83.6) | 76.7 | (70.9–82.0) | 0.629 |
| Heart rate (beats/min) | 86.4 | (76.8–97.0) | 86.4 | (76.6–97.0) | 85.4 | (80.2–95.0) | 0.940 |
| Blood product transfusion (unit) | |||||||
| Packed red blood cell | 7.0 | (4.0–12.0) | 7.5 | (3.0–12.7) | 6.0 | (5.0–12.0) | 0.818 |
| Fresh frozen plasma | 7.0 | (4.0–10.0) | 7.0 | (4.0–10.0) | 6.0 | (4.0–9.0) | 0.115 |
| Platelet concentrates | 4.0 | (0.0–10.0) | 4.0 | (0.0–10.0) | 2.0 | (0.0–10.0) | 0.565 |
| Cryoprecipitate | 0.0 | (0.0–0.0) | 0.0 | (0.0–0.0) | 0.0 | (0.0–0.0) | 0.452 |
| Fluid infusion (mL/kg/h) | 10.0 | (7.6–12.9) | 10.0 | (7.6–12.9) | 10.6 | (7.4–14.8) | 0.523 |
| Urine output (mL/kg/h) | 1.4 | (0.7–2.2) | 1.4 | (0.7–2.2) | 1.4 | (0.7–2.1) | 0.799 |
| Drug administraion | |||||||
| Bicarbonate (mEq) | 0.0 | (0.0–60.0) | 0.0 | (0.0–57.5) | 40.0 | (0.0–100.0) | 0.019 |
| Insulin (unit) | 10.0 | (2.0–25.0) | 10.0 | (3.0–25.0) | 6.0 | (0.0–25.0) | 0.142 |
| Frusemide (mg) | 10.0 | (0.0–20.0) | 10.00 | (0.0–20.0) | 10.0 | (0.0–20.0) | 0.281 |
| Mean lactate (mmol/L) | 4.1 | (3.3–5.4) | 4.0 | (3.3–5.4) | 4.5 | (3.3–5.4) | 0.806 |
| Mean NLR | 12.7 | (8.1–19.2) | 12.1 | (7.9–18.8) | 16.0 | (12.8–23.2) | 0.002 |
| Donor graft findings | |||||||
| Age (years) | 32.0 | (25.0–42.0) | 32.0 | (25.0–42.0) | 29.0 | (24.0–36.0) | 0.519 |
| Gender (Male) | 128 | (38.2%) | 114 | (38.0%) | 14 | (40.0%) | 0.818 |
| Body mass index (kg/m2) | 23.3 | (21.1–25.5) | 23.4 | (21.1–25.5) | 21.5 | (19.9–26.1) | 0.125 |
| Graft volume/SLV ratio* | 55.9 | (47.6–68.7) | 56.1 | (48.0–68.2) | 53.9 | (43.2–70.2) | 0.372 |
| Graft fatty percentage (%) | 3.0 | (0.0–5.0) | 4.0 | (0.0–5.0) | 3.0 | (0.0–5.0) | 0.989 |
| Graft fatty type | 0.572 | ||||||
| No fat | 93 | (27.8%) | 82 | (27.3%) | 11 | (31.4%) | |
| Microvesicular | 11 | (3.3%) | 10 | (3.3%) | 1 | (2.9%) | |
| Macrovesicular | 216 | (64.5%) | 196 | (65.3%) | 20 | (57.1%) | |
| Mixed | 15 | (4.4%) | 12 | (4.0%) | 3 | (8.6%) | |
| Total ischemic time (min) | 92.7 | (69.0–117.0) | 92.7 | (68.0–117.0) | 92.0 | (74.0–109.0) | 0.746 |
| Average of hepatic vascular hemodynamic on postoperative days 1, 3, and 5 | |||||||
| Hepatic arterial resistive index | 0.6 | (0.6–0.6) | 0.6 | (0.5–0.6) | 0.6 | (0.6–0.7) | 0.122 |
| Portal venous flow (mL/min) | 2057.0 | (1495.0–2613.6) | 1884.8 | (1461.7–2722.5) | 2004.6 | (1591.2–2840.1) | 0.674 |
MELD – Model for end-stage liver disease; NLR – neutrophil to lymphocyte ratio; PLR – platelet to lymphocyte ratio; AST – asparate aminotransferase; ALT – alanine aminotransferase; PRS – postreperfusion syndrome; CVP – central venous pressure; MPAP – mean pulmonary artery pressure; SVRI – systemic vascular resistive index; SLV – standard liver volume. Values are expressed as number (proportion), mean ±SD or median (IQR).
Table 2.
