Abstract
A study that compared the use of statin therapies with and without fish oil in a veteran population found an insignificant difference between the 2 arms.
The Centers for Disease Control and Prevention lists cardiovascular-related diseases as a leading cause of mortality.1 The medication class of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, more commonly known as statins, is first-line therapy to prevent negative cardiovascular outcomes and reduce premature death.2 Additional hyperlipidemia medications, such as fish oil, can be added for potential cardiovascular benefit.
Yokoyama and colleagues demonstrated that fish oil is a promising treatment for the prevention of major coronary events in patients with hypercholesterolemia.3 Furthermore, Macchia and colleagues found reductions in cardiovascular outcomes and all-cause mortality in postmyocardial infarction patients treated with fish oil and statin combination therapy.4 In contrast, the Outcomes Reduction with an Initial Glargine Intervention (ORIGIN) trial found glucose intolerant and patients with diabetes mellitus did not have improved cardiovascular outcomes with fish oil therapy.5 Likewise The Risk and Prevention Study Collaborative Group found fish oil supplementation provided no benefit for primary prevention in patients with multiple cardiovascular risk factors.6 These studies demonstrate fish oil therapy can cause diverse cardiovascular outcomes in different patient populations.
Currently, there are no studies examining the impact of fish oil and statin combination therapy on the US veteran population. The research of Yokoyama, Macchia, and The Risk and Prevention Study Collaborative Group took place in Japanese and Italian populations, which impacts their external validity.3,4,6 Furthermore, these studies had higher rates of female subjects when compared with the US veteran population. For example, 68% of female subjects in the Yokoyama study received fish oil therapy.3 Also, the ORIGIN trial subjects were restricted to patients with diabetes mellitus or who were glucose intolerant, which is not reflective of the entire veteran population.5 These differences can make it difficult to define the role of fish oil and statin combination therapy in treating cardiovascular disease and reducing mortality in the veteran population.
This study aims to help the US Department of Veterans Affairs (VA) primary care providers and clinical pharmacists address the role of fish oil and statin combination therapy in the prevention of cardiovascular disease and all-cause mortality in the veteran population. The addition of fish oil to statin therapy was compared with an established standard of care, statin monotherapy, in veterans at the Fargo Veterans Affairs Health Care System (FVAHCS).
METHODS
A retrospective chart review was conducted using the FVAHCS Computerized Patient Record System (CPRS). The institution’s review board and VA medical center approved the study. Eligible veterans with prescriptions for fish oil or statin therapy between January 1, 2000 and September 30, 2015 were randomly selected, reviewed, and sorted based on inclusion and exclusion criteria. These veterans were either placed in the fish oil and statin combination cohort or statin monotherapy cohort. Inclusion criteria required at least 1 year of statin monotherapy for inclusion in the statin cohort or at least 1 year duration of fish oil and statin combination therapy for inclusion in the fish oil and statin combination cohort (Table 1). The exclusion criteria are described in Table 2.
TABLE 1.
Inclusion Criteria
| Veterans aged ≥ 18 years in the Fargo VA Health Care System |
| Patient on VA supplied statin monotherapy longer than a year with no additional hyperlipidemia agent > 1 year, inclusion in statin monotherapy cohort |
| Patient on concomitant VA supplied fish oil and statin therapy for > 1 year without any additional hyperlipidemia agent > 1 year, inclusion in fish oil and statin combination therapy cohort |
TABLE 2.
