Key Points
Question
Are midlife risk factors for stroke and cerebrovascular disease also risk factors for late-onset epilepsy?
Findings
In this cohort study of 10 420 adults, hypertension, diabetes, smoking, APOE ε4 allele number, activity level, and alcohol use were associated with late-onset epilepsy.
Meaning
Potentially modifiable midlife vascular and lifestyle factors were associated with epilepsy in later life.
Abstract
Importance
The incidence of epilepsy is higher in older age than at any other period of life. Stroke, dementia, and hypertension are associated with late-onset epilepsy; however, the role of other vascular and lifestyle factors remains unclear.
Objective
To identify midlife vascular and lifestyle risk factors for late-onset epilepsy.
Design, Setting, and Participants
The Atherosclerosis Risk in Communities (ARIC) study is a prospective cohort study of 15 792 participants followed up since 1987 to 1989 with in-person visits, telephone calls, and surveillance of hospitalizations (10 974 invited without completing enrollment). The ARIC is a multicenter study with participants selected from 4 US communities. This study included 10 420 black or white participants from ARIC with at least 2 years of Medicare fee-for-service coverage and without missing baseline data. Data were analyzed betweeen April 2017 and May 2018.
Exposures
Demographic, vascular, lifestyle, and other possible epilepsy risk factors measured at baseline (age 45-64 years) were evaluated in multivariable survival models including demographics, vascular risk factors, and lifestyle risk factors.
Main Outcomes and Measures
Time to development of late-onset epilepsy (2 or more International Classification of Diseases, Ninth Revision codes for epilepsy or seizures starting at 60 years or older in any claim [hospitalization or outpatient Medicare through 2013]), with first code for seizures after at least 2 years without code for seizures.
Results
Of the 10 420 total participants (5878 women [56.4%] and 2794 black participants [26.8%]; median age 55 years at first visit), 596 participants developed late-onset epilepsy (3.33 per 1000 person-years). The incidence was higher in black than in white participants (4.71; 95% CI, 4.12-5.40 vs 2.88; 95% CI, 2.60-3.18 per 1000 person-years). In multivariable analysis, baseline hypertension (hazard ratio [HR], 1.30; 95% CI, 1.09-1.55), diabetes (HR, 1.45; 95% CI, 1.17-1.80), smoking (HR, 1.09; 95% CI, 1.01-1.17), apolipoprotein E ε4 genotype (1 allele HR, 1.22; 95% CI, 1.02-1.45; 2 alleles HR, 1.95; 95% CI, 1.35-2.81), and incident stroke (HR, 3.38; 95% CI, 2.78-4.10) and dementia (HR, 2.56; 95% CI, 2.11-3.12) were associated with an increased risk of late-onset epilepsy, while higher levels of physical activity (HR, 0.90; 95% CI, 0.83-0.98) and moderate alcohol intake (HR, 0.72; 95% CI, 0.57-0.90) were associated with a lower risk. Results were similar after censoring individuals with stroke or dementia.
Conclusions and Relevance
Potentially modifiable risk factors in midlife and the APOE ε4 genotype were positively associated with risk of developing late-onset epilepsy. Although stroke and dementia were both associated with late-onset epilepsy, vascular and lifestyle risk factors were significant even in the absence of stroke or dementia.
This cohort study examines data from Atherosclerosis Risk in Communities Study to assess the association of midlife vascular and lifestyle risk factors with development of late-onset epilepsy.
Introduction
The annual incidence of epilepsy is 90 to 150 per 100 000 in elderly populations,1,2,3 higher than at any other time of life,2 with a prevalence of 1.1% by age 60 years4 and cumulative incidence of 4.4% by age 85 years.3 The number of patients with late-onset epilepsy (LOE; ie, recurrent unprovoked seizures starting at 60 years or older5) will markedly increase because the population older than 65 years is projected to grow by 60% in the next 15 years.6 Stroke and neurodegenerative disease account for 30% to 50% of cases of LOE,3,7,8,9 but 25% to 50% have no known cause.3,10,11
Prior (mostly cross-sectional) studies show that some vascular risk factors, such as hypertension12,13 and elevated cholesterol,12,14 are more common in patients with adult-onset epilepsy than in those without seizures. However, whether diabetes, cigarette smoking, diet, or exercise are associated with LOE is unknown. The apolipoprotein E ε4 (APOE ε4) polymorphism is a known risk factor for Alzheimer disease, but an association has not been previously examined between APOE ε4 and LOE.
The goal of this prospective cohort study analysis was to determine whether midlife risk factors (including potentially modifiable risk factors) for stroke and cerebrovascular disease are associated with LOE, using data collected in the Atherosclerosis Risk in Communities (ARIC) Study, a large, biracial cohort followed up for more than 25 years.
Methods
Participant Inclusion
Men and women ages 45 to 64 years were recruited to the ARIC cohort between 1987 and 1989 (n = 15 792) by probability sampling from 4 US communities15: Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; Jackson, Mississippi; and Washington County, Maryland. Participants were examined in person 5 times between 1987 and 2013 and contacted yearly by telephone (with semiannual calls starting in 2012). All participants provided written informed consent, and each center’s institutional review board approved the study.
Case Identification
To identify cases of incident epilepsy, we used claims data from the ARIC hospitalization records (reported by patient or proxy and identified through surveillance of local hospital discharge lists)16 and from the Centers for Medicare and Medicaid Services (CMS) Medicare fee-for-service (FFS) data (inpatient, outpatient, and carrier claims) for 1991 to 2013. Linkage of the Medicare data to the ARIC cohort was performed using social security number, sex, and birthdate.17 Of the 15 792 ARIC cohort participants, we identified 13 270 with enrollment in Medicare FFS during the period of observation.