Perioperative characteristics between the ABO-Compatible and ABO-Incompatible living donor liver transplantation after propensity score-matching.
| Characteristics | Total subject (n=64) | ABOc (n=32) | ABOi (n=32) | p | SD | |||
|---|---|---|---|---|---|---|---|---|
| Preoperative recipient factors | ||||||||
| Age (years) | 54.0 | (49.0–57.0) | 54.0 | (49.0–57.0) | 54.0 | (49.5–57.0) | 0.954 | 5.0 |
| Gender (Male) | 48 | (75.0%) | 24 | (75.0%) | 24 | (75.0%) | >0.999 | 0.0 |
| Body mass index (kg/m2) | 24.1 | (22.2–26.3) | 24.4 | (21.7–28.6) | 23.6 | (22.4–25.3) | 0.419 | 16.1 |
| Comorbidity | ||||||||
| Diabetes mellitus | 14 | (21.9%) | 7 | (21.9%) | 7 | (21.9%) | >0.999 | 0.0 |
| Hypertension | 11 | (17.2%) | 5 | (15.6%) | 6 | (18.8%) | 0.763 | 8.3 |
| MELD score (points) | 12.0 | (8.0–20.0) | 11.5 | (8.0–20.5) | 12.0 | (8.0–20.0) | 0.565 | 11.7 |
| Hepatic decompensated complication | ||||||||
| No | 64 | (100.0%) | 32 | (100.0%) | 32 | (100.0%) | - | - |
| Inflammatory marker | ||||||||
| NLR | 2.7 | (1.7–4.1) | 1.9 | (1.6–3.1) | 3.1 | (2.2–4.8) | 0.230 | 21.6 |
| Platelet to lymphocyte ratio | 62.0 | (47.5–108.5) | 71.0 | (52.5–115.5) | 60.5 | (38.5–100.5) | 0.378 | 4.7 |
| Intraoperative recipient factors | ||||||||
| Duration of surgery (min) | 487.5 | (455.0–547.5) | 477.5 | (445.0–570.0) | 497.5 | (460.0–525.0) | 0.886 | 8.5 |
| Strong vasopressor | 8 | (12.5%) | 4 | (12.5%) | 4 | (12.5%) | >0.999 | 0.0 |
| Severe PRS | 6 | (9.4%) | 4 | (12.5%) | 2 | (6.3%) | 0.317 | 21.6 |
| Average of vital signs | ||||||||
| CVP (mmHg) | 10.2 | (8.4–11.5) | 9.7 | (8.7–11.3) | 10.6 | (8.3–12.0) | 0.891 | 3.7 |
| MPAP (mmHg) | 19.0 | (16.8–21.0) | 18.9 | (16.3–21.0) | 19.0 | (17.9–21.0) | 0.564 | 4.6 |
| Mean blood pressure (mmHg) | 76.9 | (71.0–82.2) | 77.3 | (71.8–84.6) | 76.4 | (70.7–81.2) | 0.715 | 16.5 |
| Heart rate (beats/min) | 84.6 | (80.4–97.0) | 82.4 | (77.6–98.7) | 87.6 | (81.6–95.6) | 0.648 | 14.7 |
| Blood product transfusion (unit) | ||||||||
| Packed red blood cell | 6.5 | (3.0–11.0) | 7.5 | (3.0–10.5) | 6.0 | (5.0–11.5) | 0.920 | 5.4 |
| Fresh frozen plasma | 6.0 | (4.0–9.5) | 5.0 | (4.0–10.0) | 6.0 | (4.0–8.5) | 0.744 | 0.0 |
| Platelet concentrates | 0.0 | (0.0–0.0) | 0.0 | (0.0–1.5) | 0.0 | (0.0–0.0) | 0.889 | 3.5 |
| Cryoprecipitate | 0.0 | (0.0–0.0) | 0.0 | (0.0–0.0) | 0.0 | (0.0–0.0) | - | - |
| Hourly fluid infusion (mL/kg/h) | 9.6 | (7.7–12.7) | 9.3 | (7.7–11.7) | 10.6 | (7.8–13.8) | 0.358 | 17.7 |
| Hourly urine output (mL/kg/h) | 830.0 | (497.5–1140.0) | 830.0 | (585.0–1115.0) | 830.0 | (425.0–1175.0) | 0.442 | 20.7 |
| Mean lactate (mmol/L) | 4.2 | (3.3–5.3) | 4.0 | (3.3–5.1) | 4.5 | (3.5–5.5) | 0.709 | 10.0 |
| Mean NLR | 86.5 | (81.9–89.7) | 85.3 | (80.9–88.9) | 87.5 | (84.2–90.6) | 0.344 | 17.2 |
| Donor graft factors | ||||||||
| Age (years) | 28.5 | (23.5–35.0) | 28.0 | (23.0–35.0) | 28.5 | (23.5–35.5) | 0.641 | 16.4 |
| Gender (Male) | 43 | (67.2%) | 23 | (71.9%) | 20 | (62.5%) | 0.467 | 20.1 |
| Body mass index (kg/m2) | 22.1 | (20.6–24.9) | 22.4 | (21.1–24.1) | 21.7 | (20.2–26.3) | 0.898 | 0.7 |
| Graft fatty percentage (%) | 3.0 | (0.0–5.0) | 5.0 | (0.5–5.0) | 3.0 | (0.0–5.0) | 0.815 | 17.7 |
| Graft fatty type | ||||||||
| No fatty change | 18 | (28.1) | 8 | (25.0) | 10 | (31.3) | 0.997 | 14.0 |
| Microvesicular | 2 | (3.1) | 1 | (3.1) | 1 | (3.1) | 0.0 | |