Exclusion Criteria
| Using fish oil and statin combination therapy < 1 year excludes from fish oil and statin cohort |
| Using statin therapy < 1 year excludes from either cohort |
| Using other hyperlipidemia medication (niacin, fibrate, bile acid sequestering agent, cholesterol absorption inhibitors) for > 1 year during the study period |
| Patient with clotting disorders |
| Arrhythmia diagnosis |
| Anticoagulation therapy other than aspirin |
| Use of hormone replacement therapy or hormonal contraceptive |
| Use of erythropoiesis stimulating medications |
| Illegal substance addiction |
The primary outcome was time to aggregate cardiovascular events, specifically myocardial infarction (MI), stroke, transient ischemic attack, coronary artery bypass graft, and percutaneous intervention. Adverse cardiovascular event data were obtained from the veterans’ International Classification of Disease (ICD) 9 codes. Furthermore, the secondary outcome—time to all-cause mortality—was gathered by death records in CPRS. Time to these events was compared in veterans on fish oil and statin combination therapy or statin monotherapy. The date of the cardiovascular event or death was recorded for each outcome and was obtained by reviewing provider notes that documented the incidence. If a specific day or month of incidence was not documented, July 1 was selected as the default date for the adverse cardiovascular event.
Demographics, medication adherence, diagnoses, lab values within 90 days of initiation of therapy, and primary and secondary outcomes were collected. Demographics that included age, race, and sex all were obtained via chart review. Diagnoses were gathered using ICD 9 codes. Refill history was retrieved to assess adherence. Adherence was calculated by the total days of medication therapy divided by the total days within the study. Total days in the study was calculated by the duration of therapy days between therapy initiation date and a terminating factor. Terminating factors included an adverse cardiovascular event, death, or the study termination date.
Statistics
Demographic and other cohort characterization variables were compared either by a t test, rank sum test, or Fisher exact test given the character of the variable. Kaplan Meier analysis was used to evaluate time to aggregate cardiovascular events and all-cause mortality. VA Informatics and Computing Infrastructure (VINCI) R 3.4.3 was used for data analysis. One of the few combination studies by Macchia and colleagues gave an estimate of unadjusted incident rates for patients treated with statin monotherapy vs fish oil and statin combination therapy in patients having a recent MI.4 Based on this information, a power analysis determined that a 2% difference in incidence rate of adverse cardiovascular events could be detected between the 2 cohorts with 1,000 veterans in the statin cohort, and 500 veterans in the fish oil and statin cohort assuming a time to event interval of about 7.5 years. An α level of 0.05 was set to determine statistical significance.
RESULTS
A total of 3,940 veterans with prescriptions for fish oil or statin therapy were randomly reviewed and sorted based on inclusion and exclusion criteria. This inclusion criteria resulted in 2,575 fish oil and statin combination patients and 1,365 statin monotherapy patients. Exclusion criteria produced a final total of 437 fish oil and statin combination patients and 559 statin monotherapy patients. Patient demographics are presented in Table 3. Of note, the average age at study entry was 61.5 years for fish oil and statin combination patients and 63.8 years for statin monotherapy patients (P < .001). More than 80% of the study population was white in both cohorts. Also, > 98% of the study population was male in both cohorts.
TABLE 3.
Demographics
| Variable | Fish Oil and Statin | Statin | P Value |
|---|---|---|---|
| Age at study entry, No. (y [SD]) | 437 (61.5 [11.2]) | 559 (63.8 [10.5]) | < .001 |
| Male, No. (%) | 437 (98.4) | 559 (99.3) | .23 |
| Ethnicity, No. (%) | |||
| White | 379 (86.7) | 464 (83) | |
| American Indian | 22 (5.0) | 3 (0.5) | |
| African American | 2 (0.5) | 6 (1.1) | |
| Other | 34 (7.8) | 86 (15.4) | |
All baseline laboratory data were collected within 90 days of therapy initiation (Table 4). Statin monotherapy patients had lower triglyceride levels compared with those of the fish oil and statin combination patients. However, both high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were higher in the statin monotherapy patients. As seen in Table 5, diagnosis of heart failure, hypertension, hypothyroidism, and dyslipidemia were higher in the statin monotherapy cohort, while tobacco use and pancreatitis were more prevalent in the fish oil and statin combination cohort. The Charlson Comorbidity Index for the fish oil and statin combination cohort was slightly higher than the statin monotherapy cohort (1.6 vs 1.4, P = .03). Medication adherence rates are included in Table 6.
TABLE 4.
