Key Points
Question
Do programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors accelerate tumor growth, a phenomenon defined as hyperprogressive disease?
Findings
In this multicenter cohort study including 406 patients with advanced non–small cell lung cancer (NSCLC) treated with PD-1/PD-L1 inhibitors, hyperprogressive disease was observed in 13.8% (n = 56) of the population. Patients experiencing hyperprogression had significantly worse overall survival (3.4 months) compared with patients with progression not classified as hyperprogressive disease (6.2 months).
Meaning
Hyperprogressive disease is a novel pattern of progression in patients receiving treatment with PD-1/PD-L1 inhibitors for NSCLC, of which patients and clinicians should be aware to properly select the best treatment and carefully monitor disease evolution.
This multicenter cohort study investigates whether hyperprogressive disease is observed in patients with advanced non–small cell lung cancer treated with programmed cell death 1 and programmed cell death ligand 1 inhibitors compared with single-agent chemotherapy.
Abstract
Importance
Hyperprogressive disease (HPD) is a new pattern of progression recently described in patients with cancer treated with programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors. The rate and outcome of HPD in advanced non–small cell lung cancer (NSCLC) are unknown.
Objectives
To investigate whether HPD is observed in patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors compared with single-agent chemotherapy and whether there is an association between treatment and HPD.
Design, Setting, and Participants
In this multicenter retrospective study that included patients treated between August 4, 2011, and April 5, 2017, the setting was pretreated patients with advanced NSCLC who received PD-1/PD-L1 inhibitors (8 institutions) or single-agent chemotherapy (4 institutions) in France. Measurable disease defined by Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) on at least 2 computed tomographic scans before treatment and 1 computed tomographic scan during treatment was required.
Interventions
The tumor growth rate (TGR) before and during treatment and variation per month (ΔTGR) were calculated. Hyperprogressive disease was defined as disease progression at the first evaluation with ΔTGR exceeding 50%.
Main Outcomes and Measures
The primary end point was assessment of the HPD rate in patients treated with IO or chemotherapy.
Results
Among 406 eligible patients treated with PD-1/PD-L1 inhibitors (63.8% male), 46.3% (n = 188) were 65 years or older, 72.4% (n = 294) had nonsquamous histology, and 92.9% (n = 377) received a PD-1 inhibitor as monotherapy in second-line therapy or later. The median follow-up was 12.1 months (95% CI, 10.1-13.8 months), and the median overall survival (OS) was 13.4 months (95% CI, 10.2-17.0 months). Fifty-six patients (13.8%) were classified as having HPD. Pseudoprogression was observed in 4.7% (n = 19) of the population. Hyperprogressive disease was significantly associated with more than 2 metastatic sites before PD-1/PD-L1 inhibitors compared with non-HPD (62.5% [35 of 56] vs 42.6% [149 of 350]; P = .006). Patients experiencing HPD within the first 6 weeks of PD-1/PD-L1 inhibitor treatment had significantly lower OS compared with patients with progressive disease (median OS, 3.4 months [95% CI, 2.8-7.5 months] vs 6.2 months [95% CI, 5.3-7.9 months]; hazard ratio, 2.18 [95% CI, 1.29-3.69]; P = .003). Among 59 eligible patients treated with chemotherapy, 3 (5.1%) were classified as having HPD.
Conclusions and Relevance
Our study suggests that HPD is more common with PD-1/PD-L1 inhibitors compared with chemotherapy in pretreated patients with NSCLC and is also associated with high metastatic burden and poor prognosis in patients treated with PD-1/PD-L1 inhibitors. Additional studies are needed to determine the molecular mechanisms involved in HPD.
Introduction
In the era of immuno-oncology, programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors have demonstrated a clear survival benefit as a single agent or in combination compared with standard chemotherapy in both treatment-naive patients1,2,3,4 and patients previously treated5,6,7,8 for advanced non–small cell lung cancer (NSCLC). However, progression rates reported with single-agent PD-1/PD-L1 inhibitors are in some cases equal to or higher than with conventional treatment, ranging from 33% to 44% in pretreated patients with NSCLC.5,6,7 Recently, an acceleration of tumor growth during immunotherapy, defined as hyperprogressive disease (HPD), was reported in 9% of advanced cancers9 and in 29% of patients with head and neck cancer10 treated with PD-1/PD-L1 inhibitors.
The tumor growth rate (TGR) is a tool for estimating the increase in tumor volume over time based on 2 computed tomography (CT) scan measurements.11 The TGR takes into account the sum of the target lesions defined by Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) and the interval between 2 CT scans. It can be used to quantitatively assess tumor dynamics and kinetics during treatment; specifically, it can be applied to identify the subset of patients experiencing HPD.
To explore if HPD is an unforeseen pattern of progression during IO therapy in NSCLC, we compared the TGR before and during IO therapy in a cohort of pretreated patients with advanced NSCLC. To investigate if HPD is a specific PD-1/PD-L1 inhibitor pattern, we assessed the TGR and HPD prevalence among a control cohort receiving single-agent chemotherapy.
Methods
Patients and Treatment
In this multicenter study, data were retrospectively collected from all consecutive eligible patients with advanced NSCLC treated with IO (nivolumab, pembrolizumab, atezolizumab, or durvalumab) from November 10, 2012, to April 5, 2017, in 8 French institutions. For the control cohort, equivalent data were collected in patients with advanced NSCLC failing a platinum-based regimen and treated with single-agent chemotherapy (taxanes, pemetrexed, vinorelbine tartrate, or gemcitabine chlorohydrate) from August 4, 2011, to June 13, 2016, in 4 French institutions.
