Key Points
Question
In adults in whom weaning from invasive mechanical ventilation is difficult, does early extubation using a protocolized noninvasive weaning regimen reduce the time to liberation from ventilation compared with protocolized invasive weaning?
Findings
In this randomized clinical trial that included 364 adults, the median time to liberation from ventilation for patients randomized to noninvasive weaning vs invasive weaning was 4.3 days vs 4.5 days, a difference that was not statistically significant.
Meaning
Protocolized weaning with early extubation to noninvasive ventilation compared with invasive weaning did not significantly shorten time to liberation from all forms of mechanical ventilation.
Abstract
Importance
In adults in whom weaning from invasive mechanical ventilation is difficult, noninvasive ventilation may facilitate early liberation, but there is uncertainty about its effectiveness in a general intensive care patient population.
Objective
To investigate among patients with difficulty weaning the effects of protocolized weaning with early extubation to noninvasive ventilation on time to liberation from ventilation compared with protocolized invasive weaning.
Design, Setting, and Participants
Randomized, allocation-concealed, open-label, multicenter clinical trial enrolling patients between March 2013 and October 2016 from 41 intensive care units in the UK National Health Service. Follow-up continued until April 2017. Adults who received invasive mechanical ventilation for more than 48 hours and in whom a spontaneous breathing trial failed were enrolled.
Interventions
Patients were randomized to receive either protocolized weaning via early extubation to noninvasive ventilation (n = 182) or protocolized standard weaning (continued invasive ventilation until successful spontaneous breathing trial, followed by extubation) (n = 182).
Main Outcomes and Measures
Primary outcome was time from randomization to successful liberation from all forms of mechanical ventilation among survivors, measured in days, with the minimal clinically important difference defined as 1 day. Secondary outcomes were duration of invasive and total ventilation (days), reintubation or tracheostomy rates, and survival.
Results
Among 364 randomized patients (mean age, 63.1 [SD, 14.8] years; 50.5% male), 319 were evaluable for the primary effectiveness outcome (41 died before liberation, 2 withdrew, and 2 were discharged with ongoing ventilation). The median time to liberation was 4.3 days in the noninvasive group vs 4.5 days in the invasive group (adjusted hazard ratio, 1.1; 95% CI, 0.89-1.40). Competing risk analysis accounting for deaths had a similar result (adjusted hazard ratio, 1.1; 95% CI, 0.86-1.34). The noninvasive group received less invasive ventilation (median, 1 day vs 4 days; incidence rate ratio, 0.6; 95% CI, 0.47-0.87) and fewer total ventilator days (median, 3 days vs 4 days; incidence rate ratio, 0.8; 95% CI, 0.62-1.0). There was no significant difference in reintubation, tracheostomy rates, or survival. Adverse events occurred in 45 patients (24.7%) in the noninvasive group compared with 47 (25.8%) in the invasive group.
Conclusions and Relevance
Among patients requiring mechanical ventilation in whom a spontaneous breathing trial had failed, early extubation to noninvasive ventilation did not shorten time to liberation from any ventilation.
Trial Registration
ISRCTN Identifier: ISRCTN15635197
This randomized trial compares the effects of protocolized weaning via early extubation to noninvasive ventilation vs standard weaning from invasive ventilation on time to liberation from mechanical ventilation among adults with respiratory failure ready to wean but with an unsuccessful spontaneous breathing trial.
Introduction
Invasive mechanical ventilation is a lifesaving intervention. However, prolonged ventilation is associated with increased morbidity and mortality.1,2 Optimal processes for weaning from ventilation have been studied for many years and have led to evidence-based clinical practice guidelines to facilitate early liberation from invasive mechanical ventilation.3 These guidelines recommend using spontaneous breathing trials, minimizing sedation, using weaning protocols, and early mobilization to promote liberation from ventilation.
Although most invasively ventilated patients have an uncomplicated (simple) weaning pathway, about one-third require more than 1 spontaneous breathing trial and are considered difficult to wean.1,4,5 Patients with difficulty weaning face the physical discomfort of ongoing tracheal intubation, are often unable to speak,6 and are at increased risk of ventilator-associated pneumonia.7,8 Mobilization is often delayed because of concurrent sedation and concerns about unintentional extubation.9,10 This group of patients consume a disproportionate amount of intensive care unit (ICU) resources.11
Noninvasive mechanical ventilation, which is being used increasingly as an alternative to invasive ventilation,12,13 may have a role in supporting early liberation from invasive mechanical ventilation in patients who have difficulty weaning. Although the use of noninvasive ventilation as an adjunct to weaning has been tested in previous studies, the patient populations and interventions tested are not generalizable to contemporary clinical ventilation practice.14
In this multicenter randomized clinical trial conducted in the United Kingdom, it was hypothesized that weaning protocols that directed clinicians to extubate patients who were difficult to wean to noninvasive ventilation, compared with conventional weaning protocols for invasive mechanical ventilation, would reduce the time to liberation from ventilation.
Methods
Trial Design
We conducted this randomized, allocation-concealed, controlled, open-label, multicenter trial in 41 general adult ICUs in the United Kingdom. The trial protocol was designed by the trial investigators (Supplement 1) and was approved by South Central C Research Ethics Committee (reference 12/SC/0515). It was endorsed by the UK Intensive Care Foundation. Written consent was obtained from patients, their next of kin, or a physician who was independent from the trial prior to randomization in accordance with national laws. The study included an internal pilot spanning the first 6 months of the trial, at which point progress was reviewed by the funder. The same trial protocol was used for the internal pilot as for the main study. Patients enrolled in the internal pilot were included as part of the main trial.
