Abstract
Background
Donor hypernatremia has been associated with reduced graft and recipient survival after heart, liver, kidney, and pancreas transplantation. However, it is unknown what effect donor hypernatremia has on graft and recipient outcomes after lung transplantation. The aim of this study was to investigate the relation of donor hypernatremia with the duration of postoperative mechanical ventilation, the incidence of severe primary graft dysfunction, and survival following lung transplantation.
Material/Methods
We analyzed all consecutive lung transplantations performed in adult patients at our center between 1995 and 2016. During the study period, donor hypernatremia was not considered a reason to reject lungs for transplantation. Donors were classified into 3 groups: normonatremia (sodium <145 mmol/L), moderate hypernatremia (sodium 145–154 mmol/L), or severe hypernatremia (sodium ≥155 mmol/L). Short-term outcome was defined by the duration of mechanical ventilation and incidence of primary graft dysfunction; long-term outcome was defined by 10-year mortality.
Results
Donor hypernatremia was recorded in 275 (58%) of the 474 donors. There were no differences in baseline characteristics between the 3 study groups. The duration of mechanical ventilation was similar for all groups (8±25, 7±17, and 9±15 days respectively, P=0.204). Severe primary graft dysfunction was not different between the 3 groups (29%, 26%, 28%, P=0.724). Donor hypernatremia was not associated with (graft) survival, or after correction for potential confounders.
Conclusions
Donor hypernatremia was not associated with a worse outcome in lung transplant recipients. Thus, in contrast to solid organ transplantation, donor hypernatremia is not a contraindication for lung transplantation.
MeSH Keywords: Graft Survival, Hypernatremia, Lung Transplantation, Primary Graft Dysfunction
Background
The number of lung transplantations has increased over the past 30 years. Adults who underwent primary lung transplantation (LTx) between 1990 and 2012 had a median survival of 5.7 years, with unadjusted survival rates of 88% at 3 months and 80% at 1 year [1]. The development of primary graft dysfunction (PGD) during the first 3 days is approximately 30% and PGD has been associated with significantly longer hospital length of stay, duration of mechanical ventilation, and 90-day mortality [2]. After standardization of the definition of PGD in 2005 by the International Society for Heart and Lung Transplantation (ISHLT), risk factors for grade 3 PGD were: receipt of an organ from a donor with any smoking history, elevated FiO2 during reperfusion, preoperative diagnosis of sarcoidosis, use of cardiopulmonary bypass, single LTx, large-volume blood transfusion, elevated pulmonary arterial pressures, and an overweight or obese recipient [3,4]. A systematic review and meta-analysis found that, in addition, recipient female gender, African-American race, and preoperative diagnosis of idiopathic pulmonary fibrosis were also associated with PGD [5].
For several solid transplant organs, such as heart, liver, kidney and pancreas, donor hypernatremia has been found to be associated with reduced graft survival. Post-transplant edema of the hypernatremic graft in a recipient with normal sodium levels has been proposed as one of the pathogenic mechanisms [6–9]. However, it is not known if donor hypernatremia is also associated with poor outcomes in lung transplant recipients.
In cohorts of critically ill patients, it has been shown that hypernatremia at intensive care unit (ICU) admission or hypernatremia acquired during ICU stay was associated with increased risk of mortality that was independent of age and severity of disease [10–12]. However, for patients presenting with a respiratory diagnosis, this was not found to be the case, suggesting that the general adverse effects of hypernatremia are overcome by lung protective effects in patients with lung injury [13]. The lung is different from other solid organs because of the small interstitial space and because its main function of gas exchange is a passive function. Alveolar fluid clearance through the osmotic gradient between sodium and chloride is crucial for the reabsorption of alveolar edema in cases of increased alveolar permeability.
We hypothesized that donor lungs from hypernatremic donors might display higher rates of PGD due to the difference in osmolality between hypernatremic donor and normonatremic recipients. We therefore studied the relation of donor hypernatremia with the duration of postoperative mechanical ventilation, the occurrence of severe PGD following lung transplantation, and mortality.
