Abstract
Importance
As the life expectancy of people with Down syndrome (DS) has markedly increased over the past decades, older adults with DS may be experiencing a higher incidence of aging conditions. In addition to longevity, the amyloid precursor protein gene located on chromosome 21 places individuals with DS at a high risk for developing Alzheimer disease. Yet, few studies have determined prevalence of dementia and comorbidities among older people with DS.
Objective
To determine the prevalence of dementia and aging-related comorbidities in older adult individuals with DS.
Design, Setting, and Participants
Cross-sectional analysis of 2015 California Medicare claims data. We examined 1 year of cross-sectional Medicare claims data that included 100% of Californian Medicare beneficiaries enrolled in both Medicare Part A and B in 2015. Of these 3 001 977 Californian Medicare beneficiaries 45 years or older, 878 individuals were identified as having a diagnosis of DS. Data were analyzed between April 2017 and February 2018.
Main Outcomes and Measures
The frequency of DS dementia was assessed across different age categories. The number and frequency of 27 comorbidities were compared among individuals with DS with and without dementia and by age and sex groups.
Results
A total of 353 DS individuals (40%) were identified as having dementia diagnoses (mean, 58.7 years; 173 women [49%]) and 525 without dementia diagnoses (mean, 55.9 years; 250 women [48%]). The frequency of DS dementia among those 65 years or older rose to 49%. The mean number of comorbidities per individual increased with age in general. Comorbid conditions were more numerous among those with dementia compared with those with DS without dementia (mean, 3.4 vs 2.5, respectively), especially among those younger than 65 years. In particular, 4 treatable conditions, hypothyroidism, epilepsy, anemia, and weight loss, were much more frequent in DS dementia.
Conclusions and Relevance
Older Medicare beneficiaries in California with DS, especially those with dementia, have a high level of multimorbidity including several treatable conditions. While DS follow-up has long been confined to the pediatric sphere, we found that longevity in individuals with DS will necessitate complex adult and geriatric care. More evidenced-based and standardized follow-up could support better long-term comorbidity management and dementia care among aging adults with DS.
Key Points
Question
How common are chronic comorbidity conditions, dementia, and both among aging individuals with Down syndrome older than 45 years?
Findings
In this cross-sectional study of a Medicare sample of 878 older individuals with Down syndrome (age range, 45-89 years), nearly half of older individuals with Down syndrome had a diagnosis of dementia after age 65 years. Individuals with Down syndrome had high rates of age-related conditions, especially those with Down syndrome dementia.
Meaning
Early systematic screening of treatable multimorbidity during Down syndrome adulthood and repeated tracking of cognitive decline are needed to improve elderly and dementia care in DS.
This study investigates the prevalence of dementia and aging-related comorbidities, such as hypertension, epilepsy, anemia, and weight loss, in older adult individuals with Down syndrome.
Introduction
Down syndrome (DS) is the most common genetic cause of developmental disability and cognitive impairment, with an estimated prevalence of 8.3 per 10 000 individuals in the United States (approximately 250 700 people).1 People with DS are considered to have accelerated aging physiology and conditions that often manifest by 45 years.2 During the last 3 decades, the life expectancy of people with DS has markedly increased, with a median age at death of almost 60 years3 compared with 25 years in 1983 and 49 years in 1997.4 As a consequence, people with DS may be experiencing a higher incidence of age-related comorbid conditions; however, this remains largely unstudied.
In addition to longevity, adults with DS are at a very high risk of developing Alzheimer disease (AD) owing to trisomy of chromosome 21 on which the amyloid precursor protein gene is located. Postmortem studies reveal that almost 100% of adults with DS show neuropathological findings of AD by age 40 years,5 and AD is the main cause of death in many older individuals with DS.3 However, clinical AD does not always develop systematically in those with DS, and more research is needed to study how other diseases of aging and multimorbidity are associated with DS dementia.6
The connections between increased longevity in DS, multimorbidity, and DS dementia are not well known. The investigation of a representative study of older patients with DS with and without dementia could clarify these associations and lead to a more informed care practice for these vulnerable elderly individuals. While studies on aging DS are rare and with small cohorts on DS dementia,7 secondary health claims data offer a promising opportunity to explore older adults with DS. We investigated the 2015 California Medicare data set that included beneficiaries older than 45 years to identify aging individuals with DS and to compare individuals with DS dementia with those individuals with DS without a diagnosis of dementia. The objective was to better understand the medical challenges facing aging individuals with DS.
Methods
We obtained data from the 2015 California Medicare data set including all 2015 Californian Medicare beneficiaries enrolled in both Medicare Part A and Part B. Medicare Part A covers services provided by hospitals, skilled nursing facilities, and hospice and home health agencies, whereas Part B covers services and supplies needed to diagnose or treat outpatient medical conditions and preventive health services.8 Following Centers for Medicare and Medicaid Services (CMS) Chronic Condition Warehouse guidelines,8,9 the data set was obtained by merging the 7 Medicare Standard Analytic Files for Medicare expenditures (inpatient care, outpatient care, home health agency, hospice, carrier, skilled nursing facility, and durable medical equipment) that represent aggregate claims data submitted to CMS by care clinicians on behalf of fee-for-service beneficiaries.
