Key Points
Question
How do placebo treatments affect pain processing in the brain?
Findings
This systematic meta-analysis of single-participant functional magnetic resonance imaging data of 603 healthy participants from 20 studies found that placebo treatments against experimental pain have moderate effects on pain reports, but very small effects on the neurologic pain signature, a cerebral measure of nociceptive pain.
Meaning
Placebo analgesia seems to be predominantly mediated by networks different from those underlying the primary processing of noxious stimuli.
This meta-analysis of participant-level data assesses the effect of placebo treatments on pain-associated functional neuroimaging responses in the neurologic pain signature, a multivariate brain pattern tracking nociceptive pain.
Abstract
Importance
Placebo effects reduce pain and contribute to clinical analgesia, but after decades of research, it remains unclear whether placebo treatments mainly affect nociceptive processes or other processes associated with pain evaluation.
Objective
We conducted a systematic, participant-level meta-analysis to test the effect of placebo treatments on pain-associated functional neuroimaging responses in the neurologic pain signature (NPS), a multivariate brain pattern tracking nociceptive pain.
Data Sources
Medline (PubMed) was searched from inception to May 2015; the search was augmented with results from previous meta-analyses and expert recommendations.
Study Selection
Eligible studies were original investigations that were published in English in peer-reviewed journals and that involved functional neuroimaging of the human brain with evoked pain delivered under stimulus intensity-matched placebo and control conditions. The authors of all eligible studies were contacted and asked to provide single-participant data.
Data Extraction and Synthesis
Data were collected between December 2015 and November 2017 following the Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data guidelines. Results were summarized across participants and studies in a random-effects model.
Main Outcomes and Measures
The main, a priori outcome was NPS response; pain reports were assessed as a secondary outcome.
Results
We obtained data from 20 of 28 identified eligible studies, resulting in a total sample size of 603 healthy individuals. The NPS responses to painful stimulation compared with baseline conditions were positive in 575 participants (95.4%), with a very large effect size (g = 2.30 [95% CI, 1.92 to 2.69]), confirming its sensitivity to nociceptive pain in this sample. Placebo treatments showed significant behavioral outcomes on pain ratings in 17 of 20 studies (85%) and in the combined sample (g = −0.66 [95% CI, −0.80 to −0.53]). However, placebo effects on the NPS response were significant in only 3 of 20 studies (15%) and were very small in the combined sample (g = −0.08 [95% CI, −0.15 to −0.01]). Similarly, analyses restricted to studies with low risk of bias (g = −0.07 [95% CI, −0.15 to 0.00]) indicated very small effects, and analyses of just placebo responders (g = −0.22 [95% CI, −0.34 to −0.11]) indicated small effects, as well.
Conclusions and Relevance
Placebo treatments have moderate analgesic effects on pain reports. The very small effects on NPS, a validated measure that tracks levels of nociceptive pain, indicate that placebo treatments affect pain via brain mechanisms largely independent of effects on bottom-up nociceptive processing.
Introduction
Placebo treatments are treatments with no intrinsic physical or pharmacological benefit. Nevertheless, they affect symptoms and physiology through patients’ conceptions of the therapeutic context.1,2 Placebo effects can be elicited by sham treatments, but they are also a substantial and beneficial part of the overall response to verum treatments, including those involving drugs and/or surgery.3,4 In addition to conferring clinical benefits (and harms, in the case of nocebo effects), placebo effects reduce effect sizes for drug vs placebo differences in clinical trials, which may cause an increasing number of trials to fail5 and thereby impede drug development.6,7 Thus there is an urgent need to better understand placebo effects and to develop biomarkers for active drug responses, as well as placebo responses, to improve decision making in early clinical trials.8
Though placebo treatments can affect a variety of clinical outcomes,9 placebo analgesia is the most robust and well studied.7,10,11 Placebo analgesia has been linked with multiple psychological processes, including expectations and beliefs,12 associative learning,13 and social cognition.14 Neurophysiological studies have suggested the involvement of descending inhibition of nociceptive afferents, with some studies supporting influences on spinal mechanisms15,16,17,18,19 and others supporting higher-level cortical effects that cannot be explained by nociceptive input modulation alone, indicating affective or evaluative mechanisms.20,21,22,23
Nevertheless, the mechanisms by which placebo treatments change the perception of pain remain poorly understood, in part because of 2 limitations. First, previous studies were based on small sample sizes. Second, many brain areas associated with placebo analgesia, such as the anterior midcingulate cortex, the insula, and limbic regions, are involved in a range of functions, including cognitive decision making,24 motor processes,25 and emotion.26,27 Thus, previous studies have not been able to establish whether placebo analgesia affects nociception-associated and pain-associated processing specifically or other cognitive and affective processes associated with the multidimensional experience of pain.
Recently, studies have begun to identify patterns of functional magnetic resonance imaging activity that yield objective and reliable28,29 brain measures associated with evoked pain.30,31,32,33 While they do not measure pain, which is by definition a subjective experience, they capture neurophysiological patterns associated with specific aspects of pain with high sensitivity.34,35 Among ongoing efforts, the neurologic pain signature (NPS)33 is a measure that has been shown to reliably track the intensity of evoked experimental pain across multiple studies with high sensitivity while responding only minimally to nonpainful somatic stimuli and other salient, aversive events, thus exhibiting high specificity.35,36,37,38,39 Although fully understanding the neurophysiological processes captured by the NPS is a matter of ongoing investigation, previous results suggest that the NPS predominantly reflects changes in nociceptive input and the pain that arises from it, while being insensitive to higher cognitive pain modulation.36,37,38,39
In this study, we harness these methodological advances in a systematic meta-analysis of single-participant data testing placebo effects on NPS responses. If placebo treatments predominantly affected early nociceptive processes, they would be expected to reduce activity in the NPS. If so, placebo effects and the endogenous pain-regulatory processes they engage may have pervasive effects on pain generation, making it hard to dissociate pain-associated and placebo-associated processes and outcomes. Conversely, if placebos mainly affect later-stage affective and evaluative processes, they may have little influence on NPS responses. In this case, it may be possible to develop meaningful measures of nociception that are placebo insensitive.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participant Data.40 The study protocol and hypotheses were registered (https://osf.io/n9mb3/) on December 10, 2015, which was after eligibility criteria and study search were defined, but before data collection and analysis began.