To be eligible, patients had to be 18 years or older, with histologically or cytologically confirmed stage III or IV NSCLC and available CT scans for radiological evaluation. In the single-agent chemotherapy control cohort, patients who received previous treatment with IO were excluded. The PD-L1 expression was analyzed by immunohistochemistry on tumor cells in archived biopsy specimens, when available, and the cutoff for positivity was 1%. This study was approved by the institutional review board of Gustave Roussy, and informed consent from participants was not required because of the retrospective nature.
Radiological Evaluation
At least 2 CT scans before PD-1/PD-L1 inhibitor therapy or chemotherapy (baseline and the most recent scan before baseline) and 1 CT scan during treatment were mandatory for radiological evaluation. The baseline CT had to be performed within 6 weeks before initiating treatment, and a minimum of 2 weeks between CT scans was required. All CT scans were centrally reviewed by 2 senior radiologists (L.T. and C.C.). The target lesions were defined according to RECIST version 1.1. An extensive assessment of noneligibility for radiological evaluation was performed in 1 center (Gustave Roussy) to refine inclusion of patients in subsequent centers. Therefore, patients from other centers were included only if eligible for radiological evaluation (ie, availability of the required CT scans, adequate intervals between them, and the presence of the target lesions). In cases of progression, if the patient was clinically stable, PD-1/PD-L1 inhibitors could be continued, with a subsequent evaluation at least 4 weeks later, according to immunotherapy response criteria recommendations.12 Pseudoprogression was defined as initial progression, followed by complete response or partial response or stable disease lasting more than 6 months.13
Tumor Growth Rate
The TGR was calculated according to the definition by Ferté et al14 and was computed from the sum of the largest diameters of the target lesions as per RECIST version 1.1 (eMethods in the Supplement). The TGR results were reported as a percentage increase in tumor volume per month. New lesions and nonmeasurable disease were excluded from the RECIST version 1.1 sum, and the TGR was only quantified for the target lesions.14
The TGR was measured before and after PD-1/PD-L1 inhibitors (or chemotherapy in the control cohort). The difference (ΔTGR is the TGR on treatment minus the TGR before treatment) was used to assess the association of treatment with tumor growth. Delta TGR exceeding 0% means that treatment may accelerate tumor growth.
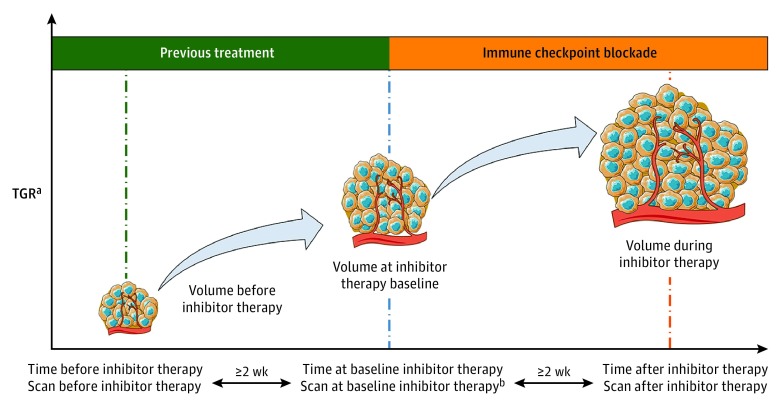
Hyperprogressive disease was defined as RECIST version 1.1 progressive disease on the first CT scan during treatment and ΔTGR exceeding 50%, corresponding to an absolute increase in the TGR exceeding 50% per month. A graphical representation of the hypothetical tumor volume variation and the HPD definition for the immunotherapy cohort is shown in Figure 1.
Figure 1. Hypothetical Tumor Volume Variation and Definition of Hyperprogressive Disease (HPD) in the Immunotherapy Cohort.
Variation of tumor growth rate (TGR) volume per month was calculated both before the start of programmed cell death (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitor therapy and during PD-1/PD-L1 inhibitor therapy. Hyperprogressive disease was defined as Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 progressive disease at the first computed tomography (CT) scan during PD-1/PD-L1 inhibitor therapy and an absolute increase in the TGR exceeding 50% per month. aNew lesions and nonmeasurable disease not included in the TGR. bThe baseline CT scan performed within 6 weeks before PD-1/PD-L1 inhibitor therapy initiation.
Statistical Analysis
Associations between HPD and categorical or continuous variables were evaluated using the Fisher exact test and the t test, respectively. Because the diagnosis of HPD depends on the timing of the radiological assessment and could induce a lead-time bias,15 a landmark analysis was performed to assess the association of HPD with overall survival (OS) using a time point at 6 weeks after PD-1/PD-L1 inhibitor or chemotherapy initiation. Patients alive at this time point and with progression on their first CT scan during PD-1/PD-L1 inhibitor therapy or chemotherapy were considered hyperprogressors or not hyperprogressors according to the diagnosis of HPD within the first 6 weeks of treatment. Overall survival curves were estimated with the Kaplan-Meier method and compared by the log-rank test. The hazard ratio (HR) was estimated using the univariate Cox proportional hazards regression model. All P values were 2 sided, and values less than .05 were considered statistically significant. Statistical analyses were performed using a software program (SAS for Windows, version 9.4; SAS Institute Inc).
Results
Immunotherapy Cohort
Overall, 406 patients (63.8% male) were included in the TGR analysis. The reasons for exclusion were evaluated in a single-center cohort (at Gustave Roussy, Villejuif, France) (n = 249) and included the following: unavailability of CT scans before baseline, at baseline, or during PD-1/PD-L1 inhibitor therapy; inadequate intervals between CT scans; or the absence of measurable disease. Of 249 patients, 76 (30.5%) were not evaluable for the TGR analysis, among whom 13.3% (33 of 249) experienced clinical progression and/or death before the first tumor evaluation during PD-1/PD-L1 inhibitor therapy (eFigure 1 in the Supplement).
