Key Points
Question
Is there an association between apolipoprotein E (APOE) ε4 and transactive response DNA-binding protein 43 (TDP-43), and if so, is it mediated by β-amyloid and tau?
Findings
In this cross-sectional study of 738 older adults with an Alzheimer disease spectrum pathological diagnosis, the APOE ε4 allele was both directly (ie, independent of β-amyloid and tau) and indirectly (ie, mediated by β-amyloid and tau) associated with TDP-43.
Meaning
The APOE ε4 allele appears to be a risk factor for TDP-43 in a model that accounts for age, β-amyloid, and tau, adding to the evidence that suggests that TDP-43 is a substantial factor in Alzheimer disease.
Abstract
Importance
Transactive response DNA-binding protein 43 (TDP-43) is associated with Alzheimer disease (AD), progressive hippocampal atrophy, and cognitive decline. The apolipoprotein E (APOE) ε4 allele is strongly associated with β-amyloid (Aβ) aggregation and risk of AD, but its association with TDP-43 is unknown.
Objective
To determine whether the APOE ε4 allele is a risk factor for TDP-43.
Design, Setting, Participants
This cross-sectional, genetic-histologic study analyzed APOE genotype, TDP-43 status (positive vs negative), Aβ status (positive vs negative), and tau neurofibrillary tangle stage (B0, Braak stage 0; B1, Braak stages I-II; B2, Braak stages III-IV; B3, Braak stage ≥ V). We fit structural equation models to map the association between APOE and TDP-43, Aβ, and tau, accounting for age and hippocampal sclerosis. We identified 751 participants with an AD pathological spectrum diagnosis and completed Aβ, tau, and TDP-43 data who were enrolled in the Mayo Clinic Alzheimer Disease Research Center, Mayo Clinic Alzheimer Disease Patient Registry, or the population based Mayo Clinic Study of Aging and died between May 12, 1999, and December 31, 2015. However, 13 were excluded from the analyses because of missing APOE data, leaving a total of 738 participants.
Main Outcomes and Measures
Transactive response DNA-binding protein 43 was the main outcome of interest. We hypothesized that the APOE ε4 allele would be significantly directly and indirectly associated with TDP-43.
Results
The 751 study participants were older (median age [interquartile range], 87 years [51-105 years]), 395 (54%) were women, and 324 (44%) were APOE ε4 carriers. The patients died between May 12, 1999, and December 31, 2015. Accounting for age, Aβ, and tau, APOE ε4 had a direct association with TDP-43 (estimate [SE], 0.31 (0.11); P = .01). The association was present among individuals with an intermediate to high likelihood of having AD (neurofibrillary tangle stage B2/B3; n = 604 [81.8%]; estimate [SE], 0.51 [0.11]; P < .001), with a similar trend for those with a low likelihood of having AD (B1; n = 134 [18.2%]; estimate [SE], 0.54 [0.32]; P = .10). We also found an indirect association of APOE ε4 with TDP-43 via Aβ and tau (estimate [SE], 0.34 [0.06]; P < .001), which was similar in magnitude to the direct association and an indirect association of APOE ε4 with hippocampal sclerosis via TDP-43 (estimate [SE], 0.65 [0.26]; P = .01).
Conclusions and Relevance
The study’s findings, which mapped a system of risk factors and outcomes, showed that the APOE ε4 allele appears to be a risk factor for TDP-43 independently of Aβ in patients with AD.
This cross-sectional study investigates the association between apolipoprotein E ε4 and transative response DNA-binding protein 43 in US patients with an Alzheimer disease spectrum pathological diagnosis.
Introduction
Alzheimer disease (AD) has been classically characterized by the aggregation of β-amyloid (Aβ) and paired helical filament tau.1 However, transactive response DNA-binding protein 43 (TDP-43), an RNA-binding protein that functions in exon skipping,2 has also recently been associated with AD.3 Indeed, TDP-43 is present in 65% to 80% of the brains of patients with AD4,5 and is associated with progressive hippocampal atrophy, a hallmark of AD,6,7 and memory loss in AD.8 Therefore, TDP-43 appears to be a substantial factor in AD pathogenesis.