Eligibility Criteria and Study Search
Criteria for study eligibility were peer-reviewed publication in the English language of an original investigation involving human participants who underwent functional neuroimaging of the brain during evoked pain and pain delivered under stimulus intensity-matched placebo and control conditions. Placebo treatment was defined as any condition where the experimental context suggested that an effective analgesic treatment was applied, including verbal suggestions and conditioning procedures that reinforced participants’ expectations of reduced pain,41 following the categorization of placebo paradigms introduced in Wager and Atlas in 2015.2 Accordingly, nonplacebo control conditions that involved no treatment, ineffective treatment, hidden treatment (in contrast to open treatment), and unconditioned treatment (in contrast to conditioned treatment) were considered eligible. We identified potentially eligible studies on Medline on May 21, 2015 and December 6, 2015. This search was augmented by results from previous meta-analyses2,42 and by requests for authors of individual placebo studies to identify additional studies missed in the online search. All 3 authors (M.Z., U.B., and T.D.W.) screened the titles and abstracts of all records retrieved; studies that provisionally met eligibility criteria were assessed for eligibility by examining the full text. (Details appear in the eMethods and eTable 1 in the Supplement.)
Data Acquisition
Authors of all eligible studies were contacted and asked to contribute participant-level neuroimaging, behavioral data (pain report), and demographic data. We requested data from the final, published analyses. Substudies with distinct participant samples and methods were treated as independent (studies 1 and 2 in Wager et al43). Reanalyses were excluded, but in the case of reanalyses with extended samples,44,45 we included the study with the largest sample. Studies with partially missing data were included and analyzed based on the available data.46,47
Outcome Definition
We obtained NPS responses33 for each individual participant in each experimental condition and contrasted pain and placebo with pain and nonplacebo control conditions. The NPS responses were calculated as the dot product of each image with the NPS pattern, yielding a weighted average of activity across the image, where the NPS specifies the weights.33 High NPS responses reflect both higher similarity of the individual images with the NPS pattern and higher-magnitude functional magnetic resonance imaging activity in the specified pattern.
To keep baseline conditions of studies with within-participant crossover designs and between-group placebo manipulations comparable, we limited our analysis to posttreatment conditions; in other words, additional baseline measurements from within-participant or mixed-design studies were excluded.46,48,49,50 Further, nonpainful51 or low-intensity43,46,48,52,53,54 stimulus conditions were excluded from analysis to maximize detection sensitivity for placebo effects.
In several studies, images were provided for separate subconditions within placebo and control categories (eg, for left-lateralized and right-lateralized stimulation,55 for strong and weak placebo conditions,56,57 or for early-heat-pain and late-heat-pain periods).16,57,58 In these cases, we summarized the NPS responses by calculating an average response under placebo and under control treatment for each participant (details appear in eTable 2 in the Supplement).
The scale of NPS responses depends on the scale of underlying imaging data and therefore on the image acquisition and analysis parameters used in the original studies. To avoid scaling issues, we based our analysis on the standardized effect size measure Hedges g, as is common in meta-analyses.59 Similarly to the Cohen d, Hedges g is based on the mean difference between conditions divided by standard deviation, but with an additional correction for small sample bias. For within-participant studies we used Hedges grm, which is based on the SD of within-participant differences corrected for within-participant correlations.60,61
Risk of Bias Assessment
We used the Cochrane risk of bias tool62 to evaluate the risk of bias for studies included in the present meta-analysis (details are in the eMethods and eTable 3 in the Supplement). We assessed biases from selection (which arises via insufficient randomization), performance (via insufficient blinding of participants or treatment providers), detection (via insufficient blinding of analysts), attrition (by missing data), reporting (via underreporting of nonsignificant studies), and sequence (which is potentially introduced by within-participant designs).
Analysis
Our main analysis followed a 3-part strategy. First, we tested the research question using all data available. Second, we conducted a conservative analysis excluding studies with high risk of bias. Third, we performed a responder analysis, in which we (1) only included participants who showed a behavioral placebo response (a pain report under placebo condition compared with the report under the control condition) above the study median, (2) excluded any experimental subconditions that may have diminished placebo effects (eg, placebo conditions deemed to be low efficacy or placebo conditions tested under pharmacological modulation), and (3) excluded any participants who were suspected outliers (the eMethods and eTable 2 in the Supplement).
Effects were summarized across studies using the generic inverse-variance weighting method with DerSimonian and Laird random effects,60 meaning studies were weighted by 1/SE2 (where SE is the standard error). We estimated heterogeneity in results using the τ statistic, which represents the standard deviation of effect sizes between studies.59 We tested against the null hypothesis of no effect at an error level of α < .05 (2-tailed), with additional inferences based on Bayes factors.63 In brief, Bayes factors represent the relative likelihood of the null and the alternative hypotheses and have the advantage (compared with P values) that support for the null hypothesis can be concluded. Further analysis details and procedures used to check image quality are provided in the eMethods in the Supplement. Analysis was completed with MATLAB 2016b (MathWorks). The analysis code is available at https://github.com/mzunhammer/PlaceboImagingMetaAnalysis.
Results
Data Acquisition
We identified 96 published articles, of which 28 were selected as eligible. These included a total of 759 participants. Data from 20 studies and 603 participants were obtained, thus including the majority of eligible studies (71%) and participants (79.4%) published until 2015. Details on data acquisition are provided in eFigure 1 and eTable 1 in the Supplement. Eligible studies that could not be obtained were generally similar to the studies obtained in terms of pain stimulation, placebo induction, and study design; notably, 8 eligible studies with patient samples could not be obtained (eTable 4 in the Supplement). An update of the study search in March 2018 indicated that at least 6 eligible studies (with a combined patient population of 196 individuals) were published after data collection (in eMethods and eTable 1 in the Supplement). Even when considering these additional studies, the present meta-analysis covers most eligible studies (59%) and participants (63.1%).
Risk of Bias
For pain ratings, the assessment of risk of bias (eResults, eTable 3, and eFigure 2 in the Supplement) indicated a high risk of performance (self-report) and detection bias, as well as unknown levels of reporting (publication) bias. For NPS responses, we found low risk of bias, because this measure does not depend on self-report and was unknown when the original studies were performed.