We defined epilepsy cases as participants with 2 or more International Classification of Diseases, Ninth Revision (ICD-9) codes for epilepsy, seizures, or convulsions (345.00-345.91 or 780.39; definition 1) in ARIC hospitalization or Medicare claims data. The use of at least 2 ICD-9 codes has a reported sensitivity of 94.4% and specificity of 91.7% for identification of epilepsy.18 Given the potential for misclassification, we also examined a second definition that required medication information (definition 2): participants with 1 or more ICD-9 codes 345.00-345.91 or 780.39, and the use of an antiepileptic drug obtained from Medicare Part D data or recorded at the fifth in-person ARIC visit (2011-2013). The use of an epilepsy-related code with use of antiepileptic drug is reported to have a sensitivity of 82% to 84% and specificity of 94% to 98%19,20 for identification of epilepsy.
We defined cases of incident LOE as epilepsy with the first epilepsy-related code at 60 years or older, with at least 2 years of available ARIC hospitalization or Medicare data prior to the first code for seizures, epilepsy, or convulsions.
Because case identification was based on ARIC hospitalization and Medicare data, we included only participants with at least 2 years of FFS coverage in primary analysis (n = 11 538). For definition 2, only participants with Medicare Part D coverage were included (n = 6872).
Participants not giving permission for DNA research were excluded from analyses using APOE ε4 genotype (n = 46). Participants with known brain tumor, multiple sclerosis, brain surgery, or brain radiation were excluded (n = 95) because were those with a claims-identified first seizure prior to age 60 years (n = 64). We included all black and all white participants in North Carolina, all black participants in Mississippi, and all white participants in Maryland and Minnesota (because of small numbers, we excluded 48 participants of other races/ethnicities and 55 black participants in Maryland and Minnesota).
Independent Variables
Information on participant sex, education, race/ethnicity, field center, age, hypertension, diabetes, hyperlipidemia, body mass index (calculated as weight in kilograms divided by height in meters squared), smoking history, activity level, alcohol use, and diet was collected from ARIC visit 1 data. Blood pressure was measured 3 times, and the second and third measurements averaged. Hypertension was defined as mean systolic blood pressure at least 140 mm Hg, diastolic blood pressure at least 95 mm Hg, or use of an antihypertensive medication at that visit. Diabetes status was defined as fasting blood glucose level of at least 126 mg/dL (to convert to millimoles per liter, multiply by 0.0555), nonfasting blood glucose level of at least 200 mg/dL, use of diabetic medications or insulin, or self-report of physician-diagnosed diabetes. Hyperlipidemia was defined as low-density lipoprotein cholesterol level of at least 200 mg/dL (to convert to millimoles per liter, multiply by 0.0259) and obesity as a body mass index of 30 or higher.
Smoking history was obtained from each participant and the pack-years of smoking calculated. The Baecke activity index was calculated from the modified Baecke questionnaire,21 and divided by sex-adjusted standardized deviation.
The ARIC Healthy Food Score has been described previously.22 Using questionnaire data, we calculated an index of diet health from food groups such as dairy, vegetables, and meats. Participants were assigned a score for each food group based on the quintile of food consumption within each group, and the total points summed. We also obtained participants’ alcohol intake and calculated daily glycemic load from questionnaire data. A mean of 1 to 7 alcoholic drinks per week was considered moderate alcohol use.
In addition to analysis of separate risk factors, we evaluated a combined “Life’s Simple Seven” (LSS) score using the number of the American Heart Association’s recommendations23 met by each participant at a poor, intermediate, or ideal level (0, 1, or 2 points each)24: controlled blood pressure, cholesterol, and glucose levels; smoking status; physical activity; healthy diet; and healthy weight (see eTable 1 in the Supplement for category definitions). The APOE ε4 allele status was ascertained previously, and participants classified on the number of ε4 alleles (TaqMan assay; Applied Biosystems).25
Incident clinical stroke information was collected on all ARIC participants, based on hospitalization records, and adjudicated by computer algorithm and independent physician reviewers26; prevalent stroke before visit 1 was obtained by self-report. Determination of dementia was made by expert panel at visit 5 using neurocognitive assessments and informant interviews from visits 2 through 5, with additional cases identified via telephone interviews (with participants and/or informants) and surveillance data.27
Data Analysis
Statistical analysis was performed using Stata, version 15.0 (StataCorp). A 2-sided P value of .05 was considered significant. We used χ2 or t tests as appropriate to compare characteristics of participants with and without LOE. We used Cox proportional hazards models to estimate the hazard ratio (HR) of developing incident LOE for each individual risk factor, using the date of the first seizure code as the event failure time, participants’ 60th birthday as the origin time, and time since 60th birthday as modeling time. Participants were censored at date of death, last ARIC contact, or last month of CMS Medicare enrollment. The final model included demographic factors and all risk factors that were significant in multivariable analysis. Owing to uniracial enrollment at some sites, race/ethnicity and field center locations were combined into a joint covariate, as is standard in ARIC studies28,29: North Carolina white, North Carolina black, Mississippi black, Minnesota white, and Maryland white participants. We also included stroke and dementia as time-varying exposures (using the date of prevalent or incident stroke26 and date of dementia diagnosis27) in a multivariable model with our covariates and created a model that censored individuals at the time of dementia or adjudicated stroke.