Because 2015 was a transition year in Medicare coding system, both International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) were used within the seven 2015 Medicare Standard Analytic Files to extract diagnosis of DS, dementia, and comorbidity conditions.10,11,12,13 All individuals with DS older than 45 years who had had at least 1 Medicare claim in 2015 were identified. Demographic variables (sex, age, and race/ethnicity) were included. Alzheimer disease and any other type of dementia were identified and pooled together in a single dementia category for the analyses (ie, AD, vascular dementia, frontotemporal dementia, Lewy body dementia, mixed dementia, senile, or presenile dementia, as well as other dementia and dementia not otherwise specified). This allowed for generation of 2 mutually exclusive DS samples: those with and without dementia. To describe multimorbidity in older adults with DS, 27 chronic comorbid conditions were evaluated in these Medicare beneficiaries. Selection of these comorbidities was based on their frequency in the general population, based on their partially curable nature, or based on their potential for improved care treatment in DS and dementia.11,12,14,15,16,17,18,19,20,21 Use of Medicare data files was reviewed by the CMS and used with permission from the Research Data Assistant Center. Use of data met federal privacy and confidentiality requirements, and data use agreement was completed following CMS guidelines. The study was approved by the University of California, San Francisco institutional review board, and patient consent was waived owing to use of deidentified data.
Statistical Analysis
Demographic data, mean number, and frequency of comorbid conditions were calculated in the whole DS sample and then with subsequent stratification by dementia status and by sex. Comparisons between groups were computed with t tests for continuous variables and χ2 or Fisher exact tests for categorical variables. We also determined frequency of dementia by age. To control for differences in age that might influence multimorbidity (eg, older age associated with a higher number of comorbidity conditions), a sensitivity analysis was conducted to check for the stability of comparative outcomes regarding comorbidity conditions in DS with and without dementia: individuals with DS dementia (n = 353) were compared with a subset of matched individuals with DS without dementia (n = 353) within the same Medicare data set. The 1:1 matching on sex (P > .99) and age (P = .60) was performed using R package Matching, version 4.9-2 2015. Statistical analyses were performed using Stata, version 14 (StataCorp) and R (R Programming). The P value level of significance was .05, and all P values were 2-sided.
Results
Among 3 001 977 Californian 2015 Medicare beneficiaries 45 years or older, 878 individuals (0.03%) were identified as having DS, including 455 men (52%) and 423 women (48%). Mean age for those beneficiaries was 57 years (range 45-89), and most were white (71%), followed by Hispanic, who were 4 times more numerous than African American individuals (Table 1).
Table 1. Demographic Characteristics of the 878 California Medicare (2015) Beneficiaries With DS With and Without Dementia.
| Demographics | No. (%) | P Value | ||
|---|---|---|---|---|
| DS (Total) (N = 878) |
DS With Dementia (n = 353) |
DS Without Dementia (n = 525) |
||
| Age, mean (SD) | 57 (7.7) | 58.7 (7.1) | 55.9 (7.5) | <.001 |
| Men | 455 (51.8) | 180 (51) | 275 (52) | .69 |
| Race/ethnicity | ||||
| White | 620 (70.6) | 261 (74) | 359 (68.4) | .08 |
| Hispanic | 171 (19.5) | 65 (18.4) | 106 (20.2) | .51 |
| African American | 44 (5) | 13 (3.7) | 31 (6) | .14 |
| Asian/Pacific Islander | 29 (3.3) | 10 (2.8) | 19 (3.6) | .52 |
| Indian/Alaska native | 5 (0.6) | 3 (0.8) | 2 (0.3) | .40 |
| Others/unknown | 9 (1) | 1 (0.3) | 8 (1.5) | .09 |
Abbreviation: DS, Down syndrome.
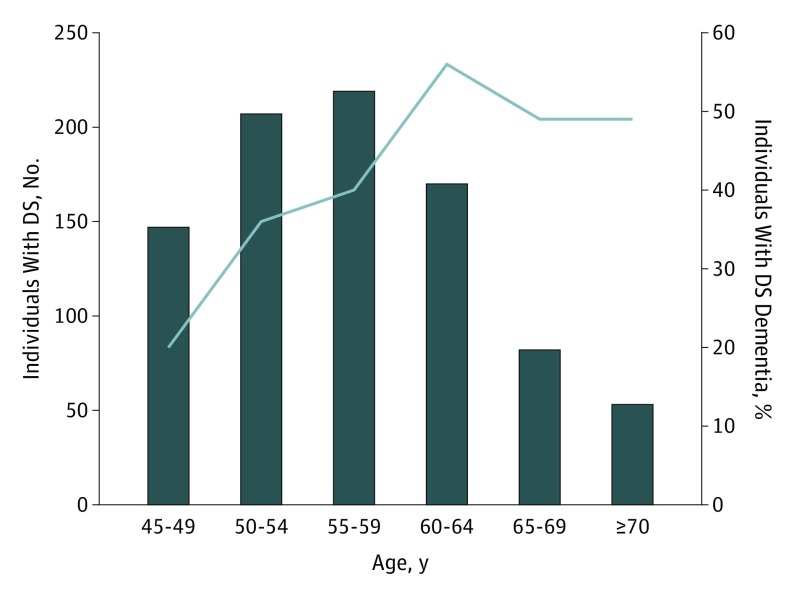
Of the 878 individuals with DS, 353 (40%) had a diagnosis of dementia (DS dementia) (Table 1). Dementia diagnosis was associated with older age (58.7 years vs 55.9 years; P < .001), but no significant differences in sex or race/ethnicity were found (Table 1). Among those with dementia, there was no difference in age by sex (men, 58.6 years vs women, 58.8 years; P = .81). As expected, the frequency of dementia showed a progressive increase with age, with a maximum of 56% among those aged 60 to 65 years, followed by a slightly lower plateau after age 65 years (Figure 1). Among the oldest individuals with DS (older than 70 years), there were 26 with dementia (12 men and 14 women) and 27 without dementia (13 men and 14 women).
