Abstract
Background
Perioperative monitoring and hemodynamic management after heterotopic thoracic cardiac xenotransplantation is challenging due to 2 independently beating hearts. Telemetry allows continuous monitoring of hemodynamic parameters of both the donor and recipient hearts. We describe our experience and report on the validity of a telemetric system during and after surgery.
Material/Methods
Wireless telemetry transmitters were implanted in 3 baboons receiving porcine donor hearts. Left ventricular pressure and ECG were assessed from the donor heart, whereas aortic pressure and temperature were assessed from the recipient. Telemetric data were validated with invasive blood pressure measurements.
Results
Telemetric blood pressure was lower than invasive blood pressure. Intraoperatively, the probe in the graft’s left ventricle measured negative end-diastolic pressures. Telemetry allowed simple discrimination between donor’s and recipient’s heart rates. Body temperature was underestimated by telemetry. Telemetric monitoring facilitates recognition of graft arrhythmias and volume demand.
Conclusions
In heterotopic thoracic cardiac xenotransplantation, telemetric implants are useful tools to continuously monitor the animals’ hemodynamic parameters and to discriminate donor and recipient organs. Accuracy is sufficient for systemic pressure measurement, but perioperative use of left ventricular end-diastolic pressure as a surrogate parameter for graft function is not advisable. Temperature measurements by telemetry do not reflect body core temperature.
MeSH Keywords: Heart Transplantation; Monitoring; Telemetry; Transplantation, Heterologous
Background
Xenotransplantation may pose an alternative to human allotransplantation; with recent advances in survival times [1–3], first clinical trials seem to be feasible in the near future [4]. In cardiac xenotransplantation, 2 non-human primate models are well established – heterotopic abdominal and orthotopic pig-to-baboon transplantation – with longest survival times of more than 900 days in the heterotopic abdominal model [1]. Recently, heterotopic thoracic heart transplantation has successfully been implemented as a third alternative. This surgical technique, described in 1974 by Barnard and Losman [5] in humans, combines the safety of heterotopic abdominal transplantation with the benefits of a working heart model [6,7].
In comparison to the abdominal approach, heterotopic thoracic transplantation is more delicate in terms of operative procedure and anesthesiological care, as the donor’s and recipient’s hearts and their great vessels are intertwined (Figure 1) and a cardiopulmonary bypass is required during surgery. Postoperatively, cardiac arrhythmias, hemodynamic instability, and volume shifts are common challenges that can jeopardize the experiment. During the first days after cardiac transplantation, the xenograft is at high risk of perioperative xenograft dysfunction (PCXD), which may lead to insufficiency and loss of the transplanted organ for reasons yet unknown [8]. Both hearts need to be closely monitored: the recipient’s organ is indispensable for life support, whereas the function of the donor organ is of major interest during a xenotransplantation experiment. Invasive blood pressure measurement and external ECG facilitate hemodynamic monitoring intraoperatively but must be discontinued after the procedure when the animal is waking from anesthesia.
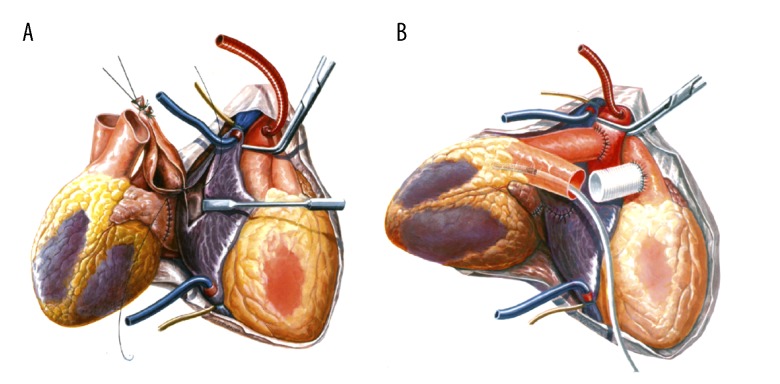
Figure 1.
Heterotopic thoracic cardiac xenotransplantation with the recipient baboon heart to the right and the porcine donor heart to the left. (A) The 2 left atria have been connected, anastomosis between the right atria has begun. (B) Aortic end-to-side anastomosis is finished; the 2 main pulmonary arteries are to be joined end-to-side by interposition of a vascular graft (modified from Reichart B, Jamieson S, Heart and Heart-Lung Transplantation, 1990, Figures 69 and 72).