We tested for interactions between the primary risk factors and race/ethnicity (hypothesizing stronger associations in black participants, based on previous studies showing higher rates of epilepsy in African American individuals4,30) and sex (hypothesizing no interactions). We also estimated HRs for development of LOE by tertiles of LSS score (described previously), adjusted for sex, race/ethnicity, center, age at visit 1, and education.23
We performed sensitivity analyses including participants regardless of Medicare FFS information. We analyzed only patients with LOE onset at age 67 years or older (n = 479), the first age at which participants were eligible for the diagnosis of LOE using only Medicare enrollment at age 65 years (rather than using ARIC hospitalization data or Medicare enrollment at younger than 65 years) and also using 2 years after the first ICD-9 code as the analysis origin. We assessed the influence of missing data using multiple imputation for missing variables. We also performed a sensitivity analysis that used a proportional hazards model, with death as a competing risk to LOE.31
Results
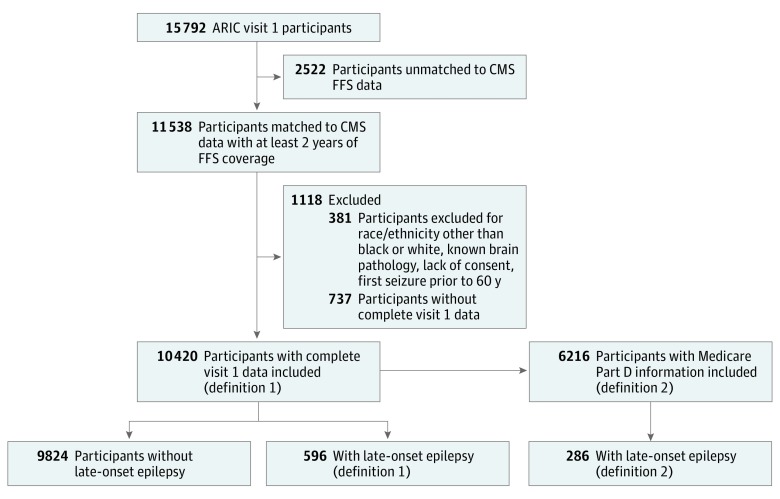
We identified 596 cases (ages 60-91 years at first seizure; 348 women) of LOE, using definition 1, of 10 420 eligible participants with FFS coverage and all baseline data (Figure 1). This represents an incidence rate of 3.33 cases of LOE per 1000 person-years after age 60 years (95% CI, 3.08-3.61). We identified 286 cases using definition 2, of 6216 participants Medicare Part D participation (incidence 2.58 per 10 000 person-years; 95% CI, 2.30-2.90). Participant characteristics are detailed in Table 1.
Figure 1. Identification of Cases of Late-Onset Epilepsy.
Definition 1 includes participants with 2 or more International Classification of Diseases, Ninth Revision (ICD-9) codes for seizures, epilepsy, or convulsions, with at least 2 years of Atherosclerosis Risk in Communities (ARIC) hospitalization or Medicare data prior to first seizure-related code, and first seizure at 60 years or older. Definition 2 includes participants with at least 1 epilepsy-related ICD-9 code and at least 1 antiepileptic drug prescription and includes only participants with Medicare Part D information available. CMS indicates Centers for Medicare and Medicaid Services; FFS, fee-for-service, RR, risk ratio.
Table 1. Characteristics of Participants With and Without Late-Onset Epilepsy.
| Variable | No. (%) (n = 10 420) | Incidence Rate of LOE per 1000 Person-Years (95% CI) |
|---|---|---|
| Incidence ages, y | ||
| 60-75 | NA | 2.39 (2.14-2.65) |
| ≥75 | NA | 6.92 (6.12-7.82) |
| Baseline variables | ||
| Race/ethnicity | ||
| White | 7626 (73.19) | 2.88 (2.60-3.18) |
| Black | 2794 (26.81) | 4.71 (4.12-5.40) |
| Sex | ||
| Male | 5878 (56.41) | 3.19 (2.82-3.61) |
| Female | 4542 (43.59) | 3.44 (3.10-3.82) |
| Education | ||
| ≤HS degree | 2558 (24.55) | 3.91 (3.36-4.53) |
| ≥HS | 7862 (75.45) | 3.15 (2.86-3.46) |
| No hypertension | 7309 (70.14) | 2.79 (2.51-3.10) |
| Hypertension | 3111 (29.86) | 4.62 (4.07-5.23) |
| No diabetes | 9212 (88.41) | 3.04 (2.78-3.32) |
| Diabetes | 1208 (11.59) | 5.70 (4.73-6.86) |
| Exercise | ||
| <Median Baecke score | 5048 (48.45) | 3.87 (3.48-4.30) |
| ≥Median Baecke score | 5372 (51.55) | 2.83 (2.50-3.19) |
| Alcohol use, mean drinks/wk | ||
| None | 6939 (66.59) | 3.62 (3.30-3.98) |
| 1-7 | 2243 (21.53) | 2.47 (2.02-3.01) |
| >7 | 1238 (11.88) | 3.29 (2.60-4.18) |
| Smoking, pack-years | ||
| Never | 4569 (43.85) | 3.31 (2.94-3.73) |
| <25 | 3007 (28.85) | 2.98 (2.54-3.49) |
| ≥25 | 2844 (27.29) | 3.76 (3.24-4.37) |
| APOE ε4 status | ||
| 0 alleles | 7207 (69.17) | 2.87 (2.59-3.19) |
| 1 allele | 2928 (28.10) | 4.13 (3.61-4.74) |
| 2 alleles | 285 (2.74) | 7.05 (5.01-9.92) |
| ARIC healthy food score | ||
| <Median score | 5064 (48.60) | 3.54 (3.15-4.00) |
| ≥Median score | 5356 (51.40) | 3.12 (2.85-3.54) |
| Not obese | 7464 (71.63) | 3.04 (2.76-3.36) |
| Obese | 2956 (28.37) | 4.08 (3.56-4.70) |
| Glycemic loada | ||
| <Median | 5127 (49.20) | 3.36 (3.00-3.77) |
| ≥Median | 5293 (50.80) | 3.31 (2.95-3.72) |
| No hyperlipidemia | 9527 (91.43) | 3.30 (3.03-3.59) |
| Hyperlipidemia | 893 (8.57) | 3.66 (2.83-4.75) |
| Variables over follow-up | ||
| No incident or prevalent stroke | 9541 (91.56) | 3.05 (2.80-3.33) |
| Any strokeb | 879 (8.44) | 7.61 (5.83-9.94) |
| No incident or prevalent dementia | 9466 (90.84) | 2.57 (2.33-2.84) |
| Any dementiab | 954 (9.16) | 8.28 (7.20-9.52) |
Abbreviations: APOE ε4, apolipoprotein E ε4; ARIC, Atherosclerosis Risk in Communities Study; HS, high school; LOE, late-onset epilepsy; NA not applicable.