Figure 1. Prevalence of Dementia in Individuals With Down Syndrome (DS).
Number of individuals with DS (histogram) and frequency of DS dementia among these individuals with DS by age group.
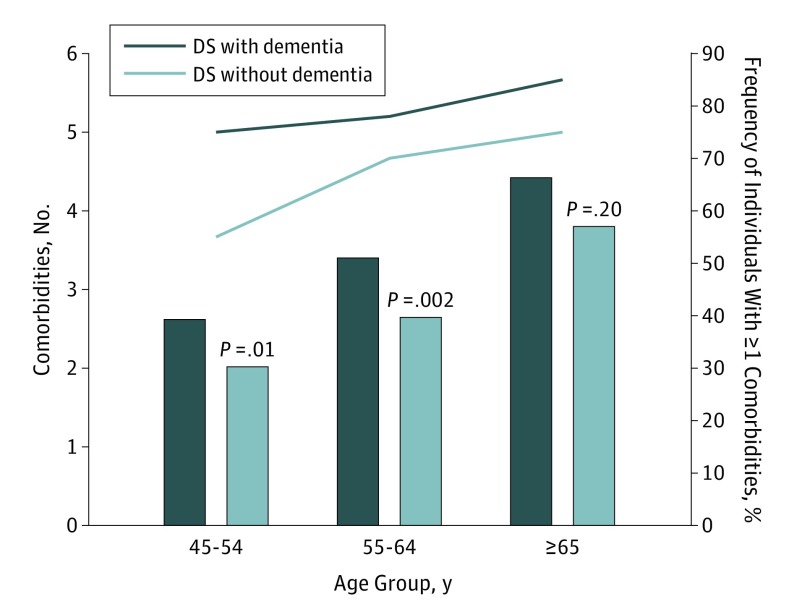
Table 2 shows that individuals with DS of all ages with dementia (n = 353) had a mean of 3.4 comorbidities (in addition to dementia), whereas individuals with DS without dementia (n = 525) had a mean of 2.5 comorbidities (P < .001). When stratifying by age (Figure 2), the number of comorbidities between DS with and without dementia was primarily different among those younger than 65 years. This result remained after matching DS dementia (n = 353) with DS without dementia (n = 353) by age and sex: individuals with DS with dementia had more comorbidities than those without dementia when younger than 65 years (mean, 3.1 vs mean, 2.3, respectively; P < .001), but this difference was no longer significant among those 65 years and older.
Table 2. Comparison of Comorbidity Conditions in Individuals With DS With and Without Dementia.
