Key Points
Question
Is monthly high-dose vitamin D supplementation associated with cancer prevention?
Findings
In this post hoc analysis of Vitamin D Assessment (ViDA) randomized clinical trial that included 5108 community adults in New Zealand, the cumulative incidence of cancer for a median follow-up period of 3.3 years was 6.5% among participants receiving 100 000 IU of vitamin D3 monthly and 6.4% among participants receiving placebo.
Meaning
Monthly high-dose vitamin D supplementation may not be associated with cancer prevention and should not be used for this purpose.
Abstract
Importance
Previous randomized clinical trials have reported inconsistent results on the effect of vitamin D supplementation on cancer incidence.
Objective
To examine whether high-dose vitamin D supplementation received monthly, without calcium, is associated with a reduction in cancer incidence and cancer mortality in the general population.
Design, Setting, and Participants
This is a post hoc analysis of data from the Vitamin D Assessment (ViDA) study, a randomized, double-blind, placebo-controlled trial that recruited participants from family practices and community groups in Auckland, New Zealand, from April 5, 2011, through November 6, 2012, with follow-up completed December 31, 2015. Participants were adult community residents aged 50 to 84 years. Of 47 905 adults invited from family practices and 163 from community groups, 5110 participants were randomized to receive vitamin D3 (n = 2558) or placebo (n = 2552). Two participants withdrew consent, and all others (n = 5108) were included in the primary analysis. Data analysis was by intention to treat.
Interventions
Oral vitamin D3, in an initial bolus dose of 200 000 IU and followed by monthly doses of 100 000 IU, or placebo for a median of 3.3 years (range, 2.5-4.2 years).
Main Outcomes and Measures
Post hoc primary outcome was the number of all primary invasive and in situ malignant neoplasms (excluding nonmelanoma skin cancers) diagnosed from randomization until the study medication was discontinued on July 31, 2015.
Results
Of the 5108 participants included in the analysis, the mean (SD) age was 65.9 (8.3) years, 58.1% were male, and 4253 (83.3%) were of European or another race/ethnicity, with the remainder being Polynesian or South Asian. Mean (SD) baseline deseasonalized 25-hydroxyvitamin D concentration was 26.5 (9.0) ng/mL. In a random sample of 438 participants, the mean follow-up 25-hydroxyvitamin D concentration consistently was greater than 20 ng/mL higher in the vitamin D group than in the placebo group. The primary outcome of cancer comprised 328 total cases of cancer (259 invasive and 69 in situ malignant neoplasms) and occurred in 165 of 2558 participants (6.5%) in the vitamin D group and 163 of 2550 (6.4%) in the placebo group, yielding an adjusted hazard ratio of 1.01 (95% CI, 0.81-1.25; P = .95).
Conclusions and Relevance
High-dose vitamin D supplementation prescribed monthly for up to 4 years without calcium may not prevent cancer. This study suggests that daily or weekly dosing for a longer period may require further study.
Trial Registration
anzctr.org.au Identifier: ACTRN12611000402943
This post hoc analysis of the Vitamin D Assessment randomized clinical trial assesses the association of monthly high-dose vitamin D supplementation vs placebo on cancer risk among New Zealand adults.
Introduction
The hypothesis that vitamin D may protect against the incidence of cancer arose from ecological studies1,2,3,4 published since the 1980s that reported inverse associations between sun exposure, the major source of vitamin D, and the incidence of several types of cancer. Subsequent meta-analyses of cohort studies5,6,7 have provided further evidence, with low baseline 25-hydroxyvitamin D (25[OH]D) concentrations associated with the increased risk of cancer during follow-up, particularly the increased risk of colorectal cancer. In contrast, the recent evidence from mendelian randomization studies is inconsistent, with genetically low 25(OH)D concentrations being associated with the increased risk of cancer mortality and ovarian cancer in 2 studies,8,9 but not with several types of cancer in a third study.10
Randomized clinical trials of vitamin D supplementation have also provided inconsistent results. Results from the Women’s Health Initiative11,12,13 did not show a protective effect of daily vitamin D and calcium supplementation against the incidence of colorectal, breast, and all invasive cancers, results that could have been due to the low vitamin D dosage of 400 IU/d. In contrast, 2 subsequent trials by 1 research group,14,15 which administered a higher vitamin D dosage of 2000 IU/d with calcium, reported a reduced incidence of all types of cancer in the treatment arm. A consistent finding in both studies was an approximately 1-year lag from the time of randomization for the benefit of vitamin D to appear on survival curves, although this analysis was not prespecified.