One unit of glycemic load reflects the equivalent effect on blood glucose as consuming 1 g of glucose.
At any time during follow-up or at baseline; time-varying covariates.
Risk Factors for LOE
The incidence of LOE was higher among black participants compared with white participants, 4.71 vs 2.88 per 1000 person-years (95% CI, 4.12-5.40 and 95% CI, 2.60-3.18; incidence rate ratio, 1.66; 95% CI, 1.41-1.95). In univariate Cox analyses, hypertension, diabetes, obesity, activity level, alcohol use, smoking history, and APOE ε4 allele status were each significantly associated with LOE, as were stroke and dementia. There was a dose-dependent effect for number of APOE ε4 alleles. The ARIC Healthy Food Score, glycemic load, and hyperlipidemia were not significantly associated with LOE.
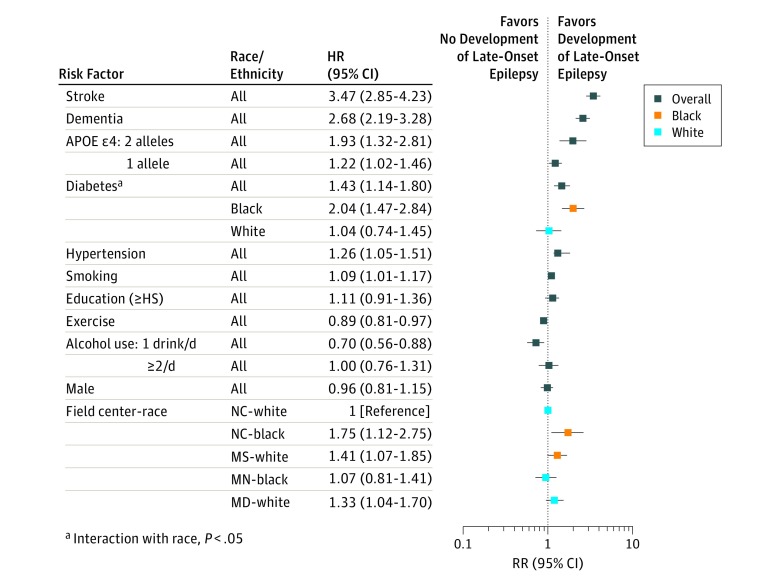
The final multivariable model included demographics, hypertension, diabetes, smoking history, alcohol use, and APOE ε4 status (which had a dose-dependent effect) (Table 2). Stroke and dementia were also significantly associated with LOE when included as time-varying exposures (Figure 2). Higher activity levels and a mean of 1 to 7 alcoholic beverages per week were associated with a lower risk of LOE. Obesity was not significantly associated with LOE in multivariable analysis. Race/ethnicity and field center was significantly associated with a higher risk of LOE, with higher observed rates in Mississippi and North Carolina black participants compared with North Carolina white participants.
Table 2. Adjusted Hazard Ratios for Development of Late-Onset Epilepsy (Multivariable Model)a.