| Comorbidity | No. (%) | P Value | ||
|---|---|---|---|---|
| DS (Total) (N = 878) |
DS With Dementia (n = 353) |
DS Without Dementia (n = 525) |
||
| Mean (SD); range | 2.9 (2.5); 0-15 | 3.4 (2.7); 0-15 | 2.5 (2.2); 0-11 | <.001 |
| Frequency | ||||
| Cardiovascular | ||||
| Hypertension | 196 (22.3) | 85 (24) | 111 (21.1) | .31 |
| Congestive heart failure | 107 (12.2) | 48 (13.4) | 59 (11.2) | .29 |
| Cardiovalvular disease | 41 (4.7) | 16 (4.5) | 25 (4.8) | .88 |
| Peripheral vascular disease | 36 (4.1) | 15 (4.2) | 21 (4) | .86 |
| Arrhythmia | 32 (3.6) | 16 (4.5) | 16 (3) | .25 |
| Myocardial infarction | 19 (2.2) | 10 (2.8) | 9 (1.7) | .26 |
| Neuropsychiatric | ||||
| Epilepsy | 259 (29.5) | 145 (41.1) | 114 (21.7) | <.001 |
| Mood disorder | 87 (9.9) | 48 (13.6) | 39 (7.4) | .003 |
| Anxiety | 84 (9.6) | 43 (12.2) | 41 (7.7) | .03 |
| Psychosis | 43 (4.9) | 25 (7.1) | 18 (3.4) | .01 |
| Cerebrovascular disease | 34 (3.9) | 16 (4.5) | 18 (3.4) | .41 |
| Alcohol use | 22 (2.5) | 15 (4.2) | 7 (1.3) | .007 |
| Traumatic brain injury | 16 (1.8) | 6 (1.7) | 10 (1.9) | .82 |
| Metabolism endocrine | ||||
| Hypothyroidism | 402 (45.8) | 183 (52) | 219 (41.7) | .003 |
| Anemia | 257 (29.3) | 122 (34.6) | 135 (25.7) | .005 |
| Dyslipidemia | 192 (21.9) | 89 (25.2) | 103 (19.6) | .05 |
| Diabetes | 101 (11.5) | 47 (13.3) | 54 (10.3) | .17 |
| Obesity | 73 (8.3) | 28 (7.9) | 45 (8.6) | .74 |
| Weight loss | 71 (8.1) | 45 (12.7) | 26 (5) | <.001 |
| Osteoporosis | 65 (7.4) | 32 (9.1) | 33 (6.3) | .12 |
| Other conditions | ||||
| Chronic pulmonary disease | 165 (18.8) | 72 (20.4) | 93 (17.7) | .32 |
| Chronic renal failure | 89 (10.1) | 45 (12.6) | 44 (8.4) | .04 |
| Ulcer disease | 34 (3.9) | 12 (3.4) | 22 (4.2) | .55 |
| Tumor and hematologic disease | 33 (3.8) | 6 (1.7) | 27 (5.1) | .009 |
| Liver disease | 31 (3.5) | 13 (3.7) | 18 (3.4) | .84 |
| Rheumatism | 5 (.57) | 2 (.57) | 3 (.57) | >.99 |
| Tobacco use | 4 (.46) | 2 (.57) | 2 (.38) | >.99 |
Abbreviation: DS, Down syndrome.
Figure 2. Comorbidities in Individuals With Down Syndrome (DS) With and Without Dementia.
By age group: mean number of comorbidities (among 27 conditions) in individuals with DS and frequency of individuals with at least 1 of 4 comorbidities (hypothyroidism, epilepsy, anemia, or weight loss) among these DS subgroups, respectively.
Six of the 27 comorbid conditions had a high prevalence (more than 18% each) in DS in general including, from most to less frequent, hypothyroidism, epilepsy, anemia, dyslipidemia, hypertension, and chronic pulmonary disease (Table 2). Of these, 4 treatable conditions (hypothyroidism, epilepsy, anemia, and weight loss) were much more common among those with dementia than without; this result remained in the matched subsample. A total of 276 individuals (78%) with DS dementia had at least 1 of those 4 diseases compared with 64% in individuals without DS dementia (Figure 2). We did note other significant differences between individuals with DS dementia and individuals without DS dementia in neuropsychological conditions (higher frequencies of mood, psychosis, anxiety, and alcohol use).
When stratifying by sex, men showed a higher frequency than women for anemia (33% [n = 150 of 455] vs 25% [n = 107 of 423]; P = .01), hypertension (26% [n = 118 of 455] vs 18% [n = 78 of 423]; P = .008), and myocardial infarction (3.5% [n = 16 of 455] vs 0.7% [n = 3 of 423]; P = .004). Women showed a higher frequency than men for hypothyroidism (49.6% [n = 210 of 423] vs 42.2% [n = 192 of 455]; P = .03), osteoporosis (10.4% [n = 44 of 423] vs 4.6% [n = 21 of 455]; P = .001), and obesity (10.9% [n = 46 of 423] vs 5.9% [n = 27 of 455]; P = .008). Stratification by sex and dementia status (Table 3) suggested that men with dementia had higher frequency of hypothyroidism, osteoporosis, chronic renal failure, mood disorders, alcohol use, and lower frequency of tumor and hematologic disease, while women with dementia had higher frequency of anemia and anxiety.
Table 3. Comparison of Comorbidity Conditions in Individuals With DS According to Sex and Dementia Status.