Patients
Adult patients who had received invasive mechanical ventilation through an endotracheal tube continuously for more than 48 hours and were ready to commence weaning were considered for enrollment. Exclusion criteria were pregnancy, presence of a tracheostomy, contraindications to noninvasive ventilation, profound neurological deficit, home ventilation prior to admission, treatment limitations, need for further surgery or sedation, or no noninvasive ventilator available. Readiness to wean was assessed by the treating clinician before randomization according to prespecified criteria.15 Patients judged ready to start weaning underwent a spontaneous breathing trial (eAppendix in Supplement 2). Patients in whom the spontaneous breathing trial failed were defined as difficult to wean and were eligible for randomization. After obtaining consent, eligible patients were randomized using web-based secure electronic randomization designed by the study statistician. The minimization method was used to randomize patients in a 1:1 (noninvasive or invasive) allocation. The stratifying factors used in the minimization algorithm were center, presence or absence of chronic obstructive pulmonary disease (COPD), and postoperative/nonoperative reason for ICU admission, and these ensured equal balance between treatment groups. Chronic obstructive pulmonary disease was defined by a preadmission diagnosis of COPD requiring pharmacological treatment, evidence of a ratio of forced expiratory volume in the first second to forced vital capacity of less than 0.7 (FEV1/FVC <0.7) and an FEV1 less than 80% of predicted, or presence of respiratory symptoms. Patients admitted to the ICU after surgery were defined as the postoperative group. Following the spontaneous breathing trial, pressure support ventilation was reestablished using the previous settings. If necessary, the level of pressure support was further titrated to achieve patient comfort and a respiratory rate less than 30/min.
Noninvasive Ventilation Weaning Protocol
When a treating clinician judged that a patient was ready to wean, the patient underwent extubation and immediately was provided with noninvasive ventilation via face mask. The noninvasive ventilator was configured to deliver an equivalent level of inspiratory positive airway pressure to the level of pressure support that was being provided by the invasive ventilator and expiratory positive airway pressure equivalent to the level of positive end-expiratory pressure. The level of inspiratory positive airway pressure was then titrated to achieve patient comfort and a respiratory rate less than 30/min. Every 2 hours, the patient was assessed for signs of distress or fatigue. In the absence of distress or fatigue, the treating clinician either removed the noninvasive ventilation mask to allow a self-ventilation trial or reduced the level of positive airway pressure by 2 cm H2O. The noninvasive weaning protocol was discontinued when the patient tolerated 12 hours of unsupported spontaneous ventilation.
Invasive Ventilation Weaning Protocol
Every 2 hours, a clinician assessed a patient for signs of distress or fatigue. In the absence of distress or fatigue, pressure support was reduced by 2 cm H2O. This cycle was repeated every 2 hours as tolerated. If at any point the patient developed signs of distress or fatigue, then reversible causes were sought and corrective treatments initiated as appropriate. If this failed to resolve the situation, the level of pressure support was increased by 2 cm H2O. Spontaneous breathing trials were repeated daily to assess extubation readiness. This cycle continued until either the patient underwent extubation after a successful spontaneous breathing trial or a tracheostomy was performed.
In both groups, the fraction of inspired oxygen was titrated to maintain arterial oxygen saturations greater than 90%. Both active weaning protocols were implemented between 8 am and 10 pm. Unless a patient developed signs of fatigue or distress, ventilator settings remained unchanged overnight.
The protocol encouraged use of a ventilator bundle (head-up position; oral decontamination; sedation hold; peptic ulcer prophylaxis) and recommended deferral of tracheostomy until after 7 days of ventilation. Guidance was provided for the criteria for reintubation, but the decision to reintubate was made by patients’ physicians. The decision to initiate antibiotic therapy and other treatments was at the discretion of patients’ physicians.
Outcome Measures
The primary outcome was time from randomization to successful liberation from ventilation, defined as the time point at which a patient was alive and free of ventilator (invasive or noninvasive) support for more than 48 hours. Secondary outcomes were duration of invasive ventilation and total ventilator days (invasive and noninvasive); proportion of patients receiving antibiotics for presumed respiratory infection; total days receiving antibiotics; rate of reintubation; mortality at 30, 90, and 180 days; time to meeting ICU discharge criteria; and rate at which patients fulfilled predefined criteria indicating the need for reintubation irrespective of whether they underwent reintubation. The predefined criteria were cardiac or respiratory arrest, respiratory pauses with loss of consciousness or gasping for air, severe psychomotor agitation inadequately controlled by sedation, persistent inability to remove respiratory secretions, heart rate of 50/min or lower or respiratory rate of 140/min or higher with loss of alertness, hemodynamic instability unresponsive to fluids and vasoactive drugs, requirement for surgery or other interventional procedure requiring deep sedation or anesthesia, proportion of patients receiving a tracheostomy, and mortality at 30, 90, and 180 days after randomization. Post hoc key process variables (weaning pathway, sedation use, length of ICU stay) are also reported. Outcomes were extracted from the ICU hospital clinical records and from questionnaires returned by patients. Because of the nature of the intervention and clinical record designs (which typically record mode of ventilation alongside respiratory variables), it was not possible for those assessing core ventilation outcomes to be blinded to treatment allocation. Adverse events were defined as development of skin or mucosal damage, vomiting, gastric distension, non–respiratory tract infection, and cardiac dysrhythmias. Health-related quality of life was assessed by the EQ-5D-5L (Euro Quality of Life 5 Dimensions Questionnaire with 5 levels of severity for each of the 5 dimensions) and the Short Form 12 at baseline (estimated retrospectively) and 90 and 180 days after randomization. All reported outcomes are postrandomization results.
