Key Points
Question
Which neoadjuvant chemotherapy regimen is associated with the best outcomes for patients with muscle-invasive bladder cancer?
Findings
This cross-sectional analysis of a cohort of 1113 patients who underwent cystectomy found that among those who underwent neoadjuvant chemotherapy, downstaging and complete response were significantly better for patients receiving dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin than for those receiving gemcitabine with cisplatin or gemcitabine with carboplatin.
Meaning
Although gemcitabine with cisplatin is the most frequently prescribed neoadjuvant chemotherapy regimen for patients with muscle-invasive bladder cancer, for eligible patients, treatment with dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin may lead to better outcomes.
Abstract
Importance
Neoadjuvant chemotherapy (NAC) followed by radical cystectomy improves survival compared with cystectomy alone for patients with bladder cancer. Although gemcitabine with cisplatin has become a standard NAC regimen, a dose-dense combination of methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) is being adopted at some institutions.
Objective
To assess the association of neoadjuvant ddMVAC vs standard regimens with downstaging and overall survival among patients treated with radical cystectomy for bladder cancer.
Design, Setting, and Participants
Cross-sectional analysis of data extracted from the medical records of a consecutive sample, after exclusions, of 1113 patients with bladder cancer of whom 824 had disease stage T2 or greater, who were treated with cystectomy at the Moffitt Cancer Center in Tampa, Florida, a tertiary care cancer center, between January 1, 2007, and May 31, 2017. Data were collected between November 14, 2016, and July 21, 2017, and analyzed between August 21, 2017, and December 8, 2017. Patients were compared based on type of NAC. Those who did not receive NAC were included as controls.
Main Outcomes and Measures
Comparative rates and the association of any downstaging, complete response, and overall survival with ddMVAC and other NAC regimens and surgery alone. Outcomes were examined using Kaplan-Meier, adjusted logistic, Cox regression, and propensity-weighted models.
Results
Of the 1113 patients who underwent cystectomy for bladder cancer, 861 (77.4%) were male, the median (interquartile range) age was 67 (60-74) years, 1051 (94.4%) were white, 27 (2.4%) black, 37 (3.3%) Hispanic/Latino, and 35 (3.1%) other race/ethnicity. Of 824 patients with muscle-invasive bladder cancer, 332 (40%) received NAC. Downstaging rates were 52.2% for ddMVAC, 41.3% for gemcitabine-cisplatin, and 27.0% for gemcitabine with carboplatin, and complete response (pT0N0) rates were 41.3% for ddMVAC, 24.5% for gemcitabine-cisplatin, and 9.4% for gemcitabine-carboplatin (2-sided P < .001). Adjusted analysis comparing ddMVAC with gemcitabine-cisplatin demonstrated a higher likelihood of downstaging (odds ratio [OR], 1.84; 95% CI, 1.10-3.09) and complete response (OR, 2.67; 95% CI, 1.50-4.77) with ddMVAC. Similar results were achieved with propensity score matching (OR, 1.52; 95% CI, 0.99-2.35). Patients who received ddMVAC had better overall survival than those treated with other chemotherapy regimens, although the observed survival benefit did not reach statistical significance in adjusted or propensity-matched models (hazard ratio, 0.44; 95% CI, 0.14-1.38; P = .16).
Conclusions and Relevance
This study suggest that neoadjuvant ddMVAC followed by cystectomy is associated with a higher complete response (ypT0N0) rate than standard NAC. These data highlight and suggest the need to further investigate ddMVAC vs standard NAC in a prospective, randomized fashion.
This cross-sectional analysis compares downstaging, responses, and survival outcomes in patients with muscle-invasive bladder cancer treated with cystectomy who underwent neoadjuvant chemotherapy with gemcitabine with cisplatin, gemcitabine with carboplatin, or dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin.
Introduction
Neoadjuvant chemotherapy (NAC) given before cystectomy is a standard treatment for muscle-invasive bladder cancer, and its use is supported by the findings of numerous randomized clinical trials and meta-analyses, which have found a 6% survival benefit at 10 years.1,2,3 Accordingly, treatment guidelines from a number of organizations, including the American Urological Association/Society of Urologic Oncology, the National Comprehensive Cancer Network, and the European Association of Urology, recommend NAC as preferred first-line therapy in the management of invasive and advanced disease.4,5,6 However, despite unequivocal evidence supporting its efficacy, the rates of adoption and routine use of NAC have been modest.7,8,9
Various chemotherapy regimens are used in clinical practice; however, most trials examining the efficacy of multiagent NACs have been based on combinations including methotrexate, vinblastine, and cisplatin with or without the addition of an anthracycline (eg, doxorubicin or epirubicin).3,10,11,12 Although a previous landmark trial reported significant improvement in complete pathologic response (38% vs 15%; P < .001) and median overall survival (77 vs 46 months; P = .05) for patients treated with neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy compared with those treated with cystectomy alone,1 serious toxic effects such as fatigue, nausea, and vomiting were common with MVAC, and more than 50% of patients experienced myelosuppression and mucositis.13 These adverse events have limited the widespread use of MVAC to date; as a result, the combination of gemcitabine with cisplatin is more commonly used before cystectomy.14,15 In the setting of renal insufficiency, the combination of gemcitabine with carboplatin is also used, despite little evidence of its effectiveness.
Neoadjuvant phase 2 trial results suggest that dose-dense MVAC (ddMVAC) or accelerated MVAC is well tolerated, safe, and has response rates similar to gemcitabine-cisplatin.16,17,18 Observational studies report similar response rates for neoadjuvant gemcitabine-cisplatin and MVAC.19,20 However, few studies comparing cancer control and survival outcomes for NAC regimens exist. The objective of this study was to examine cancer-relevant outcomes across NAC regimens and to determine the relative effectiveness of ddMVAC compared with gemcitabine-cisplatin.
