Key Points
Question
Can a consumer-targeted, pharmacist-led educational intervention reduce prescriptions for inappropriate medication among community-dwelling older adults?
Findings
In this cluster randomized trial that included 489 older adults, the percentage achieving discontinuation of a targeted inappropriate prescription at 6 months was 43% among patients receiving the intervention vs 12% receiving usual care, which represents a significant difference.
Meaning
A pharmacist-led intervention has the potential to reduce prescriptions for inappropriate medication in older adults.
Abstract
Importance
High rates of inappropriate prescribing persist among older adults in many outpatient settings, increasing the risk of adverse drug events and drug-related hospitalizations.
Objective
To compare the effectiveness of a consumer-targeted, pharmacist-led educational intervention vs usual care on discontinuation of inappropriate medication among community-dwelling older adults.
Design, Setting, and Participants
A cluster randomized trial (D-PRESCRIBE [Developing Pharmacist-Led Research to Educate and Sensitize Community Residents to the Inappropriate Prescriptions Burden in the Elderly]) that recruited community pharmacies in Quebec, Canada, from February 2014 to September 2017, with follow-up until February 2018, and randomly allocated them to intervention or control groups. Patients included were adults aged 65 years and older who were prescribed 1 of 4 Beers Criteria medications (sedative-hypnotics, first-generation antihistamines, glyburide, or nonsteroidal anti-inflammatory drugs), recruited from 69 community pharmacies. Patients were screened and enrolled before randomization.
Interventions
Pharmacists in the intervention group were encouraged to send patients an educational deprescribing brochure in parallel to sending their physicians an evidence-based pharmaceutical opinion to recommend deprescribing. The pharmacists in the control group provided usual care. Randomization occurred at the pharmacy level, with 34 pharmacies randomized to the intervention group (248 patients) and 35 to the control group (241 patients). Patients, physicians, pharmacists, and evaluators were blinded to outcome assessment.
Main Outcomes and Measures
Discontinuation of prescriptions for inappropriate medication at 6 months, ascertained by pharmacy medication renewal profiles.
Results
Among 489 patients (mean age, 75 years; 66% women), 437 (89%) completed the trial (219 [88%] in the intervention group vs 218 [91%] in the control group). At 6 months, 106 of 248 patients (43%) in the intervention group no longer filled prescriptions for inappropriate medication compared with 29 of 241 (12%) in the control group (risk difference, 31% [95% CI, 23% to 38%]). In the intervention vs control group, discontinuation of inappropriate medication occurred among 63 of 146 sedative-hypnotic drug users (43.2%) vs 14 of 155 (9.0%), respectively (risk difference, 34% [95% CI, 25% to 43%]); 19 of 62 glyburide users (30.6%) vs 8 of 58 (13.8%), respectively (risk difference, 17% [95% CI, 2% to 31%]); and 19 of 33 nonsteroidal anti-inflammatory drug users (57.6%) vs 5 of 23 (21.7%), respectively (risk difference, 35% [95% CI, 10% to 55%]) (P for interaction = .09). Analysis of the antihistamine drug class was not possible because of the small sample size (n = 12). No adverse events requiring hospitalization were reported, although 29 of 77 patients (38%) who attempted to taper sedative-hypnotics reported withdrawal symptoms.
Conclusions and Relevance
Among older adults in Quebec, a pharmacist-led educational intervention compared with usual care resulted in greater discontinuation of prescriptions for inappropriate medication after 6 months. The generalizability of these findings to other settings requires further research.
Trial Registration
ClinicalTrials.gov Identifier: NCT02053194
This cluster randomized trial investigates the effects on medication discontinuation of a program in which pharmacists educated older patients taking benzodiazepines, sedative-hypnotics, first-generation antihistamines, and selective NSAIDs about why the drugs are inappropriate for their age groups and offered potential alternative treatment options.
Introduction
Inappropriate prescriptions continue to be frequently dispensed to older adults. It has been estimated that 29.0% of Medicare beneficiaries aged 65 years and older in the United States in 20151 and 31.1% of older adults in Canada in 20162 filled a prescription for at least 1 medication included in the 2015 American Geriatrics Society Beers Criteria list of drugs to avoid in older adults.3 Labeled as inappropriate because of the risk of harm and availability of safer treatments, inappropriate prescriptions can lead to adverse drug events, falls, cognitive impairment, and emergency hospitalizations.3,4
Deprescribing is the act of reducing or stopping medication that is no longer necessary or that may cause harm.5 Primary care physicians express a lack of time, poor awareness of the harms of medications, and fear of withdrawal symptoms or patient criticism as barriers to deprescribing.6,7 Pharmacists can assist physicians in optimizing medication management in older adults.8,9 Medication reviews by a pharmacist followed by direct communication to the prescribing physician have been shown to result in safer prescribing practices.10,11,12 Patients can also initiate the deprescribing process. In a randomized clinical trial of 303 long-term users of benzodiazepine medications, providing education about the risks of benzodiazepine use compared with providing usual care resulted in an additional 23% of patients discontinuing their medication within 6 months.13 Patients who elected not to taper their medication cited physician or pharmacist discouragement as the major impediment.13,14 Streamlining communication and deprescribing efforts among patients, physicians, and pharmacists may augment shared accountability for safer prescribing while maintaining patient trust.15
The objective of the D-PRESCRIBE (Developing Pharmacist-led Research to Educate and Sensitize Community Residents to the Inappropriate Prescriptions Burden in the Elderly) cluster randomized trial was to determine the effectiveness of a pharmacist-led intervention to educate older adults and their physicians about reducing inappropriate prescriptions.