| Macrovesicular | 39 | (60.9) | 21 | (65.6) | 18 | (56.3) | 19.1 | |
| Mixed | 5 | (7.8) | 2 | (6.3) | 3 | (9.4) | 11.5 | |
| Total ischemic time (min) | 92.7 | (72.5–115.0) | 94.5 | (70.5–120.0) | 91.5 | (73.0–108.5) | 0.654 | 11.4 |
| Preop liver graft/SLV ratio (%) | 53.4 | (44.1–70.3) | 51.2 | (44.1–67.9) | 54.1 | (44.6–70.3) | >0.999 | 1.3 |
| Average of hepatic vascular hemodynamic on postoperative days 1, 3, and 5 | ||||||||
| Hepatic arterial resistive index | 0.7 | (0.6–0.7) | 0.7 | (0.6–0.7) | 0.7 | (0.6–0.7) | 0.204 | 16.5 |
| Portal venous flow (mL/min) | 1942.5 | (1584.0–2663.2) | 1855.6 | (1559.8–2648.8) | 2032.0 | (1631.0–2743.0) | 0.884 | 3.9 |
MELD – model for end-stage liver disease; PRS – postreperfusion syndrome; CVP – central venous pressure; MPAP – mean pulmonary arterial pressure; NLR – neutrophil to lymphocyte ratio; SLV – standard liver volume. Values are numbers (percentages) for categorical variables and mean ±SD, median (IQR) others.
Liver graft volumes according to ABO incompatibility before and after propensity score matching
ALV and RLV on POD 21 were smaller in the ABOi group than in the ABOc LDLT before PS matching. ALV in the ABOi group was significantly smaller on POD 21 than that in the ABOc group after PS matching, but the difference in RLV on POD 21 was marginally comparable between the groups (Table 3).
Table 3.
Comparison of liver graft regeneration between the ABO-Compatible and ABO-Incompatible living donor liver transplantation before and after propensity score matching.
| Total subject (n=335) | Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|---|
| ABOc (n=300) | ABOi (n=35) | p | ABOc (n=32) | ABOi (n=32) | p | ||
| Absolute liver graft volume (mL) | |||||||
| Preoperative day | 840.0 (736.6–1008.2) | 840.0 (736.7–1010.9) | 846.3 (706.8–1000.7) | 0.761 | 823.4 (730.9–981.7) | 857.5 (733.4–1016.2) | 0.798 |
| Postoperative day 7 | 1143.4 (1018.8–1257.5) | 1151.2 (1028.4–1259.5) | 1069.32 (925.4–1207.4) | 0.061 | 1121.9 (1014.5–1253.4) | 1061.9 (919.4–1183.2) | 0.300 |
| Postoperative day 21 | 1161.9 (1022.4–1335.0) | 1188.0 (1037.5–1367.7) | 1092.2 (966.4–1157.3) | 0.003 | 1202.0 (1107.8–1455.2) | 1098.4 (964.0–1162.0) | 0.007 |
| Relative liver graft volume (%) | |||||||
| Standard liver volume (mL) | 1530.4 (1375.4–1638.3) | 1524.8 (1373.9–1635.0) | 1559.1 (1392.8–1660.8) | 0.516 | 1585.7 (1433.3–1705.5) | 1564.6 (1395.3–1663.9) | 0.701 |
| Preoperative day | 55.9 (47.6–68.6) | 56.0 (47.9–68.2) | 53.8 (43.1–70.1) | 0.371 | 51.2 (44.1–67.9) | 54.1 (44.6–70.3) | >0.999 |
| Postoperative day 7 | 74.1 (66.3–84.6) | 74.7 (66.9–84.9) | 68.8 (62.0–82.3) | 0.022 | 71.2 (63.7–84.4) | 66.7 (62.3–80.6) | 0.846 |
| Postoperative day 21 | 78.4 (68.7–88.4) | 79.0 (69.8–89.1) | 68.8 (64.8–82.7) | 0.001 | 78.9 (70.3–88.6) | 68.9 (62.5–83.3) | 0.023 |
Values are expressed as number (proportion) and median (IQR).
Clinical outcomes according to ABO incompatibility in the propensity score-matched patients
Major events in the hospital (hospital and ICU stays, EAD development, re-operation, infection, duration of mechanical ventilation, and re-intubation) and major graft complications (acute cellular rejection, acute AMR, biliary complications, hepatic vessel thrombosis, and de novo cancer occurrence) were comparable between the groups during the follow-up period (Table 4).
Table 4.