Sample Description
Included studies are listed in the Table, and key sample characteristics are shown in eFigure 3 in the Supplement. Details on pain stimulation, placebo treatment, image acquisition, and imaging analyses are provided in the eMethods and eTables 5, 6, 7, and 8 in the Supplement. Image alignment to Montreal Neurological Institute space was satisfactory, and the coverage of the voxels making up the NPS was near optimum levels (98.4% across all participants; eMethods and eFigure 4 in the Supplement). Four participants showed mean pain ratings less than 5% of the pain scale, indicating insufficient pain stimulation; evidence for imaging artifacts was found in 12 participants (2.0%) (eMethods in the Supplement). These participants were defined as outliers and excluded from the responder analysis, but retained in the primary and the conservative analysis.
Table. Included Studies.
| Source | Participants, No. | Design | Mean Age, y | Male, No. (%) | Pain Stimulus | Treatment Type | Placebo Suggestions | Placebo Conditioning | Placebo Comparison |
|---|---|---|---|---|---|---|---|---|---|
| Atlas et al,52 2012 | 21 | Within | 25 | 10 (48) | Contact heat | Remifentanil infusion | Yes | No | Open vs hidden |
| Bingel et al,55 2006 | 19 | Within | 24 | 15 (79) | Laser | Inert topical cream | Yes | Yes | Effective vs ineffective |
| Bingel et al,49 2011 | 22 | Within | 28 | 15 (68) | Contact heat | Remifentanil infusion | Yes | Yes | Open vs hidden |
| Choi et al,56 2011 | 15 | Within | 25 | 15 (100) | Electrical | Saline infusion | Yes | Yes | Effective vs ineffective |
| Eippert et al,16 2009 | 40 | Within | 26 | 40 (100) | Contact heat | Inert topical cream | Yes | Yes | Effective vs ineffective |
| Ellingsen et al,51 2013 | 28 | Within | 26 | 19 (68) | Contact heat | Inert nasal spray | Yes | No | Treatment vs no treatment |
| Elsenbruch et al,64 2012 | 36 | Within | 26 | 15 (42) | Distension | Saline infusion | Yes | No | Effective vs ineffective |
| Freeman et al,53 2015 | 24 | Within | 27 | 12 (50) | Contact heat | Inert topical cream | Yes | Yes | Effective vs ineffective |
| Geuter et al,57 2013 | 40 | Within | 26 | 40 (100) | Contact heat | Inert topical cream | Yes | Yes | Effective vs ineffective |
| Kessner et al,50 2013 | 39 | Between | 26 | 20 (51) | Contact heat | Inert topical cream | No | Yes | Effective vs ineffective |
| Kong et al,46 2006 | 10c | Within | 27 | 6 (60) | Contact heat | Sham acupuncture | Yes | Yes | Effective vs ineffective |
| Kong et al,48 2009 | 12a | Within | 26 | 5 (42) | Contact heat | Sham acupuncture | Yes | Yes | Effective vs ineffective |
| Lui et al,65 2010 | 31 | Within | 23 | 15 (45) | Laser | Sham tens | Yes | Yes | Effective vs ineffective |
| Rütgen et al,54 2015 | 102 | Between | 25 | 32 (31) | Electrical | Inert pill | Yes | Yes | Treatment vs no treatment |
| Schenk et al,79 2014 | 32 | Within | 26 | 17 (53) | Capsaicin and heat | Topical lidocaine and inert topical cream | Yes | No | Hidden vs open (lidocaine), effective vs ineffective (inert cream) |
| Theysohn et al,45 2014 | 30 | Within | 35 | 15 (50) | Distension | Saline infusion | Yes | No | Effective vs ineffective |
| Wager et al,43 2004ab | 24 | Within | na | na | Electrical | Inert topical cream | Yes | No | Effective vs ineffective |
| Wager et al,43 2004bc | 23 | Within | na | na | Contact heat | Inert topical cream | Yes | Yes | Effective vs ineffective |
| Wrobel et al,58 2014 | 38 | Within | 26 | 22 (58) | Contact heat | Inert topical cream | Yes | Yes | Effective vs ineffective |
| Zeidan et al,47 2015 | 17a | Within | 28 | 8 (47) | Contact heat | Inert topical cream | Yes | Yes | Treatment vs no treatment |
Abbreviations: NA, not available; TENS, transcutaneous electrical nerve stimulation.
Placebo-treatment groups only.
Substudy 1.
Substudy 2.
NPS Responses to Painful Stimulation
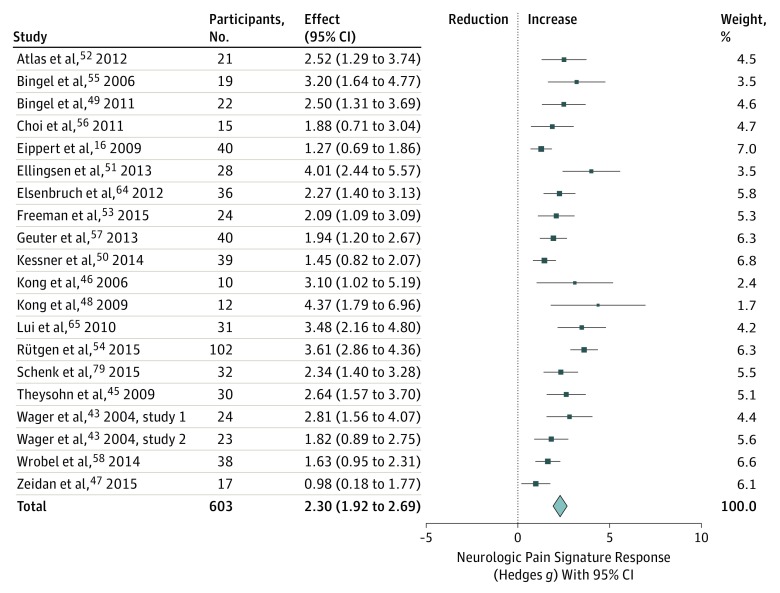
The NPS responses to painful stimulation, compared with low-level baseline of placebo and control conditions pooled, were very large (g = 2.30 [95% CI, 1.92 to 2.69]) and significant in all included studies (Figure 1). Positive NPS responses were observed in 576 individuals (95.4%) (95.6% when outliers were excluded). The estimated between-study standard deviation of effect sizes τ was 0.68, indicating that the outcomes of painful stimulation on NPS responses varied considerably among studies, which was as expected, given the broad range of different imaging and pain stimulation protocols used (Table; eFigure 3 and eTables 5, 6, 7, and 8 in the Supplement). Moreover, the NPS responded more strongly to high vs low levels of stimulus intensity in 6 of 7 studies (86%) in which this comparison was possible (g = 1.32 [95% CI, 0.86 to 1.79]; eFigure 5 in the Supplement). Together, these findings validate that the NPS was sensitive to noxious stimulation in this sample.