| Variable | Unadjusted Hazard Ratios (95% CI) | Adjusted Hazard Ratios (95% CI) | |
|---|---|---|---|
| All Participants | All Participants | Censored at Stroke and Dementia | |
| Field center/race/ethnicity | |||
| North Carolina/white | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| North Carolina/black | 2.19 (1.42-3.36) | 1.77 (1.15-2.74) | 1.41 (0.77-2.56) |
| Mississippi/black | 1.93 (1.51-2.46) | 1.37 (1.05-1.78) | 1.37 (0.99-1.90) |
| Minnesota/white | 0.95 (0.72-1.26) | 0.93 (0.70-1.24) | 1.03 (0.74-1.45) |
| Maryland/white | 1.28 (1.01-1.63) | 1.18 (0.92-1.51) | 1.31 (0.98-1.75) |
| Male | 0.93 (0.79-1.10) | 0.96 (0.80-1.15) | 0.88 (0.70-1.09) |
| Education, ≥HS | 0.82 (0.69-0.99) | 1.01 (0.84-1.68) | 1.07 (0.84-1.35) |
| Hypertension | 1.63 (1.38-1.91) | 1.41 (1.39-2.13) | 1.31 (1.06-1.63) |
| Diabetes | 1.92 (1.57-2.37) | 1.72 (1.39-2.13) | 1.52 (1.16-2.00) |
| Exercise, per sex-corrected SD | 0.85 (0.78-0.92) | 0.88 (0.81-0.97) | 0.87 (0.78-0.96) |
| Alcohol use, mean drinks/wk | |||
| 1-7 | 0.68 (0.54-0.85) | 0.75 (0.59-0.95) | 0.76 (0.57-1.00) |
| >7 | 0.94 (0.73-1.22) | 1.00 (0.76-1.32) | 0.95 (0.67-1.32) |
| Smoking, per 20 pack-years | 1.06 (0.99-1.14) | 1.12 (1.04-1.21) | 1.16 (1.05-1.27) |
| APOE ε4 status | |||
| 1 allele | 1.46 (1.23-1.73) | 1.42 (1.19-1.69) | 1.40 (1.14-1.73) |
| 2 alleles | 2.57 (1.80-3.67) | 2.36 (1.65-3.38) | 2.14 (1.36-3.39) |
| ARIC healthy food score, per SD | 0.94 (0.86-1.02) | NA | NA |
| Obesity | 1.39 (1.17-1.65) | NA | NA |
| Glycemic load, per standard deviationb | 1.00 (0.92-1.09) | NA | NA |
| Hyperlipidemia | 1.07 (0.81-1.41) | NA | NA |
Abbreviations: APOE ε4, apolipoprotein E ε4; ARIC, Atherosclerosis Risk in Communities Study; HS, high school; NA, not applicable.
All variables in table and age at visit 1 were included in the same model.
One unit of glycemic load reflects the equivalent effect on blood glucose as consuming one g of glucose.
Figure 2. Hazard Ratios (95% CIs) of Midlife Risk Factors for Late-Onset Epilepsy.
All covariates and visit 1 age are included in same model, with stroke and dementia as time-varying exposures based on date of stroke or dementia diagnosis. HS indicates high school; MD, Maryland; MS, Mississippi; MN, Minnesota; NC, North Carolina.
In models censoring individuals at the time of stroke (n = 1265) or dementia (n = 1574), hypertension, diabetes, smoking, and APOE ε4 alleles (with a dose-dependent effect) were associated with an increased risk of LOE. Moderate alcohol use and increased level of exercise were associated with a lower risk of LOE (Table 2).
Stratified Analyses
We stratified participants by race/ethnicity and sex to assess the interaction of those demographic characteristics with midlife risk factors. There was a significant interaction between race/ethnicity and diabetes. In black participants, the adjusted HR for diabetes and LOE was 1.98 (95% CI, 1.47-2.67; P < .001) while in white participants, the HR for diabetes was not statistically significant (HR, 1.01; 95% CI, 0.72-1.43; P = .004 for interaction) (Figure 2).
There was a significant interaction between sex and smoking. For smoking, the HR in women was 1.27 (95% CI, 1.14-1.42), while there was no association for men (HR, 0.98; 95% CI, 0.88-1.09; P = .001 for interaction).
Results from analysis of the smaller number of available participants using LOE definition 2 are included in eTable 2 in the Supplement. Diabetes, stroke, and dementia were significant risk factors in multivariable analysis after adjusting for demographics; effect sizes for alcohol, exercise, and APOE ε4 were similar to those seen in definition 1 though did not achieve statistical significance.
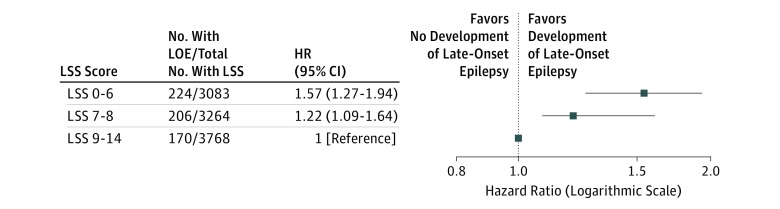
Life’s Simple Seven
We compared the HR of developing LOE in participants by LSS score (0-14),23 adjusted for sex, field center and race/ethnicity, and education level. Compared with participants in the top tertile (LSS, 9-14), the bottom tertile (LSS, 0-6) and middle tertile (LSS, 7-8) had increased risks of LOE (HR, 1.57; 95% CI, 1.27-1.94 and HR, 1.22; 95% CI, 1.09-1.64 respectively) (Figure 3).
Figure 3. Hazard Ratios (95% CIs) for Late-Onset Epilepsy by Life’s Simple Seven (LSS) Score.
Hazard ratios are adjusted for race/ethnicity, center, sex, age at visit 1, and education level. The LSS score evaluates controlled blood pressure, glucose and cholesterol levels, healthy weight, healthy diet, exercise, and smoking status. The LSS score from 0-14 is calculated from total number of healthy LSS characteristics per Folsom et al24 (eTable 1 in the Supplement).
Sensitivity Analyses
Sensitivity analyses using only LOE cases occurring at 67 years or older, and using 2 years after first claim code as the analysis origin, yielded similar results (eTable 3 in the Supplement). Analysis including all patients regardless of Medicare FFS coverage was similar. Results after multiple imputation for missing variables were similar (eTable 4 in the Supplement). Using death as a competing risk yielded similar effects for hypertension, alcohol, APOE ε4, stroke, and dementia; effects of exercise, smoking, and diabetes did not remain statistically significant (eTable 5 in the Supplement).