Statistical Analysis
The original sample size was 920 patients, but after a formal review requested by the funder, the sample size was revised to reflect a shorter than anticipated period of weaning. A median duration of weaning of 2.9 days and a difference of 1 day provided an associated hazard ratio of 1.53 and a minimum sample size of 280 with 90% power at an α=.05 significance level. One day was defined by the investigators and patient and public representatives as the minimal clinically important difference. The sample size was inflated by 23% to account for the rate of loss to follow-up seen up to the interim review of the data. It also accounted for the shape parameter, p, which was estimated by the data to be 0.918 and which allowed for nonconstant hazards (as modeled by the Weibull distribution), resulting in a final sample size of 364 (182 patients in each group). Revision of the sample size meant that the primary outcome would be analyzed using a Cox proportional hazards model as opposed to the competing risks regression model that was prespecified in the protocol.
The primary analysis method was intention to treat. Analysis of the primary outcome, time from randomization to liberation from ventilation, and other time to event outcomes used a Cox proportional hazards regression model to estimate hazard ratios and 95% confidence intervals. In addition, we used a competing risks regression model to account for the competing risk of death. Prior to the competing risk regression analysis, the cumulative incidence of liberation and death was plotted as basic descriptive data to understand the overall pattern over time. Mixed-effects logistic regression models were used to estimate the difference in mortality at 30, 90, and 180 days between the 2 groups, for which odds ratios and 95% confidence intervals are reported. Mixed-effects linear regression models were used to estimate mean treatment differences and 95% confidence intervals for continuous outcomes including the health-related quality-of-life measures (change from baseline). Mixed-effects negative binomial models were used to estimate incidence rate ratios and 95% confidence intervals for overdispersed count data; eg, number of days on invasive ventilation with zero inflation where several participants had no days on invasive ventilation. The study was not powered to detect treatment differences in secondary outcomes; hence, secondary analyses are considered exploratory.
We performed a per-protocol analysis and 2 predefined subgroup analyses (presence or absence of COPD; postoperative/nonoperative reason for ICU admission). It was not possible to perform the third planned subgroup analysis (physician-led vs nurse-led weaning) because all sites used a multiprofessional approach involving both physicians and nurses. Multiple imputation by chained equations was used to impute missing primary outcome data, and the imputed data set was analyzed as a sensitivity analysis.
All of the analyses used mixed-effects models adjusted for age, sex, center, post–spontaneous breathing trial Paco2, presence or absence of COPD, and postoperative/nonoperative reason for ICU admission, where center was included as a random effect in the models. Modeling assumptions were assessed for all models fitted. The proportional hazards assumption was assessed for the Cox proportional hazards regression model and the competing risks model using plots of the log(−log) survival function and the Schoenfeld residuals and by assessing the influence of time-varying covariates. Linear, logistic, and negative binomial regression models were checked to ensure that the assumptions of linearity and constant variance were satisfied using residual plots. In addition to this, all the covariates included in the model were assumed to be independent of the outcome. All statistical tests were 2-sided using a P<.05 significance threshold. Statistical analyses were performed using Stata version 15.1 (StataCorp).
Results
Patients
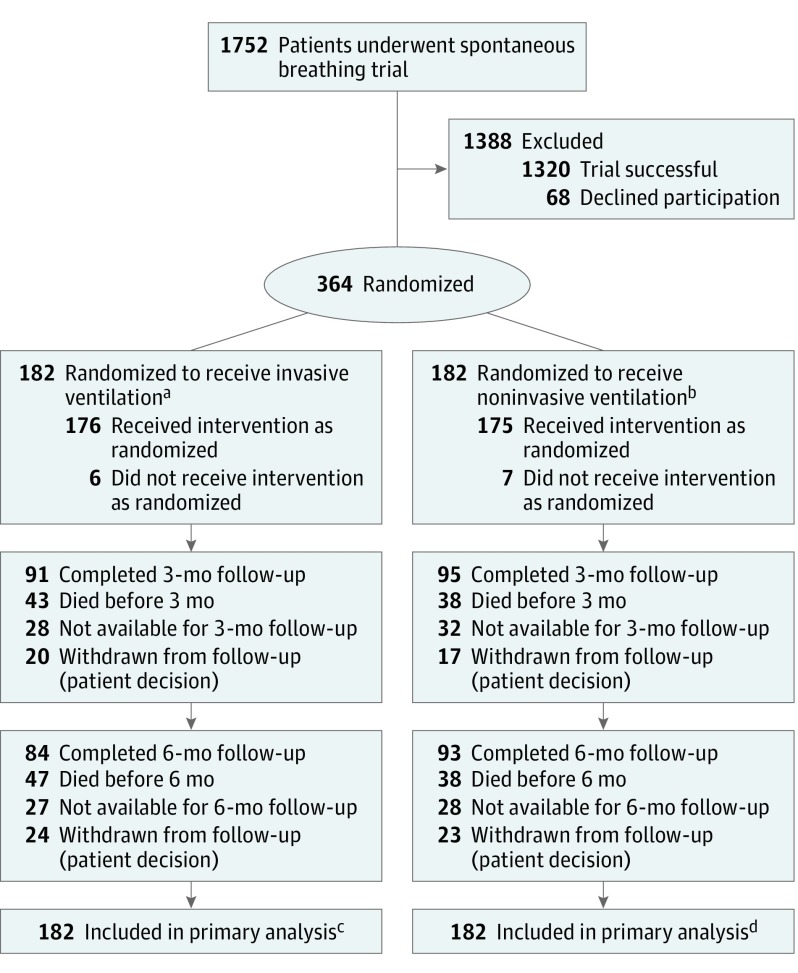
Figure 1 shows the flow of patients through the trial. Recruitment took place between March 2013 and October 2016, during which 364 patients were recruited from across 41 hospitals. There were 182 patients randomized to each group. Most patients received their randomized intervention (invasive group, 96.7% [176/182]; noninvasive group, 96.1% [175/182]).