Methods
A 2-group, parallel, pragmatic, cluster-randomized clinical trial was conducted in Quebec, Canada. The trial protocol is available and has been published (Supplement 1).16 Approval for the study was obtained on September 17, 2013, from the research ethics board of the Institut universitaire de gériatrie de Montréal in Quebec. Patients and pharmacists provided signed informed consent to participate in the trial.
Pharmacy and Participant Enrollment
Pharmacies (the cluster units) were recruited through a partnership with 3 different pharmacy chains within a 100-km radius of the research center in Montreal, Quebec, Canada. Cluster randomization was used to prevent contamination between patients using the same pharmacy.
Pharmacists received a message from the director of each pharmacy chain encouraging collaboration with the research team and were contacted by a research assistant in a systematic fashion. Eligible pharmacies had a clientele that consisted of at least 20% older adults (ie, adults aged ≥65 years). Consenting pharmacies received a list of eligible patients identified via a computer algorithm applied to the central pharmacy’s drug claims administrative data. The list was randomized and separated by medication class. Using this list, pharmacists sequentially contacted each patient by phone until a minimum of 3 patients from each medication class agreed to be contacted by a research assistant for an in-home interview, or until all patients on the list were contacted. Data were collected on pharmacy characteristics, including the mean number of prescriptions dispensed per day and the percentage of older adult patients.
Four types of Beers Criteria medications were selected to be targeted for the trial: all benzodiazepines and the sedative-hypnotic “Z-drugs” zopiclone and zolpidem; first-generation antihistamines; glyburide; and selective nonsteroidal anti-inflammatory drugs (NSAIDs) (Supplement 1 lists all included medications and the rationale for inclusion).16 Eligible patients were adults aged 65 years and older who had filled a prescription for 1 of the targeted medications for at least 3 consecutive months before screening. Patients with prescriptions for more than 1 targeted medication received only 1 intervention for the duration of the trial, based on the first drug that appeared on the list provided to each pharmacist. Exclusion criteria for patients were a diagnosis of severe mental illness or dementia (determined via antipsychotic and/or cholinesterase inhibitor or memantine prescriptions), significant cognitive impairment (score <24 on the Mini-Mental State Examination17), inability to communicate in English or French, and residence in an assisted-living facility.
Patients who expressed interest in participation were scheduled for an in-home interview with a research assistant to provide consent and have baseline data collected on demographics; drug duration and indication; self-reported health status, using the first item of the Short-Form 12-item (version 2) Health Survey (SF-12v2)18; and frailty, assessed by the 13-item Vulnerable Elders Survey (VES-13).19 A score of at least 3 on the VES-13 predicts a 4.2-fold increase in mortality or functional decline in a 2-year period.19 Recruitment occurred between February 2014 and September 2017 (latest date of follow-up in February 2018). Pharmacists and patients received no financial compensation from the research team for participating in the trial.
Randomization and Allocation Concealment
A 1:1 allocation ratio was assigned by an independent statistician and random number generator using nonstratified block sizes of 2 for participating pharmacies. Blinding pertained to both the individual and cluster levels. The trial was labeled as a “medication safety study for older adults.” Because the intervention was educational in nature, masking was not possible; however, pharmacists and patients were blinded to group allocation by being told that the intervention would be delivered sometime during the next year. Pharmacists were blinded to other participating pharmacies.
Intervention
Pharmacies in the intervention group were provided with patient educational brochures and the prototype pharmaceutical opinion by research assistants during visits to the pharmacies, and encouraged to distribute educational materials to both patients and their prescribers. Educational material for patients consisted of a drug-specific brochure, distributed by pharmacists in person or by mail, containing information about why the medication may be inappropriate, potential alternative treatment options, and, for patients prescribed sedative-hypnotics, a visual tapering protocol20 (available at https://www.deprescribingnetwork.ca/patient-handouts). Educational material for physicians was packaged in the form of an evidence-based pharmaceutical opinion that pharmacists could use or adapt during communication with each participant’s physician21 (available at https://www.deprescribingnetwork.ca/pharmaceutical-opinions). In Quebec, the pharmaceutical opinion is a legal and reimbursable pharmacist-initiated act aimed at facilitating pharmacist-physician communication.22 A standardized evidence-based pharmaceutical opinion was designed with elements requested by physicians and pharmacists during testing, including a clear rationale for why deprescribing was being recommended; evidence about drug harms; credible sources of recommendations; a choice of safer therapeutic alternatives; and personalized participant data.21 The intervention materials were distributed to each participating pharmacist in the intervention group immediately after randomization. Pharmacists in the control group provided usual care for 6 months. Usual care consisted of patients receiving normal care delivered in everyday practice without any educational materials supplied to pharmacists.
Outcomes
The primary outcome was complete cessation of prescription fills for any of the 4 medication classes 6 months after randomization, measured at the level of the participant. Discontinuation was disaggregated by drug class, defined as the absence of any prescription renewal at 6 months, sustained for at least 3 consecutive months, and lack of substitution to another inappropriate drug class. Prescription renewals were determined using pharmaceutical drug claims records. Two investigators, blinded to group allocation, independently assessed outcomes.