Discussion
Our analysis of incident LOE in this biracial community-based cohort demonstrated an association of LOE with midlife vascular risk factors, as well as lifestyle risk factors. Moreover, the increased risk of LOE in persons with APOE ε4 carrier status, midlife hypertension, diabetes, and smoking persisted after adjusting for demographics, as did the lower risk of LOE in participants with moderate alcohol intake and with higher midlife physical activity level. Results also remained similar when participants were censored at time of stroke or dementia diagnosis. These findings show that midlife vascular and lifestyle risk factors may contribute to the development of epilepsy later in life. Associations differed somewhat by race/ethnicity, with a greater risk for LOE in black participants with diabetes.
Our LOE incidence rates are comparable with other studies of LOE and to estimates worldwide.7 Faught et al4 found an incidence of 2.4 per 1000 person-years in a sample of nationwide Medicare beneficiaries, while Choi et al7 found an incidence of 2.5 per 1000 person-years in a study of participants in the Cardiovascular Health Study. Previous cross-sectional, case-control, and smaller cohort studies have also found an association between hypertension,13,32 stroke,7,14 dementia,33 and LOE. While diabetes,34 smoking,35 physical activity,36 and alcohol use37 are known to be associated with risk of ischemic stroke (and have been associated with post-stroke epilepsy38), to our knowledge, no association between these risk factors at midlife and LOE or in the absence of stroke has previously been demonstrated. The APOE ε4 genotype is the major genetic risk factor for Alzheimer disease,39 which is associated with epilepsy40; however, no prior association between APOE ε4 and LOE has previously been shown. Given the prospectively collected nature of these risk factors and the ascertainment of later development of epilepsy, we show a temporal association between previously known and unknown risk factors and LOE. The association of LOE with vascular and lifestyle risk factors persisted after participants with diagnosis of clinical stroke or dementia were included or censored, suggesting that these risk factors may contribute to LOE even in the absence of dementia or clinical stroke.
The incidence of LOE in our study was higher in black than in white participants: 4.71 per 1000 person-years in black participants (95% CI, 4.12-5.40) vs 2.88 per 1000 person-years (95% CI, 2.60-3.18) in white participants. Previous studies have also shown a higher incidence in black (4.1 in 10004 and 4.44 in 10007) than in white (2.3 in 10004 and 2.17 in 10007) individuals.30 The larger proportion of black participants in this study (25% of ARIC participants) may account for the slightly higher incidence of LOE in this study than in others. The reasons for the different incidences of LOE by race/ethnicity may be owing to differing effects of comorbidities, such as diabetes, for which we found a significantly higher effect in black individuals than in white individuals.
Mechanisms associated with LOE might include microvascular disease, which contributes to gliosis and blood-brain barrier breakdown, implicated in epileptogenesis in animal studies and in human tissue.41,42,43 In animal studies, APOE ε4 increases microvascular injury44 and exacerbates diabetes-related microvascular disease.45 That these risk factors are also highly associated with ischemic stroke could imply that subclinical vascular injuries account for some of the observed associations between the risk factors and LOE. An alternative hypothesis is that amyloid deposition may play a role in the pathogenesis of LOE, even in the absence of dementia. Previous work has shown that increasing numbers of vascular risk factors are linked to higher levels of estimated brain amyloid deposition in patients without dementia.46 Other studies have demonstrated higher levels of amyloid-β accumulation in patients with the APOE ε4 genotype and higher levels of amyloid-β in dual-allele APOE ε4 carriers than in single-allele carriers.47,48,49,50,51 The potential role of amyloid in epileptogenesis is described in animal models and in patients with AD40,52,53 and has been hypothesized in patients with epilepsy; in cognitively normal patients with temporal lobe epilepsy who undergo resection, there is a higher age-related incidence of amyloid plaques compared with control individuals without epilepsy.54 The APOE ε4 carrier status has also been associated with an increased risk of seizures after traumatic brain injury and hemorrhage.55,56 The dose-dependent association of APOE ε4 allele genotype and LOE identified here could suggest that amyloid deposition is a possible mechanism for LOE even in individuals without dementia.
These results have a number of important implications. First, they imply that (as for stroke and cardiovascular disease) it is possible that lifestyle modifications and treatment of earlier-life conditions could mitigate these risk factors associated with LOE. Second, they raise the possibility of identifying patients at risk for LOE who may benefit from clinical trials of targeted interventions for prevention.57 Third, while our data do not directly show an association with cerebral amyloid, given the previously demonstrated association between amyloid and these vascular risk factors46 and the association demonstrated here between APOE ε4 and LOE, they suggest the possibility of a role of amyloid deposition in LOE.
Strengths and Limitations
The strengths of our study are the longitudinal design, large sample size, inclusion of representative population-based cohort of black and white adults, rigorous methods of adjudicating stroke cases, and length of follow-up. This study also has limitations. Given our use of claims data, there is undoubtedly some misclassification of cases, as is typical for studies using claims codes. We have attempted to mitigate this limitation by using 2 different definitions to identify epilepsy cases. It is possible that individuals with more vascular risk factors might have more frequent medical care and thus be more likely to be identified as having LOE. In primary analysis, we only included patients with Medicare FFS coverage, which may introduce some bias; however, sensitivity analysis including participants regardless of Medicare FFS showed no change. Additionally, because we included only risk factors hypothesized a priori to be associated with LOE, we are most likely overlooking other important contributions to the development of LOE.