Figure 1. Neurologic Pain Signature Response to Noxious Stimulation vs Baseline in All Studies.
Placebo and control conditions were pooled and studies weighted according to inverse variance. Total z = 11.74 (P < .001); heterogeneity χ219 = 57.15 (P < .001); τ2 = 0.47; I2 = 6.76%.
Placebo Effects on Pain Ratings and NPS Responses
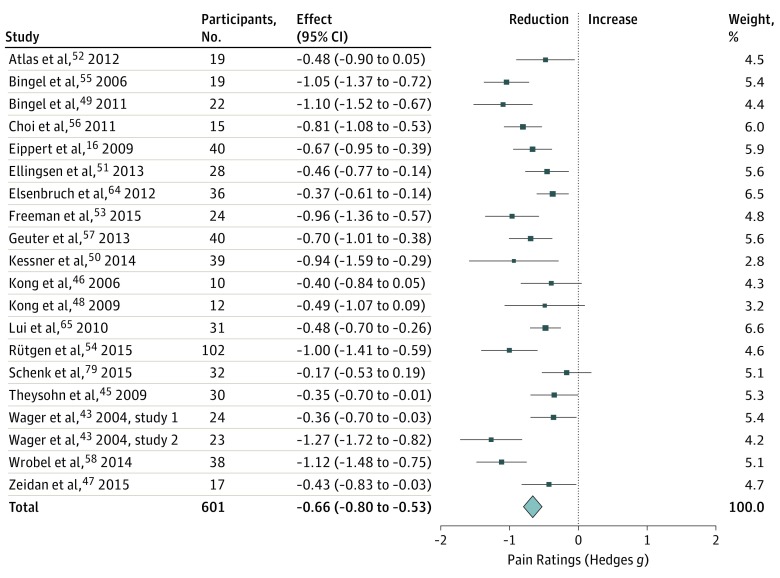
Placebo treatments, compared with matched control conditions, showed moderate66 analgesic effects on pain ratings (g = −0.66 [95% CI, −0.80 to −0.53]; Figure 2), which corresponds to a reduction of −11.3 (95% CI, −14.0 to −8.56) units on a 101-point visual analogue scale. The Bayes factor (BN[0, 0.5]) obtained for a normal null-prior (g = 0 [SD, 0.5], 2-tailed) was 9.4 × 1018, indicating overwhelming support for the hypothesis of nonnull placebo effects on pain ratings.63 Effect sizes varied considerably among studies (τ = 0.24), which may be explained by the variation in placebo paradigms used (Table; eTable 6 in the Supplement).
Figure 2. Changes in Pain Ratings After Experimental Placebo Treatment.
Studies were weighted according to inverse variance. Total z = 9.65 (P < .001); heterogeneity χ219 = 55.35 (P < .001); τ2 = 0.06; I2 = 65.68%.
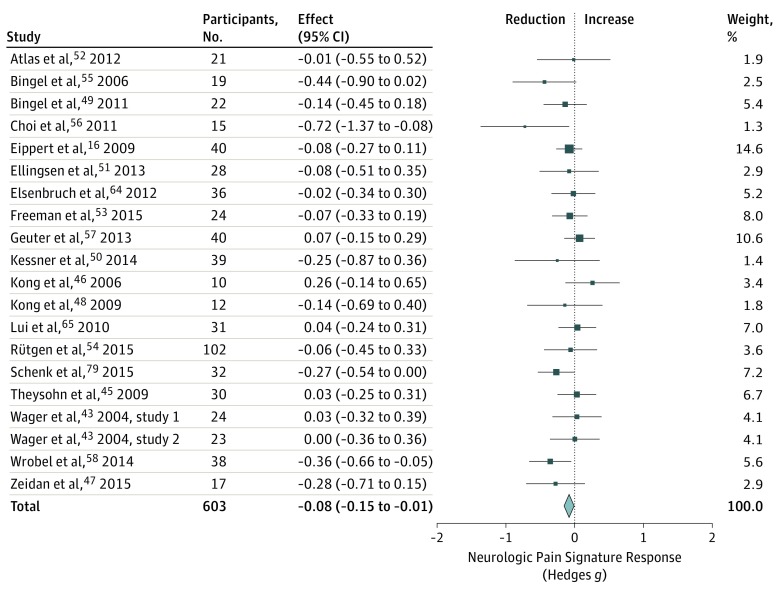
In contrast, effects of placebo treatments on NPS responses were small (g = −0.08 [95% CI, −0.15 to −0.01]; Figure 3), with little between-study heterogeneity (τ = 0.015). Thus, placebo effects on the NPS were 12.1% as large as effects on pain ratings, and 3.7% as large as the effects of painful stimulation on the NPS. The Bayes factor was less than 1 (BN[0, 0.5] = 0.805), indicating that these data provide very weak support in favor of the null hypothesis of no effect.63
Figure 3. Changes in Neurologic Pain Signature Responses After Experimental Placebo Treatments.
Studies were weighted according to inverse variance. Total z = −2.19 (P = .03); heterogeneity χ219 = 19.15 (P = .45); τ2 = 0.00; I2 = 0.77%.
A conservative analysis excluding all studies with high risk of bias and therefore including 15 studies with 429 participants yielded similar results (placebo − control on NPS, g = −0.07 [95% CI, −0.15 to 0.00]; BN(0, 0.5) = 0.787; eFigure 6 in the Supplement). Somewhat larger effects were found in the responder analysis, which included only participants showing a behavioral placebo response greater than the study median and excluded potentially ineffective placebo treatments and outliers (196 participants from 18 studies were included). In this sample of so-called placebo responders, the Bayes factor (BN[0, 0.5] = 113.8) indicated robust support for the hypothesis of a nonnull placebo effect on NPS responses. However, the effects remained in the small range (g = −0.22 [95% CI, −0.34 to −0.11]; eFigure 7 in the Supplement). Thus, even in placebo responders, effects of placebo on the NPS were only 4% to 14% as large as the overall NPS response to painful stimulation (Figure 1).