Material and Methods
We performed a retrospective cohort study of all consecutive LTxs performed between 1995 and 2016 at the University Medical Center Groningen, The Netherlands. The data acquisition in this study was performed in accordance with guidelines as outlined in Dutch legislation. The study was approved by the medical ethics committee (IRB) of our institution (Medisch Ethische Toetsingcommissie, METc 2015.589). As a retrospective study of routinely collected and anonymized data, informed consent was not required by our IRB. Patients aged <16 years, with combined heart-lung transplantation, combined lung-liver transplantation, re-transplantation, and bridge to LTx with extracorporeal life support (n=18) were excluded. Donor characteristics (age, sex, smoking, cause of death, and PaO2/FiO2 ratio) were taken from the Eurotransplant report form, including the highest level of serum sodium from the donor on the day of donation. The 3 study groups were based on donor hypernatremia defined as: normonatremia as serum sodium ≥145 mmol/L, moderate donor hypernatremia as 145–154 mmol/L, and severe donor hypernatremia as ≥155 mmol/L, which corresponds with the threshold used in previous studies in patients after cardiac or pancreatic islets transplantations [6,8].
During the study period, the donor sodium level was not considered a criterion in selecting lungs suitable for transplantation. Recipient characteristics (age, sex, diagnosis, pre-operative mechanical ventilation), surgical variables (type of LTx, ischemia time, cardiopulmonary bypass), and postoperative variables, including duration of mechanical ventilation post LTx, PGD score, ICU stay, and hospital stay, were collected. PGD was scored 0 to 3 according to ISHLT guidelines at 24, 48, and 72 hours post-transplantation. Severe PGD was defined as PGD grade 3 at any of these time points [14]. We also obtained mortality and complete long-term graft survival data for 10 years.
Statistical analysis
Demographic data on categorical variables are shown as absolute numbers as well as percentages. Unless otherwise indicated, values are expressed as means ±SD. The unpaired Student’s t-test, and the Kruskal-Wallis and Fisher’s exact tests (all 2-tailed) were used to compare the 3 groups. Kaplan-Meier curves for graft survival were constructed and compared with the log-rank test. Graft failure was defined as re-transplantation or death. Risk factors for PGD were used in Cox regression analysis to determine predictors for survival. A value of P<0.05 was considered to be statistically significant. IBM SPSS software version 23 was used.
Results
There were 474 recipients included in this study, with a mean ±SD age of 48±12 years; 48% of recipients were men. Of these, 69 recipients (15%) were admitted to the ICU pre-transplantation, and 28 recipients (6%) were mechanically ventilated pre-transplantation; 386 recipients (81%) underwent bilateral LTx.
Donor age was mean ±SD of 43±14 years. Donor serum sodium levels were available for all donors and ranged from 123–182 mmol/L, with a mean of 147±8 mmol/L.
There were no significant differences in demographic donor- and recipient-related variables among recipients with normal, moderate, and severe donor sodium levels (Table 1). Outcomes between the 3 groups were similar (Table 2). The change of preservation solution from Euro-Collins to Perfadex showed no relation with outcome parameters. For all patients, the time on mechanical ventilation was 8±20 days, grade 3 PGD at any moment occurred in 28% of patients, length of stay in the ICU was 12±18 days, length of stay in hospital was 42±28 days. There were 17 patients (3.6%) who had a re-transplantation. Overall, the 10-year mortality was 38%. Kaplan-Meier time-to-event curves for the 3 groups were not different for patient or graft survival (Figures 1, 2). Results from the Cox regression analysis are presented in Tables 3 and 4. In this model, hypernatremia was added to the available risk factors for (graft) survival, including the use of cardiopulmonary bypass, single LTx, body mass index (BMI) <22 kg/m2, number of transfused red blood cells in the first 24 hours postoperatively, and any donor smoking history. In this multivariate analysis, the only significant predictor for patient and graft survival was the number of red blood cells transfused in the first 24 hours (P=0.007 and P=0.009, respectively).
Table 1.
Demographic data.
