Key Points
Question
Does anlotinib improve overall survival and progression-free survival in third-line or further treatment of advanced non–small cell lung cancer?
Findings
In this randomized clinical trial that included 437 patients with advanced non–small cell lung cancer, substantial improvement in overall survival and progression-free survival was noted in patients who received anlotinib compared with those given placebo. Substantial improvement in objective response rate and disease control rate was also observed among the anlotinib group.
Meaning
In third-line or further treatment of Chinese patients with advanced non–small cell lung cancer, anlotinib prolonged overall survival, suggesting that anlotinib is well tolerated and is a potential later therapy for patients with this disease.
Abstract
Importance
Anlotinib is a novel multitarget tyrosine kinase inhibitor for tumor angiogenesis and proliferative signaling. A phase 2 trial showed anlotinib to improve progression-free survival with a potential benefit of overall survival, leading to the phase 3 trial to confirm the drug’s efficacy in advanced non–small cell lung cancer (NSCLC).
Objective
To investigate the efficacy of anlotinib on overall survival of patients with advanced NSCLC progressing after second-line or further treatment.
Design, Setting, and Participants
The ALTER 0303 trial was a multicenter, double-blind, phase 3 randomized clinical trial designed to evaluate the efficacy and safety of anlotinib in patients with advanced NSCLC. Patients from 31 grade-A tertiary hospitals in China were enrolled between March 1, 2015, and August 31, 2016. Those aged 18 to 75 years who had histologically or cytologically confirmed NSCLC were eligible (n = 606), and those who had centrally located squamous cell carcinoma with cavitary features or brain metastases that were uncontrolled or controlled for less than 2 months were excluded. Patients (n = 440) were randomly assigned in a 2-to-1 ratio to receive either 12 mg/d of anlotinib or a matched placebo. All cases were treated with study drugs at least once in accordance with the intention-to-treat principle.
Main Outcomes and Measures
The primary end point was overall survival. The secondary end points were progression-free survival, objective response rate, disease control rate, quality of life, and safety.
Results
In total, 439 patients were randomized, 296 to the anlotinib group (106 [36.1%] were female and 188 [64.0%] were male, with a mean [SD] age of 57.9 [9.1] years) and 143 to the placebo group (46 [32.2%] were female and 97 [67.8%] were male, with a mean [SD] age of 56.8 [9.1] years). Overall survival was significantly longer in the anlotinib group (median, 9.6 months; 95% CI, 8.2-10.6) than the placebo group (median, 6.3 months; 95% CI, 5.0-8.1), with a hazard ratio (HR) of 0.68 (95% CI, 0.54-0.87; P = .002). A substantial increase in progression-free survival was noted in the anlotinib group compared with the placebo group (median, 5.4 months [95% CI, 4.4-5.6] vs 1.4 months [95% CI, 1.1-1.5]; HR, 0.25 [95% CI, 0.19-0.31]; P < .001). Considerable improvement in objective response rate and disease control rate was observed in the anlotinib group over the placebo group. The most common grade 3 or higher adverse events in the anlotinib arm were hypertension and hyponatremia.
Conclusions and Relevance
Among the Chinese patients in this trial, anlotinib appears to lead to prolonged overall survival and progression-free survival. This finding suggests that anlotinib is well tolerated and is a potential third-line or further therapy for patients with advanced NSCLC.
Trial Registration
ClinicalTrials.gov identifier: NCT02388919
This randomized clinical trial examines the efficacy of anlotinib compared with placebo as a third-line or further therapy on overall survival of adult patients with advanced non–small cell lung cancer.
Introduction
Lung cancer is the most commonly diagnosed cancer and accounts for 20% of cancer-related mortality worldwide.1 An estimated 610 200 patients (22% of cancer-related mortality) died from lung cancer in 2015 in China.2 Non–small cell lung cancer (NSCLC) comprises 80% to 85% of lung cancers, and most patients present with locally advanced or metastatic disease at time of diagnosis.3 Recent advancements in the understanding of NSCLC malignant neoplasm molecular pathways have led to the development of targeted therapeutics.4,5,6 With the advent of novel treatments, such as targeted therapy, it is becoming increasingly common for patients to control their disease through multiple lines of therapy.
