This exploratory analysis of 1690 patients enrolled in the Field Administration of Stroke Therapy-Magnesium Trial assesses the frequency, predictors, and outcomes of the neurological deterioration that occurs among patients during the ultra-early period after ischemic stroke or intracranial hemorrhage.
Key Points
Question
What are the frequency, predictors, and outcomes of neurological deterioration in the ultra-early period after ischemic stroke and intracranial hemorrhage?
Findings
In this exploratory analysis of 1690 patients enrolled in the double-blind, placebo-controlled, randomized Field Administration of Stroke Therapy-Magnesium Trial, ultra-early neurological deterioration occurred in 1 in 3 patients with intracranial hemorrhage and in 1 in 16 patients with acute cerebral ischemia. Ultra-early neurological deterioration was associated with markedly reduced functional independence and increased mortality.
Meaning
Reducing ultra-early neurological deterioration during prehospital and early postarrival is an important target to improve outcomes among patients with acute stroke.
Abstract
Importance
Studies of neurological deterioration in stroke have focused on the subacute period, but stroke treatment is increasingly migrating to the prehospital setting, where the neurological course has not been well delineated.
Objective
To describe the frequency, predictors, and outcomes of neurological deterioration among patients in the ultra-early period following ischemic stroke or intracranial hemorrhage.
Design, Settings, and Participants
Exploratory analysis of the prehospital, randomized Field Administration of Stroke Therapy-Magnesium (FAST-MAG) Trial conducted from 2005 to 2013 within 315 ambulances and 60 stroke patient receiving hospitals in Southern California. Participants were consecutively enrolled patients with suspected acute stroke who were transported by ambulance within 2 hours of stroke onset.
Main Outcomes and Measures
The main outcome was neurological deterioration, defined as a worsening of 2 or more points on the Glasgow Coma Scale (GCS), a level of consciousness scale ranging from 3 to 15, with higher scores indicating more alertness. Imaging outcomes were ischemic or hemorrhagic injury extent identified during the first brain imaging scan. Outcomes at 3 months included global disability level (assessed using the modified Rankin Scale [mRS]; range, 0-6, with higher numbers indicating greater disability) and mortality.
Results
Among the 1690 patients (99.4%), the mean (SD) age was 69.4 (13.5) years, and 43% were female. Final diagnoses were acute cerebral ischemia in 1237 patients (73.2%), intracranial hemorrhage in 386 patients (22.8%), and neurovascular mimic in 67 patients (4.0%). The median (interquartile range [IQR]) minutes between the last well-known time and GCS assessments were 23 (14-42) minutes for prehospital, 58 (46-79) minutes for ED arrival, and 149 (120-180) minutes for early ED course assessments. From prehospital to early postarrival, ultra-early neurological deterioration (U-END) occurred in 200 of 1690 patients (11.8%), more often among patients with intracranial hemorrhage than among those with acute cerebral ischemia (119 of 386 [30.8%] vs 75 of 1237 [6.1%], P < .001). Patterns of U-END were prehospital U-END without early recovery in 30 of 965 patients (3.1%), stable prehospital course but early ED deterioration in 49 of 965 patients (5.1%), and continuous deterioration in both prehospital and early ED phases in 27 of 965 patients (2.8%). Ultra-early neurological deterioration was associated with worse 3-month outcomes, including increased global disability (mRS score, 4.6 vs 2.4; P < .001), reduced functional independence (mRS score 0-2, 32 of 200 [16.0%] vs 844 of 1490 [56.6%]; P < .001), and increased mortality (87 of 200 [43.5%] vs 176 of 1490 [11.8%]; P < .001).
Conclusions and Relevance
Ultra-early neurological deterioration occurs in 1 in 8 ambulance-transported patients with acute cerebrovascular disease, including 1 in 3 patients with intracranial hemorrhage and 1 in 16 patients with acute cerebral ischemia, and is associated with markedly reduced functional independence and increased mortality. Averting U-END may be a target for future prehospital therapeutics.
Trial Registration
ClinicalTrials.gov Identifier: NCT00059332
Introduction
For both acute cerebral ischemia (ACI) and acute intracranial hemorrhage (ICH), neurological progression following first symptom onset is a common and feared complication. However, the frequency, predictors, and outcomes of neurological deterioration in the ultra-early time window (3-4 hours), including the prehospital period, have not been well characterized. Instead, studies have generally examined neurological deterioration across extended time windows after hospital arrival, including at any time during the initial hospitalization,1,2 or have examined early neurological deterioration (END), defined as within 48 hours of admission.3,4 Although 1 study investigated prehospital deterioration among patients with intracerebral hemorrhage,5 we know of no systematic studies of ultra-early neurological deterioration (U-END) in both the prehospital and initial emergency department (ED) phase encompassing both patients with ACI and those with acute ICH.
Many interventions for treatment of acute stroke are most effective when initiated as soon as possible after stroke onset.6,7,8,9,10,11 As a result, both delivery of standard treatments and research studies of experimental treatments are increasingly migrating to the early postarrival ED time period or directly to the prehospital phase of care.12,13,14,15,16 The earliest point from which neurological deterioration can be systematically studied is the initial assessment by emergency medical services personnel prior to transport. The prehospital phase of stroke typically lasts 25 to 40 minutes from emergency medical services arrival on the scene to completion of transport to the ED. While in the ED, another 30 to 60 minutes are spent in triage, initial stabilization, transport to neuroimaging, and imaging interpretation prior to starting stroke subtype–specific therapy.17 There is an urgent need to characterize the frequency, predictors, and outcomes of neurological deterioration among patients in these earliest time windows for use as a baseline against which faster delivery methods and novel treatments can be assessed, to identify patients at greatest risk of U-END so that they can receive the fastest standard treatment, and to enrich enrollment in clinical trials of progression-averting research interventions.