Telemetric monitoring is now widely used to survey and record hemodynamic parameters in animal studies [9], and has already been employed in the heterotopic abdominal xenotransplantation model [10]. In heterotopic thoracic cardiac xenotransplantation, continuous monitoring of the recipient’s systemic blood pressure and the donor heart’s left ventricular pressure and ECG by telemetry may offer a simple means of assessing graft function and the animal’s condition, thus guiding perioperative care.
Here, we describe our experience with monitoring 2 independently working hearts in a single animal via telemetry during the early perioperative period. We describe hemodynamic challenges in the management of the heterotopic thoracic transplantation model and report on the validity of the telemetric system.
Material and Methods
Animals
Three piglets (Sus scrofa) were used as donors for heterotopic thoracic xenogeneic heart transplantation; the recipients were 3 baboons (Papio anubis and Papio hamadryas). Care of the animals was in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication No. 85-23, 1985) and the German Law for the Care of Experimental Animals (German Legislation for the Welfare of Laboratory Animals, article 5, §7–§9a, revised 2006).
The donor pigs were transgenic for hCD46 and had a homozygous GGTA1 (alpha1,3-galactosyltransferase 1) knockout. The outcome of the transplantation experiments, anesthesia, and the heterotopic thoracic transplantation technique have been described elsewhere [6]. Briefly, recipient baboons and donor pigs were premedicated with intramuscular injections of ketamine hydrochloride (Ketavet®; Pfizer Deutschland GmbH, Berlin, Germany) and midazolam (Midazolam-ratiopharm®; ratiopharm GmbH, Ulm, Germany). General anesthesia was induced and maintained with propofol (Propofol®-Lipuro 2%; B. Braun Melsungen AG, Melsungen, Germany) and fentanyl (Fentanyl-Janssen; Janssen-Cilag GmbH, Neuss, Germany). After explantation of the donor heart, the recipient’s thorax was opened at the midline. The donor heart was placed into the right chest, with the opening within the left atrium facing anteriorly. The 2 left atria and then the right atria were connected. The implantation continued with aortic end-to-side anastomosis. The 2 main pulmonary arteries were joined end-to-side by interposition of a vascular graft (Figure 1). The operation was performed with cardiopulmonary bypass.
Postoperatively, the animals were weaned from ventilation and put into their cages when adequately awakened from anesthesia. A continuous infusion of fentanyl, ketamine hydrochloride, and metamizole (Novaminsulfon-ratiopharm®; ratiopharm GmbH, Ulm, Germany) was applied to ensure analgesia. During the further course of the experiment, the animals were housed individually, and a baboon jacket with a tethering system connected to a central venous line was used to apply immunosuppression.
Implants and catheters
After induction of general anesthesia, a 5-French PiCCO-catheter (Pulsion Medical Systems, Munich, Germany) was installed in the right femoral artery to allow continuous pressure measurements and hemodynamic assessment. Data were processed via PiCCOWin software (Pulsion Medical Systems, Munich, Germany). The arterial line was removed before waking from anesthesia.
A wireless telemetry transmitter DSI PhysioTel® Multiplus D70-PCTP (Data Sciences International, St. Paul, MN, USA) was used for monitoring. The system provided 4 channels: 2 pressure, 1 biopotential, and 1 temperature channel. One pressure probe was placed through the apex into the left ventricle of the donor heart before declamping of the aorta. During reperfusion of the 2 hearts, the second pressure sensor was placed inside the recipient’s ascending aorta through the punch hole that was used during cardiopulmonary bypass to administer cardioplegic solution. Pressure catheters were secured with 2 purse-string sutures with pledgets. Both biopotential leads were sutured onto the left ventricle of the graft before terminating the cardiopulmonary bypass to derive the graft’s ECG. Temperature was measured at the transmitter housing, which was placed in a subcutaneous pouch on the right medioclavicular line at the level of the 5th to 6th rib at the end of the operation. Measurements were transmitted continuously for the duration of the experiment, starting immediately after implantation. All 4 channels were displayed in real time on a computer screen in the operating room and recorded for off-line analysis (Dataquest A.R.T.™ system, Data Sciences International/DSI, St. Paul, MN, USA). The animal cages were equipped with 2 receivers to strengthen signal quality (RMC-1, DSI, St. Paul, MN, USA). An additional mobile receiver was used during surgery.