Anlotinib (AL3818) hydrochloride is a novel multitarget tyrosine kinase inhibitor (TKI) for tumor angiogenesis and proliferative signaling.7 The prime targets of anlotinib include receptor tyrosine kinases vascular endothelial growth factor receptor 1 to 3, epidermal growth factor receptor (EGFR), fibroblast growth factor receptor 1 to 4, platelet-derived growth factor receptor α and β, and stem cell factor receptor.7,8,9 In phase 2 of the ALTER randomized clinical trial, 117 patients with advanced NSCLC who progressed after second-line or further treatment were enrolled from 13 centers; 60 of these patients were assigned to the anlotinib arm and 57 patients to the placebo arm. An improvement in the primary end point of progression-free survival (PFS) was observed (median PFS, 4.8 months for anlotinib arm vs 1.2 months for placebo arm; hazard ratio [HR], 0.32; P < .001), with potential benefit in the secondary end point of overall survival (OS) (median OS, 9.3 months for anlotinib arm vs 6.3 months for placebo arm; P = .08).10 Therefore, we conducted a multicenter, double-blind, randomized phase 3 of the ALTER trial to confirm the efficacy of anlotinib as a third-line or further treatment in patients with advanced NSCLC (stage IIIB to IV). Overall survival was the primary end point of the phase 3 study.
Methods
Study Design
This multicenter, double-blind, randomized phase 3 clinical trial was designed to evaluate the efficacy and safety of anlotinib in patients with advanced NSCLC (ClinicalTrials.gov identifier: NCT02388919). Patients from 31 grade-A tertiary hospitals in China were enrolled between March 1, 2015, and August 31, 2016. All patients provided written informed consent before entering the trial. The trial was conducted according to the principles of the Declaration of Helsinki11 and Good Clinical Practice requirements. All 31 institutions obtained approval from the research ethics board of each site. See Supplement 1 for the trial protocol.
Patients
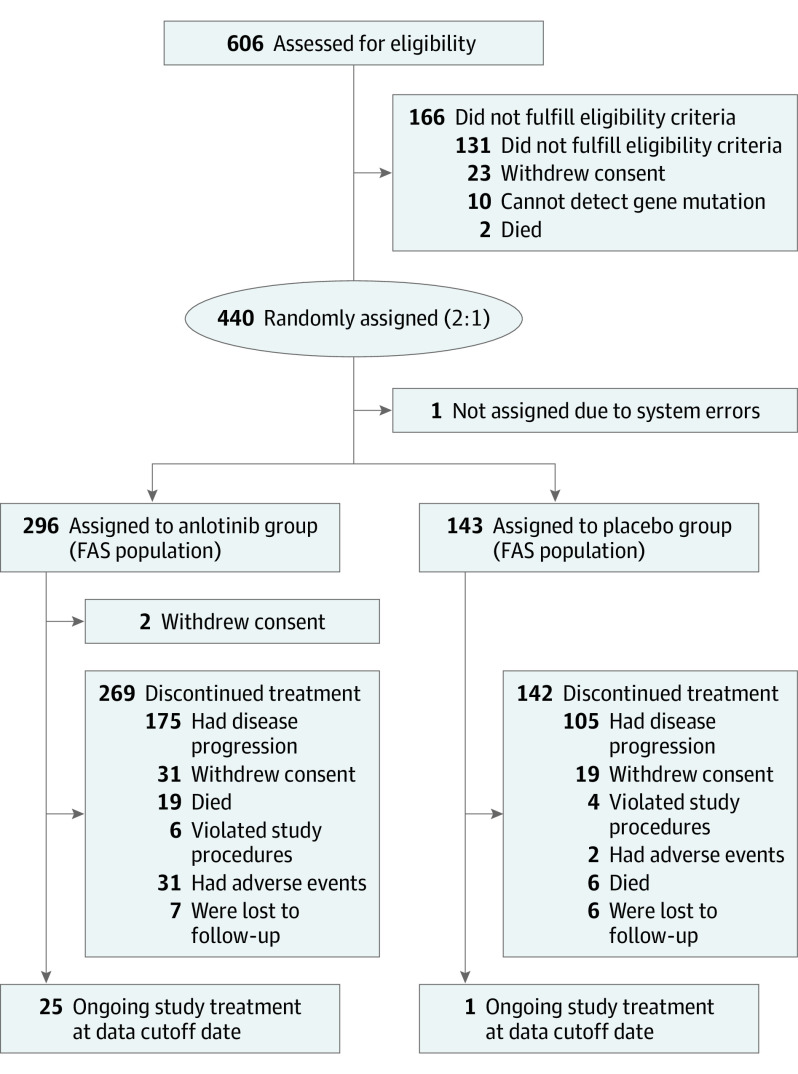
Eligible patients included those aged 18 to 75 years who had histologically or cytologically confirmed NSCLC. Other inclusion criteria were an Eastern Cooperative Oncology Group Performance Status score of 0 or 1 (score range: 0-5, with the highest score indicating death); life expectancy of 3 months or more; and disease progression after at least 1 line of chemotherapy and TKI therapy for all patients with driver alterations (EGFR [OMIM 131550] mutation or ALK [OMIM 105590] rearrangement) as well as disease progression after at least 2 lines of chemotherapy for all patients without driver alterations. Patients were excluded if they had centrally located squamous cell carcinoma with cavitary features or brain metastases that were uncontrolled or controlled for less than 2 months. The full list of inclusion and exclusion criteria are listed in eTable 1 in Supplement 2. See Figure 1 for the patient diagram.