Secondary outcomes included assessment of the delivery of the educational brochures to the patients by their pharmacists; the prevalence, timing, and type of pharmaceutical opinions sent by the pharmacists to the patients’ primary care physicians; the effect of the educational material on participants’ beliefs about the use of their inappropriate medications and their intent to discuss cessation with their physician or pharmacist; the effect of the pharmaceutical opinion on the prescribing physician’s behavior; participant-physician encounters to discuss inappropriate prescriptions; and participant improvement in self-efficacy to discontinue medication. These data were collected from patients and pharmacists 6 months after randomization during in-person interviews. The effect of the intervention on participants’ beliefs about the use of inappropriate medications and self-efficacy to discontinue medication are not reported here. Data on the timing of the distribution of the pharmaceutical opinion were not available. Because tapering was recommended for patients receiving the sedative-hypnotic drug class only, sedative-hypnotic users were asked whether a tapering conversation occurred, if tapering was attempted, and if there were barriers to deprescribing.
Sample Size
Sample size was based on the hypothesis that the intervention would achieve a rate of discontinuation at least as great as that achieved by the effect of an educational brochure alone distributed to patients compared with usual care in a previous study (27% vs 5%, respectively).13 The current study was powered to detect a minimal 20% difference in prescription renewals over usual care with 80% power, an α of .05, and an intracluster correlation of 0.05.23,24 Seventeen pharmacies per group were required to meet sample size calculations (51 patients per group per drug class, 3 patients recruited per pharmacy per drug class). To compare the added benefit of the pharmaceutical opinion with the participant educational material alone, as tested in a previous trial, an additional 3 patients from each pharmacy were recruited to the sedative-hypnotic group to detect a minimal 12.5% absolute difference.13
Statistical Methods
Baseline characteristics of the patients in the intervention and control groups were compared using absolute difference and 95% CIs. To assess the primary outcome, risk differences were estimated with 95% CIs via generalized estimating equations (GEEs) with an identity link and an exchangeable correlation structure.25 The participant served as the unit of analysis, the pharmacy as the cluster, and discontinuation as a dichotomous outcome assessed for each participant 6 months after randomization. Empirical standard errors were used. Intention-to-treat analyses were performed. Per-protocol analyses were also conducted (eTable in Supplement 2). The number needed to treat was calculated as the inverse of the difference in absolute event rates between the intervention and control groups.26 Subgroup and interaction analyses for each specific medication class were conducted using the same methods. Analyses were performed to estimate risk differences for different demographic variables using interaction terms in the GEE model for age (<80 years vs ≥80 years), sex (male vs female), education (high school or less vs college/university or more), health status (SF-12v2 score of fair and poor vs good to excellent), frailty status (VES-13 score <3 vs ≥3), previous attempt at tapering (yes vs no), duration of prescription (< 5 years vs ≥5 years), and number of medications (<10/d vs ≥10/d).
Missing outcome data were handled using multiple imputation by predictive mean matching for age, sex, education, health status, frailty status, previous attempt to taper, duration of medication use, and number of medications. Tests of significance were conducted using a 2-sided threshold of .05. Given the potential for type I error and the lack of adjustments for multiple comparisons, secondary outcomes should be interpreted as exploratory. Statistical analyses were run using SPSS version 25 and R version 3.4.4 (R Foundation) for GEE and multiple imputation by chained equations.
Results
Study Participants and Follow-up
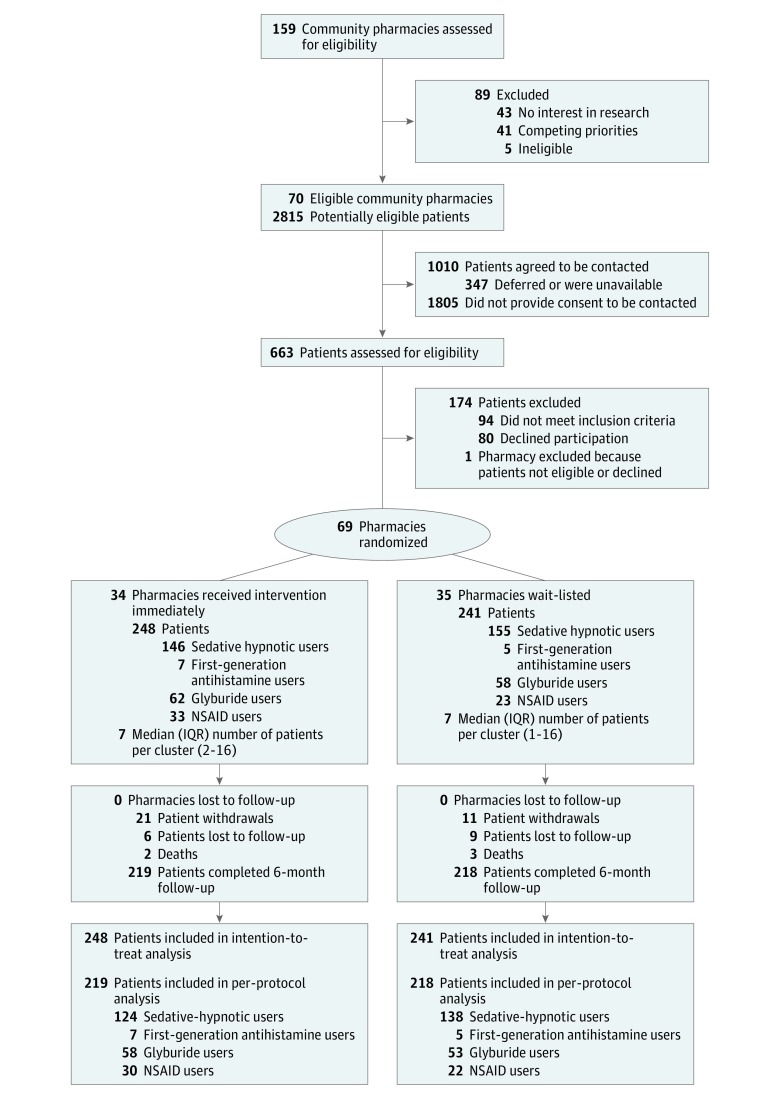
Of the 159 community pharmacies that were approached to participate, 70 (44%) consented over a 3-year period. Among the clientele of these pharmacies, 2815 potentially eligible patients were identified; 1010 provided consent to be contacted by the research team and 663 accepted an in-person interview. In total, 489 eligible patients from 69 clusters were included. Figure 1 depicts the study flow of the clusters and the patients for the trial as well as reasons for withdrawal. Outcome data on drug renewal at 6 months were unavailable when patients died, moved, or changed to a different pharmacy that was not enrolled in the trial (n = 15). Two deaths occurred in the intervention group, and, in the control group, 3 patients died and 10 moved away. The median (interquartile range [IQR]) number of patients per cluster was 7 (1-16).
