Key Points
Question
Do loss of function variants in LRRK1 and/or LRRK2 increase the risk of developing Parkinson disease?
Findings
This case-control cohort study screened 11 095 participants with Parkinson disease and 12 615 controls using next-generation sequencing data for loss of function variants in LRRK1 and LRRK2. No significant enrichment in cases with Parkinson disease (0.205% and 0.117%, respectively) was found compared with controls (0.139% and 0.087%, respectively).
Meaning
LRRK1 and LRRK2 loss of function variants do not increase the risk of Parkinson disease; kinase inhibition or allele-specific targeting of mutant LRRK2 remain viable therapeutic strategies in a subset of patients with Parkinson disease.
This case-control cohort study used next-generation sequencing data to determine whether for loss of function variants in LRRK1 and LRRK2 are associated with the etiology of Parkinson disease.
Abstract
Importance
Pathogenic variants in LRRK2 are a relatively common genetic cause of Parkinson disease (PD). Currently, the molecular mechanism underlying disease is unknown, and gain and loss of function (LOF) models of pathogenesis have been postulated. LRRK2 variants are reported to result in enhanced phosphorylation of substrates and increased cell death. However, the double knockout of Lrrk2 and its homologue Lrrk1 results in neurodegeneration in a mouse model, suggesting that disease may occur by LOF. Because LRRK2 inhibitors are currently in development as potential disease-modifying treatments in PD, it is critical to determine whether LOF variants in LRRK2 increase or decrease the risk of PD.
Objective
To determine whether LRRK1 and LRRK2 LOF variants contribute to the risk of developing PD.
Design, Setting, and Participants
To determine the prevailing mechanism of LRRK2-mediated disease in human populations, next-generation sequencing data from a large case-control cohort (>23 000 individuals) was analyzed for LOF variants in LRRK1 and LRRK2. Data were generated at 5 different sites and 5 different data sets, including cases with clinically diagnosed PD and neurologically normal control individuals. Data were collected from 2012 through 2017.
Main Outcomes and Measures
Frequencies of LRRK1 and LRRK2 LOF variants present in the general population and compared between cases and controls.
Results
Among 11 095 cases with PD and 12 615 controls, LRRK1 LOF variants were identified in 0.205% of cases and 0.139% of controls (odds ratio, 1.48; SE, 0.571; 95% CI, 0.45-4.44; P = .49) and LRRK2 LOF variants were found in 0.117% of cases and 0.087% of controls (odds ratio, 1.48; SE, 0.431; 95% CI, 0.63-3.50; P = .36). All association tests suggested lack of association between LRRK1 or LRRK2 variants and PD. Further analysis of lymphoblastoid cell lines from several heterozygous LOF variant carriers found that, as expected, LRRK2 protein levels are reduced by approximately half compared with wild-type alleles.
Conclusions and Relevance
Together these findings indicate that haploinsufficiency of LRRK1 or LRRK2 is neither a cause of nor protective against PD. Furthermore, these results suggest that kinase inhibition or allele-specific targeting of mutant LRRK2 remain viable therapeutic strategies in PD.
Introduction
Parkinson disease (PD) is a neurodegenerative disease that affects multiple brain regions, including dopaminergic neurons in the substantia nigra, and results in a complex disease with motor and nonmotor symptoms.1 Parkinson disease has a large genetic component, with LRRK2 being one of the most commonly mutated genes in the autosomal dominant and sporadic forms.2,3,4 One pathogenic LRRK2 variant (p.G2019S) is estimated to be present in approximately 1% of all cases with PD and up to 39% of cases in some populations.5,6,7 The LRRK2 locus has also been associated with idiopathic PD by genome-wide association studies, indicating that LRRK2 is a pleomorphic risk locus where multiple variants can exist that modify the risk of PD, presumably by overlapping mechanisms.4
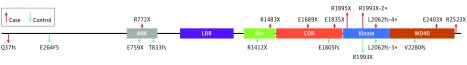
LRRK2 encodes a large multidomain protein (Figure 1) that has been suggested to play roles in many cellular processes, including vesicular trafficking, microtubule binding, autophagy and mitophagy.8 Pathogenic LRRK2 variants lead to enhanced phosphorylation of substrates and increased cell death, suggesting that kinase inhibitors block this gain of function and may be used therapeutically for PD.9,10 However, a recent report11 suggested that knockout of Lrrk2 and its homologue, Lrrk1, results in loss of dopaminergic neurons and α-synuclein pathologic change in a mouse model. Although LRRK1 variants have not been associated with PD or PD age of onset by genome-wide association studies or other analyses, LRRK1 could act as a modifier of LRRK2-associated PD owing to its sequence similarity. LRRK2 variants may cause PD through a type of loss of function (LOF), mediated by haploinsufficiency or a dominant negative mechanism, which would invalidate kinase inhibitors as a potential treatment. To investigate this possibility in humans, we identified LOF variants in LRRK1 and LRRK2 in a large series of cases with PD and control individuals and examined protein expression from several LRRK2 LOF variant carriers.