Associations Between Placebo Effects on Pain Reports and NPS Responses
For studies with crossover designs, which included within-participant testing of both placebo and control treatments, we performed a meta-analysis of within-study correlations between placebo effects on pain report and NPS responses across individuals. A Bayes factor of 894.5 (BN[0, 0.5]) indicated robust support for the hypothesis of a nonnull correlation, with greater placebo analgesia associated with greater NPS downregulation (Pearson r = 0.23 [95% CI, 0.13 to 0.33]; P < .001; eFigure 8 in the Supplement). While statistically significant, the effect was in the small to moderate range, suggesting that placebo analgesia is weakly associated with NPS downregulation.
Comparing Placebo Effects on the NPS With Effect of Reduced Stimulus Intensity
These findings suggest that the effects of placebo are small in terms of effective changes in nociceptive input. To further quantify placebo effects on the NPS in terms of equivalent changes in noxious stimulus intensity, we compared placebo effects with effects of fixed increases in physical stimulus intensity. For this purpose, we used a convenience sample of 3 available out-of-sample studies67,68,69 that tested the effects of fixed increments in noxious heat (steps of 0.4°C in a range from 45.9°C to 47.5°C67 and steps of 1°C in a range from 46°C to 48°C69) or noxious pressure (reduction of 1.5 kg/cm2 from a baseline of 6.0 kg/cm2).68 These independent studies were used because stimulus intensities in our meta-analysis sample varied across individuals and produced behavioral effects several times larger than those of placebo, making estimation of equivalent stimulus intensity differences unreliable (for example, compare Figure 2 and eFigure 5 in the Supplement). Effect sizes (g) for stimulus intensity on pain ratings were −0.63 (95% CI, −0.68 to −0.58) at −0.4°C, −1.10 (95% CI, −1.30 to −0.90] at −1.0°C, and −1.24 (95% CI, −1.66 to −0.81) at −1.5 kg/cm2 (eFigure 9 in the Supplement), compared with −0.66 (95% CI, −0.80 to −0.53) for placebo.
Placebo effects on NPS responses in the full meta-analysis sample were 5.0 and 8.9 times smaller than the effect of reducing heat by −0.4°C67 and −1.0°C,69 respectively, and 10.9 times smaller than the effect of reducing painful nail bed pressure by −1.5 kg/cm2,68 (eFigure 9 in the Supplement), corresponding to an effective reduction in noxious heat of approximately 0.1°C and in noxious pressure of approximately 0.14 kg/cm2. Thus, placebo effects on the NPS are small compared with modest reductions in stimulus intensity, despite similar effects on pain ratings.
Comparing Placebo and Remifentanil Effects on the NPS
Two of the studies in the sample used analgesic, nonsedative doses of the μ-opioid agonist remifentanil49,52 at similar doses (with brain remifentanil concentrations of 0.76 ng/mL52 and 0.80 ng/mL49). This allowed comparison of the effects of opioids and placebo treatment on NPS responses within these studies. Remifentanil (g = −0.77 [95% CI, −1.39 to −0.18]) and placebo treatments (g = −0.79 [95% CI, −1.39 to −0.18]) induced comparable analgesic effects at the behavioral level. However, the effects of remifentanil on the NPS (−1.10 [95% CI, −1.44 to −0.76] g) were about 10 times larger than the mean effect of placebo treatments in these studies (g = −0.11 [95% CI, −0.38 to 0.16]; eFigure 10 in the Supplement). These estimates indicate that the effects of placebo treatment on NPS responses are small compared with the analgesic effects of opioids, despite comparable behavioral effects.
Discussion
This large-scale meta-analysis of participant-level data revealed that placebo treatments have moderate effects on subjective reports of pain, but minimal effects on responses in the NPS, a central nervous system marker that tracks the intensity of nociceptive pain. These findings are based on most of the neuroimaging data on placebo analgesia published in the field until 2015. Results were consistent across a variety of pain-induction and placebo-induction methods and across 3 parallel analyses varying in risk of bias, including an analysis limited to placebo responders.
These results extend our understanding of placebo analgesia by suggesting that the effects of placebos on cerebral pain stimulus intensity processing are limited. We did observe small reductions in NPS response, which scaled with individual analgesia. Such effects may reflect descending inhibition of nociceptive systems, consistent with that findings of placebo, nocebo, and cognitive effects on spinal functional magnetic resonance imaging signals and brainstem nuclei involved in descending modulation that have been reported in previous studies.15,17,18,19,70 However, the very small size of the effects on the NPS argues against a strong and pervasive early influence and point to stronger influences of other systems independent of the NPS. Our results emphasize that placebo analgesia is a phenomenon not based on a single mechanism but rather multiple mechanisms that have yet to be fully understood.
Importantly, the NPS is not a complete model of pain and pain-associated functionality and was not intended as such.33 Lack of effects on the NPS does not imply that placebo effects do not influence pain perception or pain-associated behavior. Indeed, the insensitivity of the NPS to manipulations that affect reported pain35,36,37,38,39 implies that there must be other processes that contribute to pain report. However, the high sensitivity of the NPS to variation in nociceptive input (details may be found in Figure 1; eFigure 5 and eFigure 9 in the Supplement; and previous publications33,35,36,37,38,39), and its sensitivity to known analgesics (eg, the μ-opioid agonist remifentanil; eFigure 10 in the Supplement), suggest that if placebo treatments had pervasive early effects on pain processing, they should have been reflected in the NPS. They did not, leading us to infer that the placebo treatments studied here affect processes that are largely consequent to activation of nociceptive systems. Such processes include cognitive evaluation,71 pain affect, pain-associated decisionmaking, and mesolimbic reward processing.72 These processes are likely important for behavior and subjective well-being in their own right, and indeed, placebo treatments can impact long-term symptom perception and functionality in clinically meaningful ways,73,74 whether they impact nociceptive pain signaling or not.
