This cohort study of female participants with stage 0 to IIA breast cancer treated with breast-conserving surgery and whole-breast irradiation assesses whether radiation-induced toxicities of normal breast tissue 3 years after radiotherapy are associated with the transforming growth factor β gene variant C−509T.
Key Points
Question
Does an association exist between C−509T polymorphism of the TGFB1 gene and radiotherapy-induced toxicity of normal breast tissue among women with pathologically confirmed stage 0 to IIA breast cancer treated with breast-conserving surgery and whole-breast irradiation?
Findings
This prospective cohort study evaluating 174 participants in a randomized clinial trial found grade 2 or higher breast fibrosis 3 years after radiotherapy in 13.8% of patients with the C−509T variant allele vs 3.8% of patients without the variant allele, a significant difference.
Meaning
The C−509T variant allele may be used prospectively as a genetic marker to identify patients at elevated risk for fibrosis following radiotherapy.
Abstract
Importance
Whether genetic factors can identify patients at risk for radiation-induced fibrosis remains unconfirmed.
Objective
To assess the association between the C−509T variant allele in the promoter region of TGFB1 and breast fibrosis 3 years after radiotherapy.
Design, Setting, and Participants
This is an a priori–specified, prospective, cohort study nested in an open-label, randomized clinical trial, which was conducted in community-based and academic cancer centers to compare hypofractionated whole-breast irradiation (WBI) (42.56 Gy in 16 fractions) with conventionally fractionated WBI (50 Gy in 25 fractions) after breast-conserving surgery. In total, 287 women 40 years or older with pathologically confirmed stage 0 to IIA breast cancer treated with breast-conserving surgery were enrolled from February 2011 to February 2014. Patients were observed for a minimum of 3 years. Outcomes were compared using the 1-sided Fisher exact test and multivariable logistic regression.
Exposures
A C-to-T single-nucleotide polymorphism at position −509 relative to the first major transcription start site (C−509T) of the TGFB1 gene.
Main Outcomes and Measures
The primary outcome was grade 2 or higher breast fibrosis as assessed using the Late Effects Normal Tissue/Subjective, Objective, Medical Management, Analytic scale (range, 0 to 3) three years after radiotherapy.
Results
Among 287 women enrolled in the trial, TGFB1 genotype and 3-year radiotherapy-induced toxicity data were available for 174 patients, of whom 89 patients (51%) with a mean (SD) age of 60 (8) years had at least 1 copy of C−509T. Grade 2 or higher breast fibrosis was present in 12 of 87 patients with C−509T (13.8%) compared with 3 of 80 patients without the allele variant (3.8%) (absolute difference, 10.0%; 95% CI, 1.7%-18.4%; P = .02). The results of multivariable analyses indicated that only C−509T (odds ratio, 4.47; 95% CI, 1.25-15.99; P = .02) and postoperative cosmetic outcome (odds ratio, 7.09; 95% CI, 2.41-20.90; P < .001) were significantly associated with breast fibrosis risk.
Conclusions and Relevance
To date, this study seems to be the first prospective validation of a genomic marker for radiation fibrosis. The C−509T allele in TGFB1 is a key determinant of breast fibrosis risk. Assessing TGFB1 genotype may facilitate a more personalized approach to locoregional treatment decisions in breast cancer.
Trial Registration
ClinicalTrials.gov identifier: NCT01266642
Introduction
For women with breast cancer, whole-breast irradiation (WBI) after breast-conserving surgery optimizes breast preservation rates and breast cancer–specific survival, yet it can also result in problems with normal tissues, including fibrosis and adverse cosmetic outcomes.1,2,3,4,5 Unfortunately, excellent cosmesis is not universally attained following WBI, reflecting variability among patients in the extent to which the normal tissues of the breast are damaged by radiation therapy. Identifying factors associated with the risk of radiotherapy toxicities of normal tissue could be used to tailor local treatment decisions, such as radiation dosing and target volumes, to an individual’s genomic profile.6
Several studies support the hypothesis that the radiosensitivity of the breast is under genetic control.6,7,8,9,10,11,12,13,14,15,16 One such genomic candidate is the C−509T (rs1800469) variant allele of TGFB1 (GenBank NC_000019.10). A pleiomorphic cytokine, TGF-β plays an important role in normal and pathophysiological fibrogenesis by stimulating fibroblast migration, proliferation, and collagen release.17,18 The C−509T polymorphism has been associated with elevated gene expression and plasma levels of TGF-β,19 and the presence of this polymorphism has been associated with increased risk of radiation-induced fibrosis in some11,12,15,16,20 but not all21,22 retrospective studies. A recent prospective validation study failed to find an association between radiotherapy toxicities and TGFB1 polymorphisms, including C−509T, adding uncertainty to the predictive value of these genetic markers.23,24 However, the existing literature is limited by the use of multiple fibrosis scales that are variably sensitive to clinically significant changes. The objective of the present study was to prospectively assess whether an association existed between the TGFB1 C−509T variant allele and radiotherapy-induced toxicity of normal tissue following WBI. We hypothesized that this variant allele increases the risk of breast fibrosis as assessed using the Late Effects Normal Tissue/Subjective, Objective, Medical Management, Analytic (LENT/SOMA) scale.25 The LENT/SOMA fibrosis subscale is a physician-reported 4-point ordinal grading system that has demonstrated improved sensitivity to the severity of fibrosis compared with the Radiation Therapy Oncology Group (RTOG) late radiation morbidity scale in a validation study evaluating a population of breast cancer survivors.26 We also sought to explore whether any association existed between the TGFB1 C−509T variant allele and other patient- and physician-reported outcomes relevant to WBI. We tested our hypothesis in a cohort of 175 women who had participated in a randomized clinical trial comparing 2 WBI dosing regimens.