Figure 1. Flow of Pharmacy and Patient Enrollment Through the Study of Educational Interventions for Discontinuation of Inappropriate Prescriptions in Older Adults.
IQR indicates interquartile range; NSAID indicates nonsteroidal anti-inflammatory drug.
Among 489 patients who were randomized (mean [range] age, 75 [66-96]; 322 [66%] women, 113 [23%] aged ≥80 years, and 132 [27%] met criteria for frailty), 437 (89%) completed the trial (219 [88%] in the intervention group vs 218 [91%] in the control group) (Table 1). The mean number of prescriptions dispensed per day per pharmacy and the percentage of older adults among each pharmacy’s patients did not differ between the intervention and control group (Table 1).
Table 1. Baseline Characteristics of Patients and Pharmacies Included in a Study to Deprescribe Inappropriate Prescriptions in Older Adults .
| Variables | No. (%) | Absolute Difference (95% CI) |
|
|---|---|---|---|
| Intervention Group (n = 248) |
Control Group (n = 241) |
||
| Patients | |||
| Female | 153 (61.7) | 169 (70.1) | 8.4% (−0.1% to 16.7%) |
| Male | 95 (38.3) | 72 (29.9) | |
| Age, mean (SD) [range], y | 74.6 (6.6) [66-96] | 74.8 (6.3) [66-95] | 0.2 (−1.0 to 1.3) |
| College/university education or more | 114 (46.0) | 105 (43.6) | 2.4% (−6.3% to 11.1%) |
| Self-reported healtha | |||
| Excellent | 19 (7.7) | 30 (12.4) | 4.8 (−0.1 to 10.3) |
| Very Good | 88 (35.5) | 68 (28.2) | 7.3 (−0.1 to 15.4) |
| Good | 101 (40.7) | 100 (41.5) | 0.8 (−7.9 to 9.4) |
| Fair | 37 (14.9) | 35 (14.5) | 0.4 (−6.0 to 6.7) |
| Poor | 3 (1.2) | 8 (3.3) | 2.1 (−0.7 to 5.3) |
| Mini-Mental State Examination score,b mean (SD) [range] | 28.9 (1.2) [25-30] | 28.9 (1.3) [25-30] | 0.07 (−0.29 to 0.17) |
| Frailc | 66 (26.6) | 65 (27.0) | 0.4% (−7.5% to 8.2%) |
| Previously attempted cessation of targeted medication | 76 (30.7) | 91 (37.8) | 7.1% (−1.3% to 15.4%) |
| Inappropriate medication class | |||
| Sedative-hypnotics | 146 (58.9) | 155 (64.3) | 5.4% (−3.2% to 13.9%) |
| First-generation antihistamines | 7 (2.8) | 5 (2.1) | 0.7% (−2.3 to 3.9%) |
| Glyburide | 62 (25.0) | 58 (24.1) | 0.9 % (−6.7% to 8.5%) |
| NSAIDS | 33 (13.3) | 23 (9.5) | 3.8% (−1.9% to 9.5%) |
| Duration of drug use, mean (SD) [range], y | |||
| Sedative-hypnotics | 9.3 (8.4) [0.3-41] | 11.8 (10.3) [0.3-50] | 2.5 (0.3 to 4.7) |
| Median (IQR), y | 6.0 (3.0-13.0) | 10.0 (4.0-15.0) | |
| ≥5 y | 91 (62.3) | 111 (71.6) | 9.3 (−1.3 to 19.7) |
| First-generation antihistamines | 4.3 (4.5) [1-10] | 13.3 (14.0) [5-25] | 9 (2.0 to 16.1) |
| Median (IQR), y | 4.5 (1.0-5.0) | 14.0 (6.5-20.0) | |
| ≥5 y | 3 (42.9) | 5 (100) | 57.1 (3.1 to 84.2) |
| Glyburide | 9.5 (9.9) [0.3-40] | 9.9 (9.9) [1-25] | 0.4 (−2.2 to 3.0) |
| Median (IQR), y | 10.0 (4.0-14.0) | 10.0 (5.0-15.0) | |
| ≥5 y | 43 (69.3) | 38 (65.6) | 3.8 (−12.6 to 20.2) |
| NSAIDs | 9.1 (6.0) [0.4-40] | 5.6 (3.0) [0.3-30]) | 3.6 (−1.9 to 9.0) |
| Median (IQR), y | 6.0 (2.0-10.0) | 3.0 (1.5-8.5) | |
| ≥5 y | 19 (57.6) | 6 (26.1) | 31.5 (5.1 to 51.8) |
| Number of different medications/d | |||
| Mean (SD) [range] | 8.7 (3.8) [1-28] | 8.6 (4.0) [1-27] | 0.1 (−0.6 to .8) |
| Median (IQR), d | 8.5 (6.0-11.0) | 8.0 (6.0-11.0) | |
| Patients taking ≥10 different medications/d | 98 (39.5) | 91 (37.7) | 1.8 (−6.8 to 10.3) |
| Pharmacies | |||
| Prescriptions/d, mean (SD) [range] | 411.4 (186.2) [140-1000] |
350.1 (183.6) [60-900] |
61.3 (−27.6 to 150.2) |
| Percentage of adults aged ≥65 years served, mean (SD) [range] | 52.9 (17.3) [20-85] | 46.1 (18.2) [20-85] | 6.8 (−1.7 to 15.3) |
| Patients per pharmacy enrolled, mean (SD) [range] | 7.3 (3.3) [2-16] | 6.9 (3.7) [1-16] | 0.4 (−0.2 to 1.0) |
Abbreviations: IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug.