Figure 1. Participant Flow Through a Randomized Clinical Trial of Protocolized Early Extubation to Noninvasive Weaning vs Protocolized Invasive Weaning Among Patients With Respiratory Failure.
aThirty-four patients in the invasive ventilation group died during their inpatient stay. Three were withdrawn from the study during their inpatient stay (1 refused participation after being retrospectively approached for consent; 2 withdrew for personal reasons).
bThirty-three patients in the noninvasive ventilation group died during their inpatient stay. Three were withdrawn from the study during their inpatient stay for personal reasons.
cOne hundred sixty participants achieved liberation from ventilation. Twenty-two participants were censored (19 died, 2 were withdrawn from follow-up, and 1 was discharged without achieving liberation from ventilation and lost to follow-up).
dOne hundred fifty-nine participants achieved liberation from ventilation. Twenty-three participants were censored (22 died and 1 was discharged without achieving liberation from ventilation and lost to follow-up).
Participant follow-up ended in April 2017. Overall baseline and physiological characteristics of patients were well matched (Table 1). For most patients, pneumonia (35.7%) or postsurgery respiratory failure (21.4%) was the main reason for mechanical ventilation.
Table 1. Baseline Characteristics.
| Characteristics | Invasive Weaning (n = 182) | Noninvasive Weaning (n = 182) |
|---|---|---|
| Age, mean (SD), y | 61.8 (15.8) | 64.3 (13.6) |
| Male, No. (%) | 94 (51.6) | 90 (49.5) |
| Evidence of delirium (CAM-ICU positive), No. (%)a | 17 (9.3) | 23 (12.6) |
| Body mass index, mean (SD)b | 27.7 (6.6) | 28.2 (6.9) |
| Duration of ventilation prior to randomization, median (IQR), d | 4.7 (3.0-7.4) | 5.3 (3.3-8.1) |
| Antibiotics for respiratory infection, No. (%) | 100 (55) | 98 (54) |
| APACHE II score, mean (SD)c | 18.8 (6.2) | 18.9 (6.6) |
| Admission diagnosis, No. (%) | ||
| Pneumonia/respiratory infection | 73 (40.1) | 57 (31.3) |
| Postsurgery respiratory failure | 39 (21.4) | 39 (21.4) |
| Cardiac | 18 (9.9) | 27 (14.8) |
| Nonrespiratory infection | 21 (11.5) | 16 (8.8) |
| Neuromuscular | 8 (4.4) | 7 (3.9) |
| COPD/asthma exacerbation | 7 (3.9) | 7 (3.9) |
| Traumatic injuries | 5 (2.8) | 3 (1.6) |
| Gastrointestinal bleeding | 3 (1.7) | 7 (3.9) |
| Pancreatitis | 1 (0.5) | 4 (2.2) |
| Stroke | 1 (0.5) | 0 |
| Otherd | 6 (3.2) | 15 (8.2) |
| Ventilation parameters prior to spontaneous breathing trial | ||
| Exhaled minute volume, median (IQR), L/min | 10.5 (8.2-13.1) | 10.2 (8.4-12.6) |
| Total respiratory rate, median (IQR), /min | 21 (17-27) | 21 (16-27) |
| Positive end-expiratory pressure, median (IQR), cm H2O | 5 (5-8) | 5 (5-8) |
| Pressure support, median (IQR), cm H2O | 11 (8-15) | 11 (9-15) |
| P:F ratio, median (IQR), mm Hge | 242.2 (200.6-315) | 227.5 (196.9-280.7) |
| Spontaneous tidal volume, median (IQR), mL/kg | 8.2 (6.5-9.8) | 7.9 (6.4-9.5) |
| Arterial blood gas measures prior to spontaneous breathing trial | ||
| Paco2, mean (SD), mm Hg | 42.8 (10.2) (n=181) | 42.6 (8.9) (n=180) |
| pH, mean (SD) | 7.4 (0.06) (n=182) | 7.4 (0.06) (n=181) |
| Hemoglobin, mean (SD), g/dL | 9.7 (1.7) (n=182) | 9.6 (1.6) (n=181) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
CAM-ICU is the Confusion Assessment Method for Screening for Evidence of Delirium in Intensive Care (http://www.icudelirium.org).