Off-line analysis and statistics
Telemetric data was analyzed from implantation of the telemetry system until removal of the femoral arterial catheter, using the Ponemah Physiology Platform (DSI, St. Paul, MN, USA). Data was reduced by calculating median values of aortic and ventricular pressures, ECG, and temperature every 12 s; pressure measurements from the femoral artery were treated accordingly. Pulse rates were automatically derived from both telemetric and femoral arterial pressure curves by Ponemah and PiCCOWin software. In addition, pulse rates were counted manually by a researcher blinded to the results of the automated software. Automated measurements deviating less than 5% from manual counts were defined as accurate measurements.
Data was processed with Excel (Microsoft, Redmond, Washington, USA) and analyzed with GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, California, USA). Bland-Altman-Plots were created for pressure measurements. Data sets were tested for statistically significant differences by unpaired t test.
Results
Transmitter units, pressure sensors, and biopotential leads were implanted without complication in all 3 animals. The transmitters worked continuously for the period of survival (14, 9, and 16 days) and did not come close to the estimated battery life of 2 months. During the course of the first experiment, the quality of the pressure and ECG waveforms varied depending on the animal’s distance from and positioning relative to the antennas: at a distance of more than 1 meter, the telemetry signal was lost completely; at shorter distances, the signal was still lost sporadically. To improve signal quality, 1 antenna was placed directly underneath the operating table during surgery. Two more antennas were fixed perpendicularly to the outside of the cage, one to the side and the other on the top. This provided sufficient overall signal quality. A third mobile antenna was used for procedures outside the cage.
Perioperative individual mean values of recipient’s systemic and donor’s left ventricular (LV) pressures, pulse rates, and temperature measurements are shown in Table 1. Bland-Altman-plots of telemetric aortic and femoral arterial pressure values showed an overall bias of −7.7 mmHg (±6.9 mmHg, 95% limits of agreement −21.2 and 5.8 mmHg) (Figure 2). Pressure measurements taken by telemetry were lower than those by arterial line (83±19 mmHg vs. 91±16 mmHg; p<0.001) (Figure 3); this also resulted in false-negative values of left ventricular end-diastolic pressures (LVEDP) in the graft (−5±7 mmHg) (Figure 4).
Table 1.
Mean values and standard deviations from the recipient’s systemic and the graft’s left ventricular pressure, heart rate, and body temperature from animals #1–3. Pressure measurements were derived from the femoral arteria, the aorta, and the graft’s left ventricle, heart rates from analysis of the respective pressure probes and the pericardial ECG signal, and temperature from sensors in the femoral arterial line and the implant’s casing, respectively.
| Animal #1 | Animal #2 | Animal #3 | All animals | |
|---|---|---|---|---|
| Systemic and LVgraft pressures | ||||
| Pmean femoral artery [mmHg] | 73±7 | 101±13 | 96±12 | 91±16 |
| Pmean aorta [mmHg] | 61±7 | 89±14 | 97±10 | 83±18 |
| Psys LVgraft [mmHg] | 60±17 | 79±20 | 85±12 | 75±20 |
| LVEDPgraft [mmHg] | −10±3 | −9±1 | 5±3 | −5±7.4 |
| Pmean LVgraft [mmHg] | 13±3 | 27±7 | 36±6 | 26±11 |
| Heart rates recipient | ||||
| HR femoral artery [bpm] | 78±23 | 114±18 | 87±28 | 93±28 |
| Accuracy [%] | 58.3 | 80.1 | 63.6 | 67.6 |
| HR aorta [bpm] | 72±21 | 116±19 | 80±27 | 89±28 |
| Accuracy [%] | 82.1 | 91 | 83.9 | 85.8 |
| Heart rates graft | ||||
| HR epicardial ECG [bpm] | 117±14 | 125±27 | 110±12 | 118±20 |
| Accuracy [%] | 81.1 | 87.5 | 79.2 | 82.6 |
| HR LVgraft [bpm] | 114±10 | 121±18 | 112±17 | 115±16 |
| Accuracy [%] | 95.6 | 96.2 | 91.1 | 94.3 |
| Body temperatures | ||||
| Temperature, arterial catheter [°C] | 38.8±0.3 | 38.2±0.4 | 36.5±0.8 | 37.2±0.9 |
| Temperature, telemetry casing [°C] | 33.1±0.3 | 37.1±0.9 | 33.0±1.8 | 34.5±2.3 |
Psys – systolic pressure; LVEDP – left ventricular end-diastolic pressure; HR – heart rate.
Figure 2.