Given the limited trial evidence for the association of vitamin D supplementation with the incidence of cancer, we performed a post hoc analysis of a large community-based randomized clinical trial to investigate whether vitamin D supplementation is associated with a reduction in cancer incidence. The original primary aim of this trial was to assess the effect of vitamin D supplementation on the incidence of cardiovascular disease.16 We also included cancer mortality as a secondary outcome given evidence from a recent meta-analysis suggesting that vitamin D supplements are associated with a reduction in cancer mortality, but not cancer incidence.17
Methods
Study Design
The Vitamin D Assessment (ViDA) study was a randomized, double-blind, placebo-controlled clinical trial carried out in Auckland, New Zealand, from March 1, 2011, to July 31, 2015. Full details of the study methods have been published.18 Inclusion criteria were as follows: (1) age from 50 to 84 years; (2) the ability to give informed consent; (3) resident of Auckland, New Zealand, at the time of recruitment; and (4) anticipated residence in New Zealand for the 4-year study period. Exclusion criteria were as follows: (1) current use of vitamin D supplements including cod liver oil (>600 IU/d if aged 50-70 years; >800 IU/d if aged 71-84 years)19; (2) diagnosis of psychiatric disorders that would limit ability to comply with the study protocol; (3) a history of hypercalcemia, nephrolithiasis, sarcoidosis, parathyroid disease, or gastric bypass surgery; (4) enrollment in another study that could affect participation; or (5) a baseline serum-corrected calcium level greater than 10.0 mg/dL (to convert serum calcium to millimoles per liter, multiply by 0.25). The Multiregion Ethics Committee in Wellington, New Zealand, approved the study, which was registered with the Australian New Zealand Clinical Trials Registry. Written informed consent was obtained from all participants during the baseline assessment.
Participant Recruitment and Baseline Assessment
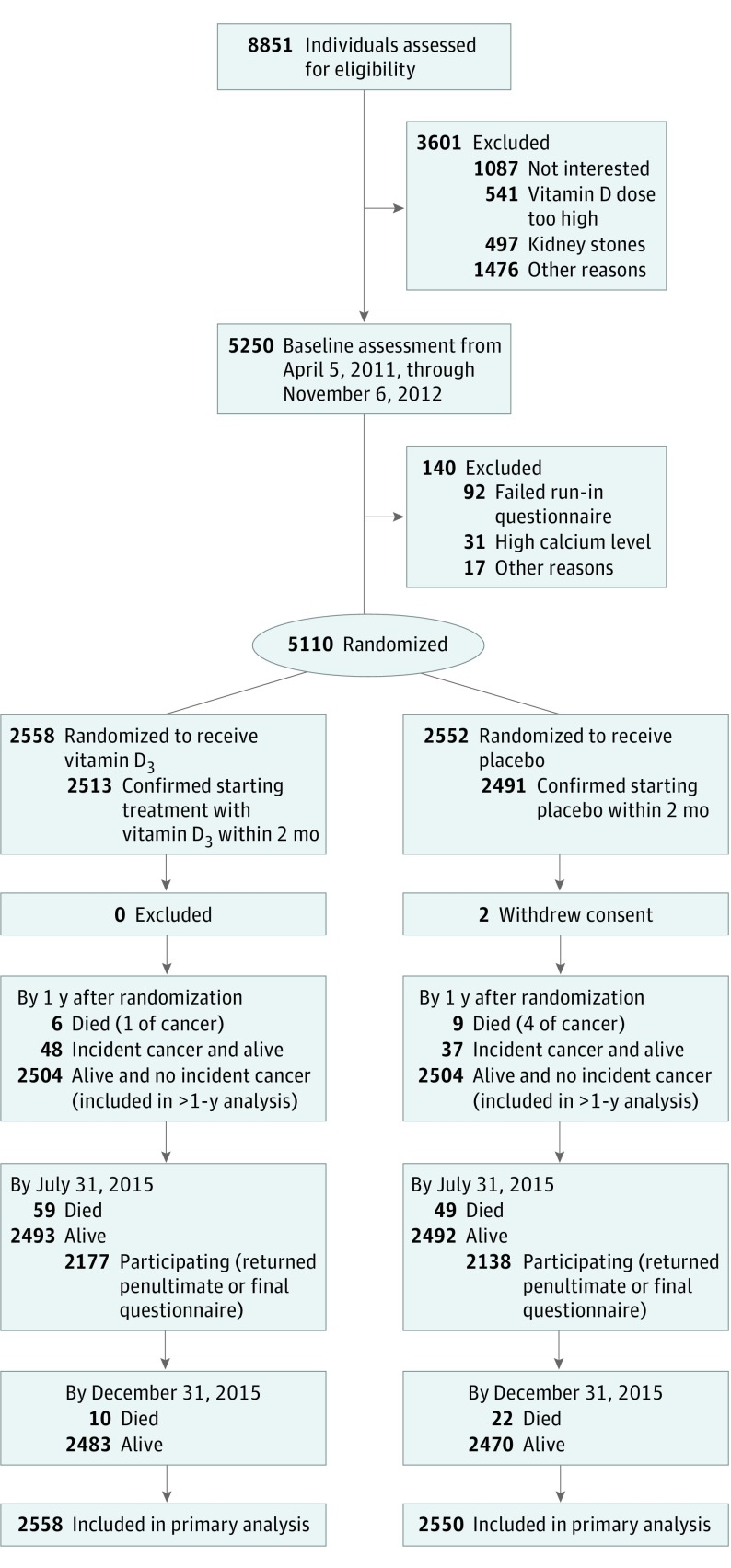
Participants were recruited from 55 family practices in Auckland; 94% of the New Zealand population is registered with family practices.20 Beginning in March 2011, a personalized letter was mailed to the homes of 47 905 potential participants inviting them to participate in the study. Of 8688 participants who replied, 5107 were interested and eligible for baseline assessments. An additional 163 potential participants from ethnic minority community groups were screened, and 143 were eligible to participate. Altogether, 5250 had a baseline assessment from April 5, 2011, to November 6, 2012 (Figure 1).