Methods
Patient Cohort
Between February 2011 and February 2014, 287 women aged 40 years or older with pathologically confirmed ductal carcinoma in situ or early invasive breast cancer (pTis, pT1, pT2, pN0, or pN1) were enrolled and randomized after breast-conserving surgery (trial protocol in Supplement 1). Patients were allocated to treatment with either hypofractionated WBI (42.56 Gy [to convert to rad, multiply by 100] in 16 fractions followed by a boost) or conventionally fractionated WBI (50 Gy in 25 fractions followed by a boost). Details regarding inclusion criteria and treatment planning have been previously reported.27 The present study was reviewed and approved by the institutional review board at MD Anderson Cancer Center (Houston, Texas). Written informed consent was obtained from all study participants.
Genotyping
A total of 227 patients consented to venipuncture. Genomic DNA from the buffy coat fraction of the blood sample was extracted using a Gentra Puregene Blood Kit (Qiagen). The DNA purity and concentration were determined by spectrophotometer measurement of absorbance at wavelengths of 260 and 280 nm. The genotype at the C−509T TGFB1 allele was determined using real-time polymerase chain reaction analyses. The reaction was conducted using an Applied Biosystems 7500 system (Life Technologies) per the manufacturer instructions, with TaqMan Genotyping Master Mix and primer probes. The presence of at least 1 T-containing allele at position −509 relative to the first major transcription start site of the TGFB1 gene was defined as the risk exposure.
Primary Outcome
The primary outcome for this study was grade 2 or higher physician-assessed fibrosis 3 years after completing radiotherapy. Fibrosis was assessed and graded by physicians using the LENT/SOMA scale25 with a structured template that included the definition of fibrosis grades as follows: grade 0, none or no difference; grade 1, barely palpable increased density; grade 2, definite increased density and firmness; grade 3, very marked density, retraction, and fixation.
Secondary Outcomes
Secondary patient-reported outcomes measured 3 years after completing radiotherapy included (1) patient-reported cosmetic outcome assessed using the Breast Cancer Treatment Outcomes Scale (BCTOS), coded as either a continuous variable28 or dichotomized at a prespecified score (≥2.5) that indicated a moderate to large change in the treated breast compared with the untreated breast; (2) patient-reported functional outcome assessed using the BCTOS; (3) patient-reported body image assessed using the Body Image Scale,29 and (4) patient-reported quality of life assessed using the Functional Assessment of Cancer Therapy–Breast Cancer (FACT-B) module trial outcome index.30 Each instrument was administered when patients returned for a follow-up visit 3 years after completing radiotherapy. Secondary physician-reported outcomes included (1) cosmetic outcome assessed by the treating physician using the RTOG/Harvard scale; (2) telangiectasia assessed using the LENT/SOMA scale; (3) toxicity-related adverse events assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4 (CTCAE v4.0); and (4) cosmetic outcome assessed by a panel of 3 physicians (including B.D.S. and S.F.S.) blinded to treatment assignment and C−509T genotype who reviewed 5-view photographs (right lateral, right anterior oblique, anteroposterior, left anterior oblique, left lateral) obtained in the standing position framed from the neck to the umbilicus. Templates with definitions of all outcomes were provided to the physicians to assess and document outcomes. The panel assessing cosmetic outcomes comprised 2 oncologists with expertise in breast cancer-focused radiation (B.D.S. and S.F.S.) and a surgeon with expertise in surgical procedures for breast cancer who were blinded to randomization arm and genotype. The RTOG/Harvard scale was used to assess cosmesis, and consensus among the 3 physicians was achieved for each patient.31
Statistical Analysis
For the primary outcome, the trial was designed to yield 83% power to detect a 15% absolute difference in risk of grade 2 or higher fibrosis using LENT/SOMA criteria 3 years after completing radiotherapy. At a 1-sided significance level of .05, assuming that 150 patients participated in this component of the study, the baseline risk of fibrosis among the participants with the wild-type gene is 5%, and the frequency of the variant allele in the population is 0.33. This prespecified difference was based on the effect size of C−509T reported in prior retrospective studies that also used the LENT/SOMA fibrosis scale.15,16 The difference in the proportions of fibrosis by genotype was evaluated using the 1-sided Fisher exact test. Univariate and multivariable logistic regression models were subsequently used to identify factors associated with the outcome. The final multivariable logistic regression model included covariates associated with the outcome at 2-sided P < .05 as well as the randomization arm regardless of its statistical significance. The Firth bias correction method was used in the logistic regression models because the proportion of at least grade 2 fibrosis was less than 10%.32 The statistical interaction of the randomization arm with the C−509T genotype was also tested in the multivariable model.