Methods
This was an exploratory analysis of patients enrolled in the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) Trial, a phase 3, National Institute of Neurological Disorders and Stroke–sponsored, placebo-controlled randomized clinical trial of field-initiated magnesium sulfate in patients with hyperacute stroke within 2 hours after the last known well time (LKWT) conducted from 2005 to 2013.16,18,19 Participating sites included 40 emergency medical system agencies, 315 ambulances, and 60 acute care receiving hospitals in Los Angeles and Orange counties in California. The study protocol was approved by the institutional review board at each prehospital and hospital study site. Enrollment occurred using explicit informed consent obtained via cellphone conversation between patients on the scene or their legally authorized representatives and enrolling physician-investigators off the scene or under exception from informed consent regulations.20,21
Neurological deterioration was defined as worsening by 2 or more points on the Glasgow Coma Scale (GCS; range, 3-15, with higher numbers indicating alertness).5 The FAST-MAG Trial patients had up to 3 serial GCS evaluations in the ultra-early time period: the first during the initial prehospital assessment (performed by paramedics), the second at the initial ED arrival (performed by ED bedside nurses), and the third early in the ED course (performed by FAST-MAG research nurses). The prehospital and early ED course GCS evaluations were mandated by the study protocol and performed in nearly all patients. The ED arrival GCS evaluation was not required by the study protocol but was commonly performed as part of routine clinical practice at participating hospitals. Accordingly, the occurrence of U-END was assessed for all patients between the prehospital and shortly postarrival GCS evaluations. For patients with a clinically documented ED arrival GCS assessment, additional analyses were performed assessing U-END occurrence in the prehospital phase (between the prehospital and ED arrival assessments) and U-END occurrence in the early postarrival phase (between the ED arrival and ED course assessments) (eFigure in the Supplement). Specific patterns of deficit progression were identified as follows: prehospital sustained indicated prehospital deterioration and then a stable early ED phase; dipper indicated prehospital deterioration and then early ED improvement; delayed indicated stable prehospital and then ED deterioration; peaker indicated prehospital improvement and then early ED deterioration; continuous major indicated prehospital deterioration and early ED deterioration; and continuous minor indicated mild (1 point) GCS score worsening in both prehospital and early ED phases, cumulatively reaching the threshold for deterioration.
Prehospital ambulance services and hospital receiving sites were directed to provide supportive treatment and blood pressure control as well as thrombolytic, endovascular, and surgical care according to the national American Heart Association/American Stroke Association guidelines for care of patients with acute ischemic stroke or intracerebral hemorrhage in addition to the study treatment of an infusion of magnesium sulfate or placebo. Throughout the study region in accordance with the predominant US emergency medical services practice, administration of antihypertensive therapies was not initiated in the field but deferred to ED arrival if needed.
All modified Rankin Scale assessments (scores range from 0 to 6, with higher numbers indicating greater disability) were performed by physician and nurse raters certified in the validated Rankin Focused Assessment method for assigning modified Rankin Scale scores. Additional study-specific training on the use of the GCS was not performed because GCS scoring is a core competency of paramedics, neurologists, and emergency department physicians and nurses.
Statistical analysis of associations used χ2 tests for binary variables and t tests for linear variables. Two-sided P ≤ .05 values were considered statistically significant. Because all analyses were considered exploratory, no adjustment for multiplicity was made. A multivariate prediction model for occurrence of neurological deterioration was derived by stepwise logistic regression using variables with univariate P ≤ .05 values as candidates. Data analyses were performed using SPSS, version 20 (SPSS Inc).
Results
Among the 1700 patients enrolled in the FAST-MAG Trial, 1690 patients (99.4%) had serial GCS assessments performed at the 2 protocol-mandated times of prehospital and early ED course (10 patients were excluded for 1 or more missing GCS scores). This population constituted the primary analytic population for the present study. Among these 1690 patients, the mean (SD) age was 69.4 (13.5) years, 725 patients (43%) were female, and the final diagnosis was ACI in 1237 patients (73.2%), acute ICH in 386 patients (22.8%), and cerebrovascular mimic in 67 patients (4.0%). The median time from LKWT to the initial paramedic GCS assessment was 23 minutes (interquartile range [IQR], 14-42 minutes), the median LKWT to ED arrival was 58 minutes (IQR, 46-79 minutes), the median LKWT to early ED course GCS assessment was 149 minutes (IQR, 120-180 minutes), and the median time from prehospital GCS assessment to early ED course GCS assessment was 117 minutes (IQR, 95-143 minutes) (Table 1).
Table 1. Time Intervals in Study Populations.
| Interval | Median (IQR) Time, min | |
|---|---|---|
| Patients With Prehospital and Early ED Course GCS Assessment (n = 1690) | Subset of Patients With Prehospital, ED Arrival, and Early ED Course GCS Assessment (n = 965) | |
| LKWT to prehospital GCS | 23 (14-42) | 24 (15-45) |
| LKWT to ED arrival | 58 (46-79) | 60 (47-81) |
| Prehospital GCS to ED arrival | 32 (27-39) | 33 (28-39) |
| ED arrival to ED arrival GCS | ND | 10 (5-22) |
| LKWT to ED arrival GCS | ND | 76 (60-100) |
| Prehospital GCS to ED arrival GCS | ND | 46 (37-59) |
| LKWT to early ED course GCS | 149 (120-180) | 150 (123-180) |
| ED arrival to early ED course GCS | 82 (62-107) | 83 (61-108) |
| ED arrival GCS to early ED course GCS | ND | 70 (45-95) |
| Prehospital GCS to early ED course GCS | 117 (95-143) | 118 (97-144) |
Abbreviations: ED, emergency department; GCS, Glasgow Coma Scale; IQR, interquartile range; LKWT, last known well time; ND, not determined.
Ultra-early neurological deterioration between prehospital paramedic evaluation and the early ED course evaluation occurred in 200 of 1690 patients (11.8%). Univariate predictors of U-END among all enrolled patients were Asian race, Hispanic ethnicity, history of hypertension, absence of history of atrial fibrillation or valvular heart disease, higher prehospital systolic and diastolic blood pressure, more severe prehospital focal motor deficits on the Los Angeles Motor Scale (LAMS), and a final diagnosis of acute ICH rather than ACI (Table 2 and eTable 4 in the Supplement). In multivariate analyses, independent predictors of U-END were prehospital GCS assessment (odds ratio [OR], 0.7 per point increase; 95% CI, 0.7-0.8) and final diagnosis of acute ICH (OR, 3.9; 95% CI, 1.6-9.5).