Randomization and Masking
Patients were randomly assigned in a 2-to-1 ratio to receive anlotinib or placebo with a block randomization scheme (block size of 4) using a double-blind, computerized, randomized list generator. Predefined stratification factors were as follows: histopathological classification (adenocarcinoma or squamous cell carcinoma or others), number of metastases (≤3 or >3), driver alterations (EGFR mutation or ALK rearrangement), and alterations status (positive or negative). Packaging of the anlotinib and placebo pills (supplied by Chia Tai Tianqing Pharmaceutical Group Co, Ltd) was identical and coded according to a random code list.
Procedures
Oral anlotinib (12 mg/d) or matched placebo was administered. Each cycle was defined as 2 weeks on-treatment followed by 1 week off-treatment.7 The treatment continued until disease progression or treatment intolerance. Dose modifications (10 mg/d or 8 mg/d) of anlotinib were allowed according to the protocol-defined dose modification criteria. Briefly, if the patient could not tolerate 12 mg/d, then the dose could be reduced to 10 mg/d or 8 mg/d. If the dose of 8 mg/d was not tolerated, then treatment was terminated. In accordance with the Response Evaluation Criteria in Solid Tumors guidelines, version 1.1, tumor assessment was performed using computed tomography within 2 weeks before treatment started. After the treatment initiation, tumors were evaluated once per cycle during the first 2 cycles and then assessed once every 2 cycles. Patient follow-up was done every 8 weeks to assess clinical outcomes, including toxicity, efficacy, and survival, until the death of the patient or until the data cutoff date (January 6, 2017), whichever came first.
Outcomes
The primary end point was OS. The secondary end points were PFS, objective response rate, disease control rate, and quality of life. Patient-reported quality of life was assessed using the European Organisation for Research and Treatment of Cancer questionnaires QLQ-C30 and QLQ-LC13 at every visit before any study-related procedures were conducted. The safety of the treatment was evaluated by the occurrence of adverse events, and the severity of the adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.02.
Statistical Analysis
The sample size was calculated on the basis of the following: an HR of 0.70 for the OS with 2-to-1 randomization; a median OS of 11.0 months for the anlotinib group vs 7.0 months for the placebo group according to results from the phase 2 trial; a significance level of P = .05; and a statistical power of 85%, with both scheduled accrual and follow-up for 12 months. A total of 450 patients were needed to be enrolled within the scheduled accrual and follow-up to achieve 291 death events.
We assessed the efficacy in the full analysis set, which was defined as all cases treated with study drugs at least once in accordance with the intention-to-treat principle. The survival curves for OS and PFS were estimated with the Kaplan-Meier method and were compared between treatment and control groups using the log-rank test. Changes in QLQ-C30 and QLQ-LC13 questionnaire scores from baseline scores were assessed by Wilcoxon rank sum test. Prespecified subgroup analysis was undertaken using univariate Cox proportional hazards regression models that, along with an interaction term (treatment and subgroup variable), tested the heterogeneity of the anlotinib and placebo subgroups. Objective response rate and disease control rate for each group were compared using Pearson χ2 or Fisher exact test when appropriate. All statistical tests were carried out on the basis of a 2-sided α = .05 and 95% CI.12 All analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Of the 606 patients screened for eligibility, 440 patients (72.6%) were enrolled. After enrollment, 1 patient (0.2%) was not assigned because of randomization system error. Overall, 439 patients were randomly assigned to either the anlotinib group (n = 296, of whom 106 [36.1%] were female and 188 [64.0%] were male, with a mean [SD] age of 57.9 [9.1] years) or the placebo group (n = 143, of whom 46 [32.2%] were female and 97 [67.8%] were male, with a mean [SD] age of 56.8 [9.1] years), but 2 patients in the anlotinib group withdrew their consent after randomization. Five patients did not fulfill eligibility criteria, but according to the intention-to-treat principle and blind review, these patients were not excluded from the full analysis set (Figure 1). The major driver alterations were EGFR mutations. Of the 437 patients, 138 (31.6%) exhibited EGFR mutations and only 7 (1.6%) harbored the ALK rearrangements. The baseline patient information is shown in eTable 2 in Supplement 2, and baseline characteristics were well balanced across the 2 groups.
Figure 1. Study Flowchart.
FAS indicates full analysis set. The data cutoff date was January 6, 2017.
The median duration of anlotinib treatment was 18 weeks, and the median duration of the placebo treatment was 6 weeks. The median (range) follow-up duration for the anlotinib group was 8.1 (0.9-22.1) months and for the placebo group was 6.0 (0.5-21.0) months.