The main characteristics of the 406 patients in the immunotherapy multicenter cohort are listed in the Table. The median follow-up was 12.1 months (95% CI, 10.1-13.8 months), the objective response rate was 18.9% (77 of 406), and 41.9% (170 of 406) of patients had progressive disease as the best response to immunotherapy (eTable 1 in the Supplement). The median progression-free survival (PFS) and OS were 2.1 months (95% CI, 1.8-3.1 months) and 13.4 months (95% CI, 10.2-17.0 months), respectively.
Table. Patient Characteristics and Association Between HPD Status and Clinical Categorical Variables for Immunotherapy-Treated Patients With NSCLC.
| Variable | No./Total No. (%) | Fisher Exact Test P Value | ||
|---|---|---|---|---|
| Total (N = 406) | Non-HPD (n = 350) | HPD (n = 56) | ||
| Age, y | ||||
| ≥65 | 188 (46.3) | 166 (47.4) | 22 (39.3) | .31 |
| <65 | 218 (53.7) | 184 (52.6) | 34 (60.7) | |
| Smoking history | ||||
| Current/former | 371 (91.4) | 319 (91.1) | 52 (92.9) | >.99 |
| None | 35 (8.6) | 31 (8.9) | 4 (7.1) | |
| Smoking exposure, pack-years | ||||
| ≤30 | 136 (33.5) | 115/312 (36.9) | 21/50 (42.0) | .53 |
| >30 | 226 (55.7) | 197/312 (63.1) | 29/50 (58.0) | |
| Missing | 44 (10.8) | 38 | 6 | |
| Histology | ||||
| Nonsquamous | 294 (72.4) | 252 (72.0) | 42 (75.0) | .75 |
| Squamous | 112 (27.6) | 98 (28.0) | 14 (25.0) | |
| Stagea | ||||
| III | 70 (17.2) | 61 (17.4) | 9 (16.1) | >.99 |
| IV | 336 (82.8) | 289 (82.6) | 47 (83.9) | |
| PD-L1 statusb | ||||
| Negative | 39 (9.6) | 32/105 (30.5) | 7/12 (58.3) | .10 |
| Positive | 78 (19.2) | 73/105 (69.5) | 5/12 (41.7) | |
| Missing | 289 (71.2) | 245 | 44 | |
| Molecular status | ||||
| ALK rearrangement | 4 (1.0) | 3/233 (1.3) | 1/36 (2.8) | .34 |
| EGFR mutation | 16 (3.9) | 16/233 (6.9) | 0 | |
| KRAS mutation | 87 (21.4) | 74/233 (31.8) | 13/36 (36.1) | |
| Wild typec | 104 (25.6) | 88/233 (37.8) | 16/36 (44.4) | |
| Other alterations | 58 (14.3) | 52/233 (22.3) | 6/36 (16.7) | |
| Missing | 137 (33.7) | 117 | 20 | |
| No. of molecular alterations | ||||
| 0-1 | 218 (53.7) | 185/227 (81.5) | 33/36 (91.7) | .16 |
| ≥2 | 45 (11.1) | 42/227 (18.5) | 3/36 (8.3) | |
| Missing | 143 (35.2) | 123 | 20 | |
| Type of treatment before PD-1/PD-L1 inhibitor therapy | ||||
| Platinum-based chemotherapy | 229 (56.4) | 192 (54.9) | 37 (66.1) | .61 |
| Chemoradiotherapy | 17 (4.2) | 17 (4.9) | 0 | |
| Pemetrexed | 17 (4.2) | 15 (4.3) | 2 (3.6) | |
| Taxanes | 44 (10.8) | 39 (11.1) | 5 (8.9) | |
| Other chemotherapy | 43 (10.6) | 37 (10.6) | 6 (10.7) | |
| Targeted therapyd | 12 (3.0) | 11 (3.1) | 1 (1.8) | |
| Tyrosine kinase inhibitorse | 37 (9.1) | 33 (9.4) | 4 (7.1) | |
| Immunotherapy | 3 (0.7) | 2 (0.6) | 1 (1.8) | |
| No prior therapy | 4 (1.0) | 4 (1.1) | 0 | |
| Response to line before PD-1/PD-L1 inhibitor therapy | ||||
| Complete response/partial response | 90 (22.2) | 75/344 (21.8) | 15/55 (27.3) | .08 |
| Stable disease | 185 (45.6) | 167/344 (48.5) | 18/55 (32.7) | |
| Progressive disease | 124 (30.5) | 102/344 (29.7) | 22/55 (40.0) | |
| Missing | 7 (1.7) | 6 | 1 | |
| PD-1/PD-L1 inhibitor therapy line, range 1-9 | ||||
| ≤2f | 218 (53.7) | 186 (53.1) | 32 (57.1) | .67 |
| >2 | 188 (46.3) | 164 (46.9) | 24 (42.9) | |
| No. of metastatic sites before PD-1/PD-L1 inhibitor therapy | ||||
| ≤2 | 222 (54.7) | 201 (57.4) | 21 (37.5) | .006 |
| >2 | 184 (45.3) | 149 (42.6) | 35 (62.5) | |
| Type of inhibitor | ||||
| PD-1 | 377 (92.9) | 325 (92.9) | 52 (92.9) | >.99 |
| PD-L1 | 29 (7.1) | 25 (7.1) | 4 (7.1) | |
| Monotherapy or combination | ||||
| Monotherapy | 380 (93.6) | 326 (92.9) | 54 (96.4) | .56 |
| Combinationg | 26 (6.4) | 24 (6.9) | 2 (3.6) | |
| ECOG performance status | ||||
| 0-1 | 360 (88.7) | 311 (88.9) | 49 (87.5) | .82 |
| ≥2 | 46 (11.3) | 39 (11.1) | 7 (12.5) | |
| Subsequent therapy | ||||
| No | 111 (27.3) | 86/215 (40.0) | 25/54 (46.3) | .44 |
| Yes | 158 (38.9) | 129/215 (60.0) | 29/54 (53.7) | |
| PD-1/PD-L1 inhibitor therapy ongoing or missing | 137 (33.7) | 135 | 2 | |
| Neutrophil count, /μL | ||||
| ≤7500 | 209 (51.5) | 188/254 (74.0) | 21/31 (67.7) | .52 |
| >7500 | 76 (18.7) | 66/254 (26.0) | 10/31 (32.3) | |
| Missing | 121 (29.8) | 96 | 25 | |
| Derived neutrophil-to-lymphocyte ratio | ||||
| ≤3 | 195 (48.0) | 174/254 (68.5) | 21/31 (67.7) | >.99 |
| >3 | 90 (22.2) | 80/254 (31.5) | 10/31 (32.3) | |
| Missing | 121 (29.8) | 96 | 25 | |
| Lactate dehydrogenase level | ||||
| ≤Upper limit of normalh | 150 (36.9) | 133/192 (69.3) | 17/27 (63.0) | .51 |
| >Upper limit of normal | 69 (17.0) | 59/192 (30.7) | 10/27 (37.0) | |
| Missing | 187 (46.1) | 158 | 29 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HPD, hyperprogressive disease; NSCLC, non–small cell lung cancer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
SI conversion factor: To convert neutrophil count to 109/L, multiply by 0.001.