Table 2. Demographic and Clinical Features Associated With Deterioration Among All Patients and Among Those With Cerebral Ischemia or ICHa.
| Feature | All Patients (n = 1690) | Patients With Cerebral Ischemia (n = 1237) | Patients With ICH (n = 386) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| U-END (n = 200) | No U-END (n = 1490) | P Value | U-END (n = 75) | No U-END (n = 1162) | P Value | U-END (n = 119) | No U-END (n = 267) | P Value | |
| Age, mean (SD), y | 68.3 (13.6) | 69.5 (13.5) | .22 | 65.7 (13.2) | 64.9 (13.9) | .59 | 65.7 (13.2) | 64.9 (13.9) | .59 |
| Female, No. (%) | 83 (41.1) | 642 (42.9) | .63 | 45 (60.0) | 515 (44.3) | .01 | 36 (30.3) | 92 (34.5) | .42 |
| Race, No. (%) | |||||||||
| White | 147 (72.8) | 1178 (78.5) | 54 (72.0) | 907 (78.1) | 90 (75.6) | 215 (80.5) | |||
| Black/African American | 24 (11.9) | 195 (13.0) | 10 (13.9) | 161 (13.9) | 11 (9.2) | 26 (9.7) | |||
| Asian | 28 (13.9) | 111 (7.4) | 10 (13.3) | 85 (7.3) | 16 (13.4) | 22 (8.2) | |||
| Other | 3 (1.5) | 14 (0.9) | 1 (1.3) | 9 (0.8) | 2 (1.7) | 4 (1.5) | |||
| Hispanic ethnicity, No. (%) | 60 (29.7) | 342 (22.8) | .03 | 18 (24.0) | 231 (19.9) | .39 | 40 (33.6) | 88 (33.0) | .90 |
| Medical history, No. (%) | |||||||||
| Hypertension | 168 (83.2) | 1151 (76.8) | .04 | 59 (78.7) | 901 (77.5) | .82 | 104 (87.4) | 200 (74.9) | .01 |
| Diabetes | 54 (26.7) | 323 (21.6) | .97 | 28 (37.3) | 253 (21.8) | <.001 | 24 (20.2) | 47 (17.6) | .55 |
| Hyperlipidemia | 92 (45.5) | 713 (47.6) | .58 | 41 (54.7) | 585 (50.3) | .47 | 48 (40.3) | 93 (34.8) | .30 |
| Atrial fibrillation | 31 (15.3) | 338 (22.6) | .02 | 23 (30.7) | 311 (26.8) | .46 | 8 (6.7) | 22 (8.2) | .61 |
| Heart disease | 82 (40.7) | 625 (41.7) | .08 | 43 (57.4) | 561 (48.3) | .12 | 36 (30.2) | 47 (17.6) | .01 |
| Prior stroke/TIA | 42 (20.8) | 392 (25.5) | .15 | 22 (29.3) | 314 (31.0) | .76 | 19 (16.0) | 48 (18.0) | .63 |
| Tobacco use | 28 (13.9) | 269 (18.0) | .15 | 7 (9.3) | 209 (18.0) | .06 | 20 (16.8) | 46 (17.2) | .92 |
| Any alcohol use | 73 (63.1) | 581 (38.8) | .47 | 4 (5.3) | 108 (9.3) | .01 | 53 (44.5) | 120 (44.9) | .94 |
| Antithrombotic prior to stroke, No. (%) | None | None | None | None | None | None | 43 (35.8) | 78 (29.2) | .20 |
| Time interval, median (IQR), min | |||||||||
| Onset to paramedic evaluation | 22.5 (15.0-36.8) |
25.0 (15.0-46.0) |
.24 | 23.0 (15.0-35.0) |
25.0 (15.0-47.0) |
.87 | 21.5 (14.0-34.3) |
25.0 (17.0-41.0) |
.18 |
| Onset to ED arrival | 57.0 (45.8-72.0) |
60.0 (47.0-82.0) |
.51 | 60.0 (49.0-76.0) |
60.0 (48.0-83.0) |
.71 | 55.0 (45.0-65.5) |
59.0 (46.5-79.5) |
.45 |
| ED arrival to early ED course GCS | 91.0 (66.0-110.0) |
83.0 (61.0-108.0) |
.05 | 85.0 (65.5-111.5) |
82.0 (60-106) |
.39 | 95.0 (68.0-110.5) |
89.0 (65.5-111.5) |
.18 |
| SBP, mean (SD), mm Hg | |||||||||
| Prehospital | 178.5 (24.4) | 173.5 (26.0) | .07 | 155.6 (23.2) | 155.8 (27.4) | .95 | 178.0 (24.4) | 173.5 (25.7) | .74 |
| ED arrival | 186.4 (32.1) | 174.1 (30.8) | <.001 | 149.4 (24.2) | 149.3 (24.7) | .95 | 186.4 (32.1) | 174.1 (30.8) | <.001 |
| Early ED course | 146.2 (27.1) | 147.9 (23.4) | .55 | 140.1 (25.2) | 145.4 (23.4) | .06 | 146.2 (27.1) | 147.9 (23.4) | .55 |
| DBP, mean (SD), mm Hg | |||||||||
| Prehospital | 100.0 (17.5) | 99.6 (19.3) | .84 | 85.5 (17.6) | 87.3 (17.6) | .59 | 100.0 (17.5) | 99.6 (19.3) | .85 |
| ED arrival | 100.3 (22.1) | 95.3 (20.9) | .03 | 77.1 (17.3) | 78.1 (16.0) | .61 | 100.3 (22.1) | 95.3 (20.9) | .34 |
| Early ED course | 77.5 (19.1) | 78.8 (16.2) | .39 | 75.1 (18.6) | 76.4 (15.5) | .52 | 77.2 (19.1) | 78.8 (16.2) | .39 |
| Severity scores | |||||||||
| Prehospital GCS | |||||||||
| Median (IQR) | 14.0 (12.0-15.0) |
15.0 (15.0-15.0) |
<.001 | 5.0 (3.5-5.0) |
4.0 (3.0-5.0) |
<.001 | 15.0 (12.0-15.0) |
15.0 (15.0-15.0) |
<.001 |
| Mean (SD) | 13.6 (1.9) | 14.3 (1.6) | <.001 | 13.2 (2.1) | 14.2 (1.8) | <.001 | 13.8 (1.8) | 14.6 (1.2) | <.001 |
| Prehospital LAMS | |||||||||
| Median (IQR) | 5.0 (4.0-5.0) |
4.0 (3.0-5.0) |
<.001 | 14.0 (11.0-15.0) |
15.0 (14.0-15.0) |
<.001 | 5.0 (4.0-5.0) |
4.0 (3.0-5.0) |
<.001 |
| Mean (SD) | 4.3 (1.0) | 3.7 (1.3) | <.001 | 4.2 (1.2) | 3.6 (1.3) | <.001 | 4.4 (0.9) | 3.9 (1.1) | <.001 |
Abbreviations: DBP, diastolic blood pressure; ED, emergency department; ICH, intracranial hemorrhage; GCS, Glasgow Coma Scale; IQR, interquartile range; LAMS, Los Angeles Motor Scale; SBP, systolic blood pressure; TIA, transient ischemic attack; U-END, ultra-early neurological deterioration.