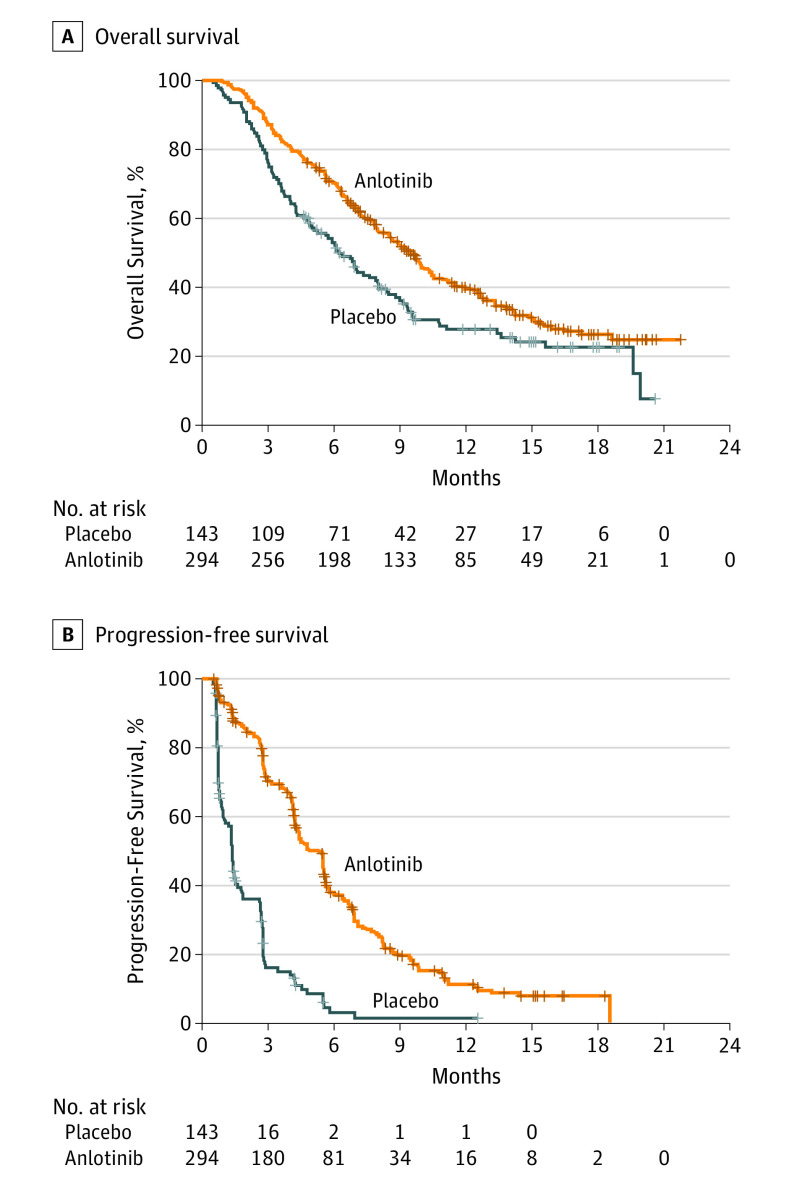
All 437 patients were included in the efficacy analysis. At the time of data cutoff, 189 of 294 patients (64.3%) in the anlotinib group died compared with the 103 of 143 patients (72.0%) in the placebo group who died. The survival rates of patients in the anlotinib group were 70.6% (95% CI, 65.4%-75.8%) at 6 months, 40.0% (95% CI, 34.0%-46.0%) at 12 months, and 26.2% (95% CI, 20.1%-32.3%) at 18 months. For those in the placebo group, the survival rates were 52.8% (95% CI, 44.6%-61.1%) at 6 months, 27.8% (95% CI, 19.9%-35.7%) at 12 months, and 22.7% (95% CI, 14.8%-30.7%) at 18 months. The median OS was 9.6 months (95% CI, 8.2-10.6) for the anlotinib group (eFigure 4 in Supplement 2), which was substantially longer than the median OS for the placebo group (6.3 months [95% CI, 5.0-8.1]; HR, 0.68 [95% CI, 0.54-0.87]; P = .002; Figure 2A). The median PFS for the anlotinib group was 5.4 months (95% CI, 4.4-5.6), which was also substantially longer than the median PFS for the placebo group (1.4 months [95% CI, 1.1-1.5]; HR, 0.25 [95% CI, 0.19-0.31]; P < .001; Figure 2B).
Figure 2. Kaplan-Meier Estimates of Overall and Progression-Free Survival.
A, For the anlotinib group, the median overall survival (OS) was 9.6 months (95% CI, 8.2-10.6); for the placebo group, the median OS was 6.3 months (95% CI, 5.0-8.1). The hazard ratio (HR) was 0.68 (95% CI, 0.54-0.87; P = .002). B, For the anlotinib group, the median progression-free survival (PFS) was 5.4 months (95% CI, 4.4-5.6). For the placebo group, the median PFS was 1.4 months (95% CI, 1.1-1.5). The HR was 0.25 (95% CI, 0.19-0.31; P < .001).