Methods
Data Sources and Study Population
Study data were obtained from the Health and Research Informatics system at H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, between November 14, 2016, and July 21, 2017, and analyzed between August 21, 2017, and December 8, 2017. The Health and Research Informatics system is an integrated institutional analytics platform that incorporates discrete data elements from clinical, administrative (billing codes), pharmacy, and cancer registry data sources. Data elements abstracted from the system included patient factors (demographic and insurance), histologic features, clinical and pathologic staging, comorbidities, treatment (type and date of surgery, chemotherapy regimen, cycle number, chemotherapy dates), and outcomes (disease status) information. An institutional cystectomy case registry was merged with Health and Research Informatics system data for a consecutive sample of patients with bladder cancer who were treated with cystectomy between January 1, 2007 and May 31, 2017. Study compliance and regulation were overseen by the Moffitt Cancer Center Scientific Review Committee and institutional review board. The study was determined to be exempt from patient informed consent.
Study Measures, Definitions, and Outcomes
Available demographic information included age, sex, race/ethnicity, marital status, education, and primary insurance. Comorbidities were captured and indexed using the Elixhauser method.21 The Elixhauser comorbidity index categorizes comorbidities based on International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes found in administrative data. Chemotherapy agents were identified at the individual patient level and then recoded into regimens annotated with start and finish dates. The most common regimens were gemcitabine-cisplatin, gemcitabine-carboplatin, and ddMVAC. Atypical or uncommon regimens, such as fluorouracil, etoposide, and paclitaxel-based chemotherapy, were categorized as other. Chemotherapy and surgery dates were used to verify neoadjuvant administration. A 6- to 8-week administration period was used to distinguish dose-dense from standard MVAC regimens, and all use of ddMVAC was confirmed with individual medical record reviews. Clinical staging was determined primarily from pathologic findings from transurethral resection of the bladder tumor, supplemented with staging imaging from radiographic studies. Pathologic staging information, including TNM classification, histology, lymph node counts, and surgical margins, was obtained from pathology reports after cystectomy. Disease status, vital status, and duration of follow-up were derived from the HRI’s cancer registry death index.
Primary outcomes of interest included pathologic downstaging and overall survival, according to NAC regimen. Downstaging was defined as either any decrease in stage or complete pathologic response (TNM classification of pT0N0 or ypT0N0). Overall survival time was measured from date of cystectomy to date of last follow-up or death from any cause. A control group comprised patients who received surgery alone, so that their downstaging and survival rates could be compared against those of patients receiving NAC. Time to cystectomy was examined as a secondary outcome across NAC groups. In addition, data on adverse events were collected from the subset of patients treated with ddMVAC NAC. For this purpose, toxic effects associated with ddMVAC were abstracted from the medical record and graded according to Common Terminology Criteria for Adverse Events (version 4.0).22
Statistical Analyses
Demographic factors and clinical variables were compared between non-NAC and NAC groups and across NAC regimens. Continuous variables were reported as mean (SD) or median (interquartile range [IQR]) values. Comparisons were performed using the χ2, t, or Wilcoxon rank sum test, as appropriate. Unadjusted and adjusted logistic regression models were used to estimate odds ratios (ORs) and 95% CIs for downstaging, using gemcitabine-cisplatin data as the reference. Multivariable models were adjusted for age, comorbidity, sex, clinical stage, chemotherapy regimen, and number of chemotherapy cycles, with gemcitabine-cisplatin data again used as the reference. Because participants were not randomly assigned to chemotherapy regimens, we also performed propensity score analyses. Logistic regression was used to compute propensity scores to calculate the predictive probabilities of receiving ddMVAC vs gemcitabine-cisplatin. Propensity scores were then included in a second set of logistic regression models to estimate adjusted ORs of any downstaging and pT0N0 for ddMVAC vs gemcitabine-cisplatin.
Kaplan-Meier curves were used to compare overall survival rates and ypT0N0 status among chemotherapy regimens and according to ypT0N0 status. To adjust for confounding factors, we performed multivariable Cox proportional hazards regression modeling and adjusted for age, comorbidity, sex, clinical stage, chemotherapy regimen, and number of cycles. Finally, we calculated a hazard ratio (HR) for ddMVAC vs gemcitabine-cisplatin in our propensity-weighted model for overall survival.
Hypothesis testing was 2-sided, and P < .05 was considered statistically significant. All statistical analyses were performed with SAS statistical software (version 9.4; SAS Institute Inc).