Transactive response DNA-binding protein 43 aggregation in AD begins in the amygdala (stage 1) and then moves to entorhinal cortex and subiculum (stage 2); the dentate gyrus of the hippocampus and occipitotemporal cortex (stage 3); the insular cortex, ventral striatum, basal forebrain, and inferior temporal cortex (stage 4); the substantia nigra, inferior olive, and midbrain tectum (stage 5); and finally to the basal ganglia and middle frontal cortex (stage 6).9,10 Evidence from a cross-sectional study showed that the presence of TDP-43 was associated with smaller hippocampal volume closest to death.8 In addition, a recent longitudinal study found that patients with an intermediate to high probability of AD who had TDP-43 aggregation had faster rates of hippocampal atrophy beginning almost a decade before death.11 Given that TDP-43 affects all of the key features of AD, it is important to better understand the risk factors of TDP-43 deposition.
Apolipoprotein E (APOE) ε4 has long been recognized as the strongest risk factor for the development of AD and is robustly linked to Aβ.12 We previously found evidence of an association between APOE ε4 and TDP-43,8,11 accounting for Braak stage, and therefore we wanted to assess the association of APOE ε4 with TDP-43 in a comprehensive disease model. A recent study found APOE ε4 was associated with TDP-43 independently of Aβ and tau in a series of regression models.13 In this study, we investigate the cross-sectional association between APOE ε4 and TDP-43 by mapping the potential associations between APOE ε4, Aβ, tau, and TDP-43. Based on earlier evidence,11 we hypothesized that tau deposition occurs before TDP-43 deposition. We fit our primary models using this framework. In addition, given the strong association between TDP-43 and hippocampal sclerosis, we explored whether the APOE ε4 allele was directly linked to hippocampal sclerosis with the assumption that hippocampal sclerosis occurs downstream of TDP-43 deposition, which additionally provides a built in sensitivity analysis.
Methods
Study Design and Participants
We conducted a cross-sectional genetic-histological study, analyzing postmortem brain tissue from 738 participants with an AD spectrum pathological diagnosis who were enrolled in the Mayo Clinic Alzheimer Disease Research Center, Mayo Clinic Alzheimer Disease Patient Registry, or the Mayo Clinic Study of Aging. The Mayo Clinic Alzheimer Disease Patient Registry and the Mayo Clinic Study of Aging are population-based studies that were designed to study incident mild cognitive impairment and dementia.14 Patient demographics are presented in Table 1. This study was approved by the Mayo Clinic institutional review board. All patients or their proxies provided written informed consent before participating in any research activity.
Table 1. Characteristics of the Study Participants.
| Characteristic | No. (%) | P Valuea | ||
|---|---|---|---|---|
| All (N = 738) | TDP+ (n = 319) | TDP− (n = 419) | ||
| Female | 395 (54) | 194 (61) | 201 (48) | <.001 |
| APOE ε4 carrier | 324 (44) | 173 (54) | 151 (36) | <.001 |
| Age at death, y | 87 (51-105) | 88 (56-105) | 86 (51-103) | <.001 |
| Tau (B1-B3)b | ||||
| B1 | 134 (18) | 33 (10) | 101 (24) | <.001 |
| B2 | 208 (28) | 63 (20) | 145 (35) | |
| B3 | 396 (54) | 223 (70) | 173 (41) | |
| Tau (Braak 0-VI) | ||||
| Braak 0 | 6 (1) | 0 (0) | 6 (1) | <.001 |
| Braak I | 34 (5) | 6 (2) | 28 (7) | |
| Braak II | 100 (13) | 25 (8) | 75 (18) | |
| Braak III | 79 (11) | 17 (5) | 62 (15) | |
| Braak IV | 129 (17) | 46 (15) | 83 (19) | |
| Braak V | 153 (21) | 82 (26) | 71 (17) | |
| Braak VI | 243 (33) | 141 (44) | 102 (24) | |
| Aβ positive | 668 (91) | 301 (94) | 367 (88) | .002 |
| Aβ (CERAD) | ||||
| 0 (normal) | 180 (24) | 42 (13) | 138 (32) | <.001 |
| 1 (sparse/possible) | 111 (15) | 33 (10) | 78 (18) | |
| 2 (moderate/probable) | 180 (24) | 68 (21) | 67 (16) | |
| 3 (frequent/definite) | 180 (24) | 174 (55) | 144 (34) | |
| Hippocampal sclerosis positive | 110 (15) | 110 (34) | 0 (0) | |
| Clinical scores closest to death, median (range) | ||||
| Years from clinical testing | 1 (0-13) | 2 (0-12) | 1 (0-13) | .07 |
| MMSE | 24 (0-30) | 21 (0-30) | 25 (3-30) | <.001 |
| Clinical dementia rating scale, sum of boxes | 6 (0-18) | 11 (0-18) | 3 (0-18) | <.001 |
Abbreviations: Aβ, β-amyloid; APOE, apolipoprotein E; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MMSE, Mini-Mental State Examination; TDP, transactive response DNA-binding protein.