See eTable 4 in the Supplement for additional baseline variables not associated with U-END and for ED arrival GCS assessment.
Among patients who received a final diagnosis of ACI, U-END occurred in 75 of 1237 patients (6.1%). Univariate predictors of U-END among patients with ACI were female sex, history of diabetes, alcohol abstinence, and more severe stroke deficits as assessed by both the GCS and the LAMS (Table 2). In addition, systolic blood pressure was nominally lower among patients with ACI and deterioration (153.6 vs 155.8, P = .06). Among patients who received a final diagnosis of ICH, U-END occurred in 119 of 386 patients (30.8%). Univariate predictors of U-END among patients with ICH were history of hypertension, coronary artery disease, myocardial infarction, and more severe stroke deficits as assessed by both the GCS and the LAMS (Table 2). In addition, systolic blood pressure at ED arrival, although not prehospital, was higher among patients with U-END.
More detailed analysis of the timing of deficit evolution was performed in the 965 patients (56.8%) who had an ED arrival GCS score documented as part of their routine care. Patients with or without a clinically performed ED arrival GCS assessment were similar in baseline characteristics and outcomes (eTable 1 in the Supplement). Among these patients, the median time from prehospital GCS assessment to ED arrival GCS assessment was 46 minutes (IQR, 37-59 minutes) and from ED arrival GCS assessment to early ED course GCS assessment was 70 minutes (IQR, 45-95 minutes) (Table 1).
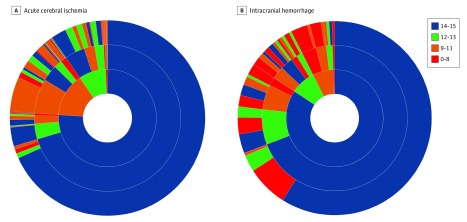
Among all patients with an ED arrival GCS assessment, 93 of 965 patients (9.6%) experienced U-END only en route to the hospital, 83 of 965 patients (8.6%) experienced U-END only between the ED arrival and early ED course GCS assessments, and 23 of 965 patients (2.4%) experienced U-END in both phases. The results of a multivariate analysis indicated that the only independent predictor of prehospital U-END was receiving a final diagnosis of ICH (OR, 3.5; 95% CI, 2.3-5.3). For postarrival U-END, receiving a final diagnosis of ICH independently increased the likelihood (OR, 6.1; 95% CI, 3.8-9.8), whereas the occurrence of prehospital U-END independently decreased the likelihood (OR, 0.64; 95% CI, 0.7-0.8). Among 713 patients with cerebral ischemia, 128 patients (18.0%) had U-END, including 64 patients (8.9%) in the prehospital phase only, 52 patients (7.3%) in the early ED phase only, and 1 (0.2%) in both phases. Among 210 patients with ICH, 82 patients (39.0%) had U-END, including 27 patients (12.9%) in the prehospital phase only, 30 patients (14.3%) in the early ED phase only, and 22 patients (10.5%) in both phases. More detailed patterns of deficit progression are shown in eTables 2 and 3 in the Supplement and in Figure 1 and Figure 2.
Figure 1. Patterns of Glasgow Coma Scale (GCS) Score Evolution Among Patients With Acute Cerebral Ischemia or Intracranial Hemorrhage.
A, Acute cerebral ischemia (n = 713). B, Intracranial hemorrhage (n = 210). Colors categorize GCS scores as indicated. Inner ring indicates proportion of patients in various GCS categories at time of paramedic prehospital examination; middle ring indicates evolution of GCS scores in these patients at time of ED arrival; and outer ring indicates further GCS score evolution at time of early postarrival ED assessment.
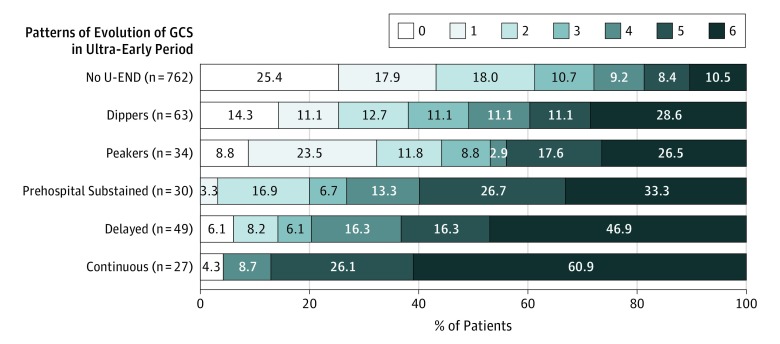
Figure 2. Three-Month Disability Outcomes (Modified Rankin Scale) Among 965 Patients With Suspected Acute Stroke Showing 6 Glasgow Coma Scale (GCS) Patterns of Evolution in the Ultra-Early Period.
U-END indicates ultra-early neurological deterioration.