Results
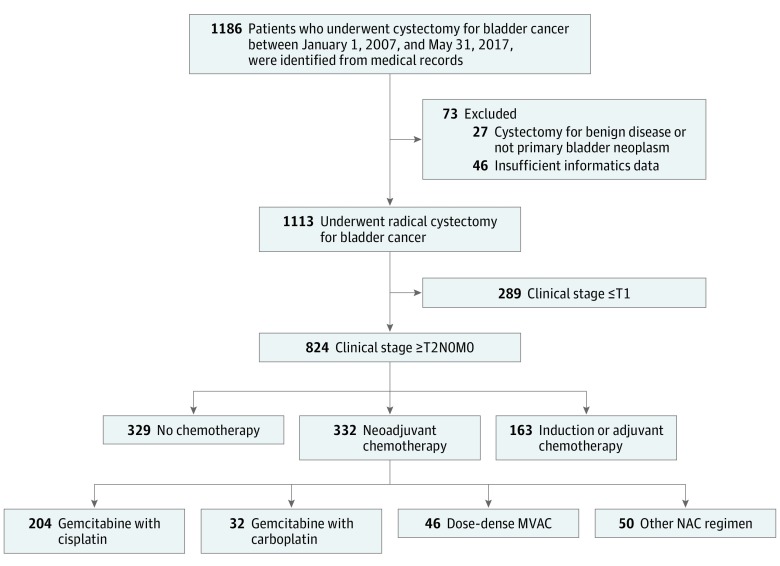
Of the 1113 patients included in the analysis, 861 (77.4%) were male, the median (IQR) age was 67 (60-74) years, and 1051 (94.4%) were white, 27 (2.4%) black, 37 (3.3%) Hispanic/Latino, and 35 (3.1%) other race/ethnicity. From a registry of 1186 patients who underwent cystectomies at the Moffitt Cancer Center from January 1, 2007, to May 31, 2017, 1113 patients were included in the analyses (Figure 1). A total of 824 patients with invasive or advanced-stage disease (≥T2N0M0) were identified within the cohort, of whom 332 (40.3%) received NAC. Of the 332 patients treated with NAC, 204 (61.4%) received gemcitabine-cisplatin, 32 (10.0%) received gemcitabine-carboplatin, and 46 (14.0%) received ddMVAC. Fifty patients (15.1%) received other agents, such as etoposide, fluorouracil, and paclitaxel regimens. Three patients received a standard MVAC regimen and were included in the Other NAC category. Owing to the broad service area of the Moffitt Cancer Center and its referral patterns, most patients received NAC at offsite locations (241 [73.0%] with outside administration; 89 [27.0%] with Moffitt Cancer Center administration).
Figure 1. Study Cohort Flowchart.
MVAC indicates methotrexate, vinblastine, doxorubicin, and cisplatin; NAC, neoadjuvant chemotherapy.
Study cohort characteristics and clinical variables, including clinical and pathologic stages and chemotherapy details, are shown in Table 1. Patients receiving NAC tended to be slightly younger and were characterized by higher clinical-stage distributions. The numbers of cycles received were similar across regimens: 146 (73.7%) of patients receiving gemcitabine-cisplatin and 42 (91.3%) of patients receiving ddMVAC received 3 or more cycles (eTable 1 in the Supplement). Clinical-stage distributions did not differ significantly among patients who received gemcitabine-cisplatin and those who received ddMVAC (clinical stage ≥T3: 56 [27.6%] received gemcitabine-cisplatin; 10 [21.7%] received ddMVAC; P = .44; eTable 1 in the Supplement). The median follow-up time was 18.6 months (95% CI, 16.5-20.7 months) for the entire cohort and 13.8 months (95% CI, 12.3-16.1 months) for those receiving NAC. When stratified by type of NAC, the median follow-up was 15 months (95% CI, 12.6-21.0 months) for those receiving gemcitabine-cisplatin, 12 months (95% CI, 8.2-19.4 months) for those receiving gemcitabine-carboplatin, 11 months (95% CI, 6.3-18.0 months) for those receiving ddMVAC, and 15.8 months (95% CI, 12.2-23.6 months) for those receiving some other NAC regimen.
Table 1. Social, Demographic, and Clinical Variables Pertaining to 1113 Patients Treated With or Without Neoadjuvant Chemotherapy.
| Variable | Patients Treated With Neoadjuvant Chemotherapy | P Value | |
|---|---|---|---|
| No | Yes | ||
| Totals, No. (%) | 781 (70.2) | 332 (29.8) | |
| Age, median (IQR), y | 68 (61-75) | 66 (58-72) | <.001 |
| Sex, No. (%) | |||
| Female | 162 (20.7) | 90 (27.1) | .02 |
| Male | 619 (79.3) | 242 (72.9) | |
| Race/ethnicity, No. (%) | |||
| White | 740 (94.8) | 311 (93.7) | .02 |
| Black | 12 (1.5) | 15 (4.5) | |
| Hispanic or Latino | 29 (3.7) | 8 (2.4) | |
| Other | 29 (3.7) | 6 (1.8) | |
| Marital status, No. (%) | |||
| Married or living together | 551 (70.7) | 237 (71.8) | .48 |
| Separated/divorced | 81 (10.4) | 29 (8.8) | |
| Single (never married) | 68 (8.7) | 36 (10.9) | |
| Widowed | 76 (9.8) | 28 (8.5) | |
| Unknown | 3 (0.4) | 0 | |
| Education, No. (%) | |||
| ≤High school | 127 (35.4) | 68 (31.6) | .26 |
| College or some college | 165 (46.0) | 94 (43.7) | |
| Graduate or professional degree | 40 (11.1) | 27 (12.6) | |
| Unknown | 27 (7.52) | 26 (12.1) | |
| Primary insurance, No. (%) | |||
| Private | 148 (19.0) | 106 (31.9) | <.001 |
| Medicare | 405 (51.9) | 178 (53.6) | |
| Medicaid | 25 (3.2) | 19 (5.7) | |
| Self-paying or uninsured | 25 (3.2) | 11 (3.3) | |
| Elixhauser comorbidity, mediana | 4 | 3 | .08 |
| Histologic type, No. (%) | |||
| Urothelial carcinoma | 622 (79.60) | 229 (69.0) | <.001 |
| Squamous cell carcinoma | 15 (1.9) | 6 (1.8) | |
| Adenocarcinoma | 10 (1.3) | 1 (0.3) | |
| Other | 134 (17.1) | 96 (28.9) | |
| Clinical AJCC stage, No. (%) | |||
| I (≤T1NxMx) | 289 (37.0) | 11 (3.3)b | <.001 |
| II (T2NxMx) | 395 (50.6) | 228 (68.7) | |
| III (T3NxMx) | 56 (7.2) | 61 (18.4) | |
| IV (T4NxMx) | 34 (4.4) | 31 (9.3) | |
| Unknown | 7 (0.9) | 1 (0.3) | |
| Pathologic AJCC stage, No. (%) | |||
| 0 (T0/Ta/isN0M0) | 205 (26.2) | 112 (33.7) | <.001 |
| I (T1N0M0) | 82 (10.5) | 14 (4.2) | |
| II (T2N0M0) | 118 (15.1) | 36 (10.8) | |
| III (T3N0M0) | 169 (21.6) | 69 (20.8) | |
| IV (T4N0-3M0-1) | 193 (24.7) | 101 (30.4) | |
| Unknown | 14 (1.8) | 0 | |
| Chemotherapy regimen, No. (%); cycles, mean (SD) No. | |||
| Gemcitabine-cisplatin | NAc | 204 (61.4); 3.7 (2.2) | <.001 |
| Gemcitabine-carboplatin | NA | 32 (9.6); 4.4 (3.6) | |
| ddMVAC | NA | 46 (13.9); 3.3 (0.9) | |
| Other | NA | 50 (15.1); 3.96 (2.1) | |
Abbreviations: AJCC, American Joint Committee on Cancer; ddMVAC, dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; NA, not applicable.