Conclusions
Midlife vascular risk factors and lifestyle factors were significantly associated with LOE, even in the absence of stroke or dementia. These findings are consistent with a possible role of vascular disease and neurodegeneration in the development of LOE.
eTable 1. Life’s Simple Seven Categories from the AHA’s Life’s Simple 7 Cardiovascular Health Metrics
eTable 2. Hazard Ratios (HRs) for Developing Late-onset Epilepsy Under Definition 2.
eTable 3. Hazard Ratios for Late-onset Epilepsy With Age of First Seizure 67 or Older, and Using 2 years After First Claim Code as Origin
eTable 4. Hazard Ratios for Late-onset Epilepsy Using Multiple Imputations for Missing Data
eTable 5. Hazard Ratios for Developing Late-Onset Epilepsy With Death as a Competing Risk
References
- 1.Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology. 2004;62(5)(suppl 2):S24-S29. doi: 10.1212/WNL.62.5_suppl_2.S24 [DOI] [PubMed] [Google Scholar]
- 2.Cloyd J, Hauser W, Towne A, et al. Epidemiological and medical aspects of epilepsy in the elderly. Epilepsy Res. 2006;68(suppl 1):S39-S48. doi: 10.1016/j.eplepsyres.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 3.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia. 1993;34(3):453-468. doi: 10.1111/j.1528-1157.1993.tb02586.x [DOI] [PubMed] [Google Scholar]
- 4.Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older U.S. Medicare beneficiaries. Neurology. 2012;78(7):448-453. doi: 10.1212/WNL.0b013e3182477edc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephson CB, Engbers JDT, Sajobi TT, et al. Towards a clinically informed, data-driven definition of elderly onset epilepsy. Epilepsia. 2016;57(2):298-305. doi: 10.1111/epi.13266 [DOI] [PubMed] [Google Scholar]
- 6.He W, Goodkind D, Kowal P. An Aging World: 2015 International Population Reports. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p95-16-1.pdf. Published 2016. Accessed January 20, 2017.
- 7.Choi H, Pack A, Elkind MSV, Longstreth WT Jr, Ton TGN, Onchiri F. Predictors of incident epilepsy in older adults: the Cardiovascular Health Study. Neurology. 2017;88(9):870-877. doi: 10.1212/WNL.0000000000003662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47(5):867-872. doi: 10.1111/j.1528-1167.2006.00554.x [DOI] [PubMed] [Google Scholar]
- 9.Rowan AJ, Ramsay RE, Collins JF, et al. ; VA Cooperative Study 428 Group . New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868-1873. doi: 10.1212/01.WNL.0000167384.68207.3E [DOI] [PubMed] [Google Scholar]
- 10.Lühdorf K, Jensen LK, Plesner AM. Etiology of seizures in the elderly. Epilepsia. 1986;27(4):458-463. doi: 10.1111/j.1528-1157.1986.tb03567.x [DOI] [PubMed] [Google Scholar]
- 11.Leppik IE. Epilepsy in the elderly: scope of the problem. Int Rev Neurobiol. 2007;81(06):1-14. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay RE, Pryor F. Epilepsy in the elderly. Neurology. 2000;55(5)(suppl 1):S9-S14. [PubMed] [Google Scholar]
- 13.Ng SK, Hauser WA, Brust JC, Susser M. Hypertension and the risk of new-onset unprovoked seizures. Neurology. 1993;43(2):425-428. doi: 10.1212/WNL.43.2.425 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Breteler MMB, de Bruyne MC, Meinardi H, Hauser WA, Hofman A. Vascular determinants of epilepsy: the Rotterdam Study. Epilepsia. 1997;38(11):1216-1220. doi: 10.1111/j.1528-1157.1997.tb01219.x [DOI] [PubMed] [Google Scholar]
- 15.The ARIC Investigators The Atherosclerosis Risk in Communities Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 16.Alonso A, Mosley TH Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80(11):1194-1201. doi: 10.1136/jnnp.2009.176818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucharska-Newton AM, Heiss G, Ni H, et al. Identification of heart failure events in medicare claims: the Atherosclerosis Risk in Communities (ARIC) study. J Card Fail. 2016;22(1):48-55. doi: 10.1016/j.cardfail.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid AY, St Germaine-Smith C, Liu M, et al. Development and validation of a case definition for epilepsy for use with administrative health data. Epilepsy Res. 2012;102(3):173-179. doi: 10.1016/j.eplepsyres.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 19.Fonferko-Shadrach B, Lacey AS, White CP, et al. Validating epilepsy diagnoses in routinely collected data. Seizure. 2017;52:195-198. doi: 10.1016/j.seizure.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8(1):1-14. doi: 10.1089/dis.2005.8.1 [DOI] [PubMed] [Google Scholar]
- 21.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936-942. doi: 10.1093/ajcn/36.5.936 [DOI] [PubMed] [Google Scholar]
- 22.Weng LC, Steffen LM, Szklo M, Nettleton J, Chambless L, Folsom AR. A diet pattern with more dairy and nuts, but less meat is related to lower risk of developing hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nutrients. 2013;5(5):1719-1733. doi: 10.3390/nu5051719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586-613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 24.Folsom AR, Olson NC, Lutsey PL, Roetker NS, Cushman M. American Heart Association’s Life’s Simple 7 and incidence of venous thromboembolism. Am J Hematol. 2015;90(5):E92. doi: 10.1002/ajh.23950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC-PET amyloid imaging study: brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87(5):473-480. doi: 10.1212/WNL.0000000000002914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones SA, Gottesman RF, Shahar E, Wruck L, Rosamond WD. Validity of hospital discharge diagnosis codes for stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2014;45(11):3219-3225. doi: 10.1161/STROKEAHA.114.006316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246-1254. doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power MC, Deal JA, Sharrett AR, et al. Smoking and white matter hyperintensity progression: the ARIC-MRI Study. Neurology. 