These findings also have implications for the objective assessment of treatment effects on pain-associated neurophysiology. Patients evaluate their pain within a complex set of personal and cultural factors,75 which poses challenges for clinical trials that use self-reported pain as a primary outcome. Whether patients feel better is paramount for overall well-being, but it does not guarantee that a treatment impacts the intended physiological mechanisms in the brain and elsewhere in the body. Objective neurophysiological measures do not replace reported pain and well-being, but they can provide measures of pharmacodynamic efficacy on specific brain targets.8,76 The present study further establishes the NPS as a brain measure that is sensitive to multiple types of evoked pain and insensitive to cognitive factors33,35,36,37,38,39 in a large and geographically diverse sample. Because the NPS was found sensitive to opioid drugs but not placebo treatment, this may make it an appealing target for evaluating pharmacodynamics and efficacy in early-stage clinical trials.8,76 Our findings suggest that brain patterns such as the NPS can be used to assess efficacy in modulating nociceptive systems for drugs or devices that are intended to modulate nociceptive input at the peripheral or spinal level or influence descending facilitation or inhibition. As suggested by Duff et al,8 the NPS and associated markers could be used to make early stop or go decisions in phase II clinical trials, before much more costly large-scale tests in phase III studies.
Limitations
Several caveats deserve mention. First, this meta-analysis included only studies testing experimental placebo treatments for evoked pain in healthy participants. They may not generalize to clinical pain, which likely involves a complex mix of nociceptive and extranociceptive processes.77 Second, we analyzed summary images from published analyses. While this helps to ensure careful quality control and is advantageous in ensuring broad generalizability of results, it likely increases interstudy heterogeneity and reduces overall effect sizes. However, these issues are unlikely to compromise our conclusions regarding placebo effects, because we compare them with strong positive controls. Third, the present meta-analysis only covers the relevant literature until mid-2015; more recent studies were not sought because of the time demands of collecting and analyzing participant-level imaging data. Advances in data sharing and standardization will hopefully make it possible to perform participant-level meta-analyses more quickly in the future.
Importantly, not all placebo manipulations are likely to be equally effective, as indicated by the heterogeneity in placebo effects on pain ratings (as shown in Figure 2 and described in previous publications10,11). Although the studies tested here were fairly homogenous in terms of placebo effect on the NPS (Figure 3), there are multiple pathways to cognitive pain modulation,37,78 and some may affect the NPS more strongly than others. Treatment contexts not studied here may still influence NPS responses.
Conclusions
In sum, we have shown that placebo treatments have only small effects on a cerebral pattern tracking nociceptive pain in what is to our knowledge the largest meta-analysis of single-participant neuroimaging data on this topic to date. This suggests that placebo analgesia is largely mediated by networks different from those underlying the primary processing of noxious stimuli. Further studies are necessary to better understand which aspects of pain processing are affected by placebo treatments, and the significance of those processes for long-term clinical outcomes and wellbeing. This work serves as a starting point for the development of brain models that track pain-associated outcomes and other clinical and behavioral endpoints.
eMethods. Supplementary methods and results.
eFigure 1. CONSORT flowchart of data-acquisition.
eFigure 2. Funnel plot of placebo effects on pain ratings.
eFigure 3. Sample characteristics.
eFigure 4. Brain-coverage.
eFigure 5. Rating (left) and Neurologic pain signature (NPS, right) responses to noxious high versus various low stimulation intensities.
eFigure 6. Very small analgesic effects of experimental placebo treatments on neurologic pain signature responses in low-risk-of bias studies (conservative analysis).
eFigure 7. Small effects of experimental placebo treatments on neurologic pain signature responses in placebo responders (responder analysis).
eFigure 8. Placebo-effects in pain ratings and NPS-response show a weak, positive association.
eFigure 9. Placebo effects on pain ratings and NPS response, compared against effects of fixed reductions in pain stimulus intensity.
eFigure 10. Placebo effects on pain ratings and NPS response, compared against effects of remifentanil.
eTable 1. Study screening, eligibility checking, and retrieval.
eTable 2. Experimental conditions selected/summarized for analysis.
eTable 3. Risk of bias assessment for NPS response according to the Cochrane risk-of-bias tool.
eTable 4. Features of eligible, but unavailable studies.
eTable 5. Pain stimulation parameters in included studies.
eTable 6. Placebo conditions in included studies.
eTable 7. Functional neuro imaging acquisition characteristics of included studies.
eTable 8. pre-processing and first-level analysis of neuroimages in included studies.
eReferences.
References
- 1.Ashar YK, Chang LJ, Wager TD. Brain mechanisms of the placebo effect: an affective appraisal account. Annu Rev Clin Psychol. 2017;13:73-98. doi: 10.1146/annurev-clinpsy-021815-093015 [DOI] [PubMed] [Google Scholar]
- 2.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16(7):403-418. doi: 10.1038/nrn3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan A, Kolts RL, Rapaport MH, Krishnan KRR, Brodhead AE, Browns WA. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749. doi: 10.1017/S0033291704003873 [DOI] [PubMed] [Google Scholar]
- 4.Jonas WB, Crawford C, Colloca L, et al. To what extent are surgery and invasive procedures effective beyond a placebo response? A systematic review with meta-analysis of randomised, sham controlled trials. BMJ Open. 2015;5(12):e009655. doi: 10.1136/bmjopen-2015-009655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuttle AH, Tohyama S, Ramsay T, et al. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain. 2015;156(12):2616-2626. doi: 10.1097/j.pain.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 6.Borsook D, Hargreaves R, Bountra C, Porreca F. Lost but making progress—where will new analgesic drugs come from? Sci Transl Med. 2014;6(249):249sr3. doi: 10.1126/scitranslmed.3008320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12(3):191-204. doi: 10.1038/nrd3923 [DOI] [PubMed] [Google Scholar]
- 8.Duff EP, Vennart W, Wise RG, et al. Learning to identify CNS drug action and efficacy using multistudy fMRI data. Sci Transl Med. 2015;7(274):274ra16. doi: 10.1126/scitranslmed.3008438 [DOI] [PubMed] [Google Scholar]