The Elixhauser comorbidity index categorizes comorbidities based on International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes found in administrative data.21
cT1 based on transurethral resection with radiographic or clinical evidence of invasive/advanced disease.
Number of cycles not applicable because these patients did not receive neoadjuvant chemotherapy.
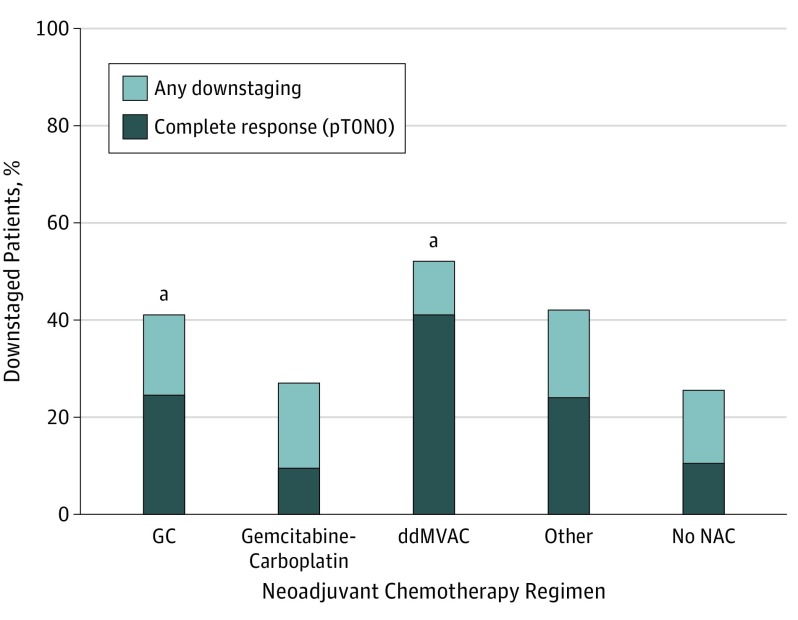
Any degree of downstaging was noted in 19 of 46 (52.2%) patients receiving ddMVAC, 92 of 204 (41.3%) receiving gemcitabine-cisplatin, and 10 of 32 (27.0%) receiving gemcitabine-carboplatin. Complete pathologic responses (pT0N0) were observed among 19 of 46 patients (41.3%) in the ddMVAC group, 50 of 204 patients (24.5%) in the gemcitabine-cisplatin group, and 3 patients (9.4%) in the gemcitabine-carboplatin group (Figure 2). A pairwise comparison of gemcitabine-cisplatin and ddMVAC significantly favored ddMVAC (χ2 = 5.20; P = .02). The pT0N0 rate for gemcitabine-carboplatin was 9.4%, and for cystectomy without NAC, it was 10.7%. Dose-dense MVAC was also associated with a significantly higher likelihood of complete pathologic response in adjusted multivariable logistic regression models (OR, 2.67; 95% CI, 1.50-4.77) and in a separate propensity-weighted model comparing gemcitabine-cisplatin with ddMVAC (OR, 1.52; 95% CI, 0.99-2.35; P = .05) (Table 2).
Figure 2. Downstaging and Complete Pathologic Response (pT0N0) Rates by Neoadjuvant Chemotherapy Group.
ddMVAC indicates dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; GC, gemcitabine-cisplatin; and NAC, neoadjuvant chemotherapy.
aP = .02 for pT0N0 downstaging and P = .10 for any downstaging.
Table 2. Univariable, Multivariable, and Propensity-Weighted Regression Analyses of Downstaging by NAC Regimen or No NAC.