| Comorbidity | Men With DS, No. (%) | Women With DS, No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Men (n = 455) |
With Dementia (n = 180) |
Without Dementia (n = 275) |
P Value | Total Women (n = 423) |
With Dementia (n = 173) |
Without Dementia (n = 250) |
P Value | |
| Mean (SD); range | 2.9 (2.6); 0-12 | 3.4 (2.8); 0-12 | 2.6 (2.4); 0-11 | <.001 | 2.7 (2.3); 0-15 | 3.3 (2.6); 0-15 | 2.4 (2); 0-11 | <.001 |
| Frequency | ||||||||
| Cardiovascular | ||||||||
| Hypertension | 118 (26) | 51 (28.3) | 67 (24) | .35 | 78 (18.4) | 34 (20) | 44 (17.6) | .59 |
| Congestive heart failure | 57 (12.5) | 25 (13.9) | 32 (11.6) | .48 | 50 (11.8) | 23 (13.3) | 27 (10.8) | .44 |
| Cardiovalvular disease | 27 (5.9) | 10 (5.6) | 17 (6.2) | .78 | 14 (3.3) | 6 (3.5) | 8 (3.2) | .89 |
| Peripheral vascular disease | 18 (4) | 6 (3.3) | 12 (4.3) | .58 | 18 (4.3) | 9 (5.2) | 9 (3.6) | .42 |
| Arrhythmia | 20 (4.4) | 9 (5) | 11 (4) | .61 | 12 (2.8) | 7 (4) | 5 (2) | .24 |
| Myocardial infarction | 16 (3.5) | 8 (4.4) | 8 (2.9) | .39 | 3 (0.7) | 2 (1.1) | 1 (0.4) | .57 |
| Neuropsychiatric | ||||||||
| Epilepsy | 127 (27.9) | 65 (36.1) | 62 (22.5) | .002 | 132 (31.2) | 80 (46.2) | 52 (20.8) | <.001 |
| Mood disorder | 42 (9.2) | 24 (13.3) | 18 (6.5) | .01 | 45 (10.6) | 24 (13.9) | 21 (8.4) | .07 |
| Anxiety | 52 (11.4) | 24 (13.3) | 28 (10.2) | .30 | 32 (7.6) | 19 (11) | 13 (5.2) | .03 |
| Psychosis | 27 (5.9) | 15 (8.3) | 12 (4.4) | .08 | 16 (3.8) | 10 (5.8) | 6 (2.4) | .07 |
| Cerebrovascular disease | 21 (4.6) | 10 (5.6) | 11 (4) | .44 | 13 (3.1) | 6 (3.5) | 7 (2.8) | .70 |
| Alcohol | 14 (3.1) | 10 (5.6) | 4 (1.5) | .02 | 8 (1.9) | 5 (2.9) | 3 (1.2) | .28 |
| Traumatic brain injury | 10 (2.2) | 4 (2.2) | 6 (2.1) | >.99 | 6 (1.4) | 2 (1.2) | 4 (1.6) | >.99 |
| Metabolism endocrine | ||||||||
| Hypothyroidism | 192 (42.2) | 89 (49.4) | 103 (37.5) | .01 | 210 (49.6) | 94 (54.3) | 116 (46.4) | .11 |
| Anemia | 150 (33) | 67 (37.2) | 83 (30.2) | .12 | 107 (25.3) | 55 (31.8) | 52 (20.8) | .01 |
| Dyslipidemia | 101 (22.2) | 48 (26.7) | 53 (19.3) | .06 | 91 (21.5) | 41 (23.7) | 50 (20) | .36 |
| Diabetes | 61 (13.4) | 28 (15.6) | 33(12) | .28 | 40 (9.5) | 19 (11) | 21 (8.4) | .37 |
| Obesity | 27 (5.9) | 11 (6.1) | 16 (5.8) | .89 | 46 (10.9) | 17 (9.8) | 29 (11.6) | .57 |
| Weight loss | 42 (9.2) | 23 (12.8) | 19 (6.9) | .03 | 29 (6.9) | 22 (12.7) | 7 (2.8) | <.001 |
| Osteoporosis | 21 (4.6) | 14 (7.8) | 7 (2.5) | .009 | 44 (10.4) | 18 (10.4) | 26 (10.4) | .99 |
| Other conditions | ||||||||
| Chronic pulmonary disease | 79 (17.4) | 33 (18.3) | 46 (16.7) | .66 | 86 (20.3) | 39 (22.5) | 47 (18.8) | .35 |
| Chronic renal failure | 51 (11.2) | 27 (15) | 24 (8.7) | .04 | 38 (9) | 18 (10.4) | 20 (8) | .40 |
| Ulcer disease | 22 (4.8) | 8 (4.4) | 14 (5.1) | .75 | 12 (2.8) | 4 (2.3) | 8 (3.2) | .77 |
| Tumor and hematologic disease | 20 (4.4) | 2 (1.1) | 18 (6.5) | .005 | 13 (3.1) | 4 (2.3) | 9 (3.6) | .57 |
| Liver disease | 18 (4) | 6 (3.3) | 12 (4.4) | .58 | 13 (3.1) | 7 (4) | 6 (2.4) | .34 |
| Rheumatism | 0 | 0 | 0 | NA | 5 (1.2) | 2 (1.1) | 3 (1.2) | >.99 |
| Tobacco use | 4 (0.9) | 2 (1.1) | 2 (0.7) | .65 | 0 | 0 | 0 | NA |
Abbreviations: DS, Down syndrome; NA, not applicable.
Discussion
In a large sample of 878 older individuals with DS (age range, 45-89 years) identified from California fee-for-services Medicare claims in 2015, prevalence of dementia increased with age, and half of individuals with DS had a diagnosis of dementia when older than 65 years. Chronic comorbid conditions were more numerous among individuals with DS with dementia than without, especially among those younger than 65 years. While DS follow-up studies have long been confined to the pediatric sphere, we show how increasing longevity among those with DS poses new challenges that require complex adult and geriatric care associated with the treatment of long-term multimorbidity and dementia.