Figure 1. Flow Diagram in the Vitamin D Assessment Study for Cancer Outcome.
The baseline assessment included collecting participant written informed consent; answering questions about sociodemographic status, lifestyle (tobacco use, alcohol use for the previous 12 months, usual leisure-time physical activity,21 and sun exposure22 during the previous 3 months); receipt of vitamin D or calcium supplements; and medical history diagnosed by a physician (including type of cancer and age at cancer diagnosis). Height (±0.1 cm) and weight (±0.1 kg) were measured with participants wearing light clothing without shoes. A nonfasting blood sample was collected to screen for hypercalcemia, with the remaining serum aliquoted and stored at −80°C for the measurement of 25(OH)D at a later time.
Randomization
After the baseline assessment, participants were mailed a run-in questionnaire (a 1-page, double-sided, self-completed questionnaire) with a blinded placebo capsule enclosed in the mailing. The participants were included in the study if they returned the questionnaire within 4 weeks, confirmed in the questionnaire that they ingested the capsule, and their laboratory results confirmed that they did not have hypercalcemia (corrected calcium level ≤10.0 mg/dL). A total of 5110 participants (4972 from the family practices, 138 from the community) were randomized from June 3, 2011, to January 23, 2013, into 1 of the 2 treatment groups, designated in random blocks of 8, 10, or 12 participants, in a race/ethnicity group, and 5-year age strata. Treatment was randomized using computer generation by the study biostatistician, and all of the other staff and participants were unaware of the treatment assignment (blinded).
Intervention
A package of vitamin D3 2.5-mg (100 000 IU) or placebo softgel oral capsules (Tishcon Corporation) were mailed to homes with a 1-page questionnaire (and reply-paid envelope) for the participant to record self-reported adherence; return of the reply-paid envelope was used to monitor retention. Two capsules were sent in the initial mailing after randomization (ie, 200 000-IU bolus of two 100 000-IU capsules or placebo) and followed monthly with vitamin D3, 100 000 IU, or placebo capsules. A monthly 100 000-IU dose of vitamin D3 was chosen because pharmacokinetic research reported that this dosage-maintained serum 25(OH)D concentrations greater than 35 ng/mL for a month after ingestion.23 The aim of the study was to raise serum 25(OH)D concentrations throughout the year to between 32 and 40 ng/mL, which observational studies suggested was optimal for health.24,25,26,27
Capsules were mailed monthly over a period of several days until June 2013. For reasons of cost, after July 2013, 4 capsules were mailed every 4 months, with monthly email or letter reminders for participants to take their monthly dose. Questionnaires were mailed monthly until November 2013. From March 2014, participants were sent questionnaires, 4 monthly with 4 capsules, and 1 capsule was to be taken each month. Participants stopped taking the assigned medication on July 31, 2015.
Serum Calcium and 25(OH)D Concentrations
The serum-corrected calcium level was measured at baseline on an automated clinical chemistry analyzer (Advia 2400; Siemens Healthcare Diagnostics). Serum 25(OH)D concentrations, combining D2 and D3, were measured in baseline aliquots stored frozen at −80°C by liquid chromatography–tandem mass spectrometry (API 4000; Sciex), with 12.7% interassay coefficient of variation in a local laboratory participating in the Vitamin D External Quality Assessment Scheme program.28 In a 10% random sample, 438 of 515 invited participants (85.0%) agreed to return at 6, 12, 24, and 36 months after the initiation of the study for the collection of blood samples to measure corrected calcium levels from fresh blood and 25(OH)D concentrations from stored blood, measured in the same batch for each participant. Deseasonalized or season-adjusted 25(OH)D concentrations were calculated for participants from their individual baseline 25(OH)D concentrations and blood collection date by using a sinusoidal model with measures derived from baseline values for all participants.29 Vitamin D deficiency was defined as a deseasonalized 25(OH)D concentration less than 20 ng/mL.29
Cancer Outcomes
The New Zealand Ministry of Health maintains registries of all deaths and all primary invasive and in situ malignant neoplasms diagnosed from pathology reports (including cancer site and morphology) in New Zealand, excluding nonmelanoma skin cancers.30 The accuracy of this cancer registry is similar to clinical audits of cancer registries in the United States and Europe.31
All New Zealand residents are assigned a unique Ministry of Health National Health Index number. These numbers were collected from all study participants who gave their written informed consent for the study researchers to access their Ministry of Health data. The National Health Index numbers were used to link individuals with cancer registration data and deaths. Information collected about cancer history at the baseline assessment was used to help distinguish between prevalent and incident cases in the Cancer Registry data.30
The aim of our analysis was to replicate the outcome definitions and statistical analysis methods used by Lappe and colleagues.15 Cancer cases were defined as International Statistical Classification of Diseases and Health Related Problems, Tenth Revision (ICD-10) diagnosis codes C00-D09 or cancer deaths.
The primary outcome was the time to first cancer reported for all defined malignant neoplasms from the time of randomization until the study medication was discontinued on July 31, 2015. The primary outcome was examined in the following prespecified groups: overall (all participants), by sex, and by baseline deseasonalized 25(OH)D concentration (<20 ng/mL or ≥20 ng/mL).