| Chemotherapy Regimen | Total No. | Downstaged, No. (%) | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)a | P Value | Propensity Score Adjusted OR (95% CI)b | P Value |
|---|---|---|---|---|---|---|---|---|
| Downstaged to pT0N0 | ||||||||
| Gemcitabine-cisplatin | 204 | 50 (24.5) | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Gemcitabine-carboplatin | 32 | 3 (9.4) | 0.34 (0.09-1.09) | .07 | 0.46 (0.17-1.25) | .13 | ||
| ddMVAC | 46 | 19 (41.3) | 2.17 (1.11-4.23) | .02 | 2.67 (1.50-4.77) | <.001 | 1.52 (0.99-2.35) | .05 |
| Other | 50 | 12 (24.0) | 0.97 (0.47-2.00) | .94 | 1.44 (0.78-2.63) | .24 | ||
| None | 777 | 83 (10.7) | 0.37 (0.25-0.55) | <.001 | 0.44 (0.30-0.64) | <.001 | ||
| Any downstaging | ||||||||
| Gemcitabine-cisplatin | 204 | 92 (41.3) | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Gemcitabine-carboplatin | 32 | 10 (27.0) | 0.49 (0.21-1.13) | .10 | 0.61 (0.31-1.20) | .15 | ||
| ddMVAC | 46 | 24 (52.2) | 1.74 (0.91-3.30) | .09 | 1.84 (1.10-3.09) | .02 | 1.62 (1.05-2.50) | .03 |
| Other | 50 | 29 (42.0) | 1.15 (0.61-2.14) | .67 | 1.31 (0.79-2.16) | .30 | ||
| None | 767 | 186 (25.7) | 0.52 (0.38-0.72) | <.001 | 0.59 (0.44-0.79) | <.001 | ||
| Overall survival | Total No. (%) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Gemcitabine-cisplatin | 204 (61.5) | NA | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Gemcitabine-carboplatin | 32 (9.6) | NA | 2.01 (1.19-3.38) | .01 | 2.00 (1.16-3.44) | .01 | ||
| ddMVAC | 46 (13.9) | NA | 0.42 (0.17-1.05) | .06 | 0.42 (0.17-1.06) | .07 | 0.44 (0.14-1.38) | .16 |
| Other | 50 (15.1) | NA | 1.51 (0.96-2.38) | .07 | 1.65 (1.05-2.80) | .03 |
Abbreviations: ddMVAC, dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; HR, hazard ratio; NAC, neoadjuvant chemotherapy; NA, not applicable because downstaging is shown in rows above; and OR, odds ratio.
Adjusted for age, comorbidity, sex, clinical stage, and chemotherapy regimen. The number of cycles was included for calculation of HRs.
Weighted for age, comorbidity, sex, clinical stage, race/ethnicity, marital status, ethnicity, insurance, histology, diversion type, number of cycles of chemotherapy. Dose-dense MVAC was compared with gemcitabine-cisplatin.
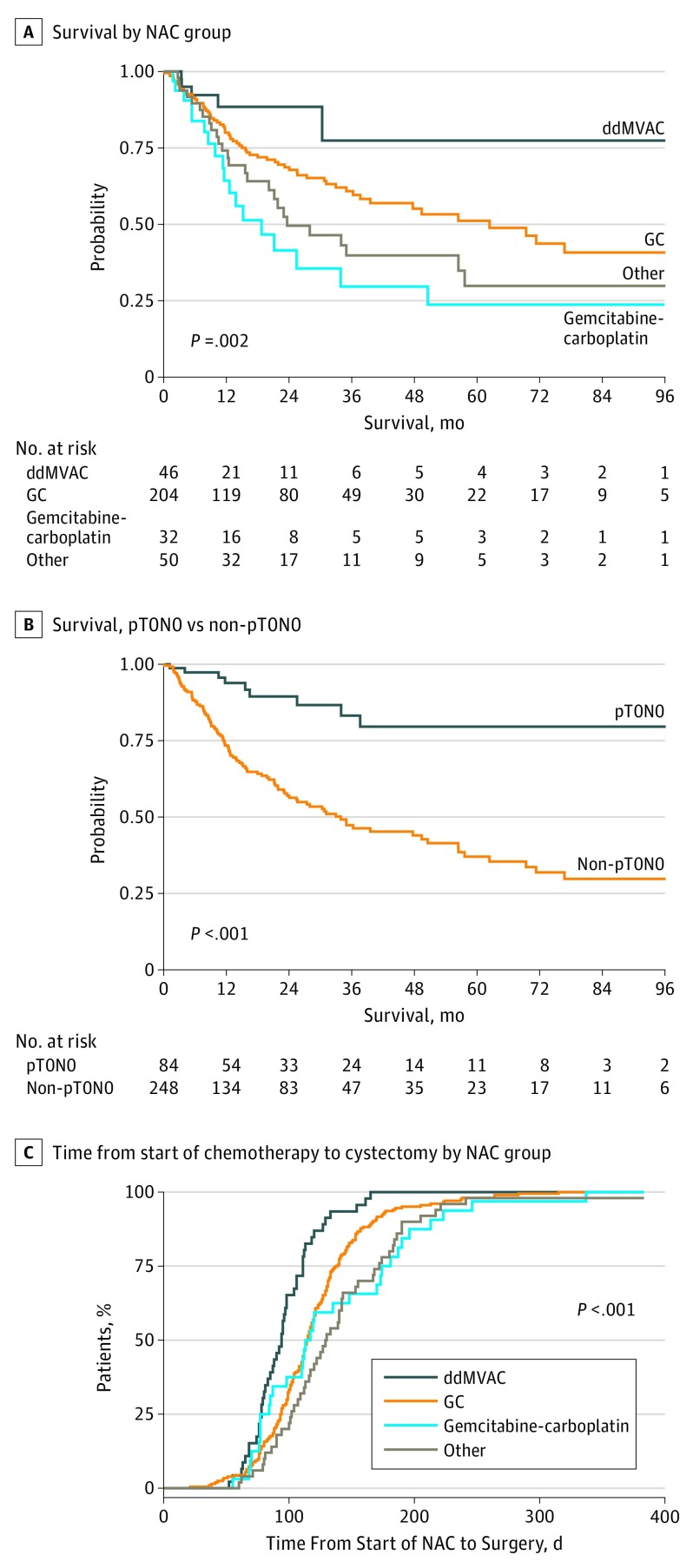
Unadjusted and adjusted survival analyses demonstrated a higher median overall survival among patients treated with neoadjuvant ddMVAC compared with those treated with other chemotherapy regimens. Two-year Kaplan-Meier survival probability estimates were 73.3% (95% CI, 48.0%-89.1%) for ddMVAC, 62% (95% CI, 53.4%-69.9%) for gemcitabine-cisplatin, and 34.8% (95% CI, 18.8%-55.1%) for gemcitabine-carboplatin (log-rank P = .002; Figure 3A). Achieving ypT0N0 was also a significant predictor of overall survival, regardless of chemotherapy type (log-rank P < .001; Figure 3B). The estimated risk of death was 60% lower for ddMVAC than for gemcitabine-cisplatin, according to adjusted (HR, 0.42; 95% CI, 0.17-1.06; P = .07) and propensity-weighted modeling (HR, 0.44; 95% CI, 0.14-1.38; P = .16) (Table 2), but these did not reach statistical significance. In contrast, the adjusted risk of death for gemcitabine-carboplatin was twice that of gemcitabine-cisplatin (HR, 2.00; 95% CI, 1.16-3.44; P = .01). The full Cox proportional hazard model for overall survival results are reported in eTable 3 in the Supplement. Seventy-eight patients with ypT0N0 (92.8%) were alive 2 years after surgery. When patients with ypT0N0 were stratified by NAC regime, 46 (92.0%) receiving gemcitabine-cisplatin, 3 (100%) receiving gemcitabine-carboplatin, 19 (100%) receiving ddMVAC, and 10 (83.3%) receiving other types of NAC were alive at 2 years.