Cohort studies of patients with DS dementia are few, and prevalence rates of dementia in DS vary according to diagnostic methods, types of inclusion and data sets, discrepancies in sample size, and participants’ age.7 Our observed overall frequency of 40% dementia among individuals with DS older than 45 years and 49% among those older than 65 years is consistent with several previous studies in which the prevalence was around 50%.22,23 However, other studies have reported higher rates, especially among prospective studies with a prevalence superior to 75% among those older than 60 years24,25 and a cumulative incidence of 79.71% by age 60 years.26 In the oldest individuals with DS within our study, the frequency of dementia declined slightly, which may reflect a survival biais.27,28 Furthermore, given that our study relies on diagnostic codes from Medicare (ICD-9-CM and ICD-10-CM codes) and often derive from primary care settings (as opposed to specialty clinics), it is possible that some symptoms of dementia might have been attributed to the preexisting developmental cognitive impairment of individuals with DS.29,30,31 As a whole, our large, population-based sample may be more representative than clinic- or institution-based studies, but our identification method is likely to be less sensitive, especially for mild dementia.
We found high frequencies of numerous chronic treatable comorbidities in this aging DS population, and in people with DS dementia in particular. Because of an accelerated aging process in DS,32 chronic comorbid conditions are observed even among middle-aged individuals with DS and with DS dementia.33 Interestingly, those with dementia younger than 65 years had a greater number of comorbidities compared with those of the same age without dementia. This suggests that a high level of multimorbidity might contribute to the heterogeneity of age at onset of DS dementia,34,35 although an alternative explanation might be that there are shared risk (and resilience) factors for dementia and other comorbidities in DS. Also, those individuals with many comorbidities are likely to be more frail and die earlier, which could account for the absence of differences in the number of comorbidities between those individuals with and without dementia older than 65 years. Adults with DS would likely benefit from integrated care because comorbidities are numerous and often preventable or treatable and because familial caregiving tends to be less existent as individuals with DS are getting older.33
We pragmatically looked for comorbidity targets that could be better treated in primary care settings to prevent or slow DS dementia onset and to lower the effect of aggravating factors of dementia. We identified 4 treatable conditions that were much higher in midlife and late-life DS dementia. Hypothyroidism was more frequent in women with DS in general, and more than half of individuals with DS dementia had hypothyroidism confirming published prevalence, ranging from 51% to 59% in DS dementia.22,24,36 Overt hypothyroid is a cause of reversible dementia37 and a recognized associated factor of poorer cognitive performance.38 Future work in DS should explore sex differences regarding thyroid dysfunction and risk of dementia and focus on the duration of sub and clinical hypothyroidism and on treatment benefits.39 Epilepsy frequency was also very high (41%) reinforcing previous research with even higher figures of 74%25 and 84%24 in DS dementia. Epilepsy is a frequent prodrome or subsequent manifestation of dementia, with new-onset epilepsy occurring within 2 years after the dementia diagnosis.40 Epilepsy is a core condition in DS that was found to predict dementia in 80% of the cases.27 Anemia was more frequent in men with DS in general, and was present in one-third of DS dementia cases, although to our knowledge, no prior publication has identified this critical association in DS. Anemia itself or the causal determinants of anemia (eg, lower serum folate concentration, iron, or B12) are associated with cognitive disorders37,41 including risk of dementia.42 Weight loss was more than twice as frequent among DS dementia (12.7%) than in individuals without dementia. In general population, weight loss is commonly associated with dementia as a prodromal factor that precedes cognitive decline43 and as an ongoing consequence of dementia.44 While being overweight is classically known in young people with DS, awareness has been raised about a mean 21% weight loss quantified during a 4-year period in DS dementia.45,46 The differences observed in comorbidity patterns according to both dementia status and sex are also of interest because they suggest that distinct follow-up screening according to sex (and thus treatment of preventable conditions) could be important for promoting better care. As a whole, whether these concurrent comorbidity factors stand as associated causes or consequences of dementia remains to be studied in the future with longitudinal data. Overall, these comorbidities are strongly associated with DS dementia and they should be screened early and better treated during the lifetime of DS people.
Limitations
The major limitation of this study is the cross-sectional nature of the study. Thus, we cannot disentangle what might be a risk factor, a prodrome, or a consequence of DS dementia. Limitations about diagnoses based on claims data also include the lack of knowledge about the duration of dementia and the duration of the comorbidities, and consequently about the chronological association between comorbidities and onset of dementia. Because we capture here Medicare and “Medi-Medi” beneficiaries (ie, individuals dually eligible for Medicare and Medical at the same time),47we might miss dementia among the poorest and severely disabled individuals with DS who would only qualify for Medical (ie, Californian Medicaid),48 as well as cases of early-onset dementia before age 45 years (because Medicare includes people older than age 45 years). However, the present top-down epidemiologic approach through the US billing system captures a large number of individuals with DS who were identified through a broad variety of health care procedures and lived throughout Californian counties. Although we examined a broad list of comorbid conditions, we were unable to look at every possible condition. Recommendations include using electronic health records and data linkage in the future to follow-up beneficiaries longitudinally and to chain health claims information to clinical data.