Health status was determined using the first item of the Short Form 12-item Health Survey.18
The Mini-Mental State Examination was used to assess cognitive function. Scores of 24-30 indicate normal cognition, while lower scores indicate severe (≤9), moderate (10-18), or mild (19-23 points) cognitive impairment.17
Frailty was assessed using the Vulnerable Elders Survey, a 13-item instrument. Patients who scored ≥3 were deemed frail.19
Outcomes
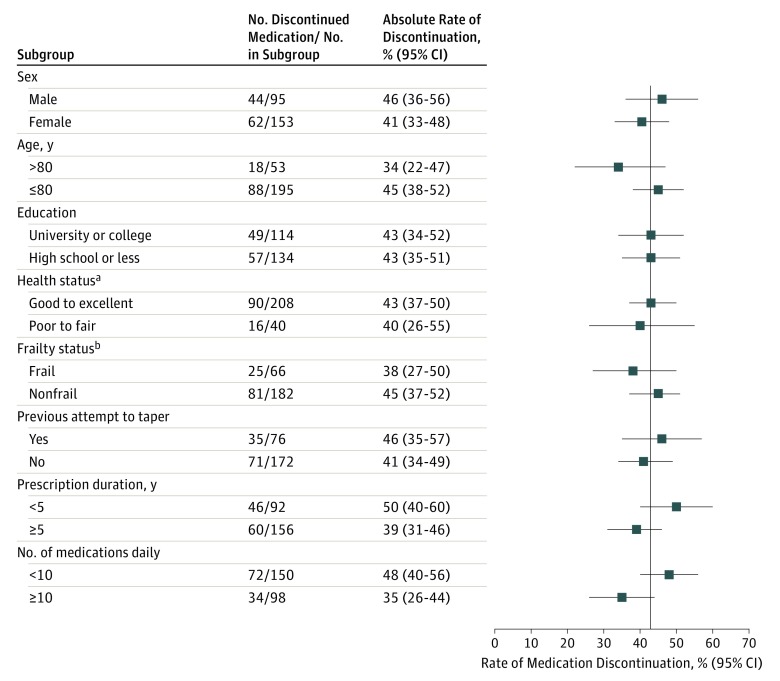
Complete cessation of prescription fills occurred among 106 of 248 patients (42.7%) in the intervention group compared with 29 of 241 (12.0%) in the control group (mean difference, 31% [95% CI, 23%-38%], number needed to treat = 3). The relative risk of discontinuation among patients who received the intervention was 3.55 (95% CI, 2.45-5.15). In the intervention vs control group, discontinuation of inappropriate medication occurred among 63 of 146 sedative-hypnotic drug users (43.2%) vs 14 of 155 (9.0%), respectively (risk difference, 34% [95% CI, 25%-43%]); 19 of 62 glyburide users (30.6%) vs 8 of 58 (13.8%), respectively (risk difference, 17% [95% CI, 2%-31%); and 19 of 33 NSAID users (57.6%) vs 5 of 23 (21.7%), respectively (risk difference, 35% [95% CI, 10%-55%]) (P for drug class interaction = .09) (Table 2). Analysis of the antihistamine drug class was not possible because of small sample size (n = 12). No significant interactions were observed between prescription discontinuation and patient age, sex, or other predisposing characteristics in the intervention group (Figure 2). No adverse effects requiring hospitalization were reported. Twenty-nine of 77 patients (38%) who attempted to taper sedative-hypnotics reported withdrawal symptoms.