Comparison of postoperative outcomes between ABO-Compatible and ABO-Incompatible living donor liver transplantation after propensity score matching.
| Characteristics | ABOc (n=32) | ABOi (n=32) | p | ||
|---|---|---|---|---|---|
| Major events in hospital | |||||
| Hospital stay (day) | 22.0 | (21.0–29.5) | 25.5 | (21.0–34.0) | 0.438 |
| Intensive care unit stay (day) | 7.0 | (6.0–7.0) | 7.0 | (6.0–7.0) | 0.956 |
| Early allograft dysfunction | 5 | (15.6%) | 5 | (15.6%) | >0.999 |
| Re-operation | 1 | (3.1%) | 3 | (9.7%) | 0.317 |
| Infection | 3 | (9.4%) | 3 | (9.7%) | >0.999 |
| Mechanical ventilation (min) | 0.0 | (0.0–391.5) | 0.0 | (0.0–217.5) | 0.626 |
| Re-intubation | 3 | (9.4%) | 1 | (3.2%) | 0.317 |
| Major graft complications | |||||
| Acute graft rejection | 6 | (18.8%) | 7 | (21.9%) | 0.782 |
| Acute cellular rejection | 4 | (12.5%) | 5 | (15.6%) | >0.999 |
| Acute antibody-mediated rejection | 2 | (5.9%) | 2 | (5.9%) | >0.999 |
| Biliary complication | 13 | (40.6%) | 12 | (37.5%) | 0.796 |
| Hepatic vessel thrombosis | – | – | – | ||
| De novo cancer occurrence | 3 | (9.4%) | 5 | (15.6%) | 0.480 |
Values are expressed as number (proportion) and median (IQR).
No difference in overall patient survival was observed between the groups during the follow-up period (Figure 1). The association between overall patient survival and ABOi LDLT was not significant based on the ABOc LDLT using a stratified Cox regression analysis (HR: 1.33; 95% CI: 0.30–5.96; p=0.706).
Figure 1.
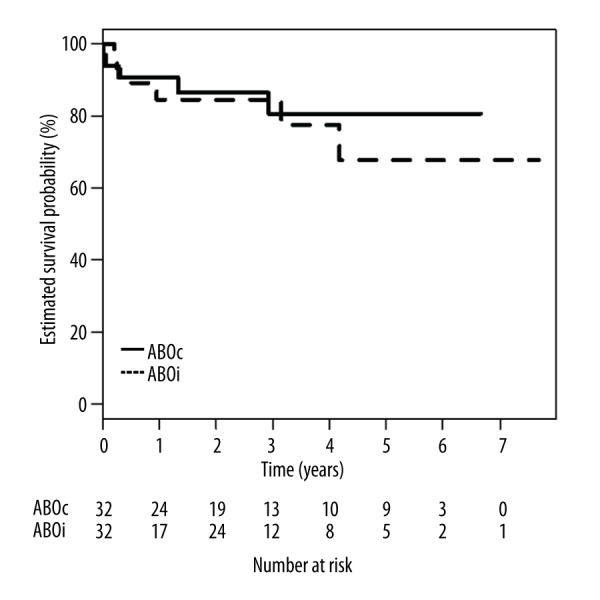
Comparison of overall patient survival between ABO-compatible and ABO-incompatible LDLT in the PS-matched patients. p=0.706, ABO-compatible vs. ABO-incompatible groups by the log-rank test.
Association between perioperative factors and liver graft volume on postoperative day 21 in patients who underwent ABO-incompatible living donor liver transplantation
In patients undergoing ABOi LDLT (Table 5), a univariate linear analysis related to the ALV on POD 21 showed that potentially significant predictors were involved in preoperative recipient factors (MELD score, hematocrit, NLR, total bilirubin, and INR), intraoperative recipient factors (central venous pressure [CVP], mean pulmonary arterial pressure, and furosemide administration), a donor graft factor (preoperative graft volume/SLV ratio), and specific ABOi LDLT factors (the preoperative serum cellular levels [%] of CD19+, which is a protein expressed on B lymphocytes, and CD4+, which is a protein expressed on T-lymphocytes; and CD4+ cell on POD 14). A multivariate linear analysis identified that preoperative graft volume/SLV ratio and CD4+ cellular level on POD 14 were independently associated with the ALV on POD 21 (R2=0.689; p=0.009). In RLV on POD 21 (Table 6), a univariate linear analysis showed that potentially valid predictors were included with preoperative recipient factors (age, male sex, BMI, hematocrit, platelet, and INR), and intraoperative recipient factors (severe PRS, CVP, FFP transfusion, and furosemide infusion), a donor graft factor (preoperative graft volume/SLV ratio), and specific ABOi LDLT factors (preoperative plasmapheresis frequency and CD4+ cellular level, and CD4+ cellular level on POD 14). A multivariate linear analysis showed that preoperative graft volume/SLV ratio and CD4+ cellular level on POD 14 were independently related to the RLV on POD 21 (R2=0.779; p<0.001).
Table 5.