- 9.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686-695. doi: 10.1016/S0140-6736(09)61706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions (review) placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010;(1):CD003974. doi: 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vase L, Petersen GL, Riley JL III, Price DD. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145(1-2):36-44. doi: 10.1016/j.pain.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 12.Atlas LY, Wager TD. How expectations shape pain. Neurosci Lett. 2012;520(2):140-148. doi: 10.1016/j.neulet.2012.03.039 [DOI] [PubMed] [Google Scholar]
- 13.Zunhammer M, Ploner M, Engelbrecht C, Bock J, Kessner SS, Bingel U. The effects of treatment failure generalize across different routes of drug administration. Sci Transl Med. 2017;9(393):1-8. doi: 10.1126/scitranslmed.aal2999 [DOI] [PubMed] [Google Scholar]
- 14.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144(1-2):28-34. doi: 10.1016/j.pain.2009.01.033 [DOI] [PubMed] [Google Scholar]
- 15.Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia—when the spine echoes what the brain expects. Pain. 2007;130(1-2):137-143. doi: 10.1016/j.pain.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 16.Eippert F, Bingel U, Schoell ED, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533-543. doi: 10.1016/j.neuron.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 17.Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. J Neurosci. 2006;26(2):559-563. doi: 10.1523/JNEUROSCI.4218-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geuter S, Büchel C. Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci. 2013;33(34):13784-13790. doi: 10.1523/JNEUROSCI.2191-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Büchel C. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science. 2017;358(6359):105-108. doi: 10.1126/science.aan1221 [DOI] [PubMed] [Google Scholar]
- 20.Wager TD, Matre D, Casey KL. Placebo effects in laser-evoked pain potentials. Brain Behav Immun. 2006;20(3):219-230. doi: 10.1016/j.bbi.2006.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martini M, Lee MCH, Valentini E, Iannetti GD. Intracortical modulation, and not spinal inhibition, mediates placebo analgesia. Eur J Neurosci. 2015;41(4):498-504. doi: 10.1111/ejn.12807 [DOI] [PubMed] [Google Scholar]
- 22.Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150(1):59-65. doi: 10.1016/j.pain.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 23.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31(2):439-452. doi: 10.1523/JNEUROSCI.3420-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49(5):757-763. doi: 10.1016/j.neuron.2006.01.032 [DOI] [PubMed] [Google Scholar]
- 25.Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci U S A. 2016;113(35):9922-9927. doi: 10.1073/pnas.1605044113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35(3):121-143. doi: 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wager TD, Atlas LY, Botvinick MM, et al. Pain in the ACC? Proc Natl Acad Sci U S A. 2016;113(18):E2474-E2475. doi: 10.1073/pnas.1600282113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo C-W, Wager TD. Neuroimaging-based biomarker discovery and validation. Pain. 2015;156(8):1379-1381. doi: 10.1097/j.pain.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo C-W, Wager TD. What reliability can and cannot tell us about pain report and pain neuroimaging. Pain. 2016;157(3):511-513. doi: 10.1097/j.pain.0000000000000442 [DOI] [PubMed] [Google Scholar]
- 30.Marquand A, Howard M, Brammer M, Chu C, Coen S, Mourão-Miranda J. Quantitative prediction of subjective pain intensity from whole-brain fMRI data using Gaussian processes. Neuroimage. 2010;49(3):2178-2189. doi: 10.1016/j.neuroimage.2009.10.072 [DOI] [PubMed] [Google Scholar]
- 31.Brodersen KH, Wiech K, Lomakina EI, et al. Decoding the perception of pain from fMRI using multivariate pattern analysis. Neuroimage. 2012;63(3):1162-1170. doi: 10.1016/j.neuroimage.2012.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JE, Chatterjee N, Younger J, Mackey S. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS One. 2011;6(9):e24124. doi: 10.1371/journal.pone.0024124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368(15):1388-1397. doi: 10.1056/NEJMoa1204471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddan MC, Lindquist MA, Wager TD. Effect size estimation in neuroimaging. JAMA Psychiatry. 2017;74(3):207-208. doi: 10.1001/jamapsychiatry.2016.3356 [DOI] [PubMed] [Google Scholar]
- 35.Woo C-W, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci. 2017;20(3):365-377. doi: 10.1038/nn.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo C-W, Roy M, Buhle JT, Wager TD. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol. 2015;13(1):e1002036. doi: 10.1371/journal.pbio.1002036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo C-W, Schmidt L, Krishnan A, et al. Quantifying cerebral contributions to pain beyond nociception. Nat Commun. 2017;8:14211. doi: 10.1038/ncomms14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker S, Gandhi W, Pomares F, Wager TD, Schweinhardt P. Orbitofrontal cortex mediates pain inhibition by monetary reward. Soc Cogn Affect Neurosci. 2016;12(4):651-661. doi: 10.1093/scan/nsw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bräscher A-K, Becker S, Hoeppli M-E, Schweinhardt P. Different brain circuitries mediating controllable and uncontrollable pain. J Neurosci. 2016;36(18):5013-5025. doi: 10.1523/JNEUROSCI.1954-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart LA, Clarke M, Rovers M, et al. ; PRISMA-IPD Development Group . Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657-1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 41.Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. J Pers Soc Psychol. 1985;48(1):47-53. doi: 10.1037/0022-3514.48.1.47 [DOI] [PubMed] [Google Scholar]
- 42.Atlas LY, Wager TD. A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions. Handb Exp Pharmacol. 2014;225:37-69. doi: 10.1007/978-3-662-44519-8_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162-1167. doi: 10.1126/science.1093065 [DOI] [PubMed] [Google Scholar]
- 44.Schmid J, Theysohn N, Gaß F, et al. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain. 2013;154(11):2372-2380. doi: 10.1016/j.pain.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 45.Theysohn N, Schmid J, Icenhour A, et al. Are there sex differences in placebo analgesia during visceral pain processing? a fMRI study in healthy subjects. Neurogastroenterol Motil. 2014;26(12):1743-1753. doi: 10.1111/nmo.12454 [DOI] [PubMed] [Google Scholar]
- 46.Kong J, Gollub RL, Rosman IS, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381-388. doi: 10.1523/JNEUROSCI.3556-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeidan F, Emerson NM, Farris SR, et al. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J Neurosci. 2015;35(46):15307-15325. doi: 10.1523/JNEUROSCI.2542-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong J, Kaptchuk TJ, Polich G, et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage. 2009;45(3):940-949. doi: 10.1016/j.neuroimage.2008.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bingel U, Wanigasekera V, Wiech K, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244 [DOI] [PubMed] [Google Scholar]