Figure 3. Survival Analyses.
A, Kaplan-Meier curves for overall survival stratified by neoadjuvant chemotherapy group. B, Kaplan-Meier curves for overall survival for patients with complete response (pT0N0) vs those without complete responses (non-pT0N0). C, Days from start of neoadjuvant chemotherapy to radical cystectomy by neoadjuvant chemotherapy group. ddMVAC indicates dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; GC, gemcitabine-cisplatin; and NAC, neoadjuvant chemotherapy.
The median (IQR) treatment time for ddMVAC was 35 (28-46) days (mean [SD] treatment time, 40.5 [17.0] days). The summary of ddMVAC adverse events (eTable 2 in the Supplement) shows that the most common grade 1 or 2 event was fatigue (n = 26) and that the most common grade 3 event was anemia requiring blood transfusion (n = 3). No grade 4 adverse events were identified. These results are consistent with previously published toxic effect reports for ddMVAC.16,18 Dose-dense MVAC also hastened readiness for surgery. The times from start of NAC to cystectomy were 95 days for ddMVAC, 119 days for gemcitabine-cisplatin, and 134 days for gemcitabine-carboplatin (Figure 3C; χ2 = 25.1; P < .001).
Discussion
Cisplatin-based chemotherapy administered before cystectomy improves survival among patients with muscle-invasive bladder cancer compared with treatment with cystectomy alone.1,2,23,24 Although most trial evidence has come from trials evaluating methotrexate, vinblastine, and cisplatin and MVAC regimens, gemcitabine-cisplatin has effectively become the standard neoadjuvant regimen, in part owing to its favorable toxic effects profile and trial results that have shown comparable metastatic disease results for gemcitabine-cisplatin and MVAC.14,15,25 Furthermore, several single-institution studies have demonstrated similar treatment effects for neoadjuvant standard MVAC and gemcitabine-cisplatin.26,27,28 Adjustments in administering MVAC, including accelerated scheduling and supportive measures to reduce the extent of cytopenia, have led to better tolerability, and as a result, 3 cycles of neoadjuvant MVAC can be safely and effectively delivered over a 6-week period.16,18 Our comparative analyses identified a significantly higher likelihood of ypT0N0 and longer survival intervals for patients who received ddMVAC compared with those treated with other regimens. We observed a complete response rate of 41.3% for patients who received an average of 3.3 cycles of ddMVAC, which was significantly higher than the rate of 24.6% for those who received an average of 3.7 cycles of gemcitabine-cisplatin. Patients treated with ddMVAC also had higher survival rates than those treated with other regimens, and although the survival association did not reach statistical significance, the magnitude of the advantage is relatively large (HR, 0.42; 95% CI, 0.17-1.06). We also confirmed a surrogate association of ypT0N0 disease status after therapy with overall survival. The association of complete pathologic response with survival for patients with nonmetastatic bladder cancer treated with chemotherapy and cystectomy has been documented in previous studies.23,29,30 Another important finding of the present study was that neoadjuvant gemcitabine-carboplatin appears essentially ineffective. Complete response rates were low (9.4%), and more concerning still, the adjusted risk of death was significantly higher than for the reference gemcitabine-cisplatin group (HR, 2.0; 95% CI, 1.19-3.38). These results raise questions regarding the role of neoadjuvant gemcitabine-carboplatin. Finally, we confirmed that the time from start of NAC to cystectomy was expedited with ddMVAC, allowing patients to complete their global treatment more quickly than with other NAC regimens.31
Reported ypT0N0 rates associated with standard neoadjuvant MVAC range from 22% to 29% in observational studies19,20,32 and from 34% to 38% in prospective randomized clinical trials.1,24 Response rates appear similar for ddMVAC; Choueiri et al17 reported downstaging to pT1N0 or lower for 19 of 39 patients (49%) treated with 4 cycles of neoadjuvant ddMVAC, and Plimack et al18 achieved pT0 in 15 of 40 (38%) of patients treated with 3 cycles of ddMVAC. However, randomized comparative studies examining pathologic and survival outcomes across different NAC regimens are lacking.17,18 The Southwest Oncology Group trial 1314 is currently randomizing patients to neoadjuvant gemcitabine-cisplatin or ddMVAC,33 although the primary goal of that study is to evaluate gene-expression marker profiles for complete response.