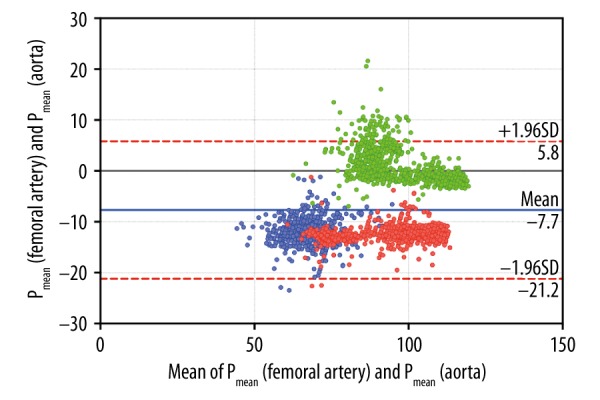
Bland-Altman plots (difference vs. average) of pressure measurements in the femoral artery (pulse curve analysis) and aorta (telemetry) from animals #1–3. Overall bias was 7.7 mmHg (±6.9 mmHg, 95% limits of agreement −5.8 and 21.2 mmHg). Data subsets of the animals are color-coded: animal #1: blue, animal #2: red, animal #3: green.
Figure 3.
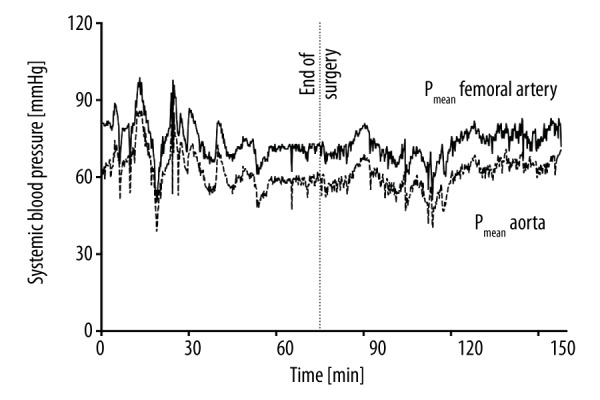
Perioperative mean systemic blood pressure of animal #1 measured in the femoral artery (upper line), and in the proximal aorta via telemetric pressure probe (lower dashed line). Mean pressure measurements taken by telemetry were lower than those taken by arterial line (61±7 mmHg vs. 73±7 mmHg, p<0.001).
Figure 4.
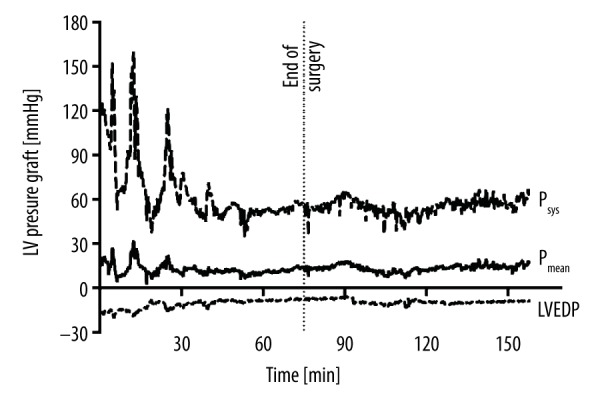
Systolic (Psys), mean (Pmean), and end-diastolic pressure (LVEDP) in the left ventricle of animal #1’s graft, measured by telemetric pressure probe. LVEDP showed negative values throughout the perioperative period.
Heart rates from the graft and the recipients’ own hearts could be distinguished using telemetric ECG, LV, and aortic pressure curves (Figure 5). Pulse rate measurements were more accurate and less prone to artefact when derived from telemetry pulse curves than from arterial line (recipient’s heart; accurate measurements: 85.8% vs. 67.6%, p<0.001), or epicardial ECG (graft; accurate measurements: 94.3% vs. 82.6%, p<0.001). Body temperatures measured by the telemetry implants were lower than those by arterial line (34.5±2.3°C vs. 37.2±0.9°C; p<0.001).
Figure 5.
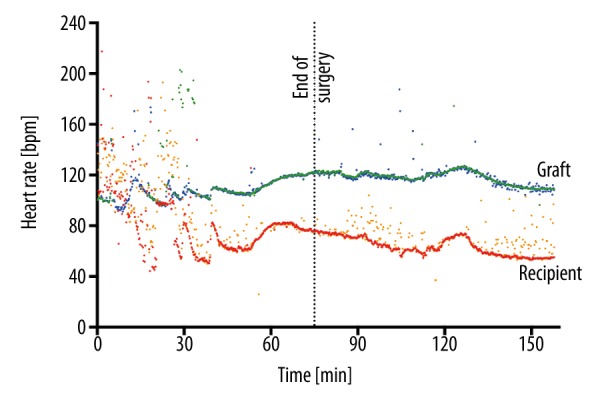
Heart rates (HR) from animal #1 in the late perioperative period. The graft’s HR (upper line) was calculated from the left ventricular (LV) pressure probe (blue), and from pericardial ECG (green). The recipient’s own HR (lower line) was calculated from the femoral arterial line (orange) and from the proximal aorta via telemetric pressure probe (red). HRs of both organs were distinctly different.