Calculated as weight in kilograms divided by height in meters squared.
The APACHE II score ranges from 0 to 71; higher scores correspond to more severe disease and higher risk of death. An APACHE II score of 10 to 19 is associated with a 25% risk of in-hospital mortality.
Other diagnoses included pulmonary hemorrhage (n = 1), bowel obstruction (n = 2), acute renal failure (n = 2), metabolic disturbance (n = 2), liver failure (n = 4), drug overdose (n = 2), respiratory failure of unknown cause (n = 5), vasculitis (n = 1), and burns (n = 2).
The P:F ratio is the partial pressure of oxygen in arterial blood divided by the fraction of inspired oxygen.
Outcomes
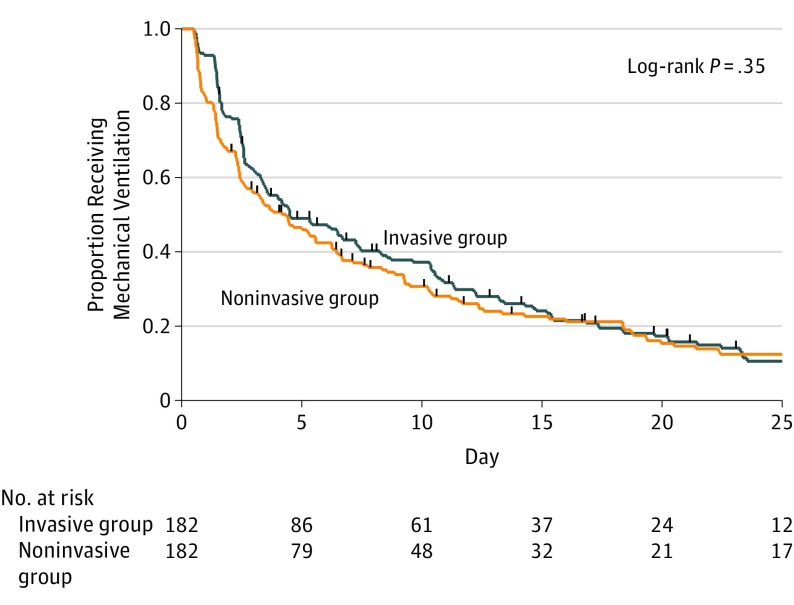
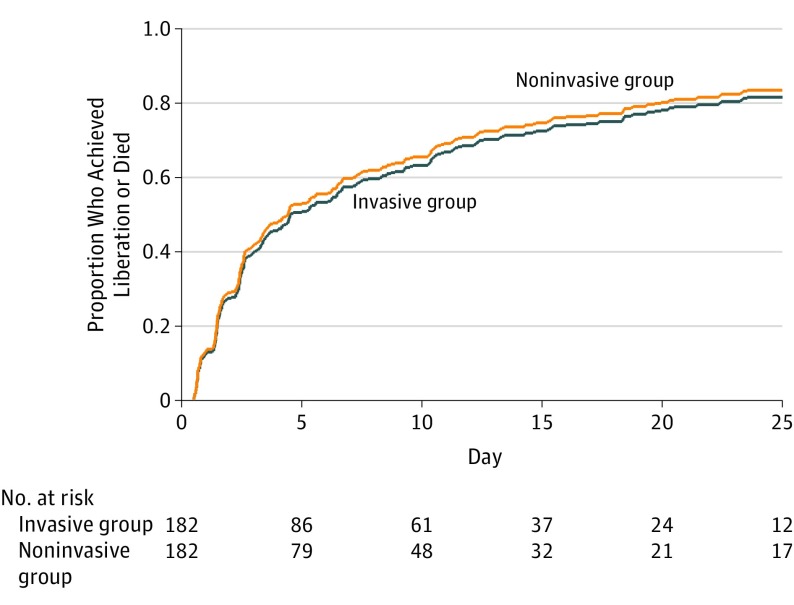
The primary outcome, time from randomization to liberation from ventilation, was a median of 4.3 days (95% CI, 2.63-5.58 days) in the noninvasive group compared with a median of 4.5 days (95% CI, 3.46-7.25 days) in the invasive group (adjusted hazard ratio, 1.1; 95% CI, 0.89-1.40) (Figure 2). The competing risks regression analysis produced a similar result (adjusted hazard ratio, 1.1; 95% CI, 0.86-1.34) (Figure 3).
Figure 2. Time to Liberation From Mechanical Ventilation by Treatment Group.
Hash marks indicate each censoring time. Median time to liberation from ventilation was 4.5 days (95% CI, 3.46-7.25 days) in the invasive group and 4.3 days (95% CI, 2.63-5.58 days) in the noninvasive group.
Figure 3. Cumulative Incidence of Liberation From Ventilation or Death by Treatment Group.
The noninvasive group required less invasive ventilation (median, 1 day vs 4 days; incidence rate ratio, 0.6; 95% CI, 0.47-0.87) and required fewer total ventilator days (median, 3 days vs 4 days; incidence rate ratio, 0.8; 95% CI, 0.62-1.0). Fewer patients in the noninvasive group received antibiotics for respiratory infection (60.4% vs 70.3%; unadjusted absolute difference, 9.9%; 95% CI, 0.17%-19.61%). The total number of days on which antibiotics were administered (respiratory and nonrespiratory) was not significantly different, with a mean of 9.1 days (SD, 12.0 days) in the noninvasive group and 10.4 days (SD, 13.2 days) in the invasive group (mean difference, 1.3 days; 95% CI, −1.31 to 3.88 days).