2015;84(8):841-848. doi: 10.1212/WNL.0000000000001283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottesman RF, Schneider ALC, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71(10):1218-1227. doi: 10.1001/jamaneurol.2014.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain SA, Haut SR, Lipton RB, Derby C, Markowitz SY, Shinnar S. Incidence of epilepsy in a racially diverse, community-dwelling, elderly cohort: results from the Einstein aging study. Epilepsy Res. 2006;71(2-3):195-205. doi: 10.1016/j.eplepsyres.2006.06.018 [DOI] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 32.Hesdorffer DC, Hauser WA, Annegers JF, Rocca WA. Severe, uncontrolled hypertension and adult-onset seizures: a case-control study in Rochester, Minnesota. Epilepsia. 1996;37(8):736-741. doi: 10.1111/j.1528-1157.1996.tb00644.x [DOI] [PubMed] [Google Scholar]
- 33.Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46(3):727-730. doi: 10.1212/WNL.46.3.727 [DOI] [PubMed] [Google Scholar]
- 34.Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol. 2005;4(12):821-826. doi: 10.1016/S1474-4422(05)70227-1 [DOI] [PubMed] [Google Scholar]
- 35.Nordahl H, Osler M, Frederiksen BL, et al. Combined effects of socioeconomic position, smoking, and hypertension on risk of ischemic and hemorrhagic stroke. Stroke. 2014;45(9):2582-2587. doi: 10.1161/STROKEAHA.114.005252 [DOI] [PubMed] [Google Scholar]
- 36.Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. doi: 10.1136/bmj.i3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambertini M, Del Mastro L, Pescio MC, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14:1. doi: 10.1186/s12916-015-0545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitkänen A, Roivainen R, Lukasiuk K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 2016;15(2):185-197. doi: 10.1016/S1474-4422(15)00248-3 [DOI] [PubMed] [Google Scholar]
- 39.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921-923. doi: 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 40.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 2017;16(4):311-322. doi: 10.1016/S1474-4422(17)30044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bar-Klein G, Lublinsky S, Kamintsky L, et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain. 2017;140(6):1692-1705. doi: 10.1093/brain/awx073 [DOI] [PubMed] [Google Scholar]
- 42.van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(pt 2):521-534. doi: 10.1093/brain/awl318 [DOI] [PubMed] [Google Scholar]
- 43.Robel S, Sontheimer H. Glia as drivers of abnormal neuronal activity. Nat Neurosci. 2016;19(1):28-33. doi: 10.1038/nn.4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512-516. doi: 10.1038/nature11087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ly H, Verma N, Wu F, et al. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol. 2017;82(2):208-222. doi: 10.1002/ana.24992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottesman RF, Schneider ALC, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443-1450. doi: 10.1001/jama.2017.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bangen KJ, Clark AL, Werhane M, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Cortical amyloid burden differences across empirically-derived mild cognitive impairment subtypes and interaction with APOE ɛ4 genotype. J Alzheimers Dis. 2016;52(3):849-861. doi: 10.3233/JAD-150900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Risacher SL, Kim S, Nho K, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI) . APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11(12):1417-1429. doi: 10.1016/j.jalz.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122-131. doi: 10.1002/ana.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(16):6820-6825. doi: 10.1073/pnas.0900345106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim YY, Mormino EC; Alzheimer’s Disease Neuroimaging Initiative . APOE genotype and early β-amyloid accumulation in older adults without dementia. Neurology. 2017;89(10):1028-1034. doi: 10.1212/WNL.0000000000004336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minkeviciene R, Rheims S, Dobszay MB, et al. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29(11):3453-3462. doi: 10.1523/JNEUROSCI.5215-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palop JJ, Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812-818. doi: 10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87(5):504-510. doi: 10.1007/BF00294177 [DOI] [PubMed] [Google Scholar]
- 55.Diaz-Arrastia R, Gong Y, Fair S, et al. Increased risk of late posttraumatic seizures associated with inheritance of APOE epsilon4 allele. Arch Neurol. 2003;60(6):818-822. doi: 10.1001/archneur.60.6.818 [DOI] [PubMed] [Google Scholar]
- 56.Biffi A, Rattani A, Anderson CD, et al. Delayed seizures after intracerebral haemorrhage. Brain. 2016;139(pt 10):2694-2705. doi: 10.1093/brain/aww199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Life’s Simple Seven Categories from the AHA’s Life’s Simple 7 Cardiovascular Health Metrics
eTable 2. Hazard Ratios (HRs) for Developing Late-onset Epilepsy Under Definition 2.
eTable 3. Hazard Ratios for Late-onset Epilepsy With Age of First Seizure 67 or Older, and Using 2 years After First Claim Code as Origin
eTable 4. Hazard Ratios for Late-onset Epilepsy Using Multiple Imputations for Missing Data
eTable 5. Hazard Ratios for Developing Late-Onset Epilepsy With Death as a Competing Risk