Comparative nonrandomized studies are also lacking. Galsky et al19 reported similar pT0N0 rates between neoadjuvant MVAC (19 of 66 [29%]) and gemcitabine-cisplatin (45 of 146 [30.8%]) cohorts), and no significant difference in survival. Similar downstaging rates between gemcitabine-cisplatin (ypT0 in 144 of 602 [23.9%] of cases) and MVAC (ypT0 in 45 of 183 [24.5%] of cases) were also reported in a large multi-institutional comparative analysis that included 935 patients, of whom 602 received gemcitabine-cisplatin and 183 received MVAC.20 A recent follow-up analysis on a subset of 319 cT3-4aN0M0 patients reported lower complete response within the gemcitabine-cisplatin group (32 of 219 [14.6%]) compared with ddMVAC (28 of 100 [28.0%]), and a higher risk of death (HR, 2.07; 95% CI, 1.25-3.42; P = .003).32 Of note, our observed complete response rate was substantially higher than that reported by Zargar et al,32 raising the possibility that the administration and effectiveness of MVAC may have been different or that the patient samples were fundamentally dissimilar. This does not, however, explain the lack of a more direct surrogate association between ypT0N0 response and survival in the study by Zargar et al.32 The complete response rate observed in this analysis (19 of 46 [41.3%]) is similar to that reported in the Southwest Oncology Group trial 8710 (48 of 126 [38.0%]).1 Lower complete response rates reported in previous observational studies may be a function of differences in tolerance and duration of chemotherapy. This type of clinical and treatment information may not be readily available or examined in multisite observational studies that are based on shared data, further limiting insight regarding lower-than-expected complete pathologic response rates.20,32,34
Limitations
A number of limitations warrant discussion. These include the nonrandomized retrospective design, which could result in bias and confounding. In addition to using standard adjustment methods, we included propensity-score weights to account for measured differences between patients. Propensity scores attempt to account for baseline characteristics that may influence assignment to treatment groups and allow for inclusion of multiple, measurable covariates without overfitting the regression model. The weighted combination of covariates contributes to a single propensity score applied across treatment groups to “level the playing field” before hypothesis testing.35,36,37 Unmeasured differences in patient groups could lead to residual confounding, which is a limitation in all observational comparative studies. For example, although we did control for comorbidity based on the Elixhauser method,21 there may be unmeasured factors influencing frailty or performance status that are not included in multivariable or propensity-weighted models. In addition, our study does not account for patients who received NAC and did not have surgery owing to complications or symptomatic adverse events or disease progression. The relatively small size of the ddMVAC group in our sample is a limitation, which may have lessened our statistical power. Although our adjusted analyses failed to reach a .05 significance threshold, the magnitude of the survival differences that we observed were substantial and suggest the clinical importance of the findings. With additional follow-up and a larger ddMVAC sample, the CIs around the adjusted and propensity-weighted hazard estimates will likely tighten. Furthermore, we were able to demonstrate a direct and significant relationship between ypT0N0 downstaging and survival, adding plausibility to the survival benefit of ddMVAC compared with gemcitabine-cisplatin. These limitations notwithstanding, our study findings contribute substantively to the evidence around neoadjuvant ddMVAC.
Conclusions
Our data suggest that neoadjuvant ddMVAC followed by radical cystectomy is associated with higher complete response rates and disease control than gemcitabine-cisplatin and that the pT0 rate after treatment with neoadjuvant gemcitabine-carboplatin is no different than that achieved with cystectomy alone. We also found that ddMVAC was associated with longer survival intervals and a lower risk of death than the other treatments examined, although those findings did not reach statistical significance, indicating that larger comparative studies are needed to definitively answer questions regarding survival.
eTable 1. Social and Demographic Information by Neoadjuvant Chemotherapy Type
eTable 2.Toxicity Summary for ddMVAC According to CTCAE v4.0
eTable 3. Full Cox Proportional Hazard Model for Overall Survival Results
References
- 1.Grossman HB, Natale RB, Tangen CM, et al. . Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859-866. doi: 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 2.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202-205. doi: 10.1016/j.eururo.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK; International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Finnbladder; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group . International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171-2177. doi: 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfred Witjes J, Lebret T, Compérat EM, et al. . Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462-475. doi: 10.1016/j.eururo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 5.Chang SS, Bochner BH, Chou R, et al. . Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198(3):552-559. doi: 10.1016/j.juro.2017.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiess PE, Agarwal N, Bangs R, et al. . Bladder cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(10):1240-1267. doi: 10.6004/jnccn.2017.0156 [DOI] [PubMed] [Google Scholar]
- 7.Bajorin DF, Herr HW. Kuhn’s paradigms: are those closest to treating bladder cancer the last to appreciate the paradigm shift? J Clin Oncol. 2011;29(16):2135-2137. doi: 10.1200/JCO.2010.34.0471 [DOI] [PubMed] [Google Scholar]