A higher proportion of patients underwent extubation in the noninvasive group (181/182) compared with the invasive group (143/182). Sixty-seven (37.0%) of 181 undergoing extubation in the noninvasive group underwent reintubation compared with 41 (28.7%) of 143 in the invasive group (odds ratio, 1.54; 95% CI, 0.89-2.41). For the end point of meeting the criteria for reintubation, there were 63 of 181 patients (34.8%) in the noninvasive group compared with 42 of 143 (29.4%) in the invasive group (odds ratio, 1.3; 95% CI, 0.78-2.12).
The rate of tracheostomy was 23.6% in the noninvasive group and 30.2% in the invasive group (odds ratio, 0.7; 95% CI, 0.44-1.15). Survival rates were not significantly different at 30 days (86.8% in the noninvasive group vs 86.3% in the invasive group; odds ratio, 1.1; 95% CI, 0.58-1.96) or at 180 days (78% in the noninvasive group vs 73.1% in the invasive group; odds ratio, 1.4; 95% CI, 0.85-2.27) (eTable 2 in Supplement 2). There were no significant differences in the proportions of patients who experienced adverse events and serious adverse events. Adverse events occurred in 45 patients (24.7%) in the noninvasive group compared with 47 (25.8%) in the invasive group. The distributions of adverse events and serious adverse events were similar (Table 2).
Table 2. Adverse Events.
| Adverse Events | No. (%) of Participants | Unadjusted Absolute Difference, % (95% CI) | |
|---|---|---|---|
| Invasive Weaning (n=182) | Noninvasive Weaning (n=182) | ||
| Antibiotics for presumed respiratory infection | 128 (70.3) | 110 (60.4) | 9.9 (0.2 to 19.6) |
| Reintubation | 41 (28.7) (n=143) | 67 (37.0) (n=181) | 8.3 (−1.9 to 18.6) |
| Tracheostomy | 55 (30.2) | 43 (23.6) | 6.6 (−2.5 to 15.7) |
| Death before intensive care unit discharge | 25 (13.7) | 22 (12.1) | 1.6 (−5.2 to 8.5) |
| Dysrhythmias | 22 (12.1) | 14 (7.7) | 4.4 (−1.7 to 10.5) |
| Nasal/skin/mouth sores or irritation | 14 (7.7) | 19 (10.4) | 2.7 (−3.2 to 8.6) |
| Nonrespiratory infection | 12 (6.6) | 11 (6.0) | 0.5 (−4.5 to 5.6) |
| Vomiting | 8 (4.4) | 14 (7.7) | 3.3 (−1.6 to 8.2) |
| Gastric distension | 6 (3.3) | 7 (3.9) | 0.5 (−3.3 to 4.4) |
| Barotrauma (eg, pneumothorax) | 3 (1.7) | 3 (1.7) | 0 (−2.6 to 2.6) |
Post hoc key process measures showed that patients in the noninvasive group underwent extubation earlier than those in the invasive group (median, 0.5 day [interquartile range {IQR}, 0.5-1 day]) vs 3 days [IQR, 2-10 days]; adjusted hazard ratio, 2.5; 95% CI, 2.01-3.15; P < .001). Among those requiring reintubation, the noninvasive group underwent reintubation at a median of 2 days (IQR, 0.9-3.0 days) after randomization compared with 3.2 days (IQR, 2.3-4.7 days) in the invasive ventilation group (P < .001). The noninvasive group received sedation for fewer days (mean, 4.1 [SD, 5.0] days vs 5.5 [SD, 5.1] days; incidence rate ratio, 0.7; 95% CI, 0.61-0.91) and spent less time in critical care (mean, 10.8 [SD, 8.8] days vs 12.2 [SD, 8.4] days; P = .02). The median time from randomization to tracheostomy was 5.8 days (IQR, 3.71-8.46 days) in the invasive group and 5.6 days (IQR, 3.43-8.46 days) in the noninvasive group. There was no significant difference between the 2 groups (nonparametric P = .65).
Although health-related quality of life was impaired (eTable 3 in Supplement 2), there was no significant difference between the 2 groups at 3 months or at 6 months.
The per-protocol analysis produced results similar to the primary analysis (hazard ratio, 1.1; 95% CI, 0.90-1.44). The explored subgroups showed no significant difference in treatment effect (eTable 4 in Supplement 2). The sensitivity analysis using multiple imputation for the 45 participants with missing (censored) primary outcome data found no difference between the 2 groups (hazard ratio, 1.1; 95% CI, 0.90-1.36). A further sensitivity analysis found no significant difference in outcome between the 3 highest recruiting centers (who recruited 161 patients [44%]) and the other participating centers. There were no major departures from the modeling assumptions for all of the regression models fitted.
Discussion
In this multicenter randomized trial, early extubation to noninvasive ventilation compared with protocolized invasive weaning with sequential pressure support reduction prior to extubation did not reduce the time to liberation from all forms of ventilation. Consistent with the protocol design, patients in the noninvasive ventilation group underwent extubation earlier and spent less time receiving invasive ventilation. Mortality rates, the requirement for reintubation or tracheostomy, and adverse event rates were not significantly different.