Data shown are number (percentage) or median (range). Groupwise comparison P values are from Fisher exact tests and Wilcoxon rank sum tests.
Tau: B1, Braak is between 1 or more or 2 or less; B2, Braak is between 2 or more and 4 or less; B3, Braak is greater than 4.
The patients died between May 12, 1999, and December 31, 2015. All cases were identified from the Mayo Clinic neuropathological database and 668 (90.5%) had received an AD spectrum pathological diagnosis in accordance with the National Institute on Aging–Alzheimer Association diagnostic criteria.15 We also included 70 cases (9.5%) with a Braak neurofibrillary tangle stage of 0 or greater and Aβ negative status because most are also considered a part of the AD spectrum pathological diagnosis.16 Cases with a pathological diagnosis of a frontotemporal lobar degeneration, progressive supranuclear palsy, or corticobasal degeneration were excluded. Cases without an APOE genotype (n = 13) were not included in the study.
APOE Genotyping
Apolipoprotein E genotyping was performed for all 738 patients, as previously described.17,18 For statistical analyses, APOE was coded as a binary variable (1 for ε4 carriers and 0 for noncarriers).
Pathologic Analysis
Neuropathological examinations were performed according to the recommendations of the Consortium to Establish a Registry for AD.19 Every specimen was assigned a Braak neurofibrillary tangle stage20 using a modified Bielschowsky silver stain on the basis of the distribution of neurofibrillary tangles. We categorized all cases into Braak subgroups (B0, Braak 0; B1, I ≤ Braak ≤ II; B2, II < Braak ≤ IV; B3, Braak > IV). β-Amyloid status was determined with Aβ immunohistochemistry (Clone 6F/3D, 1/10 dilution; Novacastra Vector Laboratories) and was dichotomized as negative for any type of Aβ deposition, including diffuse and cored plaques (Thal stage 0), or positive for any type of Aβ deposition (Thal stage >0) according to National Institute on Aging–Alzheimer Association diagnostic criteria.15 Degenerative-type hippocampal sclerosis (present vs not) was diagnosed if there was neuronal loss in the CA1 and/or subiculum regions of the hippocampus in the presence of TDP-43 deposition.21,22
Transactive response DNA-binding protein 43 was assessed according to our proposed TDP-43 staging scheme.9,10 Amygdala blocks were sectioned and immunostained for TDP-43 in all 738 cases (polyclonal antibody MC2085 that recognizes a peptide sequence in the 25-kDa C-terminal fragment23) with a DAKO-Autostainer (DAKO-Cytomaton) and 3, 3′-diaminobenzidine as the chromogen. Sections were lightly counterstained with hematoxylin. Sections of amygdala were screened for the presence of TDP-43–associated lesions, including neuronal cytoplasmic inclusions, dystrophic neurites, perivascular inclusions, and neuronal intranuclear inclusions. We screened the amygdala in our cases, given that this region has been determined to be the first affected region in AD by TDP-43.24
Statistical Analysis
We used counts and percentages to describe categorical variables and medians and ranges to describe continuous variables. Values in the TDP-43–negative and –positive groups were compared using Fisher exact or Wilcoxon rank sum tests.
To address our primary aim, we used structural equation models (SEMs) to map the associations between APOE ε4, age, Aβ, tau, and TDP-43. Structural equation models are a widely used technique in the behavioral sciences and can be considered a combination of factor analysis and regression models. While regression models are discussed using the terms association and coefficient, SEMs use the terms direct (unmediated), indirect (mediated), and total (direct + indirect) associations. We chose an SEM because it allows a variable to be a predictor and an outcome. We thought this was appropriate given that we do not know the order of events of protein deposition. In this study, the endogenous variables in the SEMs were 2-level (Aβ and TDP-43) or 3-level (tau) categorical variables. The statistical methods for the SEMs are detailed in the eMethods in the Supplement.