The secondary outcomes were all defined malignant neoplasms reported from more than 12 months after randomization until the study medication was discontinued on July 31, 2015; from randomization through December 31, 2015; from 1 year after randomization through December 31, 2015; and the number of cancer deaths after randomization through December 31, 2015. Each secondary outcome was also examined in the prespecified groups. The follow-up period for some secondary outcomes continued for 5 months after discontinuing supplementation (to December 31, 2015) because serum 25(OH)D concentrations remain higher in individuals receiving vitamin D supplements than in those receiving placebo for up to a year after discontinuing supplementation.32
The study protocol specified identification of new cancer cases not as an outcome for the ViDA study, but to later combine data for common cancers with data regarding cancer from other vitamin D supplementation trials. This report is a post hoc analysis of data collected for other outcomes; thus, we developed the statistical analysis plan for the cancer outcomes and registered cancer cases as a secondary outcome with the trial website on October 10, 2017, before receiving the Ministry of Health cancer data on November 8, 2017.
Statistical Analysis
Analysis of the cancer outcomes was conducted on an intention-to-treat basis using National Health Index numbers to identify cancer registrations and deaths regardless of whether participants continued to actively participate in the study by returning the home questionnaires. Cox regression proportional hazards models with robust sandwich variance estimates were used to compare the time to first cancer in the 2 treatment groups. Noncancer deaths were censored. Analyses were performed using SAS, version 9.4 (SAS Institute Inc), and a 2-sided P < .05 was considered significant. Weighted Schoenfeld residuals were used to check the proportional hazards assumption, which was not violated for any of the following variables in the model: treatment, age, sex, and race/ethnicity (all P > .05). Based on an overall cumulative incidence of 6.4% (328 cancer cases for the primary outcome), the study had 85% power to detect a risk ratio of 0.70 with 2-sided 95% CI.33
Results
Recruitment and Baseline Characteristics
From 8851 participants assessed for eligibility, 5250 participants had baseline assessments, and 5110 were randomized. We excluded 2 individuals who withdrew consent after randomization for their data to be retained by the researchers; thus, 5108 were included in the analysis of the primary outcome (Figure 1). Of the 5108 participants included in the analysis, the mean (SD) age was 65.9 (8.3) years, 2969 (58.1%) were male, and 4253 (83.3%) were European or another race/ethnicity (95.8% of whom had European ancestry), with the remainder being Polynesian or South Asian. Only 320 (6.3%) currently used tobacco, whereas 2173 (42.5%) were former smokers and 1214 (23.8%) reported being previously told by a physician that they had cancer. The mean (SD) observed 25(OH)D concentration was 25.3 (9.5) ng/mL and deseasonalized concentration was 26.5 (9.0) ng/mL. Baseline characteristics were similar between the vitamin D and placebo groups (Table 1).
Table 1. Characteristics of the Vitamin D Supplement and Placebo Groups.
| Characteristic | No. (%) of Participants | |
|---|---|---|
| Vitamin D (n = 2558) | Placebo (n = 2550) | |
| Age, y | ||
| 50-59 | 571 (22.3) | 567 (22.2) |
| 60-69 | 1112 (43.5) | 1108 (43.5) |
| 70-79 | 716 (28.0) | 722 (28.3) |
| 80-84 | 159 (6.2) | 153 (6.0) |
| Males | 1512 (59.1) | 1457 (57.1) |
| Race/ethnicity | ||
| Maori | 137 (5.4) | 135 (5.3) |
| Pacific Islander | 168 (6.6) | 166 (6.5) |
| South Asian | 126 (4.9) | 123 (4.8) |
| European or other | 2127 (83.2) | 2126 (83.4) |
| Highest educational levela | ||
| Primary school | 53 (2.1) | 42 (1.6) |
| Secondary school | 1091 (42.7) | 1036 (40.6) |
| Tertiary school | 1412 (55.2) | 1470 (57.7) |
| Paid employmenta | ||
| Yes | 1301 (50.9) | 1317 (51.6) |
| No | ||
| Retired | 1041 (40.7) | 1018 (39.9) |
| Other | 211 (8.2) | 212 (8.3) |
| Tobacco usea | ||
| Current | 164 (6.4) | 156 (6.1) |
| Former | 1101 (43.0) | 1072 (42.0) |
| Never | 1286 (50.3) | 1317 (51.6) |
| Alcohol usea | ||
| Current | 2177 (85.1) | 2211 (86.7) |
| Former | 224 (8.8) | 183 (7.2) |
| Never | 151 (5.9) | 154 (6.0) |
| Vigorous physical activity, h/wk | ||
| None | 1015 (39.7) | 1018 (39.9) |
| 1-2 | 609 (23.8) | 585 (22.9) |
| >2 | 804 (31.4) | 832 (32.6) |
| Refused or do not know | 130 (5.1) | 115 (4.5) |
| Anthropometry, mean (SD) | ||
| Weight, kg | 81.3 (16.5) | 81.2 (16.0) |
| BMI | 28.4 (5.1) | 28.5 (5.1) |
| Sun exposure, h/da | ||
| <1 | 350 (13.7) | 369 (14.5) |
| 1-2 | 1562 (61.1) | 1559 (61.1) |
| >2 | 611 (23.9) | 588 (23.1) |
| Takes supplements | ||
| Vitamin Db | 208 (8.1) | 200 (7.8) |
| Calcium | 125 (4.9) | 127 (5.0) |
| Previous cancer diagnosed by a physician | ||
| All cancers | 622 (24.3) | 592 (23.3) |
| Lung cancer | 35 (1.4) | 41 (1.6) |
| Breast cancer, women onlyc | 56 (5.4) | 54 (4.9) |
| Prostate cancer, mend | 94 (6.2) | 84 (5.8) |
| Melanoma | 107 (4.2) | 101 (4.0) |
| Nonmelanoma skin cancer | 289 (11.4) | 295 (11.6) |
| Other | 41 (1.6) | 17 (0.7) |
| Corrected serum calcium, mean (SD), mg/dL | 9.2 (0.4) | 9.2 (0.4) |
| 25(OH)D | ||
| Observed, mean (SD), ng/mL | 25.5 (9.5) | 25.2 (9.4) |
| <20 ng/mL, Observed | 746 (29.2) | 788 (30.9) |
| <20 ng/mL, Deseasonalized | 612 (23.9) | 658 (25.8) |
Abbreviations: BMI, body mass index (calculated as the weight in kilograms divided by height in meters squared); 25(OH)D, 25-hydroxyvitamin D.