Figure 1. Schematic of the LRRK2 Protein With Estimated Loss of Function (LOF) Variants Indicated.
Estimated LOF variants are found across the length of the protein in cases and controls. Variants that were identified in multiple individuals are annotated as 2×, 3×, and 4× for the number of times found in cases or controls. ANK indicates Ankryn repeats; COR, C-terminal of Roc; and LRR, leucine-rich repeats.
Methods
Next-generation sequencing data were obtained from 11 095 cases with PD and 12 615 controls in 5 exome and resequencing data sets (eTable 1 in the Supplement). Data processing was performed as previously described, with variants filtered for a minimal read depth of 20, genotype quality score of greater than 20, and an alternate allele ratio of greater than 25%.7 All LOF variant carriers were also screened for known pathogenic LRRK2 variants, and none were detected. Variants were collapsed per gene owing to low frequencies and because LOF variants should result in haploinsufficiency. In addition, combined multivariate and collapsing and optimized sequence kernel association burden tests were performed per gene.12 Lymphoblastoid cell lines (LCLs) were obtained from the Coriell Institute, Camden, New Jersey (https://www.coriell.org/) and cultured using standard practices. Details can be found in eMethods in the Supplement. Data were collected from 2012 through 2017. All samples were collected under informed consent procedures approved by the institutional review board of each collecting institution.
Statistical Analysis of LOF Variants
To evaluate whether there are statistical differences between cases and controls in the frequency of LRRK1 and LRRK2 LOF variants we performed a number of tests using R and RVTESTS (version 20171009).13 Logistic regression was performed using collapsed LOF variants per gene due to low frequencies of the variants and because LOF variants should all result in haploinsufficiency. Additionally, combined multivariate and collapsing and optimized sequence kernel association burden tests were performed per gene. For the LRRK2 tests, we used each separate dataset as covariate. Power calculations were performed with the SKAT (v1.3.2.1) package 8 in R (R Foundation) using the following parameters: significance level of P < .01, disease prevalence of 0.01, causal minor allele frequency of 0.001, and the percentage of causal single nucleotide polymorphisms among rare single nucleotide polymorphisms of 2 (eTables 2-6 in the Supplement).
Results
The study population included 11 095 cases with PD and 12 615 controls. From these, we identified 13 cases and 11 controls who carried an LRRK2 LOF variant (Table). The resulting frequency is 0.117% in cases and 0.087% in controls (odds ratio [OR], 1.48; SE, 0.431; 95% CI, 0.63-3.50; P = .36). Sixteen distinct LRRK2 LOF variants include 10 stop-gain variants, 3 frameshift insertions, and 3 frameshift deletions (Table). Six variants were novel (not present in the Genome Aggregation Database [gnomAD]; http://gnomad.broadinstitute.org/), whereas the remaining 10 were previously identified (Table). Burden and case-control association tests showed no significant difference in frequency of LRRK2 LOF variants, suggesting that LOF variants are not associated with PD (eTable 6 in the Supplement). These tests have 85% power to identify large causal effects (OR, >4.5). If LRRK2 LOF variants were a direct cause of PD, then we would expect them to have large ORs, and hence diminished power does not affect our conclusions.