For secondary patient-reported outcomes, the associations of C−509T genotype with BCTOS cosmesis and with functional status were assessed using the 2-sided Wilcoxon rank sum test. Association of genotype with patient-reported cosmetic outcome dichotomized at 2.5 or greater was assessed using the 2-sided Fisher exact test. Genotype associations with the Body Image Score and with the FACT-B trial outcome index were assessed using the 2-sided t test because both outcomes were normally distributed.
For secondary physician-reported outcomes, physician-assessed and photographically assessed cosmetic outcomes were dichotomized as either good or excellent vs fair or poor. The associations of C−509T genotype with photographically assessed cosmetic outcome and with grade 2 or higher telangiectasia were assessed using the 2-sided Fisher exact test.
All statistical analyses were conducted using SAS, version 9.3 (SAS Institute Inc).
Results
Patients
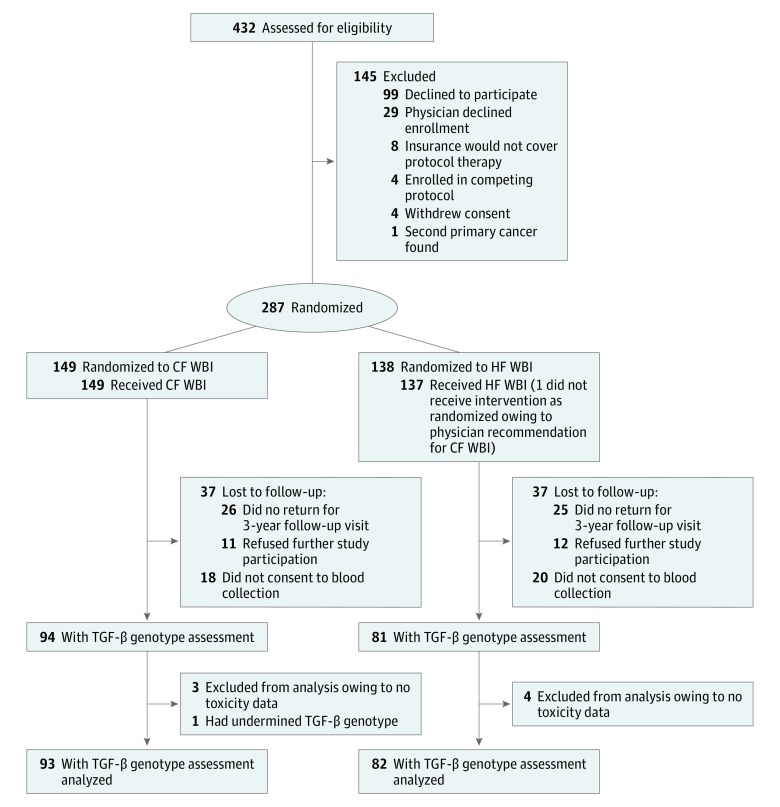
In total, 432 patients were screened for eligibility. Of these, 300 patients were registered for protocol treatment, and 287 patients were randomized and evaluable (Figure 1). Of the 149 patients allocated to conventionally fractionated WBI, all 149 patients (100%) received the allocated treatment, and 112 patients (75%) had 3-year follow-up data, of whom TGFB1 genotype was assessed in 93 patients (62%). Of the 138 patients allocated to hypofractionated WBI, 137 (99%) received the allocated treatment, and 101 (73%) had 3-year follow-up data, of whom TGFB1 genotype was assessed in 82 patients (59%). Of the 175 patients with 3-year follow-up data and TGFB1 genotype assessment, 85 (49%) had the homozygous C/C genotype (−509C), 75 (43%) had the heterozygous T/C genotype, 14 (8%) were homozygous (T/T) for the C−509T allele, and 1 (1%) had undetermined allele information (eTable 1 in Supplement 2). The variant allele (T) frequency among these 175 patients was 0.296, and the alleles were confirmed to be in Hardy-Weinberg equilibrium. There were no significant differences in any baseline patient characteristics or clinical variables between patients for whom the C−509T genotype was or was not known (eTable 2 in Supplement 2).
Figure 1. Study Enrollment Flowchart.