Spontaneous breathing trials are used to identify patients who are ready for extubation.16 The 59% to 86% of invasive ventilation patients in whom a spontaneous breathing trial fails are classified as difficult to wean.1,4,17,18,19 These patients contribute to a disproportionate amount of ICU resource utilization to achieve successful liberation.11 Noninvasive ventilation has been suggested to be a useful tool to facilitate weaning, but most previous studies recruited predominantly patients with COPD.20,21,22,23,24 In that patient group, noninvasive weaning reduced mortality, duration of invasive ventilation, reintubation, and ICU length of stay.14 The patients enrolled in the present study better reflect contemporary ICU practice, as fewer patients with COPD now undergo invasive ventilation.25,26
The rate of reintubation in this study was expected to be higher than among patients with simple weaning needs, in whom reintubation rates of 10% to 20% are reported.27 The 30% overall rate of reintubation is consistent with findings in previous studies that recruited patients with difficulty weaning.21,22,24 Because more patients underwent extubation in the noninvasive group, more were at risk of reintubation. One of the major concerns about reintubation is the association with increased mortality seen in some observational studies.28,29 The survival rates in the present study were not significantly different in noninvasive and invasive weaning groups, although these findings should be interpreted with caution because the study was not powered to show a difference in this outcome and was not designed to assess equivalence.
The design of this study afforded several advantages to previous studies. First, a protocolized weaning regimen in both groups allowed clear separation of the intervention from the effect of protocolization.30 Best practice guidelines (ventilation bundle, daily spontaneous breathing trials, tracheostomy insertion) reduced heterogeneity between treatment groups. Second, antibiotic use was selected as a surrogate for ventilator-associated pneumonia to limit the risk of detection bias arising from different approaches to obtaining respiratory samples for culture; this outcome is arguably more relevant than ventilator-associated pneumonia diagnosis as it better reflects antibiotic stewardship and exposure.
Limitations
The study has several limitations. First, the nature of the intervention prevented blinding of clinicians, patients, or outcome assessors. This may have led to performance and/or detection bias. Second, the noninvasive weaning protocol mandated sequential reductions in respiratory support (either a decrease in inspiratory pressure support or a break from noninvasive ventilation) as tolerated over a minimum of a 12-hour period. It is possible that this may have extended the period of ventilatory support for some patients. Third, in the invasive ventilation group, the protocol required once-daily spontaneous breathing trials. It is possible that more frequent spontaneous breathing trials may have led to earlier recognition of readiness for extubation in some patients. Fourth, the patients enrolled were a heterogeneous group of patients with differing relative contributions of respiratory, cardiac, neuromuscular, metabolic, pharmacological, and neuropsychological impairment. Whether a more physiologically based assessment process could identify a group more likely to benefit from noninvasive ventilation remains to be determined in future studies. Fifth, 44% of the patients were recruited from 3 centers, which could limit generalizability. It is possible that performance and outcomes may have improved as centers became more experienced in the use of the noninvasive weaning intervention.
Conclusions
Among patients requiring mechanical ventilation in whom a spontaneous breathing trial had failed, early extubation to noninvasive ventilation did not shorten time to liberation from any ventilation.
Trial Protocol
eAppendix. Supplemental Methods
eTable 1. Type and Outcomes From Spontaneous Breathing Trial
eTable 2. Survival Status
eTable 3. Subgroup Analyses
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Jeong BH, Ko MG, Nam J, et al. . Differences in clinical outcomes according to weaning classifications in medical intensive care units. PLoS One. 2015;10(4):e0122810. doi: 10.1371/journal.pone.0122810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Béduneau G, Pham T, Schortgen F, et al. ; Weaning According to a New Definition Study Group; Réseau Européen de Recherche en Ventilation Artificielle Network . Epidemiology of Weaning Outcome According to a New Definition: the WIND Study. Am J Respir Crit Care Med. 2017;195(6):772-783. doi: 10.1164/rccm.201602-0320OC [DOI] [PubMed] [Google Scholar]
- 3.Girard TD, Alhazzani W, Kress JP, et al. ; ATS/CHEST Ad Hoc Committee on Liberation From Mechanical Ventilation in Adults . An official American Thoracic Society/American College of Chest Physicians Clinical Practice guideline: liberation from mechanical ventilation in critically ill adults: rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am J Respir Crit Care Med. 2017;195(1):120-133. doi: 10.1164/rccm.201610-2075ST [DOI] [PubMed] [Google Scholar]
- 4.Peñuelas O, Frutos-Vivar F, Fernández C, et al. ; Ventila Group . Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am J Respir Crit Care Med. 2011;184(4):430-437. doi: 10.1164/rccm.201011-1887OC [DOI] [PubMed] [Google Scholar]
- 5.Esteban A, Frutos-Vivar F, Muriel A, et al. . Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188(2):220-230. doi: 10.1164/rccm.201212-2169OC [DOI] [PubMed] [Google Scholar]