Association between perioperative factors and absolute liver graft volume on postoperative day 21 in patients who underwent ABO-Incompatible living donor liver transplantation.
| Characteristics | Absolute liver graft volume (mL) on POD 21 | |||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| β | 95% CI | p | β | 95% CI | p | |
| Preoperative recipient factors | ||||||
| MELD score (points) | 7.31 | 0.31 to 14.30 | 0.041 | |||
| Laboratory parameters | ||||||
| Hematocrit (%) | −9.47 | −20.45 to 1.52 | 0.088 | |||
| Neutrophil/lymphocyte ratio | 18.06 | 0.40 to 35.72 | 0.045 | |||
| Total bilirubin (mg/dL) | 6.89 | 0.12 to 13.66 | 0.046 | |||
| International normalized ratio | 154.86 | −0.92 to 310.65 | 0.051 | |||
| Intraoperative recipient factors | ||||||
| Average of vital signs | ||||||
| CVP (mmHg) | 42.01 | 19.71 to 64.30 | 0.001 | |||
| MPAP (mmHg) | 12.07 | −1.88 to 26.02 | 0.087 | |||
| Frusemide (mg) | 2.84 | −0.04 to 5.72 | 0.054 | |||
| Donor graft factors | ||||||
| Preoperative graft volume/SLV ratio* | 7.15 | 2.94 to 11.34 | 0.002 | 11.65 | 4.44 to 18.86 | 0.006 |
| Specific factors in ABOi LDLT | ||||||
| Cluster of difference (CD) level (%) on one day before the surgery | ||||||
| CD 4+ | −5.61 | −10.63 to −0.58 | 0.031 | |||
| CD 19+ | 188.52 | −42.76 to 419.81 | 0.098 | |||
| CD level (%) on postoperative day 14 | ||||||
| CD 4+ | −7.54 | −13.66 to −1.40 | 0.019 | −6.87 | −13.13 to −0.62 | 0.035 |
MELD – model for end-stage liver disease; CVP – central venous pressure; MPAP – mean pulmonary arterial pressure; ABOi LDLT – ABO-incompatible living donor liver transplantation.
Table 6.
Association between perioperative factors and relative liver graft volume on postoperative day 21 in patients who underwent ABO-Incompatible living donor liver transplantation.
| Characteristics | Relative liver graft volume (%) on POD 21 | |||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| β | 95% CI | p | β | 95% CI | p | |
| Preoperative recipient factors | ||||||
| Age (years) | 0.62 | −0.08 to 1.32 | 0.080 | |||
| Gender (Male) | 14.00 | 1.35 to 26.64 | 0.031 | |||
| Body mass index (kg/m2) | −2.40 | −4.61 to −0.18 | 0.035 | |||
| Laboratory parameters | ||||||
| Hematocrit (%) | −0.93 | −1.81 to −0.05 | 0.039 | |||
| Platelet count (x103/μL) | −0.07 | −0.16 to 0.01 | 0.088 | |||
| International normalized ratio | 12.93 | 0.13 to 25.71 | 0.048 | |||
| Intraoperative recipient factors | ||||||
| Severe PRS | 23.54 | 6.63 to 40.44 | 0.008 | |||
| Average of vital signs | ||||||
| CVP (mmHg) | 1.87 | −0.30 to 4.04 | 0.089 | |||
| Blood product transfusion (unit) | ||||||
| Fresh frozen plasma | 1.65 | −0.18 to 3.47 | 0.075 | |||
| Frusemide (mg) | 0.23 | −0.00 to 0.47 | 0.055 | |||
| Donor graft factors | ||||||
| Preoperative graft volume/SLV ratio* | 0.84 | 0.59 to 1.08 | 0.000 | 0.76 | 0.49 to 1.03 | 0.000 |
| Specific factors in ABOi LDLT | ||||||
| Preoperative frequency of plasmapheresis | −1.37 | −2.95 to 0.21 | 0.087 | |||
| Cluster of difference (CD) level (%) on one day before the surgery | ||||||
| CD 4+ | −0.37 | −0.797 to 0.054 | 0.083 | |||
| CD level (%) on postoperative day 14 | ||||||
| CD 4+ | −0.50 | −0.95 to −0.04 | 0.032 | −0.36 | −0.62 to − 0.09 | 0.011 |
PRS – postreperfusion syndrome; CVP – central venous pressure; ABOi LDLT – ABO-incompatible living donor liver transplantation.
Discussion
The main findings in the present study were that graft volume on POD 21 was significantly smaller in recipients undergoing ABOi LDLT than in those undergoing ABOc LDLT. Larger preoperative graft volume/SLV ratio and a lower CD4+ cellular level on POD 14 were independently associated with ALV and RLV on POD 21 in patients undergoing ABOi LDLT, respectively. In the PS-matched patients, the difference in major patient and graft complications was comparable between the groups, and there was no association between ABOi LDLT and overall patient mortality against the ABOc LDLT.