After removal of the arterial line, LV and aortic pressures measured by telemetry were used to guide perioperative catecholamine therapy; intravascular volume requirements were determined by estimation of pulse pressure variations (Figure 6). Isolated cardiac arrhythmias of the graft were easily detectable by epicardial ECG and treated accordingly (Figure 7).
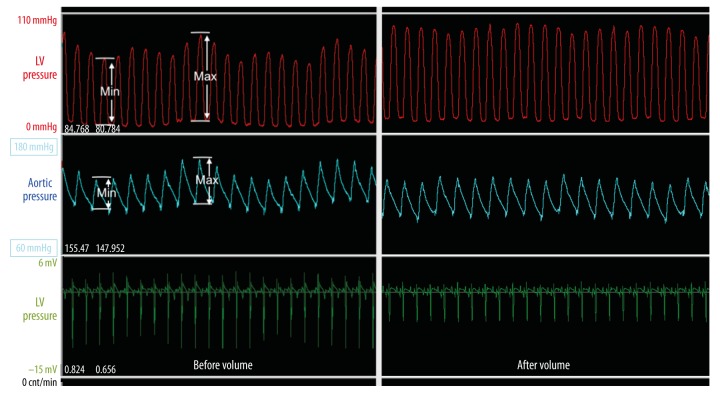
Figure 6.
Pulse pressure variation, an index of dynamic preload, in baboon #2. Amplitudes of both LV (red) and aortic (white) pressure curves showed distinct variations in size (end of surgery, left), which decreased after volume therapy (3 h after surgery, right).
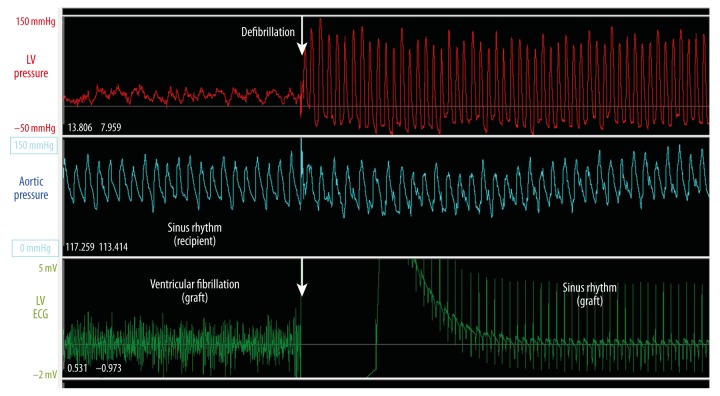
Figure 7.
Telemetric monitoring of a graft’s left ventricular pressure (above, red line), aortic pressure of the corresponding recipient’s own heart (middle, white line), and the graft’s epicardial ECG (below, green line). Perioperatively, the graft had intermittent periods of ventricular fibrillation (left), which converted to sinus rhythm (right) after external defibrillation (arrow). Fibrillation was unnoticeable by standard monitoring.
Aortic and LV pressures were stable in all animals during the first 72 h after transplantation, LVEDP measurements became positive within 12 h (Figure 8). Heart rates from donor and recipient organs could be discriminated by their respective frequencies at all times (Figure 9). Temperature measurements reached normal values within 24–48 h after transplantation (Figure 9). Because of the continuous telemetric monitoring, the animals did not need to be sedated and taken from their cages to assess vital signs and graft function.
Figure 8.