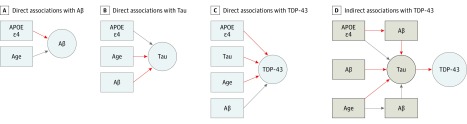
Direct, indirect, and total associations leading into the same outcome variable can be compared with each other using the regression coefficients. However, associations that lead into different outcomes cannot generally be compared because of the different scales of measurement of the outcomes. We report P values, regression estimates, and standard errors on the probit scale. We also report the predicted probabilities of TDP-43 positivity given the combinations of variables that have a direct association with TDP-43. We first fit SEMs that included all of the possible pathways between the variables in the system (Figure 1). We then removed pathways with a P value >.05 (Figure 1) and fit a more parsimonious model. Sensitivity analyses are described in the Supplement. Analyses were performed with R statistical software (version 3.1.3; R Foundation)25 and MPLUS, version 7.3 (Muthén & Muthén).26
Figure 1. Structural Equation Models (SEMs) Path Analyses Investigating the Association of Apolipoprotein E (APOE) ε4 With Transactive Response DNA-Binding Protein 43 (TDP-43).
A-C, These direct pathways were tested in the initial SEM and included all potential direct pathways. The red arrowheads were significant. The SEMs allow us to test direct associations of all variables in the model, regardless if they are the final outcome (in this case, TDP-43) or not. The estimates presented in the tables are the strength of the association depicted by the arrowheads. The estimates leading into the same box can be compared, but the estimates leading into different boxes cannot because of differing measurement scales. D, These indirect pathways were tested in the initial SEM and included all potential indirect pathways leading to TDP-43. The red arrowheads were significant. The sum of the direct and indirect pathways leads to the total effects, presented in Table 2. Aβ indicates β-amyloid.
Results
In this study of 738 pathologically confirmed cases with an AD spectrum diagnosis (median age [interquartile range], 87 years [51-105 years]), 395 (54%) were women, and 324 (44%) were APOE ε4 carriers. β-Amyloid deposition was present in 672 cases (91%) and 111 cases (15%) had hippocampal sclerosis (Table 1). Overall, 317 cases (43%) were TDP-43 positive, and 152 (48%) of the TDP-43–positive cases were women compared with 257 women (61%) in the TDP-43–negative group. Transactive response DNA-binding protein 43–positive cases were significantly older at time of death (median [interquartile range]: 88 [56-105] years) than TDP-43–negative cases (86 [51-103] years). Further, TDP-43–positive cases were more likely to have advanced neurofibrillary tangle stage pathology compared with TDP-43–negative cases. Of the participants with TDP-43, only 33 (10%) had a low probability of Alzheimer disease (B1 neurofibrillary tangle stage) compared with 223 (70%) of those with TDP-43 having a high probability of AD (B3 neurofibrillary tangle stage). Similarly, 94% of TDP-43–positive cases were Aβ-positive compared with 88% of TDP-43–negative cases. Hippocampal sclerosis was found in 34% of TDP-43–positive cases. Notably, the 54% of TDP-43–positive patients who were APOE ε4 carriers was significantly higher than the 36% APOE ε4 carriers in TDP-43–negative cases.
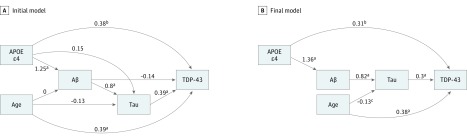
In the primary structural equation model (SEM), we did not see an association of APOE ε4 with tau, age with Aβ, or Aβ with TDP-43 (Figures 1 and 2). As described in the Methods, these associations were removed and the final model was fit without these nonsignificant associations (Figure 2). After accounting for age, Aβ, and tau, we found that APOE ε4 had a direct association with TDP-43 (estimate [SE], 0.31 [0.11]; P = .01) (Table 2 and Figures 1 and 2). We also found an indirect association of APOE ε4 with TDP-43 through Aβ and tau (Figures 1 and 2) of similar magnitude (0.34 [0.06]; P < .001) to the direct association of APOE ε4 with TDP-43. The total association of APOE ε4 with TDP-43 was also significant (0.65 [0.11]; P < .001) (Table 2). Further, the tau (0.30 [0.05]; P < .001) and age (0.38 [0.06]; P < .001) direct and overall associations with TDP-43 were also significant. Notably, we did not observe a direct association between age and Aβ (Figures 1 and 2); however, this could be partially because of the older age of the participants in the cohort in this study (the mean [SD] age of participants was age 85 [9.5] years). Table 2 shows the estimated individual and total effects of all the pathways. This SEM with TDP-43 as the outcome fits the data well (comparative fit index, 1.00; acceptable threshold, >0.95; Tucker-Lewis index, 1.00; acceptable threshold, >0.95).
Figure 2. Structural Equation Models (SEMs) Path Analyses Investigating the Association of Apolipoprotein E (APOE) ε4 on Transactive Response DNA-Binding Protein 43 (TDP-43).