| All patients N=474 |
Patients with sodium <145 mmol/l N=199 (42%) |
Patients with sodium 145–154 mmol/l N=199 (42%) |
Patients with sodium ≥155 mmol/l N=76 (16%) |
p-Values | |
|---|---|---|---|---|---|
| Donor | |||||
| Age, years | 43±14 | 44±14 | 42±15 | 42±12 | 0.234 |
| Sex, male | 223 (47%) | 91 (45%) | 94 (47%) | 38 (50%) | 0.816 |
| Smoking, yes | 173 (36%) | 73 (36%) | 67 (33%) | 33 (43%) | 0.323 |
| Smoking, pack years | 5±10 | 5±9 | 5±12 | 5±9 | 0.860 |
| Cause of death | |||||
| CVA | 134 (28%) | 64 (32%) | 49 (24%) | 21 (27%) | 0.747 |
| Traumatic brain injury | 110 (23%) | 45 (22%) | 47 (23%) | 18 (23%) | |
| SAB | 163 (34%) | 60 (30%) | 76 (38%) | 27 (35%) | |
| Hypoxic encephalopathy | 22 (5%) | 11 (5%) | 9 (4%) | 2 (2.6%) | |
| Other | 108 (23%) | 19 (9%) | 18 (9%) | 8 (10%) | |
| PaO2/FiO2 ratio | 449±100 | 451±95 | 443 ±111 | 449±100 | 0.455 |
| Type of donor, DBD | 385 (81%) | 156 (78%) | 163 (81%) | 66 (86%) | 0.262 |
| Recipient | |||||
| Age, years | 48±12 | 48±12 | 47±12 | 48±12 | 0.841 |
| Sex, male | 229 (48%) | 96 (48%) | 94 (47%) | 39 (51%) | 0.832 |
| BMI, kg/m2 | 23±4 | 22±3 | 22±3 | 22±4 | 0.775 |
| Pretransplant recipient admission to the ICU | 69 (15%) | 28 (14%) | 34 (17%) | 7 (9%) | 0.246 |
| Pretransplant mechanical ventilation | 28 (6%) | 10 (5%) | 14 (7%) | 4 (5%) | 0.673 |
| Primary disease | 0.934 | ||||
| COPD/emphysema | 236 (50%) | 101 (50%) | 95 (47%) | 40 (52%) | |
| Cystic fibrosis | 78 (16%) | 35 (17%) | 31 (15%) | 12 (15%) | |
| Idiopathic lung fibrosis | 52 (11%) | 22 (11%) | 22 (11%) | 8 (10%) | |
| Other | 108 (23%) | 41 (20%) | 51 (25%) | 16 (21%) | |
| Intra-operative variables | |||||
| Cold ischemia time (minutes) | 330±120 | 320±90 | 343±144 | 320±122 | 0.116 |
| Bilateral LTx | 386 (81%) | 165 (82%) | 164 (82%) | 57 (75%) | 0.403 |
| Use of CPB, yes | 202 (43%) | 88 (44%) | 82 (41%) | 32 (42%) | 0.827 |
Data are expressed as mean ± standard deviation for continuous variables and as numbers (percentages) for categorical variables. CVA – cerebral vascular accident; SAB – subarachnoid hemorrhage; DBD – donor after brain death; BMI – body mass index; COPD – chronic obstructive pulmonary disease; LTx – lung transplantation; CPB – cardiopulmonary bypass.
Table 2.
Outcome parameters by donor sodium value.
| Patients with sodium <145 mmol/l N=199 (42%) |
Patients with sodium 145–154 mmol/l N=199 (42%) |
Patients with sodium ≥155 mmol/l N=76 (16%) |
P-value | |
|---|---|---|---|---|
| Time on mechanical ventilation, days | 8±25 | 7±17 | 9±15 | 0.204 |
| Mechanical ventilation >24 hours | 174 (87%) | 160 (80%) | 67 (88%) | 0.097 |
| Mechanical ventilation >48 hours | 116 (58%) | 100 (50%) | 42 (55%) | 0.270 |
| Mechanical ventilation >72 hours | 88 (44%) | 74 (37%) | 35 (46%) | 0.246 |
| Severe grade 3 PGD at at ICU admission (23 missing) | 49 (25%) | 47 (25%) | 17 (22%) | 0.381 |
| Severe grade 3 PGD at 24 hours (27 missing) | 19 (10%) | 18 (9%) | 8 (11%) | 0.453 |
| Severe grade 3 PGD at 48 hours (27 missing) | 10 (5%) | 15 (8%) | 4 (5%) | 0.361 |
| Severe grade 3 PGD at 72 hours (33 missing) | 12 (6%) | 14 (7%) | 8 (11%) | 0.074 |
| ICU length of stay, days | 10±13 | 13±21 | 15±20 | 0.858 |
| Hospital length of stay, days | 42±27 | 41±29 | 45±29 | 0.651 |
| 1-year mortality | 25 (12%) | 28 (14%) | 11 (14%) | 0.344 |
Data are expressed as mean ± standard deviation for continuous variables and as numbers (percentages) for categorical variables. PGD – primary graft dysfunction; ICU – intensive care unit.