- 8.Donat SM. Integrating perioperative chemotherapy into the treatment of muscle-invasive bladder cancer: strategy versus reality. J Natl Compr Canc Netw. 2009;7(1):40-47. doi: 10.6004/jnccn.2009.0003 [DOI] [PubMed] [Google Scholar]
- 9.Porter MP, Kerrigan MC, Donato BM, Ramsey SD. Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol. 2011;29(3):252-258. doi: 10.1016/j.urolonc.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 10.Bassi P, Pagano F, Pappagallo G, et al. . Neoadjuvant M-VAC of invasive bladder cancer: G.U.O.N.E. multicenter phase III trial [abstract 567]. Eur Urol. 1998;33(suppl 1):142. [Google Scholar]
- 11.GISTV (Italian Bladder Cancer Study Group) Neoadjuvant treatment for locally advanced bladder cancer: a randomized prospective clinical trial. J Chemother. 1996;8(suppl 4):345-346. [Google Scholar]
- 12.International collaboration of trialists Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354(9178):533-540. doi: 10.1016/S0140-6736(99)02292-8 [DOI] [PubMed] [Google Scholar]
- 13.Sternberg CN, Yagoda A, Scher HI, et al. . Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium: efficacy and patterns of response and relapse. Cancer. 1989;64(12):2448-2458. doi: [DOI] [PubMed] [Google Scholar]
- 14.Loehrer PJ Sr, Einhorn LH, Elson PJ, et al. . A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10(7):1066-1073. doi: 10.1200/JCO.1992.10.7.1066 [DOI] [PubMed] [Google Scholar]
- 15.von der Maase H, Sengelov L, Roberts JT, et al. . Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602-4608. doi: 10.1200/JCO.2005.07.757 [DOI] [PubMed] [Google Scholar]
- 16.Sternberg CN, de Mulder PH, Schornagel JH, et al. ; European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group . Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19(10):2638-2646. doi: 10.1200/JCO.2001.19.10.2638 [DOI] [PubMed] [Google Scholar]
- 17.Choueiri TK, Jacobus S, Bellmunt J, et al. . Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32(18):1889-1894. doi: 10.1200/JCO.2013.52.4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. . Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32(18):1895-1901. doi: 10.1200/JCO.2013.53.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galsky MD, Pal SK, Chowdhury S, et al. ; Retrospective International Study of Cancers of the Urothelial Tract (RISC) Investigators . Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121(15):2586-2593. doi: 10.1002/cncr.29387 [DOI] [PubMed] [Google Scholar]
- 20.Zargar H, Espiritu PN, Fairey AS, et al. . Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67(2):241-249. doi: 10.1016/j.eururo.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE): Version 4.0. Bethesda, MD: US Dept of Health & Human Services, National Institutes of Health; 2010. [Google Scholar]
- 23.Sonpavde G, Goldman BH, Speights VO, et al. . Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115(18):4104-4109. doi: 10.1002/cncr.24466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitamura H, Tsukamoto T, Shibata T, et al. ; Urologic Oncology Study Group of the Japan Clinical Oncology Group . Randomised phase III study of neoadjuvant chemotherapy with methotrexate, doxorubicin, vinblastine and cisplatin followed by radical cystectomy compared with radical cystectomy alone for muscle-invasive bladder cancer: Japan Clinical Oncology Group Study JCOG0209. Ann Oncol. 2014;25(6):1192-1198. doi: 10.1093/annonc/mdu126 [DOI] [PubMed] [Google Scholar]
- 25.von der Maase H, Hansen SW, Roberts JT, et al. . Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068-3077. doi: 10.1200/JCO.2000.18.17.3068 [DOI] [PubMed] [Google Scholar]
- 26.Dash A, Pettus JA 4th, Herr HW, et al. . A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113(9):2471-2477. doi: 10.1002/cncr.23848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairey AS, Daneshmand S, Quinn D, et al. . Neoadjuvant chemotherapy with gemcitabine/cisplatin vs. methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive urothelial carcinoma of the bladder: a retrospective analysis from the University of Southern California. Urol Oncol. 2013;31(8):1737-1743. doi: 10.1016/j.urolonc.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 28.Yeshchina O, Badalato GM, Wosnitzer MS, et al. . Relative efficacy of perioperative gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. 2012;79(2):384-390. doi: 10.1016/j.urology.2011.10.050 [DOI] [PubMed] [Google Scholar]
- 29.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014;65(2):350-357. doi: 10.1016/j.eururo.2013.06.049 [DOI] [PubMed] [Google Scholar]
- 30.Rosenblatt R, Sherif A, Rintala E, et al. ; Nordic Urothelial Cancer Group . Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229-1238. doi: 10.1016/j.eururo.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 31.Alva AS, Tallman CT, He C, et al. . Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer: a multidisciplinary approach. Cancer. 2012;118(1):44-53. doi: 10.1002/cncr.26240 [DOI] [PubMed] [Google Scholar]
- 32.Zargar H, Shah JB, van Rhijn BW, et al. . Neoadjuvant dose dense MVAC versus GC in patients with cT3-4aN0M0 bladder cancer treated with radical cystectomy. J Urol. 2018;199(6):1452-1458. doi: 10.1016/j.juro.2017.12.062 [DOI] [PubMed] [Google Scholar]
- 33.Southwest Oncology Group NCI A Randomized Phase II Study of Co-Expression Extrapolation (COXEN) With Neoadjuvant Chemotherapy for Localized, Muscle-Invasive Bladder Cancer. https://clinicaltrials.gov/ct2/show/NCT02177695. Accessed December 12, 2017. [DOI] [PMC free article] [PubMed]
- 34.Zargar H, Shah JB, van de Putte EEF, et al. . Dose dense MVAC prior to radical cystectomy: a real-world experience. World J Urol. 2017;35(11):1729-1736. doi: 10.1007/s00345-017-2065-x [DOI] [PubMed] [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281. doi: [DOI] [PubMed] [Google Scholar]
- 37.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242-1258. doi: 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Social and Demographic Information by Neoadjuvant Chemotherapy Type
eTable 2.Toxicity Summary for ddMVAC According to CTCAE v4.0
eTable 3. Full Cox Proportional Hazard Model for Overall Survival Results