TNM stage (seventh edition) at advanced disease detection.
Immunohistochemistry cutoff for positivity on tumor cells of 1% or higher.
Wild type for ALK rearrangement, EGFR mutation, and KRAS mutation.
In oncogene-addicted NSCLC.
In non–oncogene-addicted NSCLC.
Four patients treated in first line for metastatic disease.
Combination of PD-1/PD-L1 inhibitor therapy and anti-EGFR monoclonal antibodies, PD-1/PD-L1 inhibitor therapy and chemotherapy, or PD-1/PD-L1 inhibitor therapy and immunotherapy (patients enrolled in clinical trials).
Upper limit of normal defined according to the cutoff of each center.
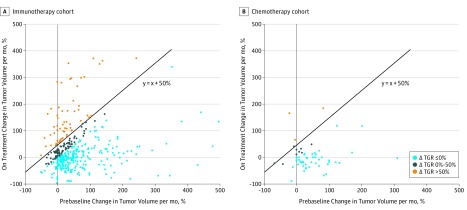
Before immunotherapy, 75 of 406 patients (18.5%) had a TGR of 0 or less (eTable 2 in the Supplement), but all were classified as having progressive disease because of the appearance of new lesions or progression in the nontarget lesions. During immunotherapy, the TGR was stable or decreased (ΔTGR ≤0) in 266 patients (65.5%) and increased (ΔTGR >0) in 140 patients (34.5%). Among them, 62 patients (15.3% of the overall population) were initially classified as having HPD (Figure 2A and Figure 3).
Figure 2. Scatterplots With Response According to Delta Tumor Growth Rate (TGR) in the Immunotherapy and Chemotherapy Cohorts.
A, Light blue spots show 266 patients with regressing or stable tumors, dark blue spots show 78 patients with progressing tumors, and orange spots show 62 patients with accelerated tumor growth during programmed cell death (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitor therapy. B, Light blue spots show 47 patients with regressing or stable tumors, dark blue spots show 9 patients with progressing tumors, and orange spots show 3 patients with accelerated tumor growth during chemotherapy. Diagonal lines separate patients with delta TGR exceeding 50% from patients with delta TGR of 50% or less.
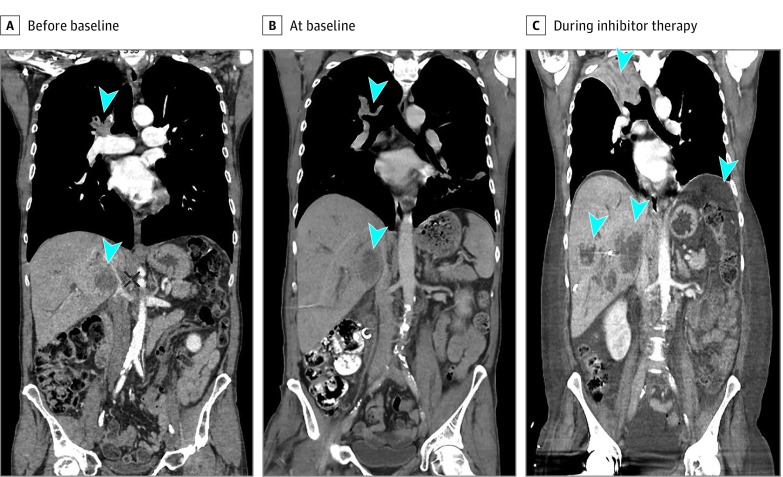
Figure 3. Case Study of a Patient With Non–Small Cell Lung Cancer With Hyperprogressive Disease During Treatment With a PD-1 Inhibitor.
Shown are computed tomographic scans before baseline (A), at baseline about 3 weeks later (B), and during programmed cell death (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitor therapy 1 month later (C) in a man in his mid-50s with stage IV (lung, liver, and bone metastases) HER2-amplified lung adenocarcinoma treated with anti–PD-1 therapy in the third line. After 2 administrations, there was evidence of extensive lung, liver, and peritoneal progression. Arrowheads show lung and liver metastases before and during anti–PD-1 treatment.