Table 2. Discontinuation of Inappropriate Medication Use in Older Adults After 6 Months.
| Discontinuation of Inappropriate Prescriptionsa | No. Discontinued/ No. in Subgroup (%) | Absolute Risk Difference, % (95% CI)b | No. Needed to Treat to Discontinue 1 Participant’s Medication | Relative Risk (95% CI) | Intracluster Correlation | |
|---|---|---|---|---|---|---|
| Intervention Group | Control Group | |||||
| All classes of drugs | 106/248 (42.7) | 29/241 (12.0) | 31 (23-38) | 3.22 | 3.55 (2.45-5.15) | 0.001 |
| Sedative-hypnotics | 63/146 (43.2) | 14/155 (9.0) | 34 (25-43) | 2.94 | 4.78 (2.80-8.14) | 0.003 |
| Glyburide | 19/62 (30.6) | 8/58 (13.8) | 17 (2-31) | 5.88 | 2.22 (1.06-4.68) | 0.003 |
| NSAIDs | 19/33 (57.6) | 5/23 (21.7) | 35 (10-55) | 2.86 | 2.64 (1.15-6.07) | 0.009 |
Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
The sample size for first-generation antihistamines was too small to warrant subgroup analysis.
95% CIs were calculated using robust standard error.
Figure 2. Absolute Discontinuation Rates of Inappropriate Medications by Subgroup of Patients in the Intervention Group.
The vertical line indicates the mean rate of medication discontinuation (42.7%).
aHealth status was determined using the first item of the Short Form 12-item Health Survey.18
bFrailty was assessed using the Vulnerable Elders Survey. Patients who scored ≥3 were deemed frail.19
Process Evaluation
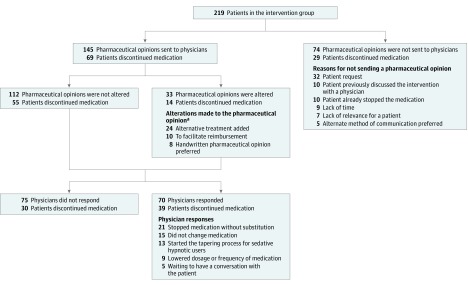
All 248 patients in the intervention group received the educational brochure. Of the 248 patients, 197 (79%) reported an encounter with their physician in which deprescribing was discussed. Among the 219 patients who completed the 6-month follow-up, 145 (66%) had pharmaceutical opinions sent to their physicians. Discontinuation of medication occurred in 69 cases (47.6%) in which a pharmaceutical opinion was sent compared with 29 cases (39.2%) in which a pharmacist opinion was not sent (risk difference, 8% [95% CI, −6% to 22%]). Reasons why pharmacists refrained from sending the pharmaceutical opinion varied (Figure 3). Of the 145 pharmaceutical opinions, 112 (77.2%) were unmodified from the original template. Prescription discontinuation occurred in 55 of the 112 cases (49.1%) in which the pharmaceutical opinion was unaltered compared with 14 of the 33 cases (42.4%) with modifications (risk difference, 7% [95% CI, −12% to 24%]). Physician response to the pharmaceutical opinion was not significantly associated with discontinuation: 39 of 70 cases (55.7%) with responses compared with 30 of 75 (40.0%) without (risk difference, 16% [95% CI, −0.5% to 31%]).
Figure 3. Process and Pattern of Pharmacist-Physician Communication of Pharmacies in the Intervention Group After 6 Months.
aMore than one reason for modifying the pharmaceutical opinion could apply.
Substitutions occurred among 37 individuals who discontinued a targeted medication. A second-generation antihistamine was substituted for a first-generation antihistamine (n = 1); glyburide was replaced with gliclazide (n = 12), sitagliptin (n = 6), and saxagliptin (n = 6); NSAIDs were replaced with acetaminophen (paracetamol) (n = 2), tramadol (n = 2), and low doses of pregabalin (n = 3); and sedative-hypnotics were replaced with zopiclone (n = 2), lorazepam (n = 1), oxazepam (n = 1), and diazepam (n = 1), which were considered failed deprescribing attempts.
Sedative-Hypnotic Tapering Process
Of the 146 sedative-hypnotic users in the intervention group, 115 (79%) discussed deprescribing with their physician and/or pharmacist after receiving the intervention. Seventy-seven sedative-hypnotic users (53%) initiated tapering, with 58 (75%) subsequently discontinuing their prescription. Almost all tapering failures were attributed to intolerance for withdrawal symptoms. Among the 69 patients (47%) who did not elect to attempt tapering, the most commonly cited reasons linked to dependence (18 [38%]), lack of concerns about harms (14 [29%]), and comfort with taking a small dose (11 [23%]). Five patients (10%) reported physician or pharmacist discouragement for initiating tapering.
Discussion
In this 6-month trial of a pharmacist-led educational intervention to reduce prescriptions for inappropriate medications in older adults, prescription renewals ceased among 43% of patients who received the educational intervention compared with 12% who received usual care, with a number needed to treat of 3 for all targeted medication classes.
These results should be considered in relation to other community-based randomized trials seeking to diminish rates of inappropriate prescriptions among older adults. Allard et al examined the effect of medication review by an independent geriatric team on the number of inappropriate prescriptions in primary care, and found that mailed recommendations to physicians resulted in a nonsignificant mean decrease of 0.24 prescriptions per patient.27 Tamblyn et al evaluated alerts to physicians via computerized prescription software with information about patient-specific risk of falls due to psychotropic medications.27 Results showed that physicians reduced the dosage of or discontinued the medication in 24.6% of cases, but no significant group difference occurred in the change of drug dose compared with usual care. Computer-generated alerts that triggered community pharmacists to call primary care physicians with recommendations to discontinue inappropriate prescriptions yielded a successful contact rate of 56%, and a medication change or discontinuation rate in 24% of these cases.28 Hanlon et al delivered in-person outpatient education during a clinic-based pharmacist-led medication review of physician practices, resulting in a 24% reduction in inappropriate medication scores vs 6% during usual care.29 An educational brochure recommending benzodiazepine discontinuation mailed directly to patients led to a sedative-hypnotic cessation rate of 27% at 6 months compared with 5% of controls.13 When physicians purposefully selected motivated patients to work with an outpatient psychologist to taper long-term benzodiazepine use for the management of insomnia, 80% of patients successfully tapered benzodiazepines by 6 months.30 Taken together, these trials indicate that efforts that directly involve patients in decision-making processes to deprescribe are more effective than pharmacist communication to physicians or prescribing software alerts alone.