Conclusions
Because longevity in DS has been increasing rapidly in the past decades, there is a high need for more updated medical and epidemiology research studies in this population.49 Aging with DS means that individuals with DS have to deal with complex long-term multimorbidity, and in half of the cases, with comorbid DS dementia. More evidenced-based and standardized care follow-up (similarly to those now operational in younger patients with DS)35 could support better comorbidity management in the aging DS population and ultimately better dementia diagnosis and specific DS care.
References
- 1.Presson AP, Partyka G, Jensen KM, et al. . Current estimate of Down Syndrome population prevalence in the United States. J Pediatr. 2013;163(4):1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covelli V, Raggi A, Meucci P, Paganelli C, Leonardi M. Ageing of people with Down’s syndrome: a systematic literature review from 2000 to 2014. Int J Rehabil Res. 2016;39(1):20-28. [DOI] [PubMed] [Google Scholar]
- 3.Englund A, Jonsson B, Zander CS, Gustafsson J, Annerén G. Changes in mortality and causes of death in the Swedish Down syndrome population. Am J Med Genet A. 2013;161A(4):642-649. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359(9311):1019-1025. [DOI] [PubMed] [Google Scholar]
- 5.Strengthening connections between Down syndrome and AD. Lancet Neurol. 2013;12(10):931. [DOI] [PubMed] [Google Scholar]
- 6.Lott IT, Head E. Alzheimer disease and Down syndrome: factors in pathogenesis. Neurobiol Aging. 2005;26(3):383-389. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C, Mobley W, Hardy J, Williams G, Corbett A. Dementia in Down’s syndrome. Lancet Neurol. 2016;15(6):622-636. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services What’s Medicare? https://www.medicare.gov/sign-up-change-plans/decide-how-to-get-medicare/whats-medicare/what-is-medicare.html. Accessed February 12, 2018.
- 9.Centers for Medicare and Medicaid Services, Chronic Condition Data Warehouse Technical guidance: calculating medicare population statistics. https://www.ccwdata.org/web/guest/condition-categorieshttp://www.ccwdata.org/index.htm. Accessed February 12, 2018.
- 10.Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. [DOI] [PubMed] [Google Scholar]
- 11.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonelli M, Wiebe N, Fortin M, et al. ; Alberta Kidney Disease Network . Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healthcare Cost and Utilization Project (H-CUP project) https://www.hcup-us.ahrq.gov/ 2008. Accessed December 2017.
- 14.Lehnert T, Heider D, Leicht H, et al. . Review: health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev. 2011;68(4):387-420. [DOI] [PubMed] [Google Scholar]
- 15.Hill JW, Futterman R, Duttagupta S, Mastey V, Lloyd JR, Fillit H. Alzheimer’s disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002;58(1):62-70. [DOI] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality (AHRQ) Multiple Chronic Conditions Chartbook: medical expenditure panel survey data. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/prevention-chronic-care/decision/mcc/mccchartbook.pdf; Accessed February 12, 2018.
- 17.Erdem E, Prada SI, Haffer SC. Medicare payments: how much do chronic conditions matter? Medicare Medicaid Res Rev. 2013;3(2):mmrr.003.02.b02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187-194. [DOI] [PubMed] [Google Scholar]
- 19.Beydoun MA, Beydoun HA, Gamaldo AA, et al. . Nationwide inpatient prevalence, predictors, and outcomes of Alzheimer’s disease among older adults in the United States, 2002-2012. J Alzheimers Dis. 2015;48(2):361-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob L, Breuer J, Kostev K. Prevalence of chronic diseases among older patients in German general practices. Ger Med Sci. 2016;14:Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suehs BT, Davis CD, Alvir J, et al. . The clinical and economic burden of newly diagnosed Alzheimer’s disease in a medicare advantage population. Am J Alzheimers Dis Other Demen. 2013;28(4):384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Real de Asua D, Quero M, Moldenhauer F, Suarez C. Clinical profile and main comorbidities of Spanish adults with Down syndrome. Eur J Intern Med. 2015;26(6):385-391. [DOI] [PubMed] [Google Scholar]
- 23.Margallo-Lana ML, Moore PB, Kay DW, et al. . Fifteen-year follow-up of 92 hospitalized adults with Down’s syndrome: incidence of cognitive decline, its relationship to age and neuropathology. J Intellect Disabil Res. 2007;51(Pt. 6):463-477. [DOI] [PubMed] [Google Scholar]
- 24.Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46(8):849-853. [DOI] [PubMed] [Google Scholar]
- 25.Visser FE, Aldenkamp AP, van Huffelen AC, Kuilman M, Overweg J, van Wijk J. Prospective study of the prevalence of Alzheimer-type dementia in institutionalized individuals with Down syndrome. Am J Ment Retard. 1997;101(4):400-412. [PubMed] [Google Scholar]