- 6.Rose L, Dainty KN, Jordan J, Blackwood B. Weaning from mechanical ventilation: a scoping review of qualitative studies. Am J Crit Care. 2014;23(5):e54-e70. doi: 10.4037/ajcc2014539 [DOI] [PubMed] [Google Scholar]
- 7.Inglis TJ, Millar MR, Jones JG, Robinson DA. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol. 1989;27(9):2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook DJ, Walter SD, Cook RJ, et al. . Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129(6):433-440. doi: 10.7326/0003-4819-129-6-199809150-00002 [DOI] [PubMed] [Google Scholar]
- 9.Schaller SJ, Anstey M, Blobner M, et al. ; International Early SOMS-Guided Mobilization Research Initiative . Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388(10052):1377-1388. doi: 10.1016/S0140-6736(16)31637-3 [DOI] [PubMed] [Google Scholar]
- 10.Costa DK, White MR, Ginier E, et al. . Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients: a systematic review. Chest. 2017;152(2):304-311. doi: 10.1016/j.chest.2017.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner DP. Economics of prolonged mechanical ventilation. Am Rev Respir Dis. 1989;140(2 Pt 2):S14-S18. doi: 10.1164/ajrccm/140.2_Pt_2.S14 [DOI] [PubMed] [Google Scholar]
- 12.Keenan SP, Sinuff T, Burns KE, et al. ; Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group . Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183(3):E195-E214. doi: 10.1503/cmaj.100071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns KE, Meade MO, Premji A, Adhikari NK. Noninvasive positive-pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2013;(12):CD004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh TS, Dodds S, McArdle F. Evaluation of simple criteria to predict successful weaning from mechanical ventilation in intensive care patients. Br J Anaesth. 2004;92(6):793-799. doi: 10.1093/bja/aeh139 [DOI] [PubMed] [Google Scholar]
- 16.Boles J-M, Bion J, Connors A, et al. . Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033-1056. doi: 10.1183/09031936.00010206 [DOI] [PubMed] [Google Scholar]
- 17.Esteban A, Alía I, Tobin MJ, et al. ; Spanish Lung Failure Collaborative Group . Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med. 1999;159(2):512-518. doi: 10.1164/ajrccm.159.2.9803106 [DOI] [PubMed] [Google Scholar]
- 18.Esteban A, Alía I, Gordo F, et al. ; Spanish Lung Failure Collaborative Group . Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. Am J Respir Crit Care Med. 1997;156(2 pt 1):459-465. doi: 10.1164/ajrccm.156.2.9610109 [DOI] [PubMed] [Google Scholar]
- 19.Funk GC, Anders S, Breyer MK, et al. . Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur Respir J. 2010;35(1):88-94. doi: 10.1183/09031936.00056909 [DOI] [PubMed] [Google Scholar]
- 20.Ferrer M, Esquinas A, Arancibia F, et al. . Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med. 2003;168(1):70-76. doi: 10.1164/rccm.200209-1074OC [DOI] [PubMed] [Google Scholar]
- 21.Girault C, Bubenheim M, Abroug F, et al. ; VENISE Trial Group . Noninvasive ventilation and weaning in patients with chronic hypercapnic respiratory failure: a randomized multicenter trial. Am J Respir Crit Care Med. 2011;184(6):672-679. doi: 10.1164/rccm.201101-0035OC [DOI] [PubMed] [Google Scholar]
- 22.Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med. 1999;160(1):86-92. doi: 10.1164/ajrccm.160.1.9802120 [DOI] [PubMed] [Google Scholar]
- 23.Prasad SB, Chaudhry D, Khanna R. Role of noninvasive ventilation in weaning from mechanical ventilation in patients of chronic obstructive pulmonary disease: an Indian experience. Indian J Crit Care Med. 2009;13(4):207-212. doi: 10.4103/0972-5229.60173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou SH, Zhou R, Chen P, et al. . Application of sequential noninvasive following invasive mechanical ventilation in COPD patients with severe respiratory failure by investigating the appearance of pulmonary-infection-control-window [in Chinese]. Zhong Nan Da Xue Bao Yi Xue Ban. 2006;31(1):120-124. [PubMed] [Google Scholar]
- 25.Davidson AC, Banham S, Elliott M, et al. ; British Thoracic Society/Intensive Care Society Acute Hypercapnic Respiratory Failure Guideline Development Group; British Thoracic Society Standards of Care Committee . BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(suppl 2):ii1-ii35. doi: 10.1136/thoraxjnl-2015-208209 [DOI] [PubMed] [Google Scholar]
- 26.Chandra D, Stamm JA, Taylor B, et al. . Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152-159. doi: 10.1164/rccm.201106-1094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thille AW, Cortés-Puch I, Esteban A. Weaning from the ventilator and extubation in ICU. Curr Opin Crit Care. 2013;19(1):57-64. doi: 10.1097/MCC.0b013e32835c5095 [DOI] [PubMed] [Google Scholar]
- 28.Rothaar RC, Epstein SK. Extubation failure: magnitude of the problem, impact on outcomes, and prevention. Curr Opin Crit Care. 2003;9(1):59-66. doi: 10.1097/00075198-200302000-00011 [DOI] [PubMed] [Google Scholar]
- 29.Gao F, Yang LH, He HR, et al. . The effect of reintubation on ventilator-associated pneumonia and mortality among mechanically ventilated patients with intubation: a systematic review and meta-analysis. Heart Lung. 2016;45(4):363-371. doi: 10.1016/j.hrtlng.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 30.Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev. 2014;(11):CD006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Supplemental Methods
eTable 1. Type and Outcomes From Spontaneous Breathing Trial
eTable 2. Survival Status
eTable 3. Subgroup Analyses
Data Sharing Statement