A, These pathways were tested in the initial SEM and included all potential pathways. B, This SEM was the final model used. It became more parsimonious after eliminating pathways that were not significant at the P < .05 level. The arrowhead linking APOE ε4 to TDP-43 represents a direct association, and the arrowheads from APOE ε4 to Aβ to tau to TDP-43 represent indirect associations (mediation by Aβ and tau). Their sum represents the total association of APOE ε4 with TDP-43. The numbers on the arrowheads are estimated regression coefficients on the probit scale. The numbers leading into the same box can be compared, but the numbers leading into different boxes cannot because of differing measurement scales. Aβ indicates β-amyloid.
aP < .05.
bP < .01.
cP <.001.
Table 2. Probit Regression Results From the Primary Structural Equation Model.
| Association | Estimate (SE) | z Value | P Value |
|---|---|---|---|
| Direct Associations | |||
| TDP-43 | |||
| APOE ε4a | 0.31 (0.11) | 2.77 | .01 |
| Tau | 0.30 (0.05) | 5.87 | <.001 |
| Age | 0.38 (0.06) | 6.05 | <.001 |
| Intercept | −0.49 (0.07) | 6.91 | <.001 |
| Tau | |||
| Age | −0.13 (0.06) | −2.07 | .04 |
| Aβ | 0.82 (0.12) | 6.98 | <.001 |
| Intercept tau 1 | 0.81 (0.09) | −8.9 | <.001 |
| Intercept tau 2 | −0.36 (0.08) | 4.29 | <.001 |
| Amyloid | |||
| APOE ε4 | 1.36 (0.16) | 8.62 | <.001 |
| Intercept | 1.00 (0.08) | −13.18 | <.001 |
| Indirect Associations With TDP-43 | |||
| APOE ε4 with Aβ with tau | 0.34 (0.06) | 5.32 | <.001 |
| Age with tau | −0.04 (0.02) | −1.98 | .05 |
| Aβ with tau | 0.25 (0.04) | 5.74 | <.001 |
| Total Associations With TDP-43 | |||
| APOE ε4 | 0.65 (0.11) | 6.06 | <.001 |
| Tau | 0.30 (0.05) | 5.87 | <.001 |
| Age | 0.34 (0.06) | 5.5 | <.001 |
| Aβ | 0.25 (0.04) | 5.74 | <.001 |
Abbreviations: Aβ, β-amyloid; APOE, apolipoprotein E; TDP, transactive response DNA-binding protein.
Apolipoprotein E, Aβ, tau, and TDP-43 were coded as positive (0) or negative (1).
A second SEM model that assumed TDP-43 was not associated with the other variables (ie, APOE, age, Aβ, and tau) did not fit the data well. In the original model, the aggregate association of TDP-43 with the other variables was highly significant (P < .001), suggesting that TDP-43 is an integral part of AD pathogenesis in this older cohort (ie, accounting for age, Aβ, and tau).
We additionally observed a direct association of APOE ε4 on Aβ (1.316 [0.16]; P < .001; Figures 1 and 2). Indeed, our findings confirm the known associations between APOE ε4 and Aβ and tau. We did not find a direct association of APOE ε4 on tau (Figures 1 and 2); there was only a significant indirect association of APOE ε4 with tau through Aβ (Figure 1). We did find a direct association of age with tau (−0.132 [0.06]; P = .04).
The APOE ε4 allele had a similar association with TDP-43 regardless of neurofibrillary tangle stages. When we stratified the association of APOE ε4 by low (B1; n = 134 cases) vs intermediate-high (B2 and B3; n = 604 cases) probability of developing AD, and after adjusting for age and Aβ, we found similar direct associations in both models via comparable regression coefficients. The P values in B1 cases were at a trend level only (B2 and B3, estimate [SE], 0.51 [0.11]; P < .001; B1, estimate [SE], 0.54 [0.32]; P = .10).
The results of these SEMs can be translated to predicted probabilities (Table 3). For example, an 85-year-old non–APOE ε4 carrier individual with B1 tau stage has a 42% predicted probability of being TDP-43 positive. By contrast, if the same individual is instead an APOE ε4 carrier, their probability of being TDP-43 positive increases to 55%. This 13% probability increase for APOE ε4 carrier vs noncarrier status was similar for the B2 (13%) and B3 (11%) neurofibrillary tangle stages. To summarize this concisely, we can say that 85-year-old APOE ε4 carriers are roughly 10% more likely to have TDP-43 in the brain at death compared with APOE ε4 noncarriers across B1, B2, and B3 stages. Similarly, APOE ε4 carriers are roughly 10% more likely to have Aβ in their brains at death compared with noncarriers. This suggests that the associations with the ε4 allele on Aβ and TDP-43 is likely of similar magnitude.