- 26.McCarron M, McCallion P, Reilly E, Mulryan N. A prospective 14-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2014;58(1):61-70. [DOI] [PubMed] [Google Scholar]
- 27.Tyrrell J, Cosgrave M, McCarron M, et al. . Dementia in people with Down’s syndrome. Int J Geriatr Psychiatry. 2001;16(12):1168-1174. [DOI] [PubMed] [Google Scholar]
- 28.Coppus A, Evenhuis H, Verberne GJ, et al. . Dementia and mortality in persons with Down’s syndrome. J Intellect Disabil Res. 2006;50(pt 10):768-777. [DOI] [PubMed] [Google Scholar]
- 29.Burt DB, Loveland KA, Primeaux-Hart S, et al. . Dementia in adults with Down syndrome: diagnostic challenges. Am J Ment Retard. 1998;103(2):130-145. [DOI] [PubMed] [Google Scholar]
- 30.Zigman WB, Devenny DA, Krinsky-McHale SJ, et al. . Alzheimer’s disease in adults with Down syndrome. Int Rev Res Ment Retard. 2008;36:103-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devenny DA, Krinsky-McHale SJ, Sersen G, Silverman WP. Sequence of cognitive decline in dementia in adults with Down’s syndrome. J Intellect Disabil Res. 2000;44(pt 6):654-665. [DOI] [PubMed] [Google Scholar]
- 32.Devenny DA, Wegiel J, Schupf N, et al. . Dementia of the Alzheimer’s type and accelerated aging in Down syndrome. Sci Aging Knowledge Environ. 2005;2005(14):dn1. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee S. Multimorbidity: older adults need health care that can count past one. Lancet. 2015;385(9968):587-589. [DOI] [PubMed] [Google Scholar]
- 34.Wallace RA, Dalton AJ. What can we learn from study of Alzheimer’s disease in patients with Down syndrome for early-onset Alzheimer’s disease in the general population? Alzheimers Res Ther. 2011;3(2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson A, Lynch SA, Wilkinson S, Hunter M. Adults with Down’s syndrome: the prevalence of complications and health care in the community. Br J Gen Pract. 2007;57(534):50-55. [PMC free article] [PubMed] [Google Scholar]
- 36.McCarron M, Gill M, McCallion P, Begley C. Health co-morbidities in ageing persons with Down syndrome and Alzheimer’s dementia. J Intellect Disabil Res. 2005;49(pt 7):560-566. [DOI] [PubMed] [Google Scholar]
- 37.Kuruppu DK, Matthews BR. Young-onset dementia. Semin Neurol. 2013;33(4):365-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuels MH. Cognitive function in untreated hypothyroidism and hyperthyroidism. Curr Opin Endocrinol Diabetes Obes. 2008;15(5):429-433. [DOI] [PubMed] [Google Scholar]
- 39.Whooten R, Schmitt J, Schwartz A. Endocrine manifestations of Down syndrome. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gholipour T, Mitchell S, Sarkis RA, Chemali Z. The clinical and neurobehavioral course of Down syndrome and dementia with or without new-onset epilepsy. Epilepsy Behav. 2017;68:11-16. [DOI] [PubMed] [Google Scholar]
- 41.Schultz BM, Freedman ML. Iron deficiency in the elderly. Baillieres Clin Haematol. 1987;1(2):291-313. [DOI] [PubMed] [Google Scholar]
- 42.Jeong S-M, Shin DW, Lee JE, Hyeon JH, Lee J, Kim S. Anemia is associated with incidence of dementia: a national health screening study in Korea involving 37,900 persons. Alzheimers Res Ther. 2017;9(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii M, Iadecola C. Metabolic and non-cognitive manifestations of Alzheimer’s disease: the hypothalamus as both culprit and target of pathology. Cell Metab. 2015;22(5):761-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alhurani RE, Vassilaki M, Aakre JA, et al. . Decline in weight and incident mild cognitive impairment: Mayo Clinic Study of Aging. JAMA Neurol. 2016;73(4):439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasher VP, Metseagharun T, Haque S. Weight loss in adults with Down syndrome and with dementia in Alzheimer’s disease. Res Dev Disabil. 2004;25(1):1-7. [DOI] [PubMed] [Google Scholar]
- 46.Stancliffe RJ, Lakin KC, Larson SA, et al. . Demographic characteristics, health conditions, and residential service use in adults with Down syndrome in 25 U.S. states. Intellect Dev Disabil. 2012;50(2):92-108. [DOI] [PubMed] [Google Scholar]
- 47.Social Security Administration Disability planner: benefits for a disabled child. https://www.ssa.gov/planners/disability/dqualify10.html. Published November 2017. Accessed February 12, 2018.
- 48.Disability and Down syndrome: filing for social security benefits. https://www.disabilitysecrets.com/conditions-page-2-30.html. Published November 2017. Accessed February 12, 2018.
- 49.US Department of Health and Human Services, Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Down Syndrome Working Group Down Syndrome Directions National Institutes of Health Research Plan on Down Syndrome 2007, revised plan 2014. https://www.nichd.nih.gov/publications/pubs/Document/DSResearchPlan_2014.pdf. Accessed February 12, 2018.