Table. Overview of Identified LRRK2 and LRRK1 LOF Variantsa.
| Gene | Complementary DNA Change | Protein Change | Chromosome No.: Location, bp (hg19) | Exon | No. of Cases | No. of Controls | gnomAD Frequency |
|---|---|---|---|---|---|---|---|
| LRRK2 | c.110dupA | p.Q37fs | 12:40619042 | 1 | 0 | 1 | 0 |
| LRRK2 | c.790_791insAATTT | p.E264fs | 12:40637435 | 7 | 0 | 1 | 0 |
| LRRK2 | c.G2275T | p.E759X | 12:40677710 | 19 | 0 | 1 | 8.138E-6 (2 alleles) |
| LRRK2 | c.C2314T | p.R772X | 12:40677749 | 19 | 1 | 0 | 5.419E-5 (15 alleles) |
| LRRK2 | c.2499_2500del | p.T833fs | 12:40677934 | 19 | 0 | 1 | 1.635E-5 (4 alleles) |
| LRRK2 | c.C4234T | p.R1412X | 12:40702952 | 30 | 0 | 1 | 4.067E-6 (1 allele) |
| LRRK2 | c.C4447T | p.R1483X | 12:40704362 | 31 | 1 | 0 | 8.133E-6 (2 alleles) |
| LRRK2 | c.G5065T | p.E1689X | 12:40714885 | 35 | 1 | 0 | 0 |
| LRRK2 | c.5414delA | p.E1805fs | 12:40716217 | 37 | 0 | 1 | 0 |
| LRRK2 | c.G5503T | p.E1835X | 12:40716306 | 37 | 1 | 0 | 4.069E-6 (1 allele) |
| LRRK2 | c.C5683T | p.R1895X | 12:40722188 | 39 | 1 | 0 | 1.08E-05 (3 alleles) |
| LRRK2 | c.C5977T | p.R1993X | 12:40734124 | 41 | 2 | 1 | 0 |
| LRRK2 | c.6184_6188del | p.L2062fs | 12:40740629 | 42 | 4 | 3 | 0.0001625 (45 alleles) |
| LRRK2 | c.G6838/TTAAins | p.V2280fs | 12:40749984 | 46 | 0 | 1 | 4.149E-6 (1 allele) |
| LRRK2 | c.G7207T | p.E2403X | 12:40758669 | 49 | 1 | 0 | 0 |
| LRRK2 | c.C7567T | p.R2523X | 12:40761550 | 51 | 1 | 0 | 1.647E-5 (4 alleles) |
| LRRK1 | c.1730_1739del | p.Y577fs | 15:101561378 | 13 | 1 | 0 | 0 |
| LRRK1 | c.2287_2293del | p.E763fs | 15:101566224 | 17 | 1 | 0 | 0 |
| LRRK1 | c.C3259T | p.Q1087X | 15:101588822 | 22 | 0 | 1 | 0 |
| LRRK1 | c.5888_5889del | p.A1963fs | 15:101608893 | 34 | 0 | 1 | 0 |
| LRRK1 | c.4790_4797del | p.A1597fs | 15:101601486 | 30 | 3 | 6 | 0.001014 (280 alleles) |
Because LRRK1 is not a known cause of PD, it was only covered in the whole-exome sequencing data set; therefore, our analysis is based on 2440 cases and 5774 controls. We identified 5 cases and 8 controls carrying an LRRK1 LOF variant, resulting in a frequency of 0.205% in cases and 0.139% in controls (OR, 1.48; SE, 0.571; 95% CI, 0.45-4.44; P = .49). These frequencies are slightly higher than those for LRRK2, but with fewer samples we cannot directly evaluate whether this proportion is more than expected by chance. Five distinct LRRK1 LOF variants included 1 stop-gain and 4 frameshift deletions (Table). Four variants were novel, whereas one was previously identified in gnomAD at a frequency of 0.101%. Five homozygous carriers of this allele have been described in gnomAD, implying that presumably healthy individuals lack the normal LRRK1 protein. Burden and case-control association tests for PD show no significant difference in LRRK1 LOF variant frequency (eTable 6 in the Supplement). Owing to the smaller sample size, LRRK1 association tests had 70% power to identify large effects (OR, >6.0). Although the ORs are reduced compared with those for LRRK2, we would still expect that causative LOF variants would have a large OR. Therefore, this study is powered well enough to detect those differences between cases and controls.