The occurrence of U-END was associated with worse early stroke outcomes (Table 3). Early intubation (intubation by the time of the early postarrival ED assessment) and in-hospital mortality were more frequent among patients with U-END than among those without U-END. Among patients with ACI, the extent of the acute ischemic change on first imaging, assessed using the Alberta Stroke Program Early Computed Tomographic Score, was higher among patients with U-END than among patients without U-END. Among patients with intraparenchymal hemorrhages, hematoma volume on first imaging was higher among patients with U-END than among patients without U-END.
Table 3. Outcomes Associated With or Without U-END Among All Patients and Among Those With Cerebral Ischemia or Intracranial Hemorrhage.
| Outcome | All Patients (n = 1690) | Patients With Cerebral Ischemia (n = 1237) | Patients With Intracranial Hemorrhage (n = 386) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| U-END (n = 200) | No U-END (n = 1490) | P Value | U-END (n = 75) | No U-END (n = 1162) | P Value | U-END (n = 119) | No U-END (n = 267) | P Value | |
| Early outcome | |||||||||
| Early ED course GCS score, median (IQR) | 8 (8-11) | 15 (15-15) | <.001 | 9 (6-11) | 15 (15-15) | <.001 | 12 (9-15) | 15 (14-15) | <.001 |
| Early ED course LAMS score, median (IQR) | 5 (5-5) | 3 (1-5) | <.001 | 5 (5-5) | 3 (1-5) | <.001 | 5 (5-5) | 5 (3-5) | <.001 |
| Early ED course NIHSS score, mean (SD) | 28.3 (9.2) | 9.1 (7.4) | <.001 | 24.1 (9.3) | 8.4 (9.3) | <.001 | 31.4 (7.8) | 12.8 (7.0) | .01 |
| Early intubation, No. (%) | 82 (40.8) | 6 (0.4) | <.001 | 14 (18.4) | 1 (0.1) | <.001 | 66 (55.5) | 5 (1.9) | <.001 |
| Mortality during initial hospitalization, No. (%) | 57 (28.2) | 66 (4.4) | <.001 | 17 (22.4) | 39 (3.4) | <.001 | 39 (32.8) | 26 (9.7) | <.001 |
| ASPECTS on first imaging, mean (SD) | ND | ND | ND | 7.6 (2.7) | 8.6 (2.4) | .01 | ND | ND | ND |
| Intracerebral hematoma volume on first imaging, mean (SD), cm3,a | ND | ND | ND | ND | ND | ND | 46.5 (48.5) | 22.0 (30.6) | <.001 |
| 90-d Outcome | |||||||||
| mRS score 0-1, No. (%) | 16 (8.0) | 591 (40.0) | <.001 | 14 (18.7) | 523 (45.0) | <.001 | 0 (0) | 34 (12.7) | <.001 |
| mRS score 0-2, No. (%) | 32 (16.0) | 844 (56.6) | <.001 | 18 (24.0) | 695 (60.0) | <.001 | 10 (8.4) | 106 (39.7) | <.001 |
| mRS score, mean (SD) | 4.6 (1.8) | 2.4 (2.1) | <.001 | 4.1 (2.1) | 2.2 (2.0) | .48 | 5.0 (1.2) | 3.4 (1.7) | <.001 |
| Mortality, No. (%) | 87 (43.5) | 176 (11.8) | <.001 | 29 (38.7) | 124 (10.7) | <.001 | 58 (48.7) | 45 (16.9) | <.001 |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomographic Score; ED, emergency department; GCS, Glasgow Coma Scale; IQR, interquartile range; LAMS, Los Angeles Motor Scale; mRS, modified Rankin Scale; ND, not determined; NIHSS, National Institutes of Health Stroke Scale; U-END, ultra-early neurological deterioration.
Row values are for the 381 patients with intracerebral hemorrhage from among the 386 patients with intracranial hemorrhage of any kind.
The occurrence of U-END was also strongly associated with worse final clinical outcome (Table 3). For example, among all 1690 patients, rates of good functional outcome (modified Rankin Scale score of 0-2) at 3 months after stroke were 16.0% (32 of 200) among individuals with U-END and 56.6% (844 of 1490) among individuals without U-END (P < .001). Rates of good functional outcome among patients with cerebral ischemia were 24.0% (18 of 75) among those with U-END vs 59.8% (695 of 1162) among those without U-END (P < .001), and among patients with ICH, the rates were 8.4% (10 of 119) among those with U-END vs 39.7% (106 of 267) among those without U-END (P < .001). Three-month mortality rates among patients with U-END were 87 of 200 (43.5%) vs 176 of 1490 (11.8%) among those without U-END (P < .001), including 29 of 75 patients (38.7%) with cerebral ischemia and with U-END vs 124 of 1162 patients (10.7%) without U-END (P < .001) and 58 of 119 patients (48.7%) with ICH and with U-END vs 45 of 267 patients (16.9%) without U-END (P < .001). The distribution of 3-month disability outcomes among patients by detailed U-END pattern categories is shown in Figure 2.
Discussion
This study found that neurological deterioration as assessed with the GCS in the ultra-early period was frequent among ambulance-transported patients with acute cerebrovascular disease, occurring in 12% of patients. Ultra-early neurological deterioration was substantially more common among patients with ICH, occurring in nearly 1 in 3 of such patients, and less common but still notable among patients with ACI, occurring in about 1 in 16 of these patients. Among all patients with acute cerebrovascular disease, independent predictors of U-END were ICH stroke subtype and lower initial prehospital GCS score.
To our knowledge, this study is the first prospective, multicenter investigation of clinical deterioration in the prehospital and early ED course during the first minutes and hours after symptom onset in a broad cerebrovascular disease cohort. For ACI, prior studies of neurological deterioration have focused solely on later in-hospital time windows. For example, in a systematic meta-analysis and later large case-series studies, the 8 studies reporting on the frequency of neurological deterioration due to stroke progression had observation periods that were not always well demarcated but that appeared to begin 3 to 72 hours after stroke onset and end 24 to 168 hours after stroke onset.22,23,24,25,26,27,28,29 In these case series, neurological worsening occurred in a median of 9.6% (IQR, 5.1%-18.1%) of patients. In the present study in a much earlier and more compressed window of median observation times between 0.4 and 2.5 hours after onset, U-END occurred in 6.1% of patients with ischemic stroke.