CF indicates conventionally fractionated; HF, hypofractionated; and WBI, whole-breast irradiation.
Of the 174 patients who had nonmissing C−509T genotype data, 89 (51%) had at least 1 T allele. The T allele was present in 45 of 93 patients (48%) allocated to conventionally fractionated WBI and 44 of 81 patients (54%) allocated to hypofractionated WBI (P = .43). The distribution of C−509T was not equal across race/ethnicity, with the T allele present in 62 of 130 non-Hispanic white (48%), 18 of 24 Hispanic white (75%), and 6 of 17 black (35%) patients (P = .01). With the exception of taxane-based chemotherapy (administered to 34 of 89 patients [38%] with the C−509T allele compared with 18 of 86 patients [21%] homozygous for −509C [P = .01]), all other baseline patient, tumor, and treatment characteristics, including body mass index, cup size, tumor stage, tumor markers, and overall administration of chemotherapy, were well matched between groups (eTable 3 in Supplement 2). Examination of baseline postsurgical, preradiotherapy patient- and physician-reported outcomes revealed no differences in any measured outcome by C−509T genotype (eTable 4 in Supplement 2).
Primary End Point of Fibrosis
Three-year LENT/SOMA fibrosis and telangiectasia data were available for 167 of the 174 patients (96%) with C−509T genotype information. The overall rate of grade 2 or higher fibrosis was significantly higher among patients with a C−509T allele (12 of 87 patients [13.8%]) than among patients without the T allele (3 of 80 patients [3.8%]) (absolute difference, 10.0%; 95% CI, 1.7%-18.4%; P = .02) (eTable 5 in Supplement 2). No difference was noted in the rate of grades 2 to 3 telangiectasia by genotype (1.1% vs 3.8%; P = .35). The 3-year physician-assessed cosmesis data were available from the treating physician for 169 of the 174 patients (97%) with C−509T genotype data and from the physician panel for 169 patients (97%). The prevalence of treating physician–assessed fair or poor cosmesis (32.2% vs 23.2%; P = .19) or panel physician–assessed fair or poor cosmesis (34.9% vs 30.1%; P = .51) did not differ significantly for those with or without the C−509T allele, respectively.
For univariate logistic regression of factors associated with the outcome, only C−509T genotype (odds ratio [OR], 3.67; 95% CI, 1.07-12.60; P = .04) and baseline postsurgical, preradiotherapy panel physician-assessed cosmesis (OR, 6.42; 95% CI, 2.30-17.91; P < .001) were significantly associated with fibrosis risk (eTable 6 in Supplement 2). The C−509T genotype was not associated with baseline panel physician–assessed cosmesis (median [interquartile range] score of 2.00 [1.00-2.00] for patients with −509C vs 2.00 [1.00-2.00] for patients with C−509T; Wilcoxon rank sum test P = .88) (eTable 4 in Supplement 2). The final multivariable model included the C−509T genotype, baseline panel physician-assessed cosmesis, and randomization arm. The association between C−509T allele and fibrosis remained statistically significant after multivariable adjustment (OR, 4.47; 95% CI, 1.25-15.99; P = .02) (Table). Fair to poor baseline panel physician-assessed cosmesis also remained statistically significant in the multivariable model (OR, 7.09; 95% CI, 2.41-20.90; P < .001); however, there was no interaction with genotype (for wild-type genotype, OR, 3.08; 95% CI, 0.36-26.5 [referent good to excellent cosmesis] vs C−509T genotype, OR, 3.19; 95% CI, 0.25-41.2 [referent good to excellent cosmesis]; P = .33 for interaction). The randomization arm was not associated with fibrosis risk (OR, 1.42; 95% CI, 0.55-3.68; P = .47) and did not interact with the C−509T allele (for wild-type genotype, OR, 0.13; 95% CI, 0.01-2.87 [referent conventionally fractionated WBI] vs C−509T genotype, OR, 10.86; 95% CI, 0.39-300.00 [referent conventionally fractionated WBI]; P = .16 for interaction).
Table. Multivariable Logistic Regression to Determine Grades 2 to 3 Fibrosis Among 205 Patients.
| Variable | Level | OR (95% CI) | P Value |
|---|---|---|---|
| C−509T genotype | C/C | 1 [Reference] | .02 |
| C/T or T/T | 4.47 (1.25-15.99) | ||
| Randomization arm | CF WBI | 1 [Reference] | .97 |
| HF WBI | 1.02 (0.38-2.77) | ||
| Panel physician–assessed cosmesis | 1-2 (Excellent-good) | 1 [Reference] | <.001 |
| 3-4 (Fair-poor) | 7.09 (2.41-20.90) |
Abbreviations: CF, conventionally fractionated; HF, hypofractionated; OR, odds ratio; WBI, whole-breast irradiation.