SI conversion factors: To convert serum calcium to millimoles per liter, multiply by 0.25; to convert 25(OH)D to nanomoles per liter, multiple by 2.496.
Percentages do not equal 100% because of missing data or do not know responses.
Vitamin D dosage: 600 IU/d or less if aged 50 to 70 years; 800 IU/d or less if aged 71 to 84 years.
Vitamin D group (n = 1046) and placebo group (n = 1093).
Vitamin D group (n = 1512) and placebo group (n = 1457).
Follow-up and Adherence
Figure 1 shows the flow diagram of follow-up after randomization. Fifteen deaths occurred within the year after randomization, 108 deaths occurred by the end of the active follow-up period on July 31, 2015, and 32 deaths occurred by the end of the passive follow-up period on December 31, 2015. This yielded a total of 155 deaths (75 deaths in the vitamin D group and 80 in the placebo group) for the entire follow-up period.
Within 2 months after randomization, most participants (5004 of 5108 [98.0%]) confirmed by questionnaire that they had started taking the study capsule; only 21 of 2558 (0.8%) of the vitamin D group and 49 of 2550 (1.9%) of the placebo group never confirmed that they were taking the medication during the active follow-up period from randomization to July 31, 2015 (median, 3.3 years; range, 2.5-4.2 years). During the last 5 months of active follow-up, 4315 of 4985 participants (86.6%) were actively involved in the trial, as indicated by the 4032 (80.9%) who returned the final July 2015 questionnaire and an additional 283 (5.7%) who returned the penultimate March 2015 questionnaire.
Participant adherence to taking the study capsule, as reported in the home questionnaires, was 85 280 capsules during 100 535 person-months (84.8%) in the vitamin D group and 83 387 capsules during 100 401 person-months (83.1%) in the placebo group up to July 31, 2015. This high adherence was confirmed by the mean observed 25(OH)D concentrations of the randomly selected participants who gave blood samples at 6 months and up to 36 months after randomization. Observed 25(OH)D concentrations ranged from 48 to 54 ng/mL in the vitamin D group, being consistently greater than 20 ng/mL higher than the mean in the placebo group (eFigure in the Supplement). Mean (SD) serum calcium levels throughout the follow-up period in this subsample were similar for the vitamin D vs placebo groups: 9.2 (0.4) vs 9.2 (0.4) mg/dL at 6, 12, and 24 months, and 9.6 (0.4) vs 9.6 (0.4) mg/dL at 36 months. No participants in this subsample developed hypercalcemia related to taking the study capsules.
Cancer Outcomes
There were 375 participants who had a first cancer registration after randomization (60 of whom died) and another 29 who died of cancer diagnosed before randomization, giving a total of 404 participants with a cancer outcome up to December 31, 2015. Types of cancer are provided in Table 2. The most common cancers were melanoma in situ (n = 71) and malignant melanoma (n = 55), followed by prostate cancer (n = 64), colorectal cancer (n = 38), breast cancer (n = 36), and lymphoid and hematopoietic cancers (n = 36).
Table 2. Number of Cancer Registrations and Deaths During Follow-up to December 31, 2015, by Type of Cancer.