Overall, 19 patients (4.7%) had progressive disease, followed by complete response and/or partial response or stable disease longer than 6 months, and were thus classified as pseudoprogressors (eTable 1 in the Supplement). Six pseudoprogressors were initially classified as having HPD on the first CT scan. Excluding these 6 patients from the 62 patients with HPD, the definitive rate of HPD was 13.8% (56 patients). Hyperprogressive disease was significantly associated with more than 2 metastatic sites before PD-1/PD-L1 inhibitors compared with non-HPD (62.5% [35 of 56] vs 42.6% [149 of 350]; P = .006) (Table). No significant differences were observed according to the baseline tumor burden, the number of previous lines of therapy (eFigure 2 in the Supplement), or age (Table).
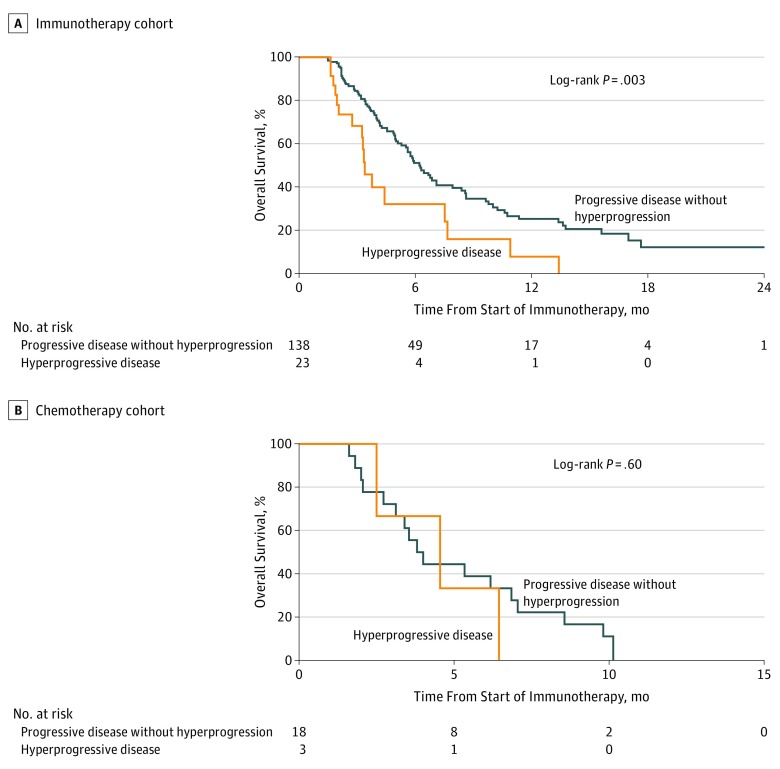
In the landmark survival analysis, patients experiencing HPD within the first 6 weeks of beginning PD-1/PD-L1 inhibitor therapy (n = 23) had significantly lower OS compared with other patients with progressive disease (ie, non-HPD patients with progressive disease at the first evaluation [n = 138]) (median OS, 3.4 months [95% CI, 2.8-7.5 months] vs 6.2 months [95% CI, 5.3-7.9 months]; HR, 2.18 [95% CI, 1.29-3.69]; P = .003) (Figure 4A). As a sensitivity analysis, 2 other landmark time points were tested. With a time point at 4 weeks, the difference in OS remained significant. However, when choosing a time point of 8 weeks, the difference in OS did not reach statistical significance.
Figure 4. Overall Survival for Hyperprogressive Disease (HPD) Compared With Progressive Disease Without Hyperprogression in the Immunotherapy and Chemotherapy Cohorts (6-Weeks Landmark Analysis).
A, Fourteen patients and 2 patients with pseudoprogression were excluded from the progressive disease without hyperprogression and HPD cohorts, respectively. The median overall survival is shown for 138 patients with progressive disease without hyperprogression at the first evaluation vs for 23 patients with diagnosed HPD within the first 6 weeks of treatment. B, The median overall survival is shown for 18 patients with progressive disease without hyperprogression at the first evaluation vs for 3 patients with diagnosed HPD within the first 6 weeks of treatment.
Chemotherapy Cohort
Overall, 59 patients were included in the TGR analysis. The reasons for exclusion were evaluated in a single-center cohort (at Gustave Roussy) (n = 77) (eFigure 3 in the Supplement). The main characteristics of the 59 patients are listed in eTable 3 in the Supplement. The median follow-up was 26.3 months (95% CI, 22.6-35.5 months), the objective response rate was 10.2% (6 of 59), and 30.5% (18 of 59) of patients had progressive disease as the best response (eTable 1 in the Supplement). The median PFS and OS were 3.9 months (95% CI, 3.1-4.8 months) and 8.6 months (95% CI, 6.2-13.4 months), respectively. No pseudoprogression was observed.
The TGR analysis is summarized in eTable 2 in the Supplement. Delta TGR was greater than 0 in 12 patients; among them, 3 patients were classified as having HPD (Figure 2B). A landmark analysis at 6 weeks showed a median OS of 4.5 months (95% CI, 2.5-6.5 months) in patients diagnosed as having HPD (n = 3) and 3.9 months (95% CI, 2.7-6.9 months) in other patients with progressive disease (ie, non-HPD patients with progressive disease at the first evaluation [n = 18]) (P = .60) (Figure 4B).
Discussion
In this study of pretreated patients with advanced NSCLC, HPD was observed in 13.8% (56 of 406) of patients treated with PD-1/PD-L1 inhibitors compared with 5.1% (3 of 59) of patients treated with single-agent chemotherapy. Our rate of HPD is concordant with the few relevant previously reported studies. Champiat et al9 identified HPD in 9% of 131 patients with advanced cancer treated with PD-1/PD-L1 inhibitors in phase 1 trials; only 13 patients had lung cancer, and none were classified as having HPD. Hyperprogressive disease was identified more frequently (29%) by Saâda-Bouzid et al10 among 34 patients with recurrent or metastatic head and neck cancer. This higher rate could have occurred because of the tumor type and/or their different definition of HPD.