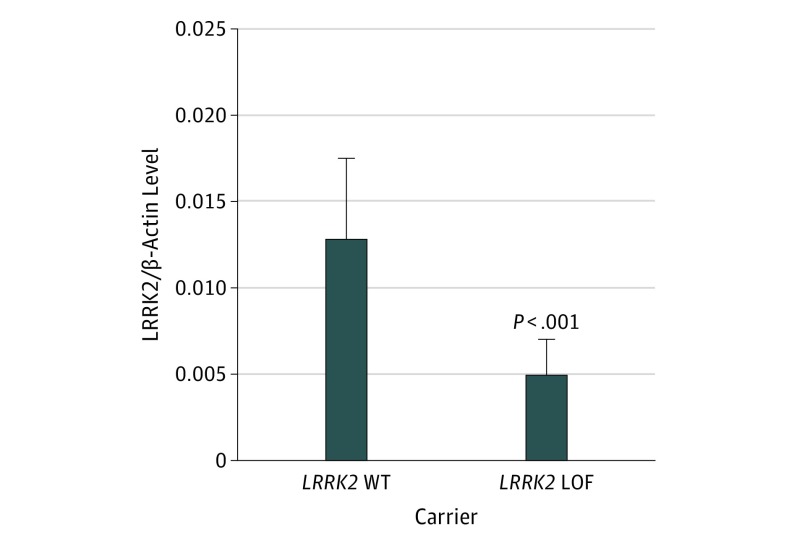
We compared LRRK2 protein levels in individuals with and without LRRK2 LOF variants using LCLs from the Coriell Institute. Three LCLs carried a LRRK2 LOF variant, and 5 LCLs were normal at LRRK2. We examined protein levels using Western blot analysis and found that normalized LRRK2 protein levels are reduced by approximately half in estimated LOF lines compared with lines without LRRK2 variants (eFigures 1 and 2 in the Supplement). A statistical comparison of mean normalized protein levels in normal LCLs compared with LOF carrier samples shows a significant difference in LRRK2 protein levels (Figure 2), indicating that these are true LOF variants.
Figure 2. Mean Normalized LRRK2 Protein Levels in Loss of Function (LOF) LRRK2 Carriers.
Protein levels are significantly reduced compared with LRRK2 WT carriers. WT indicates wild-type. Error bars indicate SD. Comparison used unpaired t test with Welch correction.
Discussion
We have analyzed a large set of cases with PD and controls for LOF variants in LRRK1 and LRRK2. Our results show that LRRK1 and LRRK2 LOF variants occur in the general population at an estimated frequency of approximately 0.1% to 0.2%. Interestingly, no significant differences were seen in LOF variant prevalence between cases with PD and controls. We have also shown that LRRK2 protein levels are diminished in LCLs from LRRK2 LOF carriers compared with individuals with normal LRRK2. We add more evidence to support the view that LRRK1 is unlikely to cause disease on its own, and more importantly, that pathogenic LRRK2 variants are likely to act through a gain of function rather than an LOF mechanism to cause PD.
Limitations
Among the limitations in this study, not all DNA samples were available for Sanger sequencing validation. However, because most of these variants are present in gnomAD, they are likely authentic variants. We also used stringent filters for sequencing depth to minimize false-positive findings. Although the analysis was completed in a relatively large data set, we lacked power to detect smaller effect sizes. However, causal variants typically have large effect sizes; therefore, our results imply that LRRK1 and LRRK2 LOF variants do not cause PD. We cannot exclude the possibility of small effects on PD risk.
Conclusions
Recognizing that LOF variants in LRRK2 are not damaging to an individual indicates that kinase inhibitors and allele-specific oligos could be used as PD therapies. Previous studies14,15 have shown that mutant alleles of LRRK2 can be selectively knocked down in various cell lines using allele-specific oligos. Additional studies of chronic LRRK2 kinase inhibition in mice and nonhuman primates16,17 have shown that multiple inhibitors are effective at reducing LRRK2 phosphorylation in the brain with no adverse effects in the kidneys; however, a lysosomal phenotype has been reported in treated nonhuman primate lungs that is similar to reports from Lrrk2 knockout mice. Our results support the expansion of these studies in clinical trials and in cells from LRRK2 variant carriers.