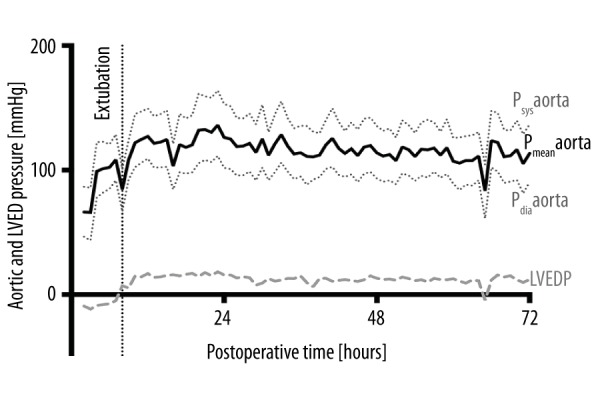
Systolic (Psys), mean (Pmean), and diastolic pressure (Pdia) in the proximal aorta and end-diastolic pressure (LVEDP) in the left ventricle of animal #2’s graft in the 72 h following transplantation, measured by telemetric pressure probe. Both recipient and donor hearts had normal functions. After extubating and moving the animal from supine into upright position, LVEDP changed from negative to positive values, indicating a hydrostatic effect responsible for perioperative measurement errors.
Figure 9.
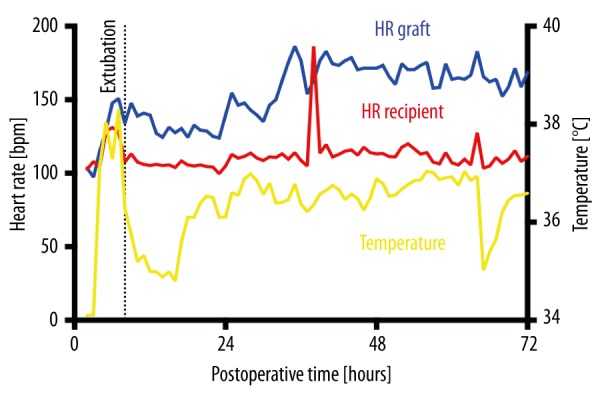
Heart rates (HRs) and body temperature of animal #2 in the 72 h following transplantation. HRs were calculated from the left ventricular (LV, blue) and aortic pressure probe (red), temperature was measured by the implant’s casing (yellow). The different HRs of donor and recipient hearts were clearly discriminable. Temperature rose immediately after operation due to external warming, then dropped again when moving the animal into its cage. Temperature equilibrium was only reached 30 h after surgery.
Discussion
Following successful implantation of the telemetric device, aortic and LV pressures as well as ECG and temperature could be monitored continuously in the 3 animals via wireless transmission and recorded for further analysis.
Pressure measurements in the ascending aorta via telemetry showed an overall bias of −7.7 mmHg in the Bland-Altman plot in comparison to measurements via femoral artery catheter (Figure 2), indicating sufficient accuracy for systemic blood pressure measurement where mean pressures usually averaged 70 to 80 mmHg. Pressure measurements taken by telemetry were lower than those obtained by arterial line; in 2 animals, the difference was more than 12 mmHg (Table 1). Lower values were also observed by the probe in the left ventricle of the graft and resulted in false-negative LVEDP. LVEDP is widely used by clinicians as a surrogate parameter of left ventricular preload and has been described as an early parameter of xenograft rejection [10]. In cardiac insufficiency due to PCXD or graft rejection, ventricular preload will increase to above normal values. However, with normal values averaging 6–12 mmHg, an inaccuracy of 12 mmHg would lead to a profound misinterpretation of LVEDP.
The telemetric pressure probes are factory-calibrated, so recalibration or zeroing is not necessary. According to the manufacturer, the initial accuracy of the D70-PCTP implant is specified as ±3 mmHg with a drift of <2 mmHg over the first month of use. Thus, inaccuracy and drift do not explain these findings observed in the hours directly after transplantation. The most probable reason is a hydrostatic effect resulting from the pressure probes and the sensor being placed on different levels [11,12]. The sensor (i.e., the device) is implanted slightly above the level of the aorta and the left ventricular apex with the animal in supine position. As the tip of the probe lies several cm below the sensor, the corresponding measurements are lower than the actual pressure. As the animal returns to an upright position after recovery from anesthesia (Figure 8), the sensor’s position is beneath the probe tips, resulting in false high measurements. For systemic pressure measurements, the resulting error is negligible, but the use of absolute LVEDP as a parameter of left ventricular preload is, at least in the early hours after transplantation, not advisable.
Discrimination between 2 asynchronously beating hearts in the heterotopic thoracic model is difficult with conventional external ECG monitoring and requires frequent sedation to allow necessary handling of the animal [6]. The implanted epicardial ECG leads of the telemetry system delivered an isolated ECG derivation of the cardiac graft, while the external leads showed mixed ECGs of the 2 asynchronous hearts. It was thus possible to screen and differentiate cardiac arrhythmias. Ventricular fibrillation of the donor heart was rapidly diagnosed in 1 animal, which would otherwise have been undetectable by monitoring systemic pressure or external ECG alone (Figure 7).