Many studies have suggested that various independent factors, including ischemia-reperfusion injury [24,25], liver graft volume [10,26], steatosis [27–29], donor age [30,31], and portal circulation [8,32], are associated with liver graft regeneration in experimental and clinical LT settings. The regeneration rate of dual-graft LDLT using both ABOi and ABOc grafts was comparable but postoperative complications (antibody-mediated rejection and biliary problems) were not presented. Dual-LDLT using ABOi and ABOc grafts is considered a feasible treatment for small-for-size syndrome [33]. In the present study, the perioperative factors related to graft regeneration were matched between the ABOc and ABOi groups using a PS-matching analysis. Our study and the study by Song et al. shared favorable ABOi LDLT findings that the ABOi LDLT was acceptable for patients with ESLD because postoperative morbidity and mortality were comparable compared with ABOc LDLT. However, in the PS-matched patients of our study, the ABO incompatibility between liver grafts and recipients affected graft regeneration capability, and probably tended to suppress growth of the graft mass even though the difference in regeneration was not large enough to result in clinical significance. Because insufficient graft regeneration is closely connected to graft dysfunction [34], patients with poor donor-graft quality could have undergone inappropriate graft regeneration in the ABOi LDLT group, resulting in delayed recovery of graft function. Poor graft regeneration was considered as a cause of graft dysfunction in patients who underwent ABOi LDLT. To assess graft regeneration, it is necessary to discriminate true graft regeneration from graft edema [35] or a portal hyperperfusion-related graft size increase [8], which is related to poor postoperative outcome. Therefore, it was helpful to investigate graft regeneration using serial volumetric assessments in patients with poor donor-graft quality and quantity, as well as inappropriate graft circulation.
The high serum level of CD4+ T-lymphocytes on POD 14 had a negative effect on increasing liver graft size on POD 21 in ABOi LDLT. Until now, little has been reported on the direct relationship between CD4+ T-lymphocyte level and graft regeneration in the LT setting. In experimental studies, T-lymphocytes play an important role in neutrophil-mediated inflammation in liver grafts after ischemia-reperfusion. Particularly, CD4+ T-lymphocytes trigger recruitment of neutrophils into the liver graft [36]. Warm ischemia-reperfusion injury in the hepatic microcirculation caused CD4+ T-lymphocytes to migrate into liver grafts. The CD4+ T-lymphocytes interact with platelets and sinusoidal endothelial cells and disrupt the microcirculation [37]. Aggressive CD4+ T-lymphocyte-induced inflammation in the graft can be associated with reduced regeneration ability of allograft cells under the ABOi condition.
The preoperative graft volume/SLV ratio was positively associated with liver graft volume on POD 21. A previous study suggested that small-for-size grafts after major hepatectomy or LT regenerate vigorously and attain a graft size similar to the initial native liver [38]. A small-for-size graft with minimal ischemic injury regenerates robustly after partial liver graft transplantation [39]. One study reported that the partial graft sizes affect graft regeneration duration, with a smaller starting graft volume delaying the regeneration progress in LDLT [11]. Partial grafts have impaired ability to recover and maintain appropriate graft function in LDLT of patients in poor clinical condition, representing a high MELD score [40]. The present study suggests that sufficient allograft volume at the transplanted point may ensure postoperative vigorous regeneration of grafts against ABO incompatibility.
During ABOi transplantation, acute AMR has been reported frequently in heart, kidney, and pancreatic allografts, manifesting in organ dysfunction and a pathological microvasculature [41,42]. During LT, AMR development is rare due to the ability of the liver to modulate immunological reactions and thus compensate for injury. In particular, by decreasing the titer to <1: 16 in plasma exchange therapy (plasmapheresis) and administering rituximab, ABOi AMR can be prevented [43,44]. Acute AMR usually develops during the first several weeks after surgery, leading to rapid liver graft failure in highly sensitized patients [45]. Liver grafts with AMR present with specific histopathological features, including monocytic, eosinophilic, or neutrophilic microvasculitis accompanied by dilatation, disruption, and edema (of the portal veins, capillaries, and inlet venules, respectively) [21]. In the present study, there was no difference in the incidence of AMR between the 2 groups, and the development of AMR was not statistically associated with the liver graft volume on POD 21. However, based on previous studies [21,43], AMR onset may be related to poor postoperative outcomes, including impaired graft regeneration in patients scheduled for ABOi LDLT. Therefore, meticulous monitoring of titers is necessary to identify whether additional treatment is needed. Further investigation of the association between partial liver graft regeneration and AMR development is also required because ABOi partial grafts are now widely used [44].
The present study has several limitations. First, although we tried to balance the confounding factors between the ABOc and ABOi groups using a PS matching analysis, hidden biases were not totally eliminated as unknown factors may have remained. Second, because of the study design, the complex effect of immunosuppressants on graft regeneration was not investigated. Patients undergoing ABOi LDLT required more careful immunosuppression therapy than those undergoing ABOc LDLT owing to their immunologic reaction related to the ABO blood barrier [46,47]. Further study would be helpful to guide the immunosuppressant levels between the graft regeneration and the ABOi-specified complications. Third, the difference in graft regeneration between ABOc and ABOi LDLT was not large enough to have clinical significance in our study; therefore, the ability to apply the study results in selecting ABOi LDLT donors is limited. Fourth, we could not assess the change in postoperative graft volumes over the long term. Future study is required to investigate the association between ABO incompatibility and long-term graft regeneration. Finally, because of the small sample size in this study, the results pertaining to patient survival, acute graft rejection, and biliary complications should be interpreted cautiously.