In our study, HPD was associated with poor survival in patients with NSCLC treated with PD-1/PD-L1 inhibitors. Hyperprogressive disease could potentially explain the initial excess of death in some phase 3 trials. For example, in the CheckMate 057 study,5 the progression rate was 44% with nivolumab and 29% with docetaxel, with an excess of 14 deaths during the first 3 months in the nivolumab arm.16 As a result, OS curves crossed at 6 months, with an initial survival benefit in favor of docetaxel. In addition, a recent retrospective study17 reported that approximately 15% of early deaths were due to disease progression during the first 3 months of nivolumab treatment in patients with advanced NSCLC. The European Medicines Agency recently included an alert in the summary of product information for nivolumab regarding treatment of patients with NSCLC with poor prognostic features or aggressive disease.18 In our study, the absence of a significant survival difference using a landmark analysis at 8 weeks is likely because of the small number of patients with HPD alive at that time point and eligible for the landmark analysis. This finding further suggests that HPD is a rapid phenomenon, which leads to early death mostly in the first 2 months of treatment.
There is no consensus on the optimal definition of HPD. Champiat et al9 defined HPD as progressive disease at the first evaluation in addition to an increase of at least 2-fold in the TGR during PD-1/PD-L1 inhibitor therapy compared with the TGR before PD-1/PD-L1 inhibitors. Saâda-Bouzid et al10 described HPD as an increase of at least 2-fold in tumor growth kinetics after PD-1/PD-L1 inhibitor initiation, which measured the variation of the sum of the largest diameters of the target lesions per unit of time during immunotherapy compared with tumor growth kinetic before PD-1/PD-L1 inhibitors. We used a stringent definition of HPD that requires a high-volume increase per month to classify a patient as a hyperprogressor. For example, a tumor with a 20% volume increase per month before immunotherapy had to have a 70% increase per month during immunotherapy to be labeled as HPD. Despite the differences in methods, the present analysis and the 2 previous studies9,10 highlight the importance of quantifying tumor growth speed to discriminate between progression due to natural history of the disease (the tumor growth speed is already high before the start of the new treatment) and progression due to the potential intrinsic association of the experimental treatment (the tumor growth speed is lower before the start of the new treatment). Unfortunately, the TGR assessment cannot be validated in published randomized studies because the radiological evaluations before the baseline CT scan data were not captured.
In our immunotherapy cohort, HPD was significantly associated with a high number of metastatic sites before PD-1/PD-L1 inhibitors, whereas no association with the baseline tumor burden was found. However, the target lesions defined by RECIST version 1.1 do not always perfectly reflect the whole tumor burden, especially in patients with nonmeasurable disease (lung lymphangitis, bone metastases, and pleural or peritoneal effusions). Furthermore, high lactate dehydrogenase levels and a derived neutrophil to lymphocyte ratio exceeding 3 were recently shown to negatively influence the survival outcome of patients with NSCLC treated with PD-1/PD-L1 inhibitors.19 In our analysis, no significant association was found between these biomarkers and HPD status; however, lactate dehydrogenase levels and neutrophil counts were not available for 46.1% (187 of 406) and 29.8% (121 of 406) of patients, respectively. Contrary to what was observed by Champiat et al,9 no significant association between HPD and age was found in our study, probably because of the different methods used to assess HPD. Recently, Kato et al20 identified EGFR mutations and MDM2 amplification as possible molecular predictors of HPD. In our cohort, none of 16 patients with EGFR-mutated lung adenocarcinoma experienced HPD. Despite the association between EGFR mutations and decreased benefit from immunotherapy in patients with NSCLC,21 the potential role of EGFR mutations in driving HPD remains unknown. The phenomenon of disease progression acceleration was previously described in oncogene-addicted NSCLC after interruption of targeted agents, such as RAF,22 ALK,23 and EGFR24 inhibitors. In the present analysis, no significant association between HPD and the type of previous therapy was found, minimizing the risk of such an association.
In 6 of the 62 patients with HPD (9.7%), initial HPD was further reclassified as pseudoprogression, a feature described in 4.7% (19 of 406) of our total population, in line with a recently published study in the same setting.25 Variable rates of pseudoprogression have been reported in patients with NSCLC (2%-19%),26,27 melanoma (4%-7%),28,29 and renal cell carcinoma (1%-15%)30,31,32 on PD-1/PD-L1 blockade. However, a comparison of these numbers should be interpreted with caution in the absence of a common definition of pseudoprogression across the studies.33 We identified HPD in only 3 of 59 patients (5.1%) treated with single-agent chemotherapy, and no pseudoprogression was described, suggesting that these patterns are new and specific to PD-1/PD-L1 inhibitors.
To our knowledge, the present study is the largest analysis exploring HPD to date and is the first conducted in a dedicated NSCLC population. In addition, we believe that this is the only study to include a control cohort of chemotherapy-treated patients with NSCLC and is thus able to assess the negative association with survivial of HPD compared with conventional disease progression during PD-1/PD-L1 inhibitor therapy. Although in some immunotherapy trials5,6,8 the first CT scan was performed at week 9, the fact that HPD drives toward early death (mainly in the first 6 weeks of treatment) prompts discussion over an anticipated first radiological evaluation during PD-1/PD-L1 inhibitor therapy to properly identify hyperprogressors. Ultimately, because of the poor OS associated with HPD, an early switch to salvage chemotherapy in these patients should be considered.