Table 3. Predicted Probabilities of Transactive Response DNA-Binding Protein (TDP-43) Positivity Using Only Variables With Direct Associations With TDP-43.
| Age, y | APOE ε4 | Tau | Predicted Probability, % |
|---|---|---|---|
| 85 | 0 | 1 | 42 |
| 85 | 1 | 1 | 55 |
| 85 | 0 | 2 | 54 |
| 85 | 1 | 2 | 66 |
| 85 | 0 | 3 | 66 |
| 85 | 1 | 3 | 76 |
Abbreviation: APOE, apolipoprotein E.
In a sensitivity analysis, we explored the possibility that APOE ε4 also had an association with hippocampal sclerosis (eFigure 1 in the Supplement). This model did not change any of the previously discussed associations with TDP-43, but it did show additional significant associations of APOE ε4 with hippocampal sclerosis. However, the association of APOE ε4 with hippocampal sclerosis was indirect through TDP-43. To state this more simply, we did not find a direct association of APOE ε4 with hippocampal sclerosis after accounting for age, Aβ, tau, and TDP-43. The first indirect association was from APOE ε4 through TDP-43 (0.65 [0.26]; P = .01) and the second indirect association was from APOE ε4 thorough Aβ, tau, and TDP-43 (0.66 [0.19]; P < .001).
In our secondary analysis that used ordinal coding of Aβ and tau, we again observed that APOE ε4 had direct (0.319 [0.11]; P = .003) and total (0.663 [0.11]; P < .001) associations with TDP-43 (eTable in the Supplement). Moreover, APOE ε4 had indirect associations with TDP-43 through age, Aβ, and tau. In contrast to the primary model, we also observed an additional significant association of APOE ε4 with tau and an association of age with Aβ. This is likely due to the increase in power that was obtained from the increased information in the ordinal rather than dichotomized variables for Aβ and tau. eTable in the Supplement shows the direct, indirect, and total associations of these pathways and eFigure 2 in the Supplement illustrates these associations.
Discussion
We mapped a system of variables in 738 patients with an AD spectrum pathological diagnosis to investigate the association between APOE ε4 and TDP-43 while accounting for age, Aβ, and tau. We found that in all of the models the APOE ε4 allele had a direct association with TDP-43 (ie, the association was independent of Aβ) in the brains of patients with AD, suggesting that the ε4 allele is a risk factor for TDP-43.
We also observed an indirect association of APOE ε4 with TDP-43 through Aβ. The direct and indirect associations of APOE ε4 on TDP-43 were of similar magnitudes. Thus, these pathways—the direct and indirect associations through Aβ and tau—could be equally significant and important in AD pathogenesis. Additionally, we confirmed that there is an association of APOE ε4 with Aβ. Moreover, we found that when stratifying by tau neurofibrillary tangle stage, the estimates remained stable. Although we had limited power in the lowest neurofibrillary tangle stage group (B1), the estimate for the association was comparable with the association for greater neurofibrillary tau tangle stages (B2 and B3). A limitation of these methods is that we cannot compare the importance or relevance of associations across outcomes. That is, we cannot directly compare the APOE ε4 association with Aβ with the APOE ε4 association with TDP-43. However, because the predicted probability of ε4 carriers having TDP-43 or Aβ in their brain at death is 10% greater than noncarriers—holding all other variables constant—it may be inferred that the separate associations of APOE ε4 with Aβ and TDP-43 are of similar magnitudes. Regardless, the findings from this study are further evidence supporting the hypothesis that TDP-43 plays an important role in AD.
Although Aβ and tau are traditional hallmarks of AD, many older adults die with a substantial burden of additional proteins and neurodegenerative processes in their brains. In fact, if we only consider Aβ and tau, there are many patients who have these 2 proteins yet do not have cognitive impairment, which has been termed resilience.27 A recent study showed, similarly, that APOE ε4 is associated with TDP-43 independently of Aβ and tau by using a series of regression models.13 This study provides further evidence of the direct, indirect, and total associations of APOE ε4 with TDP-43 by using SEMs. Given this new evidence of an association of APOE ε4 with TDP-43, it strengthens the argument that TDP-43 is a missing link in the chain of cognitive decline in AD. However, the results from this analysis should be considered within the context of the model, which accounted for age, Aβ, tau, and hippocampal sclerosis, but there may be other, as yet unmeasurable variables that play a role in this pathway.