Conclusions
In conclusion, because ABO incompatibility is associated with reduced graft regeneration within 1 month after LDLT in volume, the liver grafts for ABOi LDLT require more meticulous monitoring than those for ABOc LDLT. Graft regeneration must be investigated using a volumetric assessment in patients with poor donor-graft quality and quantity, as well as inappropriate graft circulation in those who underwent ABOi LDLT, to distinguish true graft regeneration from graft edema or portal hyperperfusion-related graft size. Preoperative sufficient graft volume is one of the important factors required to improve graft regeneration after ABOi LDLT. Because an aggressive increase in CD4+ cells is associated with poorer graft regeneration, careful management of inflammation, including the CD4+ cellular level, is required under the complex immunosuppression regimen in the ABOi LDLT group, although the pathological process of graft regeneration was uncertain under CD4+ T-lymphocyte-induced inflammation. The ABOi LDLT is a feasible option for patients with ESLD. However, when a graft with extended criteria is utilized for ABOi LDLT, close and serial graft volumetric monitoring is required regarding postoperative graft regeneration status.
Abbreviations
- ABOi
ABO-incompatible
- ABOc
ABO-compatible
- LDLT
living donor liver transplantation
- MELD
model for end-stage liver disease
- ALV
absolute liver volume
- RLV
relative liver volume
Footnotes
Conflicts of interest
None.
Source of support: The statistical consultation was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1062)
References
- 1.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:961–72. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 2.Decoster EL, Troisi R, Sainz-Barriga M, et al. Improved results for adult split liver transplantation with extended right lobe grafts: Could we enhance its application? Transplant Proc. 2009;41:3485–88. doi: 10.1016/j.transproceed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 3.White SA, Prasad KR. Liver transplantation from non-heart beating donors. BMJ. 2006;332:376–77. doi: 10.1136/bmj.332.7538.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990;336:519–23. doi: 10.1016/0140-6736(90)92082-s. [DOI] [PubMed] [Google Scholar]
- 5.Farges O, Kalil AN, Samuel D, et al. The use of ABO-incompatible grafts in liver transplantation: A life-saving procedure in highly selected patients. Transplantation. 1995;59:1124–33. [PubMed] [Google Scholar]
- 6.Egawa H, Teramukai S, Haga H, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: A Japanese multicenter study. Am J Transplant. 2014;14:102–14. doi: 10.1111/ajt.12520. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki S, Makuuchi M, Matsunami H, et al. Living related liver transplantation in adults. Ann Surg. 1998;227:269–74. doi: 10.1097/00000658-199802000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagi S, Iida T, Taniguchi K, et al. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005;11:68–75. doi: 10.1002/lt.20317. [DOI] [PubMed] [Google Scholar]
- 9.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14:504–8. doi: 10.1002/lt.21381. [DOI] [PubMed] [Google Scholar]
- 10.Olthoff KM, Emond JC, Shearon TH, et al. Liver regeneration after living donor transplantation: Adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015;21:79–88. doi: 10.1002/lt.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcos A, Fisher RA, Ham JM, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375–79. doi: 10.1097/00007890-200004150-00028. [DOI] [PubMed] [Google Scholar]
- 12.Nagy P, Kiss A, Schnur J, Thorgeirsson SS. Dexamethasone inhibits the proliferation of hepatocytes and oval cells but not bile duct cells in rat liver. Hepatology. 1998;28:423–29. doi: 10.1002/hep.510280220. [DOI] [PubMed] [Google Scholar]
- 13.Francavilla A, Carr BI, Starzl TE, et al. Effects of rapamycin on cultured hepatocyte proliferation and gene expression. Hepatology. 1992;15:871–77. doi: 10.1002/hep.1840150520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francavilla A, Starzl TE, Barone M, et al. Studies on mechanisms of augmentation of liver regeneration by cyclosporine and FK 506. Hepatology. 1991;14:140–43. doi: 10.1002/hep.1840140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae MS, Park CS, Oh SA, Hong SH. Predictive role of intraoperative plasma fibrinogen for postoperative portal venous flow in living donor liver transplantation. Ann Transplant. 2017;22:83–95. doi: 10.12659/aot.902103. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Na GH, Choi HJ, et al. Surgical outcome of right liver donors in living donor liver transplantation: Single-center experience with 500 cases. J Gastrointest Surg. 2012;16:1160–70. doi: 10.1007/s11605-012-1865-y. [DOI] [PubMed] [Google Scholar]
- 17.Egawa H, Ohmori K, Haga H, et al. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl. 2007;13:579–88. doi: 10.1002/lt.21092. [DOI] [PubMed] [Google Scholar]
- 18.Heinemann A, Wischhusen F, Puschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366–68. doi: 10.1002/lt.500050516. [DOI] [PubMed] [Google Scholar]
- 19.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–49. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 20.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–63. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 21.Demetris AJ, Bellamy C, Hubscher SG, et al. 2016 comprehensive update of the Banff Working Group on liver allograft pathology: Introduction of antibody-mediated rejection. 2016;16:2816–35. doi: 10.1111/ajt.13909. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–88. [Google Scholar]