Secondary End Points
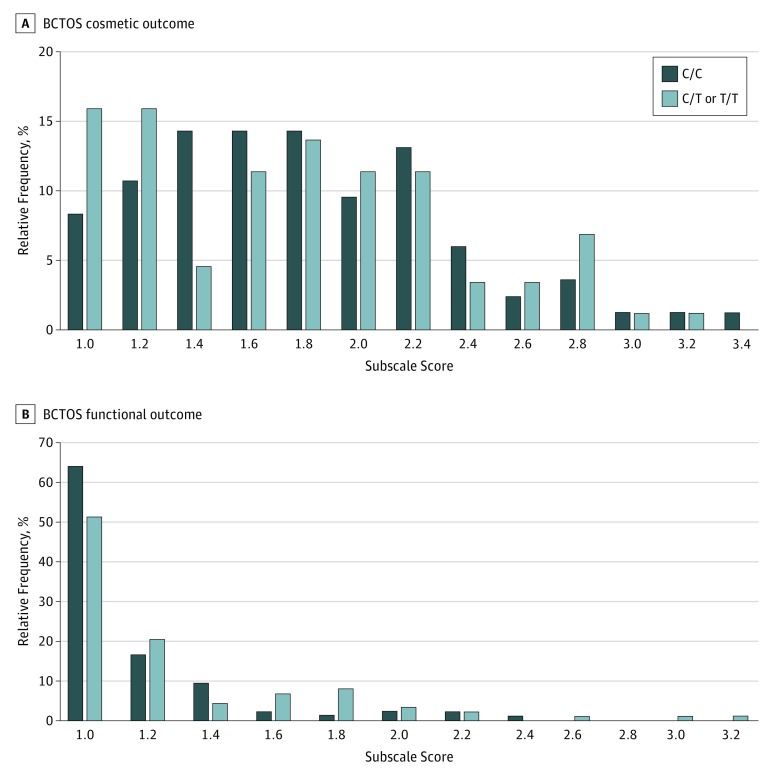
Three years after radiotherapy, patients with the C−509T allele reported worse functional outcomes on the BCTOS than did patients without the variant allele (median [interquartile range] score of 1.00 [1.00-1.14] for patients with −509C vs 1.00 [1.00-1.43] for patients with C−509T; Wilcoxon rank sum test P = .049) (eTable 7 in Supplement 2) (Figure 2). Exploratory analysis of individual items contributing to worse functional outcome revealed a trend for higher likelihood of moderate to large shoulder stiffness (BCTOS question 11) among patients with the C−509T variant allele (11.4% vs 3.4%, P = .08) (eTable 8 in Supplement 2). No differences were noted in patient-reported BCTOS cosmetic outcome (median [interquartile range] score of 1.71 [1.43-2.14] for patients with −509C vs 1.71 [1.29-2.14] for patients with C−509T; P = .61 determined by use of the Wilcoxon rank sum test) (Figure 2). The prevalence of adverse patient-assessed cosmetic outcome did not differ by genotype (12.6% vs 9.5%; P = .52). There were also no differences in Body Image Score (mean score of 1.36 [95% CI, 1.25-1.46] for patients with −509C vs 1.29 [95% CI, 1.2-1.37] for patients with C−509T; P = .29) or FACT-B trial outcome index (mean score of 78.23 [95% CI, 76.3-80.16] for patients with −509C vs 78.35 [95% CI, 76.37-80.34] for patients with C−509T; P = .93) between genotypes (eTable 7 in Supplement 2).
Figure 2. Frequency of Breast Cancer Treatment Outcomes Scale (BCTOS) Subscale Scores Among Patients With Genotypes C/C vs C/T or T/T at Position −509 of the TGFB1 Gene.
Each subscale score is the mean of the ratings of all items belonging to that subscale. Each item evaluates the difference between the treated and untreated breast on a 4-point ordinal scale: 1, indicates no difference; 2, slight difference; 3, moderate difference; 4, large difference. Scores are assorted into 0.2-U bins.
The association between 3-year CTCAE v4.0–assessed toxicities and C−509T genotype is shown in eTable 9 in Supplement 2. No patients developed higher than grade 2 toxicity. Patients with the C−509T allele had a higher prevalence of grade 2 breast atrophy than patients without the variant allele (16.9% vs 7.1%; P = .047) (eTable 9 in Supplement 2). There was no difference in the prevalence by genotype of any other CTCAE v4.0–assessed grade 2 toxicities.
Discussion
The results of the present study showed that patients with at least 1 copy of the C−509T allele in the TGFB1 gene had an increased risk of developing fibrosis after WBI. The association of C−509T with fibrosis occurred independent of the radiotherapy fractionation regimen or postsurgical, preradiotherapy cosmesis, the only other factor associated with breast fibrosis. Homozygosity at the −509C allele appeared to confer protection against fibrosis, with only 3.8% of patients with this genotype exhibiting grade 2 or higher fibrosis compared with patients with at least 1 copy of the T allele at this locus, whose prevalence of grade 2 or higher fibrosis was 13.8%. This finding mirrors prior reports that similarly describe low rates of fibrosis among patients homozygous for the −509C allele.15 To our knowledge, the present study is the first prospective validation of a genomic factor associated with late radiation fibrosis, substantiating a role for somatic genomic toxicity stratification in a clinical oncology population.