For acute ICH, most prior studies have also focused on neurological deterioration occurring within 24 hours of ED arrival.30,31,32 Current large studies analyzing deterioration among patients with ICH after hospital arrival have considered periods from 5 to 28 hours after the LKWT,31 from 1.5 to 25.5 hours,32 from up to 6 hours to 72 hours,30 and from a median of 7.3 hours to 9 to 13 hours after the LKWT.33,34 One prior single-center study did investigate the prehospital period in 98 patients with intracerebral hemorrhage and reported neurological deterioration in 22% of these patients.5 Our study confirms and extends this finding in a larger multicenter cohort, documenting deterioration in 23% of patients with ICH in the prehospital setting as well as finding that deterioration additionally occurs in 24% of patients in the first minutes to hours after ED arrival.
Among all patients with acute cerebrovascular disease transported by paramedics, there were only 2 independent predictors of neurological deterioration: more severe initial level of consciousness impairment as assessed by the GCS and a final stroke subtype diagnosis of ICH rather than ACI. Predictors of early neurological deterioration were largely different among patients with ACI and with ICH, although more severe initial deficits at the time of initial paramedic encounters were associated with deterioration in both stroke subtypes. Female sex and alcohol abstinence were associated with neurological deterioration in ACI, whereas history of hypertension and myocardial disease were associated with neurological deterioration in ICH. There was a suggestion of a differential association of blood pressure with progression among patients with ACI compared with those with ICH. Among patients with ACI, END tended to be associated with lower systolic blood pressure at the time of the first paramedic evaluation, whereas among patients with ICH, END tended to be associated with higher systolic blood pressure at ED arrival.
Our findings on the association of prehospital and ED arrival blood pressure with END among patients with ICH are of interest given the nascent conduct of randomized trials of blood pressure lowering in the field.35,36,37 Two prior observational studies have investigated aspects of this association. In a single-center study of 98 ICH cases, neurological deterioration in the field was associated with higher prehospital diastolic blood pressure but not with higher prehospital systolic blood pressure.5 In another single-center study of 536 patients with ICH, neurological deterioration in the prehospital period was not examined, but neurological deterioration after hospital arrival was associated with higher prehospital diastolic and systolic blood pressures.33 In the present study, END was associated with higher systolic blood pressure on ED arrival but not with higher prehospital blood pressures. The nonuniform but suggestive findings from these 3 observational studies support further formal trials of the therapeutic strategy of prehospital blood pressure moderation in patients likely to have ICH.
For prehospital system planning, the present study provides unique insight into the frequency of deterioration occurring between paramedic evaluation in the field and ED arrival. Our study findings indicated that emergency medical services system planners may anticipate that approximately 1 in 10 patients with stroke (including about 1 in 4 patients with acute ICH) will deteriorate between the scene and ED arrival. This frequency of prehospital deterioration reflects transport in a largely urban or suburban setting with relatively short transport times. In rural settings with longer transport times, the frequency of deterioration will be higher, as suggested by the 1 in 8 rate of deterioration in the present study between the scene and the later ED course evaluation.
The potential pathophysiologic mechanisms of U-END are varied. In ACI, causes of U-END may include reduction in collateral circulation, clot propagation, recurrent embolus, and hemorrhagic transformation of infarct. In acute intracerebral hemorrhage, causes of U-END may include hematoma expansion and obstructive hydrocephalus. The much larger hematoma volume observed on arrival imaging among patients with intraparenchymal hemorrhage and U-END, approximately twice the size of that among patients without U-END, suggests that hemorrhage expansion in the field and during the early ED course, prior to the collection of the first images, was likely an important contributor. In both ischemic and hemorrhagic stroke, U-END may also be caused by noncerebrovascular events, such as seizure, respiratory compromise, and myocardial infarction. These noncerebrovascular events were rare in the prehospital period,16 but intubation for respiratory compromise or concern did occur early in the ED course in about 6 in 10 of the patients with ICH and U-END. The high frequency of U-END emphasizes the importance of migrating clinical trials for acute stroke into the prehospital setting so that treatments can be initiated at the earliest moment of medical contact in advance of U-END.15,16,36,37,38,39,40
Limitations
Our analysis included only those patients who were enrolled in a clinical trial. Although the FAST-MAG Trial entry criteria were broad in age, stroke severity, and comorbidities,41 the study did exclude patients with prestroke disability, systolic blood pressure higher than 220, and other uncommon features. Such patients may have different frequencies of U-END. For example, patients with ICH and systolic blood pressure greater than 220 mm Hg may have higher rates of U-END. The present study, similar to prior prehospital investigations,5,33 used a change in GCS scores to identify neurological deterioration and improvement. Because the GCS is a measure of the level of consciousness rather than the degree of focal deficit, it may not capture all clinically relevant deficit changes in patients. Studies of in-hospital neurological deterioration among patients with ACI have most often focused on a prespecified increase in the National Institutes of Health Stroke Scale score,1 but assessments using this scale are too time-consuming for routine use in the field by paramedics. The GCS has good interrater reliability and is universally used by prehospital personnel, making study findings of wide applicability. As in prior studies, the present investigation excluded patients who were already comatose at the time of first paramedic evaluation, as further worsening in these patients would have been difficult to detect. Comatose patients with ICH at the time of emergency medical services arrival are uncommon, but when such patients are included, rates of deterioration in the prehospital setting will be slightly lower than the current study estimates. Although initial brain imaging was obtained in all study patients, follow-up serial imaging was not mandated; thus, the present study cannot address the frequency of postarrival infarct growth or hemorrhage expansion. The timing of the early ED course GCS assessment was nonuniform; the median time was approximately 82 minutes, but the IQR was from about 61 to 108 minutes after ED arrival, which was caused by the variability in arrival time of the responding regional study nurse. The FAST-MAG Trial collected information on the timing of the start of antihypertensive medication administration, if any, after arrival. Some data on blood pressure medication use among patients with ICH were collected in a nonmonitored manner and will be analyzed in a future exploratory analysis.