- 23.Stuart EA. Matching methods for causal inference: a review and a look forward. 2010:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olthoff KM. Molecular pathways of regeneration and repair after liver transplantation. World J Surg. 2002;26:831–37. doi: 10.1007/s00268-002-4060-6. [DOI] [PubMed] [Google Scholar]
- 25.Selzner M, Camargo CA, Clavien PA. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469–75. doi: 10.1002/hep.510300215. [DOI] [PubMed] [Google Scholar]
- 26.Francavilla A, Zeng Q, Polimeno L, et al. Small-for-size liver transplanted into larger recipient: a model of hepatic regeneration. Hepatology. 1994;19:210–16. [PMC free article] [PubMed] [Google Scholar]
- 27.Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: Disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35–42. doi: 10.1002/hep.510310108. [DOI] [PubMed] [Google Scholar]
- 28.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–13. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 29.McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: Always feasible? J Hepatol. 2011;54:1055–62. doi: 10.1016/j.jhep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Taguchi T, Fukuda M, Ohashi M. Differences in DNA synthesis in vitro using isolated nuclei from regenerating livers of young and aged rats. Mech Ageing Dev. 2001;122:141–55. doi: 10.1016/s0047-6374(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 31.Tanemura A, Mizuno S, Wada H, et al. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World J Surg. 2012;36:1102–11. doi: 10.1007/s00268-012-1496-1. [DOI] [PubMed] [Google Scholar]
- 32.Eguchi S, Yanaga K, Sugiyama N, et al. Relationship between portal venous flow and liver regeneration in patients after living donor right-lobe liver transplantation. Liver Transpl. 2003;9:547–51. doi: 10.1053/jlts.2003.50128. [DOI] [PubMed] [Google Scholar]
- 33.Song GW, Lee SG, Hwang S, et al. Dual living donor liver transplantation with ABO-incompatible and ABO-compatible grafts to overcome small-for-size graft and ABO blood group barrier. Liver Transpl. 2010;16:491–98. doi: 10.1002/lt.22016. [DOI] [PubMed] [Google Scholar]
- 34.Pan N, Lv X, Liang R, et al. Suppression of graft regeneration, not ischemia/reperfusion injury, is the primary cause of small-for-size syndrome after partial liver transplantation in mice. PLoS One. 2014;9:e93636. doi: 10.1371/journal.pone.0093636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bekheit M, Rajakannu M, Bucur P, et al. Serial volumetric assessment of large for size liver grafts after whole cadaveric liver transplant in adults: Do large liver grafts shrink in size? HPB (Oxford) 2016;18:200–6. doi: 10.1016/j.hpb.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwacka RM, Zhang Y, Halldorson J, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–89. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandoga A, Hanschen M, Kessler JS, Krombach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology. 2006;43:306–15. doi: 10.1002/hep.21017. [DOI] [PubMed] [Google Scholar]
- 38.Miyaoka Y, Miyajima A. To divide or not to divide: Revisiting liver regeneration. Cell Div. 2013;8:8. doi: 10.1186/1747-1028-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selzner N, Selzner M, Tian Y, et al. Cold ischemia decreases liver regeneration after partial liver transplantation in the rat: A TNF-alpha/IL-6-dependent mechanism. Hepatology. 2002;36:812–18. doi: 10.1053/jhep.2002.35535. [DOI] [PubMed] [Google Scholar]
- 40.Lei JY, Wang WT, Yan LN. Risk factors of SFSS in adult-to-adult living donor liver transplantation using the right liver: A single-center analysis of 217 cases. Hepatogastroenterology. 2012;59:1491–97. doi: 10.5754/hge11634. [DOI] [PubMed] [Google Scholar]
- 41.Escaned J, Flores A, Garcia-Pavia P, et al. Assessment of microcirculatory remodeling with intracoronary flow velocity and pressure measurements: Validation with endomyocardial sampling in cardiac allografts. Circulation. 2009;120:1561–68. doi: 10.1161/CIRCULATIONAHA.108.834739. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Sun Q, Zhang M, et al. Capillary dilation and rarefaction are correlated with intracapillary inflammation in antibody-mediated rejection. J Immunol Res. 2014;2014:582902. doi: 10.1155/2014/582902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011;41:317–22. doi: 10.1007/s00595-010-4437-3. [DOI] [PubMed] [Google Scholar]
- 44.Song GW, Lee SG, Hwang S, et al. ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 2016;16:157–70. doi: 10.1111/ajt.13444. [DOI] [PubMed] [Google Scholar]
- 45.Demetris AJ, Nakamura K, Yagihashi A, et al. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992;16:671–81. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kishida N, Shinoda M, Itano O, et al. Increased incidence of thrombotic microangiopathy after ABO-incompatible living donor liver transplantation. Ann Transplant. 2016;21:755–64. doi: 10.12659/aot.900915. [DOI] [PubMed] [Google Scholar]
- 47.Haga H, Egawa H, Shirase T, et al. Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation. Liver Transpl. 2004;10:16–27. doi: 10.1002/lt.20002. [DOI] [PubMed] [Google Scholar]