| Type of Cancer | First Cancer Registration After Randomization and Alive on December 31, 2015, No. | Deaths, No. | Total, No. | |
|---|---|---|---|---|
| Cancer Registration After Randomization | Cancer Registration Before Randomization | |||
| Invasive neoplasms | ||||
| Colorectal | 28 | 8 | 2 | 38 |
| Oropharynx and other digestive tract | 17 | 11 | 3 | 31 |
| Respiratory and intrathoracic organs | 9 | 12 | 0 | 21 |
| Malignant melanoma and other malignant neoplasms of skin | 50 | 1 | 4 | 55 |
| Breast | 31 | 2 | 3 | 36 |
| Prostate | 56 | 1 | 7 | 64 |
| Lymphoid and hematopoietic | 25 | 8 | 3 | 36 |
| Other | 17 | 17 | 7 | 41 |
| In situ neoplasms | ||||
| Melanoma in situ | 71 | 0 | 0 | 71 |
| Other carcinoma in situ | 11 | 0 | 0 | 11 |
| Total | 315 | 60 | 29 | 404 |
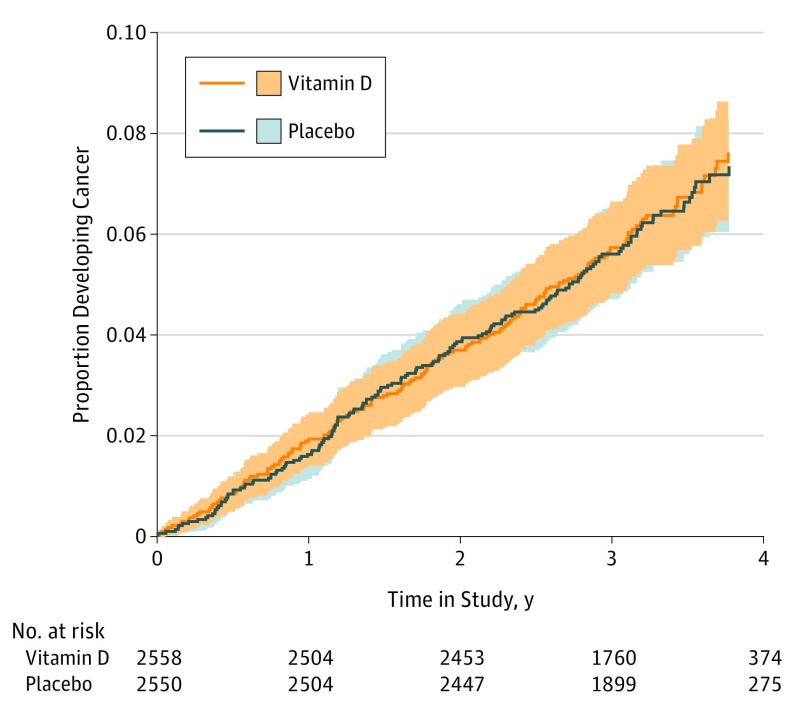
The numbers of participants with the primary and secondary outcomes in the vitamin D and placebo groups during follow-up, along with hazard ratios (HRs) adjusted for age, sex, and race/ethnicity, are provided in Table 3. There was no difference in the percentage of participants with cancer registrations from randomization to July 31, 2015 (primary end point), between the vitamin D (165 of 2558 participants [6.5%]) and placebo (163 of 2550 [6.4%]) arms (HR, 1.01; 95% CI, 0.81-1.25; P = .95). Similar results were seen in men and women (HR, 0.96; 95% CI, 0.74-1.25 vs HR, 1.09; 95% CI, 0.75-1.59; P = .57), and in participants with 25(OH)D less than 20 ng/mL vs 20 ng/mL or more (HR, 1.01; 95% CI, 0.65-1.58 vs HR, 1.04; 95% CI, 0.81-1.33; P = .80). There was no difference between the vitamin D and placebo arms in the time to first cancer registration up to July 31, 2015, including from 1 year after randomization (Figure 2). Similar results were seen for all secondary outcomes as well as for nonskin cancers (Table 3). Stratifying the sample by sex or by baseline 25(OH)D concentration produced similar results for all secondary outcomes (eTable in the Supplement).
Table 3. Proportion of Participants Having Incident Cancer or Dying of Cancer During Follow-up and Hazard Ratios Adjusted for Age, Sex, and Race/Ethnicity by Study Treatment Group.
| Cancer Outcome | Events, No. (%) | Adjusted Hazard Ratio (95% CI) | P Value (Wald χ2) | |
|---|---|---|---|---|
| Vitamin D (n = 2558) | Placebo (n = 2550) | |||
| Primary outcome: cancer registration from randomization to July 31, 2015 | ||||
| All participantsa | 165 (6.5) | 163 (6.4) | 1.01 (0.81-1.25) | .95 |
| Men | 108 (7.1) | 110 (7.5) | 0.96 (0.74-1.25) | .76 |
| Women | 57 (5.4) | 53 (4.8) | 1.09 (0.75-1.59) | .66 |
| 25(OH)D <20 ng/mLb | 37 (6.0) | 42 (6.4) | 1.01 (0.65-1.58) | .96 |
| 25(OH)D ≥20 ng/mLc | 128 (6.6) | 121 (6.4) | 1.04 (0.81-1.33) | .79 |
| Secondary outcomes: cancer registration from randomization to December 31, 2015 | 188 (7.3) | 187 (7.3) | 1.00 (0.82-1.22) | .99 |
| Cancer deaths from randomization to December 31, 2015 | ||||
| Cancer registration after randomizationd | 30 (1.2) | 30 (1.2) | 0.99 (0.60-1.64) | .97 |
| All cancer deathse | 44 (1.7) | 45 (1.8) | 0.97 (0.64-1.47) | .89 |
| Cancer registration from 1 y after randomization to the following datesf: | ||||
| July 31, 2015 | 116 (4.6) | 122 (4.9) | 0.95 (0.74-1.23) | .69 |
| December 31, 2015 | 139 (5.6) | 146 (5.8) | 0.95 (0.75-1.19) | .64 |
| Nonskin cancer registration from randomization to July 31, 2015g | 111 (4.3) | 111 (4.4) | 0.99 (0.76-1.29) | .96 |
| Invasive cancer registration from randomization to July 31, 2015h | 128 (5.0) | 131 (5.1) | 0.97 (0.76-1.24) | .80 |
Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiple by 2.496.