Past research suggests that TDP-43 is a substantial contributor in AD4,8; therefore, the finding that APOE ε4, the strongest risk factor for AD, is directly associated with TDP-43 while accounting for age, Aβ, and tau, although novel, should not be astonishing. Furthermore, APOE ε4 has been associated with other diseases, such as frontotemporal lobar degeneration,28 in which Aβ is typically absent, yet TDP-43 plays an important role. The APOE ε4 allele, along with increasing age, has been associated with copathologies in differing dementia diagnoses.29 With that said, to our knowledge, the biological mechanism of how APOE ε4 may cause or influence the deposition of TDP-43 without going through Aβ is unknown. Apolipoprotein E ε4 is thought to reduce the clearance of Aβ and recently has been shown to have the greatest association with its deposition during the early seeding stages.30 However, there are many alternative ways in which APOE ε4 could be associated with TDP-43 independently of Aβ. Apolipoprotein E ε4, for example, is also thought to be involved with reduced neurogenesis, mitochondrial function, lipid and cholesterol metabolism, vascular function, and glucose mechanism.31 It is also thought to be involved with increased aberrant brain activity, increased neuronal toxicity, and increased brain atrophy.31 Hence, any of these “non-Aβ mechanisms” could result in a decreased clearance of TDP-43 species, or increased toxicity or toxic deposition of TDP-43 species. Therefore, although we do not have a current mechanism to account for an association of APOE ε4 with TDP-43 independent of Aβ, it is a possible link that is worthy of further investigation.
In our sensitivity analysis, we further investigated whether hippocampal sclerosis occurring downstream of TDP-43 changed the observed associations and whether APOE ε4 is associated with the presence of hippocampal sclerosis. We did not see any difference in the association between APOE ε4 and TDP-43 when we included hippocampal sclerosis in the SEM. In fact, we found that an indirect association between APOE ε4 and hippocampal sclerosis was via TDP-43. We did not find a direct association of APOE ε4 with hippocampal sclerosis and we did not find an association through Aβ. This further strengthens the hypothesis that hippocampal sclerosis is a downstream effect of TDP-43 and supports an association between APOE ε4 and TDP-43 that is independent of hippocampal sclerosis.
In the secondary analysis, which coded Aβ as 4 levels and tau as 7 levels, we found that APOE ε4 had a direct association with Aβ and tau; a direct association with Aβ was also found in the primary analysis. We did not observe a direct association with tau in the primary model that coded Aβ as 2 levels and tau as 3, which may be due to the increase in power and the fact that SEMs perform better with continuous variables. The association between APOE ε4 and Aβ is well established, as APOE ε4 inhibits the clearance of Aβ32 and is the strongest risk factor for AD.33 On the other hand, our finding that the APOE ε4 allele is associated with tau is novel, and more studies will need to be conducted to further investigate this association. A recent study by Shi and colleagues34 in cell and mouse models found that APOE ε4 was associated with phosphorylated tau and tau-mediated neurodegeneration, although the tau isoform (4 repeated tau) was different from the tau isoforms that we analyzed in our models (mixed 3 + 4 repeated tau).
Limitations
One of the main limitations to this study is the relatively small sample size of the B0 and B1 groups. In addition, in the analyses we hypothesized that tau deposition occurs before TDP-43, which limited our ability to assess for an association of TDP-43 with tau.
Conclusions
Increasing evidence now suggests that TDP-43 has an important role in AD. It is associated with cognitive performance, hippocampal degeneration, and APOE ε4 independently of Aβ. Therefore, predictive models for TDP-43 will be important in understanding the AD pathogenesis, including who may be at risk of developing cognitive impairment. Although Aβ and tau are fundamental to AD development, research that attempts to entirely explain the risk of developing AD through only these proteins has stagnated and they alone are not sufficient to understand the disease. Transactive response DNA-binding protein 43 is very likely one of the missing pieces of the puzzle. It will be critical to develop methods to measure TDP-43 in vivo to better understand its risk factors, progression of pathology, and association with Aβ and tau progression and to understand the trajectories of cognitive decline.
eMethods. Structural equation models (SEMs) and sensitivity analyses.
eTable. Probit regression results from the secondary structural equation model.
eFigure 1. Sensitivity analysis of structural equation models (SEMs) path analyses investigating the effect of APOE ε4 on TDP-43, accounting for hippocampal sclerosis using penalized scores.
eFigure 2. Secondary analyses of structural equation models (SEMs) path analyses investigating the effect of APOE ε4 on TDP-43, coding Aβ and tau as ordinal variables.