The OS and PFS benefits in favor of anlotinib were observed across most predefined subgroups (Figure 3; eTable 3 in Supplement 2). Patients with EGFR mutation had an HR of 0.59 (95% CI, 0.37-0.93) for OS and an HR of 0.15 (95% CI, 0.09-0.24) for PFS. Those without EGFR mutation had an HR of 0.73 (95% CI, 0.55-0.97) for OS and an HR of 0.29 (95% CI, 0.22-0.39) for PFS. In terms of the different pathological types, patients with adenocarcinoma had an HR of 0.67 (95% CI, 0.51-0.89) for OS and an HR of 0.21 (95% CI, 0.15-0.28) for PFS; both OS and PFS benefits were obtained from anlotinib. In patients with squamous cell carcinoma and other pathological types, only improved PFS was observed (HR, 0.37 [95% CI, 0.22-0.60]).
Figure 3. Subgroup Analysis of Overall and Progression-Free Survival.
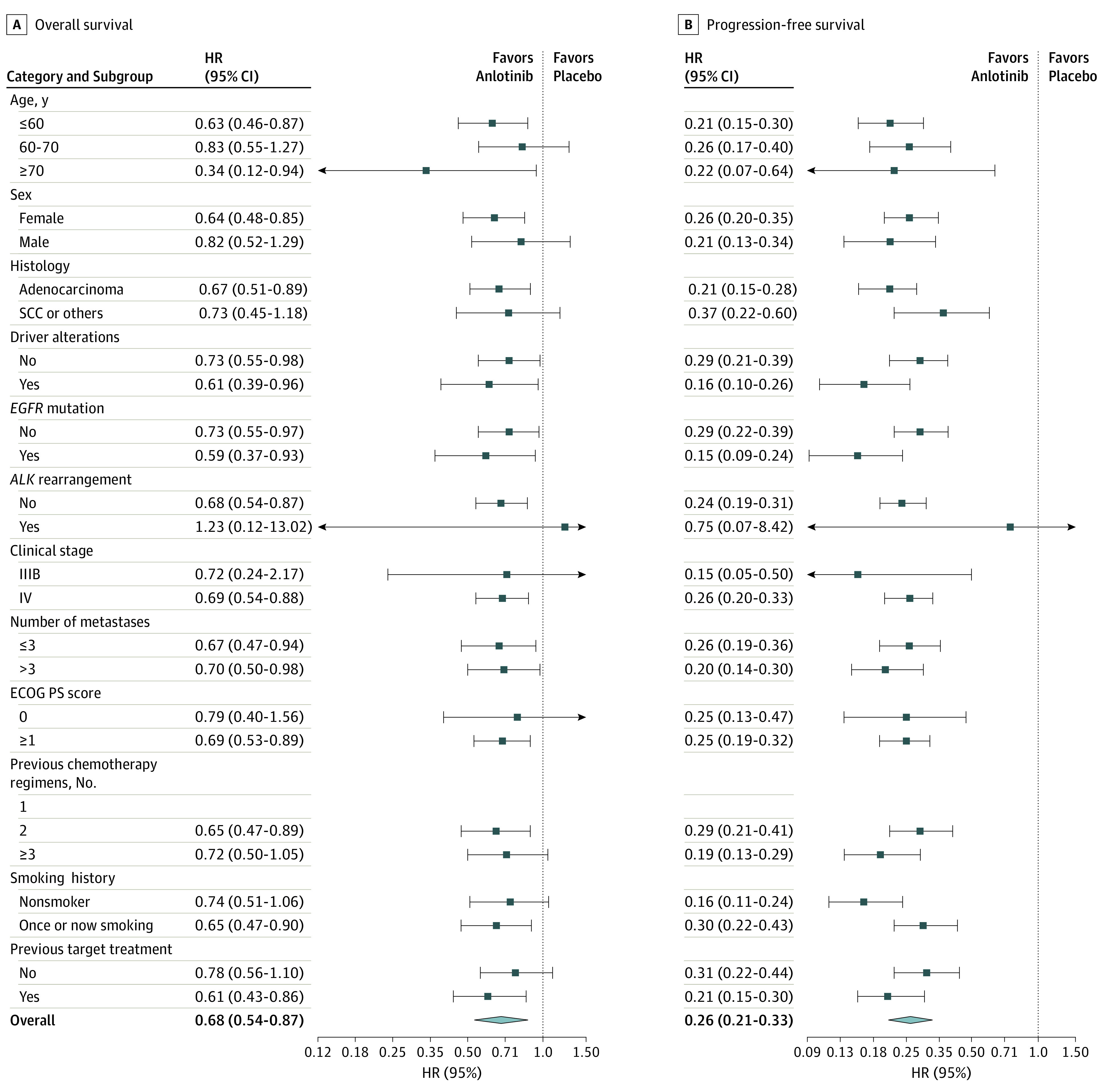
ECOG PS indicates Eastern Cooperative Oncology Group Performance Status (score range: 0-5, with the highest score indicating death); EGFR, endothelial growth factor receptor; HR, hazard rationm; and SCC, squamous cell carcinoma.