Figure 1.
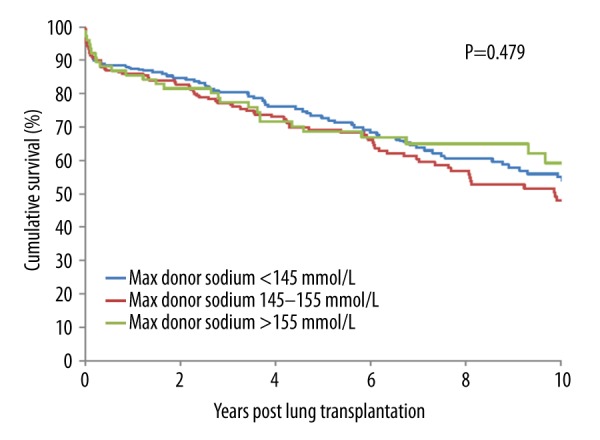
Kaplan-Meier actuarial cumulative patient survival curves for patients who underwent an LTx from donors with sodium <145 mmol/L vs. 145–154 mmol/L vs. ≥155 mmol/L.
Figure 2.
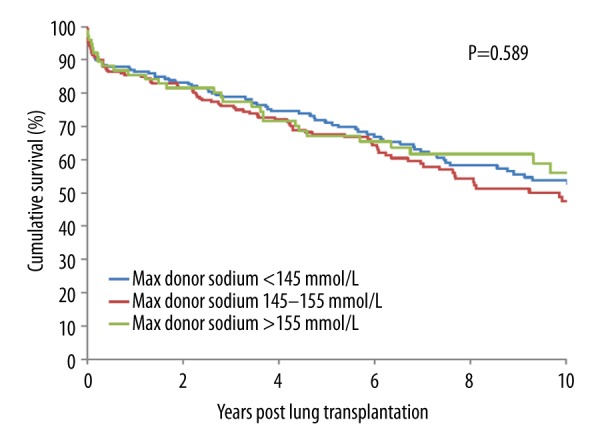
Kaplan-Meier actuarial cumulative graft survival curves for patients who underwent an LTx from donors with sodium <145 mmol/L vs. 145–154 mmol/L vs. ≥155 mmol/L.
Table 3.
Cox regression analysis of risk factors for survival.
| HR (95%CI) | P-value | |
|---|---|---|
| Donor sodium ≥155 mmol/l | 0.88 (0.58–1.32) | 0.53 |
| Total number of RBC’s in first 24 hours postoperatively | 1.02 (1.01–1.03) | 0.007 |
| Use of CPB | 0.93 (0.66–1.30) | 0.65 |
| Donor smoking, any | 0.89 (0.65–1.21) | 0.45 |
| Single LTx | 1.22 (0.83–1.78) | 0.31 |
| BMI ≥22 | 0.85 (0.63–1.16( | 0.31 |
CPB – cardiopulmonary bypass; LTx – lung transplantation; BMI – body mass index.
Table 4.
Cox regression analysis of risk factors for graft survival.
| HR (95%CI) | P-value | |
|---|---|---|
| Donor sodium ≥155 mmol/l | 0.91 (0.61–1.36) | 0.64 |
| Total number of RBC’s in first 24 hours postoperatively | 1.02 (1.00–1.03) | 0.009 |
| Use of CPB | 0.94 (0.68–1.31) | 0.72 |
| Donor smoking, any | 0.88 (0.65–1.20) | 0.42 |
| Single LTx | 1.32 (0.92–1.91) | 0.13 |
| BMI ≥22 | 0.83 (0.62–1.13) | 0.24 |
CPB – cardiopulmonary bypass; LTx – lung transplantation; BMI – body mass index.
Discussion
To our knowledge, this is the first report on the possible effect of donor hypernatremia on outcome after LTx. In contrast to observations for heart, pancreas, and liver transplantations [6–8], we found no adverse effect of donor hypernatremia on ventilation time, primary graft dysfunction, or mortality after LTx.