A key challenge in identifying genetic risk factors of radiotherapy toxicity is to precisely, robustly, and sensitively define the outcomes of interest. Late toxicity was defined differently among previous studies, likely contributing to the unresolved association between TGFB1 single-nucleotide polymorphisms and radiation fibrosis. Our study used the LENT/SOMA scale, which provides an objective and validated scale of radiation-induced fibrosis that was strongly associated with the C−509T allele in a prior retrospective study.15 Clinically significant fibrosis was defined in our study as grade 2 on the LENT/SOMA scale (ie, “definite increased density and firmness”), which is readily differentiated from grade 1 (ie, “barely palpable” fibrosis).25 Yet our results conflict with the report from the Radiogenomics: Assessment of Polymorphisms for Predicting the Effects of Radiotherapy (RAPPER) study, a prospective validation study that evaluated multiple single-nucleotide polymorphisms and outcomes in a large multicenter clinical cohort.23 In contrast to the LENT/SOMA scale described in the present study, the RAPPER study used the CTCAE, version 3, toxicity scale for fibrosis, defining grade 1 fibrosis as “increased density on palpation,” compared with the definition of grade 2 as “marked increase in density and function on palpation with or without minimal retraction.” This instrument is insensitive to the separation observed between grades 1 and 2 on the LENT/SOMA scale, as evidenced by only a single patient being scored as having grade 2 fibrosis in the RAPPER study.24 Thus, despite a large cohort size, the RAPPER study likely lacked the power to detect genetic contributions to fibrosis owing to an insensitive event definition and low event rate. The positive association reported in the present study reinforces the importance of outcome measure selection in identifying genetic risk factors for late toxicity.
Fibrosis was chosen as a clinically meaningful end point for the present study because it provides a plausible biological mechanism for multiple other adverse outcomes, including decrements in cosmesis, function, and quality of life. We found that the C−509T allele was associated with increased risk of breast fibrosis and that this allele may increase risk of worse functional outcomes and breast atrophy, specific morbidities that are expected to arise from increased fibrosis. Our exploratory analyses provided internal validation of the clinical relevance of the primary outcome. However, all cosmesis, body image, and quality of life assessments were similar, indicating that fibrosis did not drive all of the patient experience. That there was no association of C−509T with cosmesis, despite its association with fibrosis, suggests that adverse cosmetic outcome is a multifactorial process attributable not simply to susceptibility to radiation fibrosis but also to disease, host, and surgical factors. Rather, we observed a significant association between preradiotherapy cosmesis and late fibrosis risk. Cosmetic outcome after surgery can reflect challenging anatomy, inferior surgical procedures, or poor wound healing, each of which could be expected to lead to a more fibrotic healing process. We speculate that this profibrotic environment enhances the development of late fibrosis after radiotherapy.
Clinically, the most apparent application of C−509T genotyping would be to aid in postradiation fibrosis risk assessment. Our data showed that patients homozygous for the −509C allele were at very low risk for fibrosis following standard breast radiotherapy doses. As one example, the decision of whether to include a tumor bed boost in patients with favorable tumor risk features may benefit from C−509T genotype information, although such a management application should be investigated prospectively before being applied broadly. Further investigations on the role of TGFB1 genotyping in lymphedema risk assessment and breast reconstruction decisions are ongoing.
Limitations
Late toxicities were evaluated 3 years after radiotherapy, but fibrosis as well as other radiotherapy-related toxicities can increase beyond this time point. Therefore, our study may underestimate the effect size of the C−509T allele. The present study was appropriately powered for the primary end point, yet the relatively small sample size limited post hoc subgroup analyses. This is notable regarding subgroup analysis by ethnicity because we observed enrichment of the risk allele among Hispanic patients but could not evaluate whether this subpopulation had a statistically elevated risk of fibrosis. The sample size also limited our ability to assess the effect of −509T homozygosity, which was present in only 14 patients but has previously been associated in retrospective studies with much higher fibrosis risk.15 Because our study included only patients with early-stage cancer who received moderate radiation doses to the breast, it is difficult to evaluate the association between the TGFB1 genotype and the increased dose to the skin. Thus, it is unclear how the C−509T allele may affect fibrosis and functional outcomes among patients treated for locally advanced, inflammatory, or recurrent breast cancers.
Conclusions
In this prospective validation study, the C−509T allele was significantly associated with increased risk of grade 2 or higher breast fibrosis as assessed using LENT/SOMA criteria, breast atrophy, and adverse functional outcomes among patients with early-stage breast cancer 3 years after the completion of whole-breast radiotherapy. Genotyping patients to determine the presence of this polymorphism may assist with risk stratification, patient counseling, and potential therapeutic approaches for late radiotherapy toxicity.