Our post hoc analysis (eTable 4 in Supplement 2) showed that, after disease progression, more patients in the placebo group compared with the anlotinib group received subsequent treatment (93 [65.0%] vs 143 [48.6%]; P = .002), especially chemotherapy (59 [41.3%] vs 66 [22.5%]; P < .001).
The objective response rate was significantly higher in the anlotinib group compared with the placebo group (27 [9.2%] vs 1 [0.7%]; P < .001). The difference of disease control rate between anlotinib and placebo groups was also statistically significant (238 [81.0%] vs 53 [37.1%]; P < .001) (eFigure 1 in Supplement 2).
Changes in the QLQ-C30 and QLQ-LC13 scores in the first, second, fourth, and sixth treatment cycle from baseline are shown in eFigures 2 and 3 in Supplement 2. Mean increase in OLQ-LC13 total score was small in the anlotinib group in the first, second, and fourth treatment cycle. The QLQ-C30 analysis showed that patients in the anlotinib group maintained their major health status throughout the cycles from baseline.
The most common adverse events with statistical difference between the 2 groups were observed in the anlotinib group and included hypertension, fatigue, thyroid-stimulating hormone elevation, anorexia, hypertriglyceridemia, hand-foot syndrome, and hypercholesterolemia (eTable 5 in Supplement 2). During the treatment in the anlotinib group, 24 patients (8.2%) had their dose adjusted to 10 mg/d and 2 patients (0.7%) had their dose adjusted to 8 mg/d. The major reasons for dose reduction were hand-foot syndrome (n = 7) and hypertension (n = 3). Adverse events of grade 3 or higher were reported in 182 patients (61.9%) in the anlotinib group and 53 patients (37.1%) in the placebo group. Of these, the most common grade 3 or higher adverse events among the anlotinib group were hypertension (40 [13.6%]), hyponatremia (24 [8.2%]), and elevated γ-glutamyltransferase (16 [5.4%]). Twenty patients (6.8%) in the anlotinib group and 8 patients (5.6%) in the placebo group died during the 30-day follow-up period after the last dose of the study treatment was administered, and no death was found to be associated with anlotinib.
Discussion
This trial met its primary end point on the last day of data cutoff. The results showed that patients with advanced NSCLC who received anlotinib as third-line or further therapy had better OS, PFS, and objective response rate compared with patients who received placebo pills. Anlotinib was well tolerated, and the patient-reported outcome analysis revealed that patients in the anlotinib group generally maintained a reasonable quality of life.
To our knowledge, the present study is the first phase 3 trial in the third-line or beyond setting that compared a multitarget agent with placebo to show an OS benefit. In phase 2 of this trial, prolongation of PFS was achieved; however, OS was not significant between the anlotinib and placebo arms, which contrasts with the results in phase 3.10 This discrepancy may be explained by the small sample size in the phase 2 trial—only 117 patients were enrolled. In the phase 2 trial, close attention was not directed toward patients’ driver alterations, as EGFR status was unknown in 60.7% of the total population. Considering the effect on OS of driver alterations and the corresponding targeted therapies as subsequent therapy, all patients in the phase 3 study provided specimens before enrollment to detect these driver alterations.