Includes 28 deaths in the vitamin D group and 30 deaths in placebo group due to cancer.
Based on deseasonalized concentrations: vitamin D group (n = 612) and placebo group (n = 658).
Vitamin D group (n = 1946) and placebo group (n = 1892).
Vitamin D group (n = 2544) and placebo group (n = 2535). Excludes those who died of cancer diagnosed before randomization.
Includes 29 deaths (14 deaths in vitamin D group and 15 deaths in placebo group) of cancer diagnosed before randomization.
Vitamin D group (n = 2504) and placebo group (n = 2504). Excludes those who were registered with a diagnosis of cancer within 12 months of randomization.
Excludes malignant melanoma, melanoma in situ, and malignant neoplasm of skin.
Excludes in situ neoplasms.
Figure 2. Proportion of Participants Developing Cancer During Follow-up From June 3, 2011, to July 31, 2015, by Study Group.
Shaded areas represent 95% CIs for the 2 curves.
Discussion
The results of this large randomized clinical trial suggest that monthly high-dose vitamin D supplementation is not associated with reductions in cancer incidence or cancer mortality in people selected from the community in New Zealand. The cancer incidence results are consistent with findings from previous randomized clinical trials of community samples in the United States, which reported an HR of 0.98 (95% CI, 0.90-1.05; P = .54), and Great Britain, which reported an HR of 1.09 (95% CI, 0.86-1.36; P = .47),13,34 and with a recent meta-analysis of vitamin D supplementation trials.17 However, our results given in Table 3 comparing the vitamin D group with the placebo group do not confirm results from a recent Nebraska study15 reporting a 35% reduced HR for follow-up starting from 12 months after randomization (P = .047), nor the 30% reduced HR for follow-up starting from randomization (P = .06). Our results also do not confirm a recent meta-analysis of 3 trials that found that vitamin D supplementation was associated with a significant reduction in cancer mortality by 12%.17
There are several possible explanations for why our trial did not observe a similar reduction in cancer incidence from vitamin D supplementation as in the recent Nebraska study.15 First, we gave bolus doses rather than daily doses of vitamin D. Recent studies35 suggest that vitamin D is more able to enter cells than 25(OH)D for conversion to the active metabolite of vitamin D (1,25-dihydroxyvitamin D); in our study, vitamin D would only have been present in the blood circulation for several days after each monthly dose because of its short half-life.36 Thus, the Nebraska study15 may have produced a stronger continuous vitamin D exposure from their daily dose. Second, participants received vitamin D alone rather than with calcium. It is possible that both medications, acting separately or synergistically, had a greater influence than vitamin D alone, although a reduction in cancer incidence was not seen in the Women’s Health Initiative13 which also gave both supplements. Third, the cancer profile in the ViDA study had a much larger proportion of cases with melanoma (33%) (Table 2) compared with the Nebraska study15 (6%), although analyses restricted to nonskin cancer produced a similar null result. The similar results for men and women in our study (Table 3) suggests that the inclusion of women alone in the Nebraska study15 is an unlikely explanation for the difference in results between the 2 studies.
Other possible reasons for the null result in our study include an insufficient proportion of participants (25%) with vitamin D deficiency, which limited statistical power in that subgroup. However, the lower mean baseline observed 25(OH)D concentration in the ViDA study (25.3 ng/mL) compared with the Nebraska study15 (32.8 ng/mL) suggests that participants in the former study were more likely to be deficient in vitamin D. Another possible explanation for the null finding is the relatively short follow-up time of the study (median, 3.3 years; range, 2.5-4.2 y) which may have been too short to detect any association of vitamin D supplementation with the incidence of cancer.
Strengths and Limitations
Our study has important strengths. Because our sample was recruited from the community, results are likely to be relevant for the general population. Adherence to taking the study capsule was high, as confirmed by the doubling in mean 25(OH)D concentrations in the vitamin D arm of the random subsample, which was 54.1 ng/mL at 36 months’ follow-up in the vitamin D arm or 9 ng/mL higher than in the Nebraska study (eFigure in the Supplement).15 Retention and active participation in the study were high: 87% of participants returned the final 2 questionnaires. We enquired extensively at the baseline assessment about cancer history, which allowed us to identify incident cancer cases after randomization. These cases were systematically identified from the cancer registry regardless of whether participants continued to actively participate, allowing us to do intention-to-treat analyses. Although our study had 85% power to detect a risk ratio of 0.70, observed in the Nebraska study,15 our power was much lower for detecting an association between cancer and vitamin D, including the modest 12% reduction in cancer mortality reported in a recent meta-analysis of vitamin D supplementation.17
Conclusions
We showed that monthly high-dose vitamin D supplementation without calcium for up to 4 years is not associated with cancer prevention. Further research is required to study the influence of daily or weekly doses of vitamin D on the risk of cancer for longer periods of time.