Conclusions
Approximately 1 in 8 patients with suspected acute cerebrovascular disease experienced U-END as assessed with the GCS during paramedic transport and initial ED evaluation. Ultra-early neurological deterioration was more common among patients with ICH, occurring in nearly 1 in 3 of these patients, and less common but still frequent among patients with ACI, occurring in 1 in 16 of these patients. Patients with more severe initial deficits in the field had higher rates of U-END, and patients with U-END had substantially worse final functional outcomes and increased mortality. These findings suggest the desirability of initiating stroke therapies as soon as possible after stroke onset, including in the prehospital setting, to avert the occurrence of U-END.
eTable 1. Demographic and Clinical Characteristics of the Patients in the Secondary Analysis With Or Without GCS Available at Hospital Arrival
eTable 2. Patterns of Ultra-Early Neurological deterioration Among Patients With GCS Assessments Prehospital, on ED Arrival, and in the Early ED Course; Grouped by Ultra-Early Time Segment in Which U-END Occurred
eTable 3. Patterns of Ultra-Early Neurological deterioration Among Patients With GCS Assessments Prehospital, on ED Arrival, and in the Early ED Course; Grouped by Occurrence or Non-Occurrence of Net Deterioration
eTable 4. ED Arrival GCS and Additional Baseline Variables Not Associated With U-END in All, Cerebral Ischemia, and Intracranial Hemorrhage Patients
eFigure. Timeline Delineating Typical FAST-MAG Patient Enrollment and Assessment Activities During the First 2 Hours After Symptom Onset
References
- 1.Ali LK, Saver JL. The ischemic stroke patient who worsens: new assessment and management approaches. Rev Neurol Dis. 2007;4(2):85-91. [PubMed] [Google Scholar]
- 2.Ferrari J, Knoflach M, Kiechl S, et al. ; Austrian Stroke Unit Registry Collaborators . Early clinical worsening in patients with TIA or minor stroke: the Austrian Stroke Unit Registry. Neurology. 2010;74(2):136-141. doi: 10.1212/WNL.0b013e3181c9188b [DOI] [PubMed] [Google Scholar]
- 3.Dávalos A, Castillo J, Marrugat J, et al. Body iron stores and early neurological deterioration in acute cerebral infarction. Neurology. 2000;54(8):1568-1574. doi: 10.1212/WNL.54.8.1568 [DOI] [PubMed] [Google Scholar]
- 4.Leira R, Dávalos A, Silva Y, et al. ; Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society . Early neurological deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63(3):461-467. doi: 10.1212/01.WNL.0000133204.81153.AC [DOI] [PubMed] [Google Scholar]
- 5.Moon JS, Janjua N, Ahmed S, et al. Prehospital neurological deterioration in patients with intracerebral hemorrhage. Crit Care Med. 2008;36(1):172-175. doi: 10.1097/01.CCM.0000297876.62464.6B [DOI] [PubMed] [Google Scholar]
- 6.Carcel C, Wang X, Sato S, et al. ; INTERACT2 Investigators . Degree and timing of intensive blood pressure lowering on hematoma growth in intracerebral hemorrhage: Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial-2 results. Stroke. 2016;47(6):1651-1653. doi: 10.1161/STROKEAHA.116.013326 [DOI] [PubMed] [Google Scholar]
- 7.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313(8):824-836. doi: 10.1001/jama.2015.0846 [DOI] [PubMed] [Google Scholar]
- 9.Saver JL. Time is brain—quantified. Stroke. 2006;37(1):263-266. doi: 10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480-2488. doi: 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 12.Ankolekar S, Fuller M, Cross I, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: the rapid intervention with glyceryl trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke. 2013;44(11):3120-3128. doi: 10.1161/STROKEAHA.113.001301 [DOI] [PubMed] [Google Scholar]
- 13.Dowlatshahi D, Wasserman JK, Butcher KS, et al. Stroke prenotification is associated with shorter treatment times for warfarin-associated intracerebral hemorrhage. Cerebrovasc Dis. 2013;36(5-6):383-387. doi: 10.1159/000355500 [DOI] [PubMed] [Google Scholar]
- 14.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311(16):1632-1640. doi: 10.1001/jama.2014.3203 [DOI] [PubMed] [Google Scholar]
- 15.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45(1):159-167. doi: 10.1161/STROKEAHA.113.001346 [DOI] [PubMed] [Google Scholar]
- 16.Saver JL, Starkman S, Eckstein M, et al. ; FAST-MAG Investigators and Coordinators . Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015;372(6):528-536. doi: 10.1056/NEJMoa1408827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group A systems approach to immediate evaluation and management of hyperacute stroke: experience at eight centers and implications for community practice and patient care. Stroke. 1997;28(8):1530-1540. doi: 10.1161/01.STR.28.8.1530 [DOI] [PubMed] [Google Scholar]
- 18.Saver JL, Starkman S, Eckstein M, et al. ; FAST-MAG Investigators and Coordinators . Methodology of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) phase 3 trial: part 1, rationale and general methods. Int J Stroke. 2014;9(2):215-219. doi: 10.1111/ijs.12243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saver JL, Starkman S, Eckstein M, et al. ; FAST-MAG Investigators and Coordinators . Methodology of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) phase 3 trial: part 2, prehospital study methods. Int J Stroke. 2014;9(2):220-225. doi: 10.1111/ijs.12242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanossian N, Starkman S, Liebeskind DS, et al. ; FAST-MAG Trial Investigators . Simultaneous ring voice-over-Internet phone system enables rapid physician elicitation of explicit informed consent in prehospital stroke treatment trials. Cerebrovasc Dis. 2009;28(6):539-544. doi: 10.1159/000247596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saver JL; American Stroke Association . Food and Drug Administration public hearing on the conduct of emergency clinical research: testimony of the American Stroke Association. Acad Emerg Med. 2007;14(4):e57-e58. doi: 10.1197/j.aem.2006.11.023 [DOI] [PubMed] [Google Scholar]