The graft’s heart rate can be estimated by either analyzing the epicardial ECG signal or the LV pulse curve, and the recipient’s heart rate estimated by analyzing the aortic pressure signal. The epicardial positioning of the 2 ECG leads was slightly different in every animal for technical reasons. Some positions were less suitable for analysis than others, resulting in erroneous data due to failure of the software recognition algorithm to differentiate between heartbeats. The manufacturer recommends suturing only 1 lead to the epicardium, while placing the reference electrode on the diaphragm for optimal ECG quality. Regarding the recipient’s own heart rate, telemetric assessment was superior to heart rate analysis via arterial catheter. The implanted telemetric system seems to be less prone to movement and handling artefacts, or dampening and resonance effects that jeopardize the pressure signal of an arterial line [11].
Telemetric temperature measurements were significantly lower than arterial measurements. The mean difference in 1 animal was more than 5°C. Arterial line measurements are taken from the circulating blood, representing body core temperature, while telemetry implants measure temperature via their outer casing, reflecting body surface temperature due to the subcutaneous location. Differences in temperature were least pronounced in animal #2 due to external warming at the end of the operation, during which body surface temperature almost equilibrated with body core temperature. After removing the heating device before extubation, the telemetric temperature swiftly dropped back to the approximate 2-degree difference present before warming (Figure 9). Thus, telemetry markedly underestimated true body core temperatures in the perioperative period. Temperature measurements equilibrated at low normal values 24–48 h after surgery; this is possibly due to the animals wearing baboon jackets, which isolate and warm the body surface to near-core temperatures. Nevertheless, telemetric temperature measurements might not always be reliable, especially in hemodynamically compromised animals with severe inflammatory syndromes such as septic shock or graft rejection. Horvath et al. reported that telemetric temperature measurements may indicate early onset of fever [10]; in this study, intra-abdominal implants were used, which seem to better reflect core temperatures than subcutaneous implants.
During heterotopic thoracic heart transplantation, the animal is monitored by external ECG, temperature and pressure measurement via arterial and central venous lines. Before awakening from anesthesia and moving the baboon into the cage, all lines have to be removed. In these early hours after transplantation, surgical complications such as cardiac arrhythmias, hemodynamic instability, bleeding, and systemic inflammation syndromes are very common and need to be treated quickly and aggressively. Sedation is needed for non-invasive blood pressure measurement and external ECG outside the cage, and this endangers the animal due to the adverse effects of anesthetics. Telemetric monitoring is wireless, avoiding the need for sedation and the associated distress and risks that may influence graft function. Episodes of hypotension or cardiac arrhythmias can be easily recognized, facilitating early postoperative catecholamine and antiarrhythmic therapy. Indices of dynamic preload such as pressure pulse variations, which are now widely used in critically ill patients [13], can predict fluid responsiveness and guide volume management (Figure 6). Thus, telemetry serves as a monitoring tool to survey an animal experiment in the long run and is also indispensable to guarantee optimal treatment in the initial phase after cardiothoracic surgery.
There are, however, also several shortcomings and disadvantages that limit the usefulness of a telemetry system. A major shortcoming of this system is the battery, which lasts only 2 months if used continuously. None of our 3 experiments came close to the warranted battery life. To prolong battery life, the transmitter could be switched on and off manually by a magnet swipe. However, this would require sedation of the animal, and important changes in the animal’s condition might be missed while the system is on stand-by. For experiments extending beyond several months, the implant may have to be exchanged. Extension of battery life in telemetric systems would thus be greatly desirable in future.
Signal reception is strongly dependent on the distance between implant and antenna, the number of antennas used, and their exact placement. If signal quality deteriorates, measurements become invalid, reducing the software’s ability to correctly analyze the data. In practice, all hemodynamic monitoring is lost. Our antennas lost signal at a distance of approximately 1 meter from the implant. The angle between antenna and implant also influences signal quality. Two antennas mounted perpendicularly onto the outside of the cage were necessary to guarantee a sufficient signal. During surgery and for procedures outside the cage, a single antenna placed directly underneath the operation table sufficed. Improved signal range and antenna sensitivity would reduce signal disruption and simplify data collection.