Labs Within 90 Days of Starting Therapy
| Variable | Fish Oil and Statin | Statin | |||
|---|---|---|---|---|---|
| No. | Value (SD) | No. | Value (SD) | P Value | |
| AST, U/L | 246 | 26.9 (10.3) | 309 | 23.7 (10) | < .001 |
| ALT, U/L | 337 | 36.2 (17.8) | 315 | 29.5 (14.3) | < .001 |
| CPK, U/L | 109 | 116.1 (77.2) | 87 | 144.5 (295.5) | .39 |
| LDL, mg/dL | 338 | 108.2 (39) | 303 | 125.5 (42.9) | < .001 |
| HDL, mg/dL | 322 | 41.1 (11.8) | 305 | 45.2 (12.1) | < .001 |
| TG, mg/dL | 316 | 252.1 (327.0) | 297 | 159.8 (84.7) | < .001 |
| BMI | 418 | 32.0 (5.5) | 498 | 30.3 (5.2) | < .001 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; CPK, creatine phosphokinase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides.
TABLE 5.
Disease Morbidity Index
| Variable | Fish Oil and Statin, No. (%) | Statin, No. (%) | P Value | ||
|---|---|---|---|---|---|
| Alcohol use | 20 (4.6) | 20 (3.6) | .42 | ||
| Diabetes mellitus | 98 (22.5) | 130 (23.2) | .82 | ||
| Heart failure | 16 (3.7) | 43 (7.7) | < .01 | ||
| Hypertension | 162 (37.2) | 294 (52.4) | < .001 | ||
| Hypothyroidism | 19 (4.4) | 47 (8.4) | .01 | ||
| Tobacco use | 140 (32.1) | 148 (26.4) | .049 | ||
| Dyslipidemia | 223 (1.1) | 353 (62.9) | < .001 | ||
| Pancreatitis | 7 (1.6) | 0 | < .01 | ||
| Morbidity | No. | Value (SD) | No. | Value (SD) | |
| Charlson Comorbidity Index | 436 | 1.6 (1.7) | 559 | 1.4 (1.7) | .03 |
TABLE 6.
Medication Adherence
| Variable | Fish Oil and Statin | Statin | |||
|---|---|---|---|---|---|
| No. | % (SD) | No. | % (SD) | P Value | |
| Percent days covered fish oil | 436 | 72.2 (25.6) | - | - | |
| Percent days covered statin | 436 | 80.2 (30.8) | 559 | 93.4 (14.9) | < .001 |
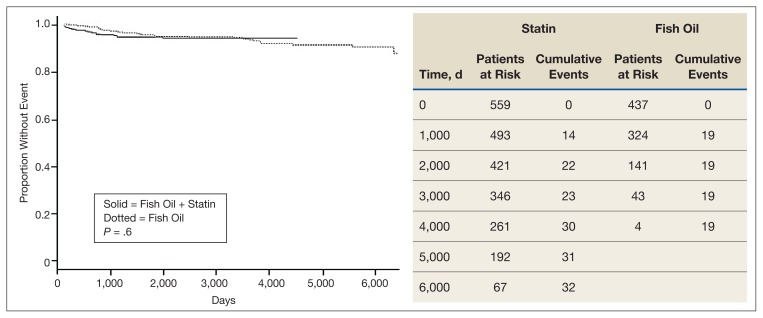
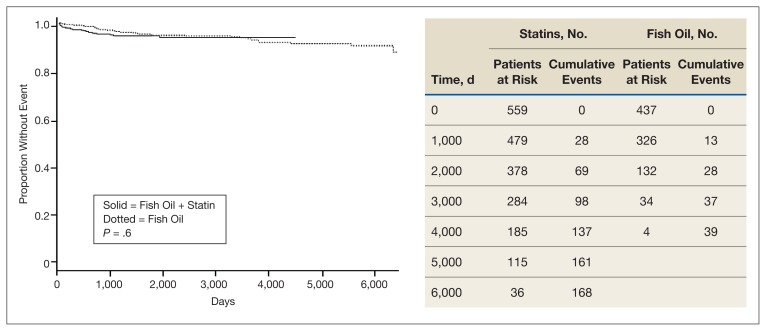
Kaplan Meier curves of the primary outcome, time to aggregate adverse cardiovascular event, and the secondary outcome, time to all-cause mortality are shown in Figure 1 and Figure 2, respectively. This shows adverse cardiovascular events and all-cause mortality for approximately 4,500 days for the fish oil and statin cohort and approximately 6,000 days for the statin monotherapy cohort. Kaplan Meier curves failed to show a statistically significant difference in time to adverse cardiovascular event (P = .6) or all-cause mortality (P = .16).