eMethods. Variant Identification and Testing
eTable 1. Overview of Included Datasets
eTable 2. Primer Sequences Used for Validation of LRRK2 Mutations in Lymphoblastoid Cell Lines
eTable 3. LRRK2 Loss of Function Mutation Carrier Specifications
eTable 4. LRRK1 Loss of Function Mutation Carrier Specifications
eTable 5. LRRK2 Protein Expression Samples Specifications Lymphoblastoid Cell Lines
eTable 6. Outcome Overview of Statistical Tests Performed
eFigure 1. Representative Western Blot Showing LRRK2 Protein Levels From 5 WT and 3 LRRK2 LOF Variant Carriers
eFigure 2. Mean Average Normalized Protein Signal From 4 Separate Western Blots
eAppendix. Detailed Information About Collaborators and Affiliated Groups and Projects
References
- 1.Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596. doi: 10.1002/ana.20834 [DOI] [PubMed] [Google Scholar]
- 2.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601-607. doi: 10.1016/j.neuron.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Paisán-Ruíz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44(4):595-600. doi: 10.1016/j.neuron.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 4.Singleton A, Hardy J. The evolution of genetics: Alzheimer’s and Parkinson’s diseases. Neuron. 2016;90(6):1154-1163. doi: 10.1016/j.neuron.2016.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesage S, Dürr A, Tazir M, et al. ; French Parkinson’s Disease Genetics Study Group . LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N Engl J Med. 2006;354(4):422-423. doi: 10.1056/NEJMc055540 [DOI] [PubMed] [Google Scholar]
- 6.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354(4):424-425. doi: 10.1056/NEJMc055509 [DOI] [PubMed] [Google Scholar]
- 7.Blauwendraat C, Kia DA, Pihlstrom L, et al. ; International Parkinson’s Disease Genomics Consortium (IPDGC); COURAGE-PD Consortium. Insufficient evidence for pathogenicity of SNCA His50Gln (H50Q) in Parkinson’s disease. Neurobiol Aging. 2018;64(159):159.e5-e159.e8. doi: 10.1016/j.neurobiolaging.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roosen DA, Cookson MR. LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol Neurodegener. 2016;11(1):73. doi: 10.1186/s13024-016-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greggio E, Jain S, Kingsbury A, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23(2):329-341. doi: 10.1016/j.nbd.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 10.Sen S, West AB. The therapeutic potential of LRRK2 and α-synuclein in Parkinson’s disease. Antioxid Redox Signal. 2009;11(9):2167-2187. doi: 10.1089/ars.2009.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giaime E, Tong Y, Wagner LK, Yuan Y, Huang G, Shen J. Age-dependent dopaminergic neurodegeneration and impairment of the autophagy-lysosomal pathway in LRRK-deficient mice. Neuron. 2017;96(4):796-807.e6. doi: 10.1016/j.neuron.2017.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ionita-Laza I, Lee S, Makarov V, Buxbaum JD, Lin X. Sequence kernel association tests for the combined effect of rare and common variants. Am J Hum Genet. 2013;92(6):841-853. doi: 10.1016/j.ajhg.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics. 2016;32(9):1423-1426. doi: 10.1093/bioinformatics/btw079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L, Shimoji M, Wang J, et al. Development of inducible leucine-rich repeat kinase 2 (LRRK2) cell lines for therapeutics development in Parkinson’s disease. Neurotherapeutics. 2013;10(4):840-851. doi: 10.1007/s13311-013-0208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Yñigo-Mojado L, Martín-Ruíz I, Sutherland JD. Efficient allele-specific targeting of LRRK2 R1441 mutations mediated by RNAi. PLoS One. 2011;6(6):e21352. doi: 10.1371/journal.pone.0021352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fell MJ, Mirescu C, Basu K, et al. MLi-2, a potent, selective, and centrally active compound for exploring the therapeutic potential and safety of LRRK2 kinase inhibition. J Pharmacol Exp Ther. 2015;355(3):397-409. doi: 10.1124/jpet.115.227587 [DOI] [PubMed] [Google Scholar]
- 17.Fuji RN, Flagella M, Baca M, et al. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med. 2015;7(273):273ra15. doi: 10.1126/scitranslmed.aaa3634 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Variant Identification and Testing
eTable 1. Overview of Included Datasets
eTable 2. Primer Sequences Used for Validation of LRRK2 Mutations in Lymphoblastoid Cell Lines
eTable 3. LRRK2 Loss of Function Mutation Carrier Specifications
eTable 4. LRRK1 Loss of Function Mutation Carrier Specifications
eTable 5. LRRK2 Protein Expression Samples Specifications Lymphoblastoid Cell Lines
eTable 6. Outcome Overview of Statistical Tests Performed
eFigure 1. Representative Western Blot Showing LRRK2 Protein Levels From 5 WT and 3 LRRK2 LOF Variant Carriers
eFigure 2. Mean Average Normalized Protein Signal From 4 Separate Western Blots
eAppendix. Detailed Information About Collaborators and Affiliated Groups and Projects