Several mechanisms explain the discrepant results between the current trial and those published previously.In the current trial, accountability for adhering to evidence-based recommendations was augmented by involving patients in pharmacist-physician communication through the use of an educational brochure. In the current study, 75% of sedative-hypnotic users who initiated tapering successfully completed the protocol vs 54% in a 2014 trial that examined the effect of patient education alone on the reduction of inappropriate benzodiazepine prescriptions among older adults.13 Only 5 patients (10%) cited physician and/or pharmacist discouragement for deprescribing in the current trial compared with 17 (33%) in the 2014 trial.13 In the current study, even when the pharmaceutical opinions were not sent, pharmacists were still exposed to the evidence-based template, which may have increased support to patients for tapering, regardless of medication class. Use of a preset computer algorithm targeting specific medication classes allowed time-efficient identification of at-risk patients by pharmacists, with 1 simple recommendation emitted per patient rather than a long list of drug-related problems, as in previous deprescribing studies.28,29,31 Benzodiazepine users were not purposively selected by their physicians to participate in the trial because of an explicit desire to taper sedative-hypnotics, but were enrolled blinded to study outcome.30
Pharmacists’ scope of practice in many jurisdictions, including in Canada, the United States, the United Kingdom, and New Zealand, permits implementation of pharmacist-led deprescribing interventions.9,32,33 Communication with patients and physicians during outpatient service can occur by phone, fax, electronically, or in person, depending on the type of practice, setting, and payer. To provide leadership and support to physicians for optimizing medication management, compensation schemes are increasingly available to pharmacists for services rendered during collaborative patient care with a physician for complex medication issues, including deprescribing.9,32,33 Pharmacists in Quebec were able to access a government-sponsored, pharmacist-initiated service fee of $19 Can for sending evidence-based pharmaceutical opinions to physicians during the course of the trial.32 Financial incentives and performance measures alone do not lead to successful deprescribing,34 but can optimize medication dispensation when combined with process redesign, evidence-based guidelines, and patient education.35
Limitations
This study has several limitations. First, difficulties occurred in the recruitment of patients using first-generation antihistamines and NSAIDs. These medications are available over-the-counter and are not entered into the drug claims database. Second, high rates of glyburide discontinuation in the control group occurred because the onset of this trial coincided with the release of new guidelines for the treatment of type 2 diabetes in the frail elderly, recommending against glyburide.36 Third, although 34% of pharmacists opted not to send the evidence-based pharmaceutical opinion to physicians, exposure to the information still occurred and may have influenced conversations between pharmacists and patients. Fourth, adverse events beyond those requiring hospitalization or those experienced during sedative-hypnotic withdrawal were not collected. It is possible that adverse health events could have resulted from discontinuing a medication, even if fitting within a guideline. No data regarding additional clinic visits or phone calls were available. Fifth, the follow-up reported here is limited to 6 months. A longer follow-up may change the success rate of the intervention with some patients either discontinuing their use later or others restarting medications. Sixth, because the evidence of deprescribing was from an analysis of pharmacy fill data at a given point in time, it is unknown whether there were other contributing factors to the reasons for stopping the medications in question.
Conclusions
Among older adults in Quebec, a pharmacist-led educational intervention resulted in greater discontinuation of inappropriate prescriptions compared with usual care after 6 months. The generalizability of these findings to other settings requires further research.
Trial Protocol
eTable
Data Sharing Statement
References
- 1.Patel R, Zhu L, Sohal D, et al. Use of 2015 Beers Criteria medications by older Medicare beneficiaries. Consult Pharm. 2018;33(1):48-54. doi: 10.4140/TCP.n.2018.48 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Institute for Health Information Drug Use Among Seniors in Canada, 2016. Ottawa, ON: Canadian Institute for Health Information; 2018. https://www.cihi.ca/sites/default/files/document/drug-use-among-seniors-2016-en-web.pdf. Accessed September 14, 2018.