Limitations
Our study has some limitations, mainly related to its retrospective nature. First, a potential underestimation of HPD may have occurred because 30.5% (76 of 249) of the patients treated in 1 center (Gustave Roussy) were excluded from the TGR analysis, mostly because of rapid progression and/or death that prevented any further evaluation or because of the absence of the target lesions. In addition, in our study, PD-L1 expression was not available for 71.2% (289 of 406) of patients because this information was not mandatory for PD-1/PD-L1 inhibitor prescription in pretreated patients with NSCLC and because the percentage of positive expression was often not provided and tested with heterogeneous methods; therefore, we were unable to precisely characterize the interplay between PD-L1 status and HPD. Similarly, tumor mutational burden (TMB) was not available because it was not routinely assessed outside of clinical trials. In our study, only 26 patients (2 classified as having HPD) were treated with immunotherapy in combination with other drugs. Recently, a significant survival benefit for first-line PD-1/PD-L1 inhibitors–chemotherapy (KEYNOTE-021 study,34 IMpower150 study,35 KEYNOTE-189 study3, KEYNOTE 407 study,36 and IMpower131 study37) and PD-1/PD-L1 inhibitor–PD-1/PD-L1 inhibitor (CheckMate 227 study4) combinations compared with platinum doublets has been reported. In high-TMB patients with NSCLC, the PFS curves of nivolumab plus ipilimumab and platinum-based chemotherapy treatments cross between 3 and 6 months; in patients receiving platinum-based chemotherapy in combination with pembrolizumab or placebo, an early separation of both PFS and OS curves has been observed.3,36 These findings suggest a considerable rate of fast progressions or early deaths, potentially due to HPD, in patients treated with double immune checkpoint blockade; in contrast, the addition of chemotherapy to PD-1/PD-L1 inhibitors may hamper PD-1/PD-L1 inhibitor resistance and HPD. Overall, whether HPD is an issue in PD-L1 or TMB selected patients with NSCLC or develops on PD-1/PD-L1 inhibitor combinatorial strategies remains an open question that should be addressed in future studies.
Finally, despite the large sample population herein, it was impossible to define a particular clinical or pathological phenotype for HPD because of the limited number of hyperprogressors. Likewise, the characterization of the molecular basis of HPD remains an unmet need. Some immune checkpoint molecules, such as PD-1 expression38 and Tim-3 expression,39 might temper T-regulatory (Treg) cell proliferation and immune suppressive functions, a phenomenon defined as “contra-suppression.”40 Furthermore, a high level of interferon γ (IFN-γ), usually released by PD-1 blockade,41 may have detrimental effects on immunity as observed in murine mycobacterial infections42 or in cancer models where increased IFN-γ was associated with activation of tumor immunosuppressive myeloid cells43 and upregulation of inhibitory metabolites44 (eg, indoleamine 2,3-dioxygenase) involved in Treg differentiation.45 Alternatively, PD-1/PD-L1 blockade may upregulate the interleukin 6, interleukin 17, and neutrophil axis, generating a potent aberrant inflammation responsible for immune escape and accelerated growth, as shown in tuberculosis46 and lung cancer47 in vivo models. Future studies with prospective assessment of tumor and blood samples from patients with HPD both before treatment and on treatment help clarify the mechanisms behind this phenomenon and its causal relation to treatment.
Conclusions
We identified HPD in 13.8% (56 of 406) of patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors and in only 5.1% (3 of 59) of patients with advanced NSCLC treated with single-agent chemotherapy. In this study, HPD was associated with a high number of metastatic sites at baseline and poor survival (3.4 months), suggesting a detrimental association of immunotherapy in a subset of patients with NSCLC. Additional studies are needed to characterize the molecular basis of HPD.
eMethods. Supplemental Methods
eFigure 1. Flowchart for delta TGR Evaluation in the Immunotherapy Cohort (From a Single Institution)
eFigure 2. Box Plots Showing Baseline Tumor Burden (A) and Number of Previous Lines (B) Distributions According to HPD Status (Immunotherapy Cohort)
eFigure 3. Flowchart for delta TGR Evaluation in the Chemotherapy Cohort (From a Single Institution)
eTable 1. Typical and Atypical Response Patterns to PD-1/PD-L1 Inhibitors and Single-Agent Chemotherapy
eTable 2. TGR Analysis in the Immunotherapy and Single-Agent Chemotherapy Cohorts
eTable 3. Patient Characteristics in the Chemotherapy Cohort
References
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Rodríguez-Abreu D, Robinson A, et al. OA 17.06 updated analysis of KEYNOTE-024: pembrolizumab vs platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS ≥50%. J Thorac Oncol. 2017;12(11):S1793-S1794. doi: 10.1016/j.jtho.2017.09.431 [DOI] [Google Scholar]
- 3.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 4.Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi: 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 9.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti–PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920-1928. doi: 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 10.Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti–PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605-1611. doi: 10.1093/annonc/mdx178 [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Roca C, Koscielny S, Ribrag V, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47(17):2512-2516. doi: 10.1016/j.ejca.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 12.Seymour L, Bogaerts J, Perrone A, et al. ; RECIST Working Group . iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caramella C, Tazdait M, Mezquita L, et al. 1164P: patterns of progression under antiPD1/PDL1 in advanced NSCLC patients allow discriminating pseudo-progression from real progression. Ann Oncol. 2017;28(suppl_5). doi: 10.1093/annonc/mdx376.029 [DOI] [Google Scholar]
- 14.Ferté C, Fernandez M, Hollebecque A, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20(1):246-252. doi: 10.1158/1078-0432.CCR-13-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963-2969. doi: 10.1200/JCO.2013.49.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters S, Cappuzzo F, Horn L, et al. OA03.05: analysis of early survival in patients with advanced non-squamous NSCLC treated with nivolumab vs docetaxel in CheckMate 057. J Thorac Oncol. 2017;12(1):S253. doi: 10.1016/j.jtho.2016.11.241 [DOI] [Google Scholar]
- 17.Inoue T, Tamiya M, Tamiya A, et al. Analysis of early death in Japanese patients with advanced non–small-cell lung cancer treated with nivolumab. Clin Lung Cancer. 2018;19(2):e171-e176. doi: 10.1016/j.cllc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Opdivo, INN-nivolumab. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003985/WC500189765.pdf. Accessed September 29, 2017.