Trial protocol
eTable 1. Frequencies of C−509T Alleles
eTable 2. Baseline Patient and Clinical Characteristics Among Patients With 3-Year Follow-up Who Were Tested or Not for C−509T Genotype
eTable 3. Baseline Patient and Clinical Characteristics and Patient-Reported or Physician-Reported Outcomes by C−509T Genotype
eTable 4. Baseline Patient and Physician-Reported Outcomes by C−509T Genotype
eTable 5. Three-Year Physician-Reported Outcomes by C−509T Genotype
eTable 6. Univariate Logistic Regression to Predict Grade 2-3 Fibrosis
eTable 7. Three-Year Patient/Physician-Reported Outcomes by C−509T Genotype
eTable 8. Baseline and 3-Year BCTOS Individual Questions by C−509T Genotype
eTable 9. Three-Year NCI CTCAE v4.0 Toxicities by C−509T Genotype
References
- 1.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ. Semin Oncol. 2001;28(4):400-418. doi: 10.1016/S0093-7754(01)90133-2 [DOI] [PubMed] [Google Scholar]
- 2.Bijker N, Meijnen P, Peterse JL, et al. ; EORTC Breast Cancer Cooperative Group; EORTC Radiotherapy Group . Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ. J Clin Oncol. 2006;24(21):3381-3387. doi: 10.1200/JCO.2006.06.1366 [DOI] [PubMed] [Google Scholar]
- 3.Emdin SO, Granstrand B, Ringberg A, et al. ; Swedish Breast Cancer Group . SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Acta Oncol. 2006;45(5):536-543. doi: 10.1080/02841860600681569 [DOI] [PubMed] [Google Scholar]
- 4.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M; UK Coordinating Committee on Cancer Research; Ductal Carcinoma in situ Working Party; DCIS trialists in the UK, Australia, and New Zealand . Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand. Lancet. 2003;362(9378):95-102. doi: 10.1016/S0140-6736(03)13859-7 [DOI] [PubMed] [Google Scholar]
- 5.Clarke M, Collins R, Darby S, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival. Lancet. 2005;366(9503):2087-2106. doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 6.West C, Rosenstein BS. Establishment of a radiogenomics consortium. Radiother Oncol. 2010;94(1):117-118. doi: 10.1016/j.radonc.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Angèle S, Romestaing P, Moullan N, et al. . ATM haplotypes and cellular response to DNA damage: association with breast cancer risk and clinical radiosensitivity. Cancer Res. 2003;63(24):8717-8725. [PubMed] [Google Scholar]
- 8.Damaraju S, Murray D, Dufour J, et al. . Association of DNA repair and steroid metabolism gene polymorphisms with clinical late toxicity in patients treated with conformal radiotherapy for prostate cancer. Clin Cancer Res. 2006;12(8):2545-2554. doi: 10.1158/1078-0432.CCR-05-2703 [DOI] [PubMed] [Google Scholar]
- 9.Ho AY, Fan G, Atencio DP, et al. . Possession of ATM sequence variants as predictor for late normal tissue responses in breast cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(3):677-684. doi: 10.1016/j.ijrobp.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 10.Isomura M, Oya N, Tachiiri S, et al. . IL12RB2 and ABCA1 genes are associated with susceptibility to radiation dermatitis. Clin Cancer Res. 2008;14(20):6683-6689. doi: 10.1158/1078-0432.CCR-07-4389 [DOI] [PubMed] [Google Scholar]
- 11.Andreassen CN, Alsner J, Overgaard J, et al. . TGFB1 polymorphisms are associated with risk of late normal tissue complications in the breast after radiotherapy for early breast cancer. Radiother Oncol. 2005;75(1):18-21. doi: 10.1016/j.radonc.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Andreassen CN, Alsner J, Overgaard M, Overgaard J. Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother Oncol. 2003;69(2):127-135. doi: 10.1016/j.radonc.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 13.Andreassen CN, Alsner J, Overgaard M, Sørensen FB, Overgaard J. Risk of radiation-induced subcutaneous fibrosis in relation to single nucleotide polymorphisms in TGFB1, SOD2, XRCC1, XRCC3, APEX and ATM. Int J Radiat Biol. 2006;82(8):577-586. doi: 10.1080/09553000600876637 [DOI] [PubMed] [Google Scholar]
- 14.Chang-Claude J, Ambrosone CB, Lilla C, et al. . Genetic polymorphisms in DNA repair and damage response genes and late normal tissue complications of radiotherapy for breast cancer. Br J Cancer. 2009;100(10):1680-1686. doi: 10.1038/sj.bjc.6605036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giotopoulos G, Symonds RP, Foweraker K, et al. . The late radiotherapy normal tissue injury phenotypes of telangiectasia, fibrosis and atrophy in breast cancer patients have distinct genotype-dependent causes. Br J Cancer. 2007;96(6):1001-1007. doi: 10.1038/sj.bjc.6603637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quarmby S, Fakhoury H, Levine E, et al. . Association of transforming growth factor beta-1 single nucleotide polymorphisms with radiation-induced damage to normal tissues in breast cancer patients. Int J Radiat Biol. 2003;79(2):137-143. doi: 10.1080/0955300021000045673 [DOI] [PubMed] [Google Scholar]