- 3.The American Geriatrics Society 2015 Beers Criteria Update Expert Panel American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 4.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012. doi: 10.1056/NEJMsa1103053 [DOI] [PubMed] [Google Scholar]
- 5.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834. doi: 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 6.Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544. doi: 10.1136/bmjopen-2014-006544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med. 2015;175(12):1942-1949. doi: 10.1001/jamainternmed.2015.5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinewine A, Fialová D, Byrne S. The role of the pharmacist in optimizing pharmacotherapy in older people. Drugs Aging. 2012;29(6):495-510. doi: 10.2165/11631720-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 9.Tannenbaum C, Tsuyuki RT. The expanding scope of pharmacists' practice: implications for physicians. CMAJ. 2013;185(14):1228-1232. doi: 10.1503/cmaj.121990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krska J, Cromarty JA, Arris F, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30(3):205-211. doi: 10.1093/ageing/30.3.205 [DOI] [PubMed] [Google Scholar]
- 11.Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging. 2009;26(12):1013-1028. doi: 10.2165/11318890-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12.Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379(9823):1310-1319. doi: 10.1016/S0140-6736(11)61817-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi: 10.1001/jamainternmed.2014.949 [DOI] [PubMed] [Google Scholar]
- 14.Martin P, Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open. 2017;7(4):e015959. doi: 10.1136/bmjopen-2017-015959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YZ, Turner JP, Martin P, Tannenbaum C. Does a consumer-targeted deprescribing intervention compromise patient-healthcare provider trust? Pharmacy (Basel). 2018;6(2):31. doi: 10.3390/pharmacy6020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin P, Tamblyn R, Ahmed S, Benedetti A, Tannenbaum C. A consumer-targeted, pharmacist-led, educational intervention to reduce inappropriate medication use in community older adults (D-PRESCRIBE trial): study protocol for a cluster randomized controlled trial. Trials. 2015;16:266. doi: 10.1186/s13063-015-0791-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE). Psychopharmacol Bull. 1988;24(4):689-692. [PubMed] [Google Scholar]
- 18.Ware J Jr, Kosinski M, Keller SDA. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 19.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691-1699. doi: 10.1046/j.1532-5415.2001.49281.x [DOI] [PubMed] [Google Scholar]
- 20.Martin P, Tamblyn R, Ahmed S, Tannenbaum C. An educational intervention to reduce the use of potentially inappropriate medications among older adults (EMPOWER study): protocol for a cluster randomized trial. Trials. 2013;14:80. doi: 10.1186/1745-6215-14-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin P, Tannenbaum C.. A prototype for evidence-based pharmaceutical opinions to promote physician-pharmacist communication around deprescribing. Can Pharm J (Ott). 2018;151(2):133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canadian Pharmacists Association. A review of pharmacy services in Canada and the health and economic evidence. Ottawa, ON: Canadian Pharmacists Association; 2016. https://www.pharmacists.ca/cpha-ca/assets/File/cpha-on-the-issues/Pharmacy%20Services%20Report%201.pdf. [Google Scholar]
- 23.Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol. 2006;35(5):1292-1300. doi: 10.1093/ije/dyl129 [DOI] [PubMed] [Google Scholar]
- 24.Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128. doi: 10.1016/0197-2456(90)90005-M [DOI] [PubMed] [Google Scholar]
- 25.Ukoumunne OC, Forbes AB, Carlin JB, Gulliford MC. Comparison of the risk difference, risk ratio and odds ratio scales for quantifying the unadjusted intervention effect in cluster randomized trials. Stat Med. 2008;27(25):5143-5155. doi: 10.1002/sim.3359 [DOI] [PubMed] [Google Scholar]
- 26.McAlister FA, Straus SE, Guyatt GH, Haynes RB; Evidence-Based Medicine Working Group . Users’ guides to the medical literature, XX: integrating research evidence with the care of the individual patient. JAMA. 2000;283(21):2829-2836. doi: 10.1001/jama.283.21.2829 [DOI] [PubMed] [Google Scholar]
- 27.Tamblyn R, Eguale T, Buckeridge DL, et al. The effectiveness of a new generation of computerized drug alerts in reducing the risk of injury from drug side effects: a cluster randomized trial. J Am Med Inform Assoc. 2012;19(4):635-643. doi: 10.1136/amiajnl-2011-000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monane M, Matthias DM, Nagle BA, Kelly MA. Improving prescribing patterns for the elderly through an online drug utilization review intervention: a system linking the physician, pharmacist, and computer. JAMA. 1998;280(14):1249-1252. doi: 10.1001/jama.280.14.1249 [DOI] [PubMed] [Google Scholar]
- 29.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428-437. doi: 10.1016/S0002-9343(97)89519-8 [DOI] [PubMed] [Google Scholar]
- 30.Curran HV, Collins R, Fletcher S, Kee SC, Woods B, Iliffe S. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33(7):1223-1237. doi: 10.1017/S0033291703008213 [DOI] [PubMed] [Google Scholar]
- 31.Allard J, Hebert R, Rioux M, Asselin J, Voyer L. Efficacy of a clinical medication review on the number of potentially inappropriate prescriptions prescribed for community-dwelling elderly people. CMAJ. 2001;164(9):1291-1296. [PMC free article] [PubMed] [Google Scholar]
- 32.Canadian Foundation for Pharmacy Changing face of pharmacy. Fees and claims data for government-sponsored pharmacist services, by province (updated September 2017). Mississauga. https://www.cfpnet.ca/bank/document_en/118-2017-changing-face-of-pharmacy.pdf. 2017. Accessed September 11, 2018.
- 33.American Pharmacists Association Billing Primer: A Pharmacist’s Guide to Outpatient Fee-for-Service Billing Washington, DC: American Pharmacists Association; 2018.
- 34.Rat C, Penhouet G, Gaultier A, et al. Did the new French pay-for-performance system modify benzodiazepine prescribing practices? BMC Health Serv Res. 2014;14(1):301. doi: 10.1186/1472-6963-14-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnipper JL, Rothschild JM. Improving medication safety. Lancet. 2012;379(9823):1278-1280. doi: 10.1016/S0140-6736(12)60078-6 [DOI] [PubMed] [Google Scholar]
- 36.Committee CDACPGE. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2013;37(suppl 1):s1-s212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable
Data Sharing Statement