Donor hypernatremia has been associated with worse outcomes in other solid organs. In one study of 181 liver grafts, it was found that donor hypernatremia caused postoperative elevated AST and ALT levels and an increased rate of early graft loss [7]. But the aforementioned study indicated that changes in hepatocytes induced by hypernatremia were reversible and the correction attenuated liver graft injury [7]. In one study, 164 recipients had a larger intraoperative increase in serum sodium associated with worse recipient short-term outcomes. The 10% of patients with preoperative hyponatremia (<130 mmol/L) seemed to be at risk for complications because of their larger shift in sodium, which was associated with higher odds of prolonged intubation and longer ICU stay and hospital length of stay [15]. Importantly, in assessment of donor livers, a peak serum sodium >155 mmol/L is considered a marginal donor criterion [16]. In a single center retrospective study on donor sodium levels, no impact was found on the outcome after heart transplantation, however, the comparatively mild donor hypernatremia in the high-risk group (162±7 mmol/L) potentially limited their results [17]. In a much larger cohort of 4641 patients after cardiac transplantation, a clear U-shaped correlation of both high (>170 mmol/L) and low (<130 mmol/L) donor sodium levels with 1-year mortality rate was found [6]. This effect was mainly evident within the first 3 months after transplantation, suggesting that hypo- and hypernatremia affect initial graft function. In the consensus statement for PGD in heart transplantation, hypernatremia was named as a donor risk factor [18]. In a small study of 80 kidney recipients from 54 brain dead donors in Poland during 2006–2008, no relationship was observed between donor serum sodium concentration and early or 1-year renal function [9]. But there was a negative correlation between donor serum sodium concentration and recipient creatinine clearance at 2, 3, and 4 years after kidney transplantation. This suggests that high sodium concentrations in donors may initiate chronic reactions, which damage the kidney [9]. A retrospective analysis of in vivo and in vitro pancreatic islet function studies in mice was performed on islets isolated from hypernatremic (serum sodium levels ≥160 mmol/L) and control (serum sodium levels ≤155 mmol/L) human pancreatic donors. In the assessment of islet efficacy and survival, it was shown that donor hypernatremia, dependent on its duration, was associated with a significant islet loss and diminished function when transplanted. The authors proposed an additional role of elevated chloride levels in hypernatremic donors, suggesting that the influx of chloride ions may also trigger cell death and loss of islets [8]. Various other mechanisms could explain the relation between donor hypernatremia and outcome after solid organ transplantation. Donor hypernatremia might be a marker of severe injury to the host and may be related to other relevant but unspecified factors. Hypernatremia may result in retention of extra amounts of sodium by the transplanted organ leading to increased edema upon reperfusion. Although the lung is obviously very sensitive to the development of edema, the impact of the preservation phase on a “hypernatremic” lung might differ from normonatremic organs. In an animal study of LPS-induced acute lung injury, it was shown that a hyperosmolar state – in this model of hypernatremia with a maximum of 165 mmol/L – led to decreased vascular permeability and less pulmonary edema [19].
We also looked at the potential effect of different preservation solutions over the study time period. In our institution, in 2001, we changed the preservation solution from Euro-Collins, a solution with a sodium concentration of 10 mmol/L, to Perfadex, a solution with a sodium concentration of 138 mmol/L. The high sodium content of Perfadex is thought to maintain the intracellular energy levels better and avoid hyperkalemia-induced pulmonary vasoconstriction. There is no proven difference in survival between the 2 solutions but the use of Perfadex has been shown to improve the PaO2/FiO2 ratio and lower the duration of mechanical ventilation [20]. In our study, we did not find a relation of this change in policy with outcomes.
Limitations of our analysis were its retrospective nature and that it was a single center study. Although we had a large cohort, it was not as large as an international registry. The Eurotransplant report form only contains data on sodium at the day of donation, so the duration of the hypernatremia in the donor could not be determined. We chose to use the peak sodium levels on the day of procurement because hypernatremia is typically only slowly corrected in the ICU [21,22]. Also, the sodium level in the recipient was not known. Measurement of pulmonary artery pressure was not routine at our center, so these data were missing from this study. During the long study period other factors (like the use of different immunosuppression schemes) may have confounded the effect of donor hypernatremia. We also did not have complete data on the use of extended donor criteria or the lung allocation score of the recipients.
Conclusions
In conclusion, donor hypernatremia was not associated with primary graft dysfunction, duration of mechanical ventilation, or long-term survival. Thus, in contrast to other solid organs, donor hypernatremia is not a contraindication for lung transplantation.
Acknowledgements
We would like to sincere thank Rens Teeuwen, Willy Steenhuis, and Marja van Kammen for their assistance in completing the data.
Footnotes
Conflict of interests
None.