References
- 1.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184-185. doi: 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- 2.Buratti E, Brindisi A, Pagani F, Baralle FE. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet. 2004;74(6):1322-1325. doi: 10.1086/420978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61(5):435-445. doi: 10.1002/ana.21154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephs KA, Whitwell JL, Tosakulwong N, et al. TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. 2015;78(5):697-709. doi: 10.1002/ana.24493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139(11):2983-2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erten-Lyons D, Dodge HH, Woltjer R, et al. Neuropathologic basis of age-associated brain atrophy. JAMA Neurol. 2013;70(5):616-622. doi: 10.1001/jamaneurol.2013.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61(4):487-492. doi: 10.1212/01.WNL.0000079053.77227.14 [DOI] [PubMed] [Google Scholar]
- 8.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127(6):811-824. doi: 10.1007/s00401-014-1269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 2016;131(4):571-585. doi: 10.1007/s00401-016-1537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Josephs KA, Murray ME, Whitwell JL, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014;127(3):441-450. doi: 10.1007/s00401-013-1211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josephs KA, Dickson DW, Tosakulwong N, et al. Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: a longitudinal retrospective study. Lancet Neurol. 2017;16(11):917-924. doi: 10.1016/S1474-4422(17)30284-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977-1981. doi: 10.1073/pnas.90.5.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HS, Yu L, White CC, et al. Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE ε4 haplotype status: a community-based cohort study. Lancet Neurol. 2018;17(9):773-781. doi: 10.1016/S1474-4422(18)30251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58-69. doi: 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duyckaerts C, Braak H, Brion JP, et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015;129(5):749-756. doi: 10.1007/s00401-015-1390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crook R, Hardy J, Duff K. Single-day apolipoprotein E genotyping. J Neurosci Methods. 1994;53(2):125-127. doi: 10.1016/0165-0270(94)90168-6 [DOI] [PubMed] [Google Scholar]
- 18.Josephs KA, Tsuboi Y, Cookson N, Watt H, Dickson DW. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch Neurol. 2004;61(10):1579-1584. doi: 10.1001/archneur.61.10.1579 [DOI] [PubMed] [Google Scholar]
- 19.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479-486. doi: 10.1212/WNL.41.4.479 [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 21.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88(3):212-221. doi: 10.1007/BF00293396 [DOI] [PubMed] [Google Scholar]
- 22.Murray ME, Cannon A, Graff-Radford NR, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128(3):411-421. doi: 10.1007/s00401-014-1302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YJ, Xu YF, Cook C, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106(18):7607-7612. doi: 10.1073/pnas.0900688106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116(2):215-220. doi: 10.1007/s00401-008-0400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(2):1-36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- 26.Muthén LK, Muthén BO Mplus user's guide. Los Angeles, CA: Muthén & Muthén; 1998-2017. https://www.statmodel.com/download/usersguide/MplusUserGuideVer_8.pdf. Accessed February 1, 2018.
- 27.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006-1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agosta F, Vossel KA, Miller BL, et al. Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proc Natl Acad Sci U S A. 2009;106(6):2018-2022. doi: 10.1073/pnas.0812697106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181-2193. doi: 10.1093/brain/awy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CC, Zhao N, Fu Y, et al. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 2017;96(5):1024-1032.e3. doi: 10.1016/j.neuron.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106-118. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verghese PB, Castellano JM, Garai K, et al. ApoE influences amyloid-β (Aβ) clearance despite minimal APOE/Aβ association in physiological conditions. Proc Natl Acad Sci U S A. 2013;110(19):E1807-E1816. doi: 10.1073/pnas.1220484110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921-923. doi: 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Yamada K, Liddelow SA, et al. ; Alzheimer’s Disease Neuroimaging Initiative . ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523-527. doi: 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Structural equation models (SEMs) and sensitivity analyses.
eTable. Probit regression results from the secondary structural equation model.
eFigure 1. Sensitivity analysis of structural equation models (SEMs) path analyses investigating the effect of APOE ε4 on TDP-43, accounting for hippocampal sclerosis using penalized scores.
eFigure 2. Secondary analyses of structural equation models (SEMs) path analyses investigating the effect of APOE ε4 on TDP-43, coding Aβ and tau as ordinal variables.