- 50.Kessner S, Wiech K, Forkmann K, Ploner M, Bingel U. The effect of treatment history on therapeutic outcome: an experimental approach. JAMA Intern Med. 2013;173(15):1468-1469. doi: 10.1001/jamainternmed.2013.6705 [DOI] [PubMed] [Google Scholar]
- 51.Ellingsen D-M, Wessberg J, Eikemo M, et al. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc Natl Acad Sci U S A. 2013;110(44):17993-17998. doi: 10.1073/pnas.1305050110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci. 2012;32(23):8053-8064. doi: 10.1523/JNEUROSCI.0383-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman S, Yu R, Egorova N, et al. Distinct neural representations of placebo and nocebo effects. Neuroimage. 2015;112:197-207. doi: 10.1016/j.neuroimage.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rütgen M, Seidel E-M, Silani G, et al. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proc Natl Acad Sci U S A. 2015;112(41):E5638-E5646. doi: 10.1073/pnas.1511269112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1-2):8-15. doi: 10.1016/j.pain.2005.08.027 [DOI] [PubMed] [Google Scholar]
- 56.Choi JC, Yi D-J, Han BS, Lee PH, Kim JH, Kim B-H. Placebo effects on analgesia related to testosterone and premotor activation. Neuroreport. 2011;22(9):419-423. doi: 10.1097/WNR.0b013e32834601c9 [DOI] [PubMed] [Google Scholar]
- 57.Geuter S, Eippert F, Hindi Attar C, Büchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67:227-236. doi: 10.1016/j.neuroimage.2012.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrobel N, Wiech K, Forkmann K, Ritter C, Bingel U. Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. Cortex. 2014;57:60-73. doi: 10.1016/j.cortex.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 59.Deeks JJ, Higgins JP Statistical algorithms in review manager 5 on behalf of the Statistical Methods Group of The Cochrane Collaboration. http://training.cochrane.org/handbook/statistical-methods-revman5. Published 2010. Accessed June 15, 2018.
- 60.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Effect sizes based on means In: Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, eds. Introduction to Meta-Analysis. Hoboken, NJ: John Wiley & Sons, Ltd; 2009. doi: 10.1002/9780470743386.ch4 [DOI] [Google Scholar]
- 61.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4(November):863. doi: 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, England: The Cochrane Collaboration; 2011. [Google Scholar]
- 63.Dienes Z. Using Bayes to get the most out of non-significant results. Front Psychol. 2014;5(July):781. doi: 10.3389/fpsyg.2014.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elsenbruch S, Kotsis V, Benson S, et al. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain. 2012;153(2):382-390. doi: 10.1016/j.pain.2011.10.036 [DOI] [PubMed] [Google Scholar]
- 65.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Neural bases of conditioned placebo analgesia. Pain. 2010;151(3):816-824. doi: 10.1016/j.pain.2010.09.021 [DOI] [PubMed] [Google Scholar]
- 66.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 67.Zunhammer M, Geis S, Busch V, Greenlee MW, Eichhammer P. Effects of intranasal oxytocin on thermal pain in healthy men: a randomized functional magnetic resonance imaging study. Psychosom Med. 2015;77(2):156-166. doi: 10.1097/PSY.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 68.López-Solà M, Woo C-W, Pujol J, et al. Towards a neurophysiological signature for fibromyalgia. Pain. 2017;158(1):34-47. doi: 10.1097/j.pain.0000000000000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnan A, Woo CW, Chang LJ, et al. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife. 2016;5:e15166. doi: 10.7554/eLife.15166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326(5951):404. doi: 10.1126/science.1180142 [DOI] [PubMed] [Google Scholar]
- 71.Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014;17(11):1607-1612. doi: 10.1038/nn.3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci. 2009;29(15):4882-4887. doi: 10.1523/JNEUROSCI.5634-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wechsler ME, Kelley JM, Boyd IOE, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365(2):119-126. doi: 10.1056/NEJMoa1103319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. 2014;84(3):623-637. doi: 10.1016/j.neuron.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 75.Anderson SR, Losin EAR. A sociocultural neuroscience approach to pain. Cult Brain. 2017;5(1):14-35. doi: 10.1007/s40167-016-0037-4 [DOI] [Google Scholar]
- 76.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia: part 2: how, where, and what to look for using functional imaging. Discov Med. 2011;11(58):209-219. [PubMed] [Google Scholar]
- 77.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581-624. doi: 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- 78.Ploner M, Bingel U, Wiech K. Towards a taxonomy of pain modulations. Trends Cogn Sci. 2015;19(4):180-182. doi: 10.1016/j.tics.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 79.Schenk LA, Sprenger C, Geuter S, Büchel C. Expectation requires treatment to boost pain relief: an fMRI study. Pain. 2014;155(1):150-157. doi: 10.1016/j.pain.2013.09.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary methods and results.
eFigure 1. CONSORT flowchart of data-acquisition.
eFigure 2. Funnel plot of placebo effects on pain ratings.
eFigure 3. Sample characteristics.
eFigure 4. Brain-coverage.
eFigure 5. Rating (left) and Neurologic pain signature (NPS, right) responses to noxious high versus various low stimulation intensities.
eFigure 6. Very small analgesic effects of experimental placebo treatments on neurologic pain signature responses in low-risk-of bias studies (conservative analysis).
eFigure 7. Small effects of experimental placebo treatments on neurologic pain signature responses in placebo responders (responder analysis).
eFigure 8. Placebo-effects in pain ratings and NPS-response show a weak, positive association.
eFigure 9. Placebo effects on pain ratings and NPS response, compared against effects of fixed reductions in pain stimulus intensity.
eFigure 10. Placebo effects on pain ratings and NPS response, compared against effects of remifentanil.
eTable 1. Study screening, eligibility checking, and retrieval.
eTable 2. Experimental conditions selected/summarized for analysis.
eTable 3. Risk of bias assessment for NPS response according to the Cochrane risk-of-bias tool.
eTable 4. Features of eligible, but unavailable studies.
eTable 5. Pain stimulation parameters in included studies.
eTable 6. Placebo conditions in included studies.
eTable 7. Functional neuro imaging acquisition characteristics of included studies.
eTable 8. pre-processing and first-level analysis of neuroimages in included studies.
eReferences.