As mentioned above, telemetry sensors cannot be zeroed during the experiment and are prone to drift, a gradual and increasing offset from the original calibration over time [12]. Initial accuracy and monthly drift (±3 mmHg and <2 mmHg for pressures and 0.35°C and 0.2°C for temperatures) seem more than acceptable for most measurements over a period of several months. However, low pressure measurements such as central venous and end-diastolic ventricular pressures may become unreliable with time, because their normal values are in the same range as accuracy and drift.
During cardiac surgery, great care has to be taken not to damage the telemetric implant. The pressure probes, and especially the long ECG leads, may make the operating field in the thoracic cage confusing. ECG leads can dislocate very easily and fluid-filled catheter probes may be kinked or jammed when closing the sternum, all of which lead to loss of function of the respective sensors. Other surgical complications such as bleeding or infection are negligible compared to the risks of the cardiac transplantation procedure itself.
Conclusions
Telemetric implants are accurate tools to continuously monitor hemodynamic parameters and guide treatment in the early postoperative period after heterotopic thoracic cardiac pig-to-baboon xenotransplantation. ECG and pulse rate measurement by telemetry may be used to discriminate donor and recipient organs, facilitating detection and rapid treatment of arrhythmias of the graft. Minor inaccuracies in pressure measurements, most probably due to hydrostatic effects of fluid-filled probes, are negligible for systemic pressure monitoring but the use of absolute LVEDP as a surrogate parameter of graft function is not advisable in the first days after transplantation. Temperatures monitored by telemetry are body surface temperatures, not body core temperatures, and need to be interpreted with caution.
Acknowledgments
We would like to thank the Institute for Molecular Animal Breeding and Biotechnology, Faculty of Veterinary Medicine, LMU, Munich, Germany, for providing the donor pigs, the German Primate Center, Göttingen, Germany, for providing the recipient baboons, and the Walter Brendel Centre of Experimental Medicine for its support.
Footnotes
Source of support: German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) TRR 127
References
- 1.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SC, Wawke W, Higginbotham LB, et al. Fc-silent anti-CD154 domain antibody effectively prevents nonhuman primate renal allograft rejection. Am J Transplant. 2017;17:1182–92. doi: 10.1111/ajt.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah JA, Patel MS, Elias N, et al. Prolonged survival following pig-to-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade. Am J Transplant. 2017;17:2178–85. doi: 10.1111/ajt.14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuurman HJ. Pig-to-nonhuman primate solid organ xenografting: Recent achievements on the road to first-in-man explorations. Xenotransplantation. 2016;23:175–78. doi: 10.1111/xen.12244. [DOI] [PubMed] [Google Scholar]
- 5.Barnard CN, Losman JG. Left ventricular bypass. S Afr Med J. 1975;49:303–12. [PubMed] [Google Scholar]
- 6.Bauer A, Postrach J, Thormann M, et al. First experience with heterotopic thoracic pig-to-baboon cardiac xenotransplantation. Xenotransplantation. 2010;17:243–49. doi: 10.1111/j.1399-3089.2010.00587.x. [DOI] [PubMed] [Google Scholar]
- 7.Abicht JM, Mayr T, Reichart B, et al. Pre-clinical heterotopic intrathoracic heart xenotransplantation: A possibly useful clinical technique. Xenotransplantation. 2015;22:427–42. doi: 10.1111/xen.12213. [DOI] [PubMed] [Google Scholar]
- 8.Byrne GW, McGregor CGA. Cardiac xenotransplantation: Progress and challenges. Curr Opin Organ Transplant. 2012;17:148–54. doi: 10.1097/MOT.0b013e3283509120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen RH, Kadner A, Adams DH. Monitoring pig-to-primate cardiac xenografts with live Internet images of recipients and xenograft telemetric signals: histologic and immunohistochemical correlations. J Heart Lung Transplant. 2000;19:591–97. doi: 10.1016/s1053-2498(00)00101-7. [DOI] [PubMed] [Google Scholar]
- 10.Horvath KA, Corcoran PC, Singh AK, et al. Left ventricular pressure measurement by telemetry is an effective means to evaluate transplanted heart function in experimental heterotopic cardiac xenotransplantation. Transplant Proc. 2010;42:2152–55. doi: 10.1016/j.transproceed.2010.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner RM. Direct blood pressure measurement – dynamic response requirements. Anesthesiology. 1981;54:227–36. doi: 10.1097/00000542-198103000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Sarazan RD. Cardiovascular pressure measurement in safety assessment studies: Technology requirements and potential errors. J Pharmacol Toxicol Methods. 2014;70:210–23. doi: 10.1016/j.vascn.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care. 2014;18:650. doi: 10.1186/s13054-014-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]