Source of support: Self financing
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: Thirty-first adult lung and heart-lung transplant report – 2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–24. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Porteous MK, Diamond JM, Christie JD. Primary graft dysfunction: Lessons learned about the first 72 h after lung transplantation. Curr Opin Organ Transplant. 2015;20:506–14. doi: 10.1097/MOT.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carby M, Bag R, Corris P, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–59. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 4.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–34. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: A systematic review and meta-analysis. PLoS One. 2014;21:e92773. doi: 10.1371/journal.pone.0092773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoefer D, Ruttmann-Ulmer E, Smits JM, et al. Donor hypo- and hypernatremia are predictors for increased 1-year mortality after cardiac transplantation. Transpl Int. 2010;23:589–93. doi: 10.1111/j.1432-2277.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 7.Totsuka E, Dodson F, Urakami A, et al. Influence of high donor serum sodium levels on early postoperative graft function in human liver transplantation: effect of correction of donor hypernatremia. Liver Transpl Surg. 1999;5:421–28. doi: 10.1002/lt.500050510. [DOI] [PubMed] [Google Scholar]
- 8.Qi M, Luis V, Bilbao S, et al. Sodium levels of human pancreatic donors are a critical factor for determination of islet efficacy and survival. Am J Physiol Endocrinol Metab. 2015;308:E362–6. doi: 10.1152/ajpendo.00443.2014. [DOI] [PubMed] [Google Scholar]
- 9.Kwiatkowska E, Bober J, Ciechanowski K, et al. Increased serum sodium values in brain-dead donor’s influences its long-term kidney function. Transplant Proc. 2013;45:51–56. doi: 10.1016/j.transproceed.2012.07.153. [DOI] [PubMed] [Google Scholar]
- 10.Lindner G, Funk GC, Schwarz C, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50:952–57. doi: 10.1053/j.ajkd.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Funk GC, Lindner G, Druml W, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36:304–11. doi: 10.1007/s00134-009-1692-0. [DOI] [PubMed] [Google Scholar]
- 12.Darmon M, Diconne E, Souweine B, et al. Prognostic consequences of borderline dysnatremia: Pay attention to minimal serum sodium change. Crit Care. 2013;17:R12. doi: 10.1186/cc11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bihari S, Peake SL, Bailey M, et al. Admission high serum sodium is not associated with increased intensive care unit mortality risk in respiratory patients. J Crit Care. 2014;29:948–54. doi: 10.1016/j.jcrc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Shah RJ, Diamond JM, Cantu E, et al. Objective estimates improve risk stratification for primary graft dysfunction after lung transplantation. Am J Transplant. 2015;15:2188–96. doi: 10.1111/ajt.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudcova J, Ruthazer R, Bonney I, Schumann R. Sodium homeostasis during liver transplantation and correlation with outcomes. Anesth Analg. 2014;119:1420–28. doi: 10.1213/ANE.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 16.Briceño J, Solórzano G, Pera C. A proposal for scoring marginal liver grafts. Transpl Int. 2000;13:S249–52. doi: 10.1007/s001470050334. [DOI] [PubMed] [Google Scholar]
- 17.Kaczmarek I, Meiser B, Groetzner J, et al. Lack of impact of donor sodium levels on outcome after heart transplantation. Transplant Proc. 2003;35:2121–22. doi: 10.1016/s0041-1345(03)00741-3. [DOI] [PubMed] [Google Scholar]
- 18.Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 2014;33:327–40. doi: 10.1016/j.healun.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Bihari S, Dixon DL, Lawrence MD, Bersten AD. Induced hypernatraemia is protective in acute lung injury. Respir Physiol Neurobiol. 2016;227:56–67. doi: 10.1016/j.resp.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Latchana N, Peck JR, Whitson B, Black SM. Preservation solutions for cardiac and pulmonary donor grafts: A review of the current literature. J Thorac Dis. 2014;6:1143–49. doi: 10.3978/j.issn.2072-1439.2014.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoorn EJ, Betjes MG, Weigel J, Zietse R. Hypernatraemia in critically ill patients: Too little water and too much salt. Nephrol Dial Transplant. 2008;23:1562–68. doi: 10.1093/ndt/gfm831. [DOI] [PubMed] [Google Scholar]
- 22.Oude Lansink-Hartgring A, Hessels L, Weigel J, et al. Long-term changes in dysnatremia incidence in the ICU: A shift from hyponatremia to hypernatremia. Ann Intensive Care. 2016;6:22. doi: 10.1186/s13613-016-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