The number of previous targeted treatment regimens, EGFR mutation, and ALK rearrangement was balanced across the 2 arms. In addition, the proportion of patients in the anlotinib group who received subsequent therapies was not larger than that in the placebo group, suggesting that the recorded OS benefit was attributable to anlotinib but not to either subsequent targeted treatment or other therapies. After data cutoff (January 6, 2017), we continued the OS follow-up until May 18, 2017. Further survival analysis showed a median OS of 9.6 months for the anlotinib group, which was 3.3 months longer than that for the placebo group. Second-line docetaxel has been shown to improve survival in NSCLC by a median of 3 months.13 However, few trials have focused on evaluating docetaxel as the third-line or further therapy, and retrospective evidence only shows an objective response rate of 2.3%.14 Recently, a meta-analysis confirmed that immune checkpoint inhibitors compared with docetaxel could substantially improve OS in NSCLC as a second-line therapy; the median OS of immune checkpoint inhibitors ranged from 9.2 to 13.8 months.15 As for the third-line or further setting, the ATLANTIC study (A Global Study to Assess the Effects of MEDI4736 [Durvalumab] in Patients With Locally Advanced or Metastatic Non Small Cell Lung Cancer) has evaluated durvalumab as a third-line or later treatment in advanced NSCLC, and the median OS from this drug ranged from 9.3 to 13.3 months in different cohorts.16
As a multiple target TKI, the target molecules of anlotinib include vascular endothelial growth factor receptor 1 to 3, EGFR, platelet-derived growth factor receptor α and β, fibroblast growth factor receptor 1 to 3, and stem cell factor receptor,7,8,9 all of which contribute to inhibitory action on tumor angiogenesis and partial tumor cell growth function. During the treatment, only 24 (8.2%) patients reduced their dose to 10 mg/d and 2 (0.7%) patients’ dose was decreased to 8 mg/d, which indicates that anlotinib was well tolerated by patients with limited toxicity. The tolerable profile of anlotinib results from the treatment schedule of 2 weeks on-treatment followed by 1 week off-treatment; the low dose of the drug, which is related to a low IC50 concentration8; the rapid absorption through the intestine; a long half-life of 116 (±47) hours; a Tmax of 7.3 (±3.3) hours; and the stable plasma concentration within the treatment window. High frequency of grade 3 toxicity at 10 mg/d for 4 consecutive weeks was observed in phase 1 of the trial.7 Therefore, high oral bioavailability and adherence may be a few of the reasons for the increased survival outcomes.
In the present study, subgroup analysis showed that the improvement in PFS and OS for patients with NSCLC was consistent with most analyzed subgroups. For example, anlotinib was found to be effective in PFS and OS for patients in both EGFR-mutated and EGFR wild-type subgroups (eFigure 5 in Supplement 2). By contrast, other multitargeted agents, such as sorafenib (Monotherapy Administration of Sorafenib in Patients With Non-Small Cell Lung Cancer [MISSION] trial), were reported to be more effective in patients with EGFR mutation.17 One potential reason for this difference might be that the improvement in OS among patients with EGFR mutation could be partly biased by the unbalanced use of EGFR TKIs between the sorafenib arm (19 [43.2%] patients) and the placebo arm (8 [17.7%] patients) during the subsequent treatments.18 For squamous lung cancer, previous discoveries have established that the fibroblast growth factor signaling pathway plays a fundamental role in cancer development by supporting tumor angiogenesis and cancer cell proliferation via different mechanisms.19,20,21 However, in previous trials, multiple target receptor TKIs, such as vandetanib, did not show efficacy in this population, although it is against fibroblast growth factor receptor.22 In the current study, considerable OS improvement was not seen in patients with squamous cell carcinoma either (HR, 0.73 [95% CI, 0.45-1.18]; P = .19), but a substantially improved PFS was achieved in this population. Because the squamous cell carcinoma subgroup included only 101 patients, further analysis of the efficacy of anlotinib in this population is planned.
The most common adverse events from the use of other TKIs—such as gefitinib, erlotinib hydrochloride, and afatinib dimaleate, which are used in first-line treatment of EGFR-mutated advanced NSCLC—are gastrointestinal (diarrhea and stomatitis/mucositis) and cutaneous (erythra, dry skin, and paronychia) conditions.23 However, one of the main concerns with any antiangiogenic treatment is bleeding, as has occurred with bevacizumab, a humanized antibody against vascular endothelial growth factor, that has caused adverse events such as hypertension, proteinuria, bleeding, and thrombosis.24 In this trial, a similar level of adverse events was observed, but the occurrence of bleeding events was low.
Limitations
This trial has some limitations. First, at the start of this trial, third-line therapy options for patients with NSCLC were nonexistent, saying nothing of any recommended salvage regimens approved in China; thus, we chose placebo as the control. A dramatic change has taken place in the past 2 years. The development of immune checkpoint inhibitors has placed docetaxel or checkpoint inhibitor as a third-line therapy for NSCLC. The PFS of third-line or further anlotinib in the present study seems to be not inferior to the results achieved by docetaxel or nivolumab in previous reports. Head-to-head comparisons of anlotinib and chemotherapy as third-line treatment are beneficial to identify the sequence of therapy strategies. Second, potential biomarkers were not reported in this study. The analysis of biomarkers, such as C-C motif ligand 2 and active circulating endothelial cells, suitable for anlotinib therapy using tissue or cancerous pleural effusion specimens is still ongoing. These results will be reported in the future.
Conclusions
Anlotinib as third-line and further therapy is well tolerated and offers significantly improved PFS and OS compared with placebo among Chinese patients in our trial. Anlotinib is a potential treatment option for the management of patients with advanced NSCLC. Future studies will look into the therapeutic strategies for anlotinib combined or compared with other therapies in NSCLC and other solid tumors.