- 19.Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncol. 2018;4(3):351-357. doi: 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242-4250. doi: 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210-216. doi: 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellema WW, Burgers SA, Smit EF. Tumor flare after start of RAF inhibition in KRAS mutated NSCLC: a case report. Lung Cancer. 2015;87(2):201-203. doi: 10.1016/j.lungcan.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 23.Kuriyama Y, Kim YH, Nagai H, Ozasa H, Sakamori Y, Mishima M. Disease flare after discontinuation of crizotinib in anaplastic lymphoma kinase-positive lung cancer. Case Rep Oncol. 2013;6(2):430-433. doi: 10.1159/000354756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011;17(19):6298-6303. doi: 10.1158/1078-0432.CCR-11-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38-47. doi: 10.1016/j.ejca.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 26.Gandara DR, Von Pawel J, Sullivan RN, et al. Impact of atezolizumab (atezo) treatment beyond disease progression (TBP) in advanced NSCLC: results from the randomized phase III OAK study [abstract]. J Clin Oncol. 2017;35(15_suppl):9001. doi: 10.1200/JCO.2017.35.15_suppl.9001 [DOI] [Google Scholar]
- 27.Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non–small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1–defined disease progression in clinical trials. Semin Oncol. 2017;44(1):3-7. doi: 10.1053/j.seminoncol.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 28.Long GV, Weber JS, Larkin J, et al. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017;3(11):1511-1519. doi: 10.1001/jamaoncol.2017.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510-1517. doi: 10.1200/JCO.2015.64.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George S, Motzer RJ, Hammers HJ, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. 2016;2(9):1179-1186. doi: 10.1001/jamaoncol.2016.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstock C, Maher VE, Zhang L, et al. FDA analysis of treatment beyond disease progression disease (PD) in patients with metastatic renal cell carcinoma (mRCC) treated with nivolumab vs. everolimus [abstract]. J Clin Oncol. 2016;34(15_suppl):4508. doi: 10.1200/JCO.2016.34.15_suppl.4508 [DOI] [Google Scholar]
- 32.Escudier B, Motzer RJ, Sharma P, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72(3):368-376. doi: 10.1016/j.eururo.2017.03.037 [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal GM, Theoret MR, Pazdur R. Treatment beyond progression with immune checkpoint inhibitors: known unknowns. JAMA Oncol. 2017;3(11):1473-1474. doi: 10.1001/jamaoncol.2017.1819 [DOI] [PubMed] [Google Scholar]
- 34.Langer CJ, Gadgeel SM, Borghaei H, et al. ; KEYNOTE-021 Investigators . Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non–small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 36.Paz-Ares L, Luft A, Tafreshi A, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). J Clin Oncol. 2018;36(15_suppl):105. doi: 10.1200/JCO.2018.36.15 [DOI] [Google Scholar]
- 37.Jotte R, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36(18_suppl):LBA9000. doi: 10.1200/JCO.2018.36.18_suppl.LBA9000 [DOI] [Google Scholar]
- 38.Franceschini D, Paroli M, Francavilla V, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119(3):551-564. doi: 10.1172/JCI36604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moorman JP, Wang JM, Zhang Y, et al. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol. 2012;189(2):755-766. doi: 10.4049/jimmunol.1200162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnaba V, Schinzari V. Induction, control, and plasticity of Treg cells: the immune regulatory network revised? Eur J Immunol. 2013;43(2):318-322. doi: 10.1002/eji.201243265 [DOI] [PubMed] [Google Scholar]
- 41.Peng W, Liu C, Xu C, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72(20):5209-5218. doi: 10.1158/0008-5472.CAN-12-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai S, Kauffman KD, Sallin MA, et al. CD4 T cell–derived IFN-γ plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog. 2016;12(5):e1005667. doi: 10.1371/journal.ppat.1005667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123-1131. doi: 10.1158/0008-5472.CAN-05-1299 [DOI] [PubMed] [Google Scholar]
- 44.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into TH17-like T cells. J Immunol. 2009;183(4):2475-2483. doi: 10.4049/jimmunol.0900986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lázár-Molnár E, Chen B, Sweeney KA, et al. Programmed death-1 (PD-1)–deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107(30):13402-13407. doi: 10.1073/pnas.1007394107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbay EA, Koyama S, Liu Y, et al. Interleukin-17A promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J Thorac Oncol. 2017;12(8):1268-1279. doi: 10.1016/j.jtho.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eFigure 1. Flowchart for delta TGR Evaluation in the Immunotherapy Cohort (From a Single Institution)
eFigure 2. Box Plots Showing Baseline Tumor Burden (A) and Number of Previous Lines (B) Distributions According to HPD Status (Immunotherapy Cohort)
eFigure 3. Flowchart for delta TGR Evaluation in the Chemotherapy Cohort (From a Single Institution)
eTable 1. Typical and Atypical Response Patterns to PD-1/PD-L1 Inhibitors and Single-Agent Chemotherapy
eTable 2. TGR Analysis in the Immunotherapy and Single-Agent Chemotherapy Cohorts
eTable 3. Patient Characteristics in the Chemotherapy Cohort