eFigure. Mean (95% CI) Observed 25-Hydroxyvitamin D Concentration During Follow-up in the Random Subsample, by Treatment Group
eTable. Subgroup Analysis for Secondary Outcomes
References
- 1.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9(3):-. doi: 10.1093/ije/9.3.227 [DOI] [PubMed] [Google Scholar]
- 2.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19(6):614-622. doi: 10.1016/0091-7435(90)90058-R [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res. 1990;10(5A):1307-1311. [PubMed] [Google Scholar]
- 4.Lefkowitz ES, Garland CF. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23(6):1133-1136. doi: 10.1093/ije/23.6.1133 [DOI] [PubMed] [Google Scholar]
- 5.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414-1424. doi: 10.1002/ijc.25439 [DOI] [PubMed] [Google Scholar]
- 6.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46(12):2196-2205. doi: 10.1016/j.ejca.2010.03.037 [DOI] [PubMed] [Google Scholar]
- 7.Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila). 2011;4(5):735-743. doi: 10.1158/1940-6207.CAPR-10-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. doi: 10.1136/bmj.g6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong JS, Cuellar-Partida G, Lu Y, et al. ; Australian Ovarian Cancer Study . Association of vitamin D levels and risk of ovarian cancer: a mendelian randomization study. Int J Epidemiol. 2016;45(5):1619-1630. doi: 10.1093/ije/dyw207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitrakopoulou VI, Tsilidis KK, Haycock PC, et al. ; GECCO Consortium; PRACTICAL Consortium; GAME-ON Network (CORECT, DRIVE, ELLIPSE, FOCI-OCAC, TRICL-ILCCO) . Circulating vitamin D concentration and risk of seven cancers: mendelian randomisation study. BMJ. 2017;359:j4761. doi: 10.1136/bmj.j4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. ; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684-696. doi: 10.1056/NEJMoa055222 [DOI] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Johnson KC, Kooperberg C, et al. ; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100(22):1581-1591. doi: 10.1093/jnci/djn360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunner RL, Wactawski-Wende J, Caan BJ, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women’s Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr Cancer. 2011;63(6):827-841. doi: 10.1080/01635581.2011.594208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586-1591. doi: 10.1093/ajcn/85.6.1586 [DOI] [PubMed] [Google Scholar]
- 15.Lappe J, Watson P, Travers-Gustafson D, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234-1243. doi: 10.1001/jama.2017.2115 [DOI] [PubMed] [Google Scholar]
- 16.Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study: a randomized clinical trial. JAMA Cardiol. 2017;2(6):608-616. doi: 10.1001/jamacardio.2017.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111(5):976-980. doi: 10.1038/bjc.2014.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scragg R, Waayer D, Stewart AW, et al. The Vitamin D Assessment (ViDA) Study: design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J Steroid Biochem Mol Biol. 2016;164:318-325. doi: 10.1016/j.jsbmb.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 20.Ministry of Health New Zealand Enrolment in a primary health organisation. https://www.health.govt.nz/our-work/primary-health-care/about-primary-health-organisations/enrolment-primary-health-organisation. Accessed February 05, 2018.
- 21.Wareham NJ, Jakes RW, Rennie KL, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation Into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6(4):407-413. doi: 10.1079/PHN2002439 [DOI] [PubMed] [Google Scholar]
- 22.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19(3):559-563. doi: 10.1093/ije/19.3.559 [DOI] [PubMed] [Google Scholar]
- 23.Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87(3):688-691. doi: 10.1093/ajcn/87.3.688 [DOI] [PubMed] [Google Scholar]
- 24.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes [published corrections appear in Am J Clin Nutr. 2006;84(5):1253 and Am J Clin Nutr. 2007;86(3):809]. Am J Clin Nutr. 2006;84(1):18-28. doi: 10.1093/ajcn/84.1.18 [DOI] [PubMed] [Google Scholar]
- 25.Scragg R, Sowers M, Bell C; Third National Health and Nutrition Examination Survey . Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813-2818. doi: 10.2337/diacare.27.12.2813 [DOI] [PubMed] [Google Scholar]
- 26.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest. 2005;128(6):3792-3798. doi: 10.1378/chest.128.6.3792 [DOI] [PubMed] [Google Scholar]
- 27.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20(7):713-719. doi: 10.1016/j.amjhyper.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 28.Vitamin D External Quality Assessment Scheme program http://www.deqas.org. Accessed March 28, 2018.
- 29.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97(6):1243-1251. doi: 10.3945/ajcn.112.054502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Health New Zealand New Zealand Cancer Registry—table of available data. https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/new-zealand-cancer-registry-nzcr/new-zealand-cancer-registry-table-available-data. Accessed February 05, 2018.
- 31.Cunningham R, Sarfati D, Hill S, Kenwright D. An audit of colon cancer data on the New Zealand Cancer Registry. N Z Med J. 2008;121(1279):46-56. [PubMed] [Google Scholar]
- 32.Martinaityte I, Kamycheva E, Didriksen A, Jakobsen J, Jorde R. Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. J Clin Endocrinol Metab. 2017;102(10):3731-3738. doi: 10.1210/jc.2017-01187 [DOI] [PubMed] [Google Scholar]
- 33.OpenEpi–Toolkit Shell for Developing New Applications http://www.openepi.com/Power/PowerRCT.htm. Accessed February 5, 2018.
- 34.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619-4628. doi: 10.1210/jc.2013-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaney RP, Armas LA. Quantifying the vitamin D economy. Nutr Rev. 2015;73(1):51-67. doi: 10.1093/nutrit/nuu004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Mean (95% CI) Observed 25-Hydroxyvitamin D Concentration During Follow-up in the Random Subsample, by Treatment Group
eTable. Subgroup Analysis for Secondary Outcomes