- 22.Balami JS, Chen RL, Grunwald IQ, Buchan AM. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011;10(4):357-371. doi: 10.1016/S1474-4422(10)70313-6 [DOI] [PubMed] [Google Scholar]
- 23.Cavallini A, Micieli G, Marcheselli S, Quaglini S. Role of monitoring in management of acute ischemic stroke patients. Stroke. 2003;34(11):2599-2603. doi: 10.1161/01.STR.0000094423.34841.BB [DOI] [PubMed] [Google Scholar]
- 24.Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke. 2008;39(2):414-420. doi: 10.1161/STROKEAHA.107.489294 [DOI] [PubMed] [Google Scholar]
- 25.Kalra L, Yu G, Wilson K, Roots P. Medical complications during stroke rehabilitation. Stroke. 1995;26(6):990-994. doi: 10.1161/01.STR.26.6.990 [DOI] [PubMed] [Google Scholar]
- 26.Kim YD, Choi HY, Jung YH, et al. The ischemic stroke predictive risk score predicts early neurological deterioration. J Stroke Cerebrovasc Dis. 2016;25(4):819-824. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 27.Pinto AN, Melo TP, Lourenço ME, et al. Can a clinical classification of stroke predict complications and treatments during hospitalization? Cerebrovasc Dis. 1998;8(4):204-209. doi: 10.1159/000015852 [DOI] [PubMed] [Google Scholar]
- 28.Rocco A, Pasquini M, Cecconi E, et al. Monitoring after the acute stage of stroke: a prospective study. Stroke. 2007;38(4):1225-1228. doi: 10.1161/01.STR.0000259659.91505.40 [DOI] [PubMed] [Google Scholar]
- 29.Siegler JE, Samai A, Semmes E, Martin-Schild S. Early neurological deterioration after stroke depends on vascular territory and stroke etiology. J Stroke. 2016;18(2):203-210. doi: 10.5853/jos.2016.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. How should we lower blood pressure after cerebral hemorrhage? a systematic review and meta-analysis. Cerebrovasc Dis. 2017;43(5-6):207-213. doi: 10.1159/000462986 [DOI] [PubMed] [Google Scholar]
- 31.Manning LS, Rothwell PM, Potter JF, Robinson TG. Prognostic significance of short-term blood pressure variability in acute stroke: systematic review. Stroke. 2015;46(9):2482-2490. doi: 10.1161/STROKEAHA.115.010075 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka E, Koga M, Kobayashi J, et al. Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the Stroke Acute Management With Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke. 2014;45(8):2275-2279. doi: 10.1161/STROKEAHA.114.005420 [DOI] [PubMed] [Google Scholar]
- 33.Fan JS, Chen YC, Huang HH, How CK, Yen DH, Huang MS. The association between on-scene blood pressure and early neurological deterioration in patients with spontaneous intracerebral haemorrhage. Emerg Med J. 2015;32(3):239-243. doi: 10.1136/emermed-2013-203114 [DOI] [PubMed] [Google Scholar]
- 34.Fan JS, Huang HH, Chen YC, et al. Emergency department neurologic deterioration in patients with spontaneous intracerebral hemorrhage: incidence, predictors, and prognostic significance. Acad Emerg Med. 2012;19(2):133-138. doi: 10.1111/j.1553-2712.2011.01285.x [DOI] [PubMed] [Google Scholar]
- 35.ISRCTN registry. ISRCTN26986053. Rapid intervention with glyceryl trinitrate in hypertensive stroke trial-2. http://www.isrctn.com/ISRCTN26986053. Accessed May 15, 2018. [DOI] [PMC free article] [PubMed]
- 36.Ebinger M, Rozanski M, Waldschmidt C, et al. ; STEMO-Consortium . PHANTOM-S: the prehospital acute neurological therapy and optimization of medical care in stroke patients–study. Int J Stroke. 2012;7(4):348-353. doi: 10.1111/j.1747-4949.2011.00756.x [DOI] [PubMed] [Google Scholar]
- 37.Shaw L, Price C, McLure S, et al. Paramedic Initiated Lisinopril for Acute Stroke Treatment (PIL-FAST): results from the pilot randomised controlled trial. Emerg Med J. 2014;31(12):994-999. doi: 10.1136/emermed-2013-202536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ClinicalTrials.gov. Field Randomization of NA-1 Therapy in Early Responders (FRONTIER). https://clinicaltrials.gov/ct2/show/NCT02315443. Accessed May 15, 2018.
- 39.Ankolekar S, Parry R, Sprigg N, Siriwardena AN, Bath PM. Views of paramedics on their role in an out-of-hospital ambulance-based trial in ultra-acute stroke: qualitative data from the Rapid Intervention With Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT). Ann Emerg Med. 2014;64(6):640-648. doi: 10.1016/j.annemergmed.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 40.Fassbender K, Balucani C, Walter S, Levine SR, Haass A, Grotta J. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol. 2013;12(6):585-596. doi: 10.1016/S1474-4422(13)70100-5 [DOI] [PubMed] [Google Scholar]
- 41.Sanossian N, Apibunyopas KC, Liebeskind DS, et al. ; FAST-MAG (Field Administration of Stroke Therapy-Magnesium) Investigators and Coordinators . Characteristics and outcomes of very elderly enrolled in a prehospital stroke research study. Stroke. 2016;47(11):2737-2741. doi: 10.1161/STROKEAHA.116.013318 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Demographic and Clinical Characteristics of the Patients in the Secondary Analysis With Or Without GCS Available at Hospital Arrival
eTable 2. Patterns of Ultra-Early Neurological deterioration Among Patients With GCS Assessments Prehospital, on ED Arrival, and in the Early ED Course; Grouped by Ultra-Early Time Segment in Which U-END Occurred
eTable 3. Patterns of Ultra-Early Neurological deterioration Among Patients With GCS Assessments Prehospital, on ED Arrival, and in the Early ED Course; Grouped by Occurrence or Non-Occurrence of Net Deterioration
eTable 4. ED Arrival GCS and Additional Baseline Variables Not Associated With U-END in All, Cerebral Ischemia, and Intracranial Hemorrhage Patients
eFigure. Timeline Delineating Typical FAST-MAG Patient Enrollment and Assessment Activities During the First 2 Hours After Symptom Onset