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria of Patients
eTable 2. Baseline Characteristics of the Study Population
eTable 3. Interaction Analysis Between Treatment and Subgroup Variables
eTable 4. Subsequent Treatment of Patients in Two Groups (FAS)
eTable 5. Adverse Events
eFigure 1. Waterfall Plot of the Percentage Change From Baseline in the Sum of Longest Tumour Diameters
eFigure 2. Mean Changes of QLQ C-30 Score at the End of (A) 1st, (B) 2nd, (C) 4th and (D) 6th Treatment Cycle From Baseline
eFigure 3. Mean Changes of OLQ LC-13 Total Score at the End of Different Treatment Cycles From Baseline
eFigure 4. Kaplan-Meier Estimate of Overall Survival Until May 2017
eFigure 5. Kaplan-Meier Estimates of Overall Survival and Progression-Free Survival in Patients with EGFR+ and EGFR-
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289. doi: 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 4.Maione P, Rossi A, Sacco PC, Bareschino MA, Schettino C, Gridelli C. Advances in chemotherapy in advanced non-small-cell lung cancer. Expert Opin Pharmacother. 2010;11(18):2997-3007. doi: 10.1517/14656566.2010.511615 [DOI] [PubMed] [Google Scholar]
- 5.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jänne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non–Small Cell Lung Cancer: the SELECT-1 randomized clinical trial. JAMA. 2017;317(18):1844-1853. doi: 10.1001/jama.2017.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. doi: 10.1186/s13045-016-0332-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:77-86. doi: 10.1016/j.gene.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 9.Taurin S, Yang CH, Reyes M, et al. Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer. 2018;28(1):152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654-661. doi: 10.1038/bjc.2017.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 12.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673. doi: 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 13.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095-2103. doi: 10.1200/JCO.2000.18.10.2095 [DOI] [PubMed] [Google Scholar]
- 14.Massarelli E, Andre F, Liu DD, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. 2003;39(1):55-61. doi: 10.1016/S0169-5002(02)00308-2 [DOI] [PubMed] [Google Scholar]
- 15.Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210-216. doi: 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garassino MC, Cho BC, Kim JH, et al. ; ATLANTIC Investigators . Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521-536. doi: 10.1016/S1470-2045(18)30144-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paz-Ares L, Hirsh V, Zhang L, et al. Monotherapy Administration of Sorafenib in Patients With Non-Small Cell Lung Cancer (MISSION) trial: a phase III, multicenter, placebo-controlled trial of sorafenib in patients with relapsed or refractory predominantly nonsquamous non-small-cell lung cancer after 2 or 3 previous treatment regimens. J Thorac Oncol. 2015;10(12):1745-1753. [DOI] [PubMed] [Google Scholar]
- 18.Mok TS, Paz-Ares L, Wu YL, et al. Association between tumor EGFR and KRAS mutation status and clinical outcomes in NSCLC patients randomized to sorafenib plus best supportive care (BSC) or BSC alone: subanalysis of the phase III MISSION trial. Ann Oncol. 2012;23(suppl 9):ixe1. doi: 10.1093/annonc/mds499 [DOI] [Google Scholar]
- 19.Tiseo M, Gelsomino F, Alfieri R, et al. FGFR as potential target in the treatment of squamous non small cell lung cancer. Cancer Treat Rev. 2015;41(6):527-539. doi: 10.1016/j.ctrv.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 20.Helsten T, Schwaederle M, Kurzrock R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications. Cancer Metastasis Rev. 2015;34(3):479-496. doi: 10.1007/s10555-015-9579-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Procopio MG, Laszlo C, Al Labban D, et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol. 2015;17(9):1193-1204. doi: 10.1038/ncb3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(33):5407-5415. doi: 10.1200/JCO.2008.17.3138 [DOI] [PubMed] [Google Scholar]
- 23.Califano R, Tariq N, Compton S, et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs. 2015;75(12):1335-1348. doi: 10.1007/s40265-015-0434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehm S, Rothermundt C, Hess D, Joerger M. Antiangiogenic drugs in oncology: a focus on drug safety and the elderly—a mini-review. Gerontology. 2010;56(3):303-309. doi: 10.1159/000262450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria of Patients
eTable 2. Baseline Characteristics of the Study Population
eTable 3. Interaction Analysis Between Treatment and Subgroup Variables
eTable 4. Subsequent Treatment of Patients in Two Groups (FAS)
eTable 5. Adverse Events
eFigure 1. Waterfall Plot of the Percentage Change From Baseline in the Sum of Longest Tumour Diameters
eFigure 2. Mean Changes of QLQ C-30 Score at the End of (A) 1st, (B) 2nd, (C) 4th and (D) 6th Treatment Cycle From Baseline
eFigure 3. Mean Changes of OLQ LC-13 Total Score at the End of Different Treatment Cycles From Baseline
eFigure 4. Kaplan-Meier Estimate of Overall Survival Until May 2017
eFigure 5. Kaplan-Meier Estimates of Overall Survival and Progression-Free Survival in Patients with EGFR+ and EGFR-