- 17.Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987;165(1):251-256. doi: 10.1084/jem.165.1.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sporn MB, Roberts AB. Transforming growth factor–β: multiple actions and potential clinical applications. JAMA. 1989;262(7):938-941. doi: 10.1001/jama.1989.03430070086036 [DOI] [PubMed] [Google Scholar]
- 19.Grainger DJ, Heathcote K, Chiano M, et al. . Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet. 1999;8(1):93-97. doi: 10.1093/hmg/8.1.93 [DOI] [PubMed] [Google Scholar]
- 20.Terrazzino S, La Mattina P, Gambaro G, et al. . Common variants of GSTP1, GSTA1, and TGFβ1 are associated with the risk of radiation-induced fibrosis in breast cancer patients. Int J Radiat Oncol Biol Phys. 2012;83(2):504-511. doi: 10.1016/j.ijrobp.2011.06.2012 [DOI] [PubMed] [Google Scholar]
- 21.Andreassen CN, Overgaard J, Alsner J, et al. . ATM sequence variants and risk of radiation-induced subcutaneous fibrosis after postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(3):776-783. doi: 10.1016/j.ijrobp.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 22.Zschenker O, Raabe A, Boeckelmann IK, et al. . Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother Oncol. 2010;97(1):26-32. doi: 10.1016/j.radonc.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 23.Barnett GC, Coles CE, Elliott RM, et al. . Independent validation of genes and polymorphisms reported to be associated with radiation toxicity. Lancet Oncol. 2012;13(1):65-77. doi: 10.1016/S1470-2045(11)70302-3 [DOI] [PubMed] [Google Scholar]
- 24.Barnett GC, Coles CE, Burnet NG, et al. . No association between SNPs regulating TGF-β1 secretion and late radiotherapy toxicity to the breast. Radiother Oncol. 2010;97(1):9-14. doi: 10.1016/j.radonc.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavy JJ, Denekamp J, Letschert J, et al. ; EORTC Late Effects Working Group . Late effects toxicity scoring. Radiother Oncol. 1995;35(1):11-15. doi: 10.1016/0167-8140(95)97448-M [DOI] [PubMed] [Google Scholar]
- 26.Hoeller U, Tribius S, Kuhlmey A, Grader K, Fehlauer F, Alberti W. Increasing the rate of late toxicity by changing the score?. Int J Radiat Oncol Biol Phys. 2003;55(4):1013-1018. doi: 10.1016/S0360-3016(02)04202-5 [DOI] [PubMed] [Google Scholar]
- 27.Shaitelman SF, Schlembach PJ, Arzu I, et al. . Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation. JAMA Oncol. 2015;1(7):931-941. doi: 10.1001/jamaoncol.2015.2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanton AL, Krishnan L, Collins CA. Form or function? part 1: subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91(12):2273-2281. doi: [DOI] [PubMed] [Google Scholar]
- 29.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37(2):189-197. doi: 10.1016/S0959-8049(00)00353-1 [DOI] [PubMed] [Google Scholar]
- 30.Brady MJ, Cella DF, Mo F, et al. . Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974-986. doi: 10.1200/JCO.1997.15.3.974 [DOI] [PubMed] [Google Scholar]
- 31.Harris JR, Levene MB, Svensson G, Hellman S. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys. 1979;5(2):257-261. doi: 10.1016/0360-3016(79)90729-6 [DOI] [PubMed] [Google Scholar]
- 32.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27-38. doi: 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Frequencies of C−509T Alleles
eTable 2. Baseline Patient and Clinical Characteristics Among Patients With 3-Year Follow-up Who Were Tested or Not for C−509T Genotype
eTable 3. Baseline Patient and Clinical Characteristics and Patient-Reported or Physician-Reported Outcomes by C−509T Genotype
eTable 4. Baseline Patient and Physician-Reported Outcomes by C−509T Genotype
eTable 5. Three-Year Physician-Reported Outcomes by C−509T Genotype
eTable 6. Univariate Logistic Regression to Predict Grade 2-3 Fibrosis
eTable 7. Three-Year Patient/Physician-Reported Outcomes by C−509T Genotype
eTable 8. Baseline and 3-Year BCTOS Individual Questions by C−509T Genotype
eTable 9. Three-Year NCI CTCAE v4.0 Toxicities by C−509T Genotype