FIGURE 1.
Days to Aggregate Adverse Vascular Event
FIGURE 2.
Days to All-Cause Mortality
DISCUSSION
Analysis of this study failed to detect a statistically significant difference for time to aggregate adverse cardiovascular events or all-cause mortality. This may be due to fewer adverse cardiovascular events and mortality in the 2 cohorts than was anticipated. Fish oil and statin combination therapy may have a small effect size in the studied population when compared with statin monotherapy, which makes it difficult to detect a difference. However, this study had a relatively large sample size, which may indicate that both therapies are associated with similar aggregate adverse cardiovascular outcomes and all-cause mortality for the included study population.
There are no studies examining fish oil and statin therapy in the veteran population and only limited studies comparing statin and fish oil combination therapy vs statin monotherapy for adverse cardiovascular outcomes and all-cause mortality. One of the few comparison studies was by Macchia and colleagues and consisted of 7,924 post MI patients in Italy. Over a 4-year period, researchers found a slight improvement in the adjusted paired-matched population for all-cause mortality in the fish oil and statin therapy cohort vs statin monotherapy (8.6% vs 13.6% P < .001).3 A benefit also was seen in the fish oil and statin cohort vs statin monotherapy in the adjusted paired-matched population for death or stroke (16.7% vs 11.5% P < .001).3
In contrast, this study did not address postmyocardial infarction patients exclusively. Rather, patients in this study had lower morbidity, which resulted in fewer adverse cardiovascular outcomes and a greater difficulty to detect a difference in this healthier population. These healthier patients may derive less benefit from primary or secondary prevention with statin and fish oil combination therapy.
In this study, there were extensive inclusion and exclusion criteria to assess the relationship between the cohorts for adverse cardiovascular events caused by atherosclerotic disease. Veterans were required to take fish oil and statin therapy or statin monotherapy for at least 1 year. Other literature has only examined clinical impact on adverse cardiovascular event outcomes if therapy was a year or longer.7 Therefore, to prevent confounders from other medications, veterans who used any hyperlipidemia agent other than fish oil and statin therapy for longer than 1 year were excluded. Extensive exclusion criteria eliminated many veterans. However, the robust exclusion of clotting disorders, arrhythmias, chronic anticoagulation other than aspirin, hormonal medication use, or illegal substance abuse prevented the potential confounder of nonatherosclerotic adverse cardiovascular events, for example, a stroke due to poorly controlled atrial fibrillation.
Comparison of demographic data showed both cohorts were of similar age, sex, and race. Of note the Fargo veteran population was primarily white (> 80% in both cohorts). This is slightly higher than the percentage of whites for all US veterans. The slight difference most likely had a minimal clinical impact. Laboratory values recorded within 90 days of initiation of therapy were largely clinically similar except for triglycerides being significantly higher in the fish oil and statin combination cohort (Table 4). This may reflect selection bias, where providers may be more likely to add fish oil therapy for the potential to further control triglycerides.
Diagnoses of hypertension, heart failure, and dyslipidemia were higher in the statin monotherapy cohort. However, body mass index, tobacco use, and pancreatitis were statistically higher in fish oil and statin combination cohort. Even though there was a statistically significant difference in disease diagnoses, this likely created a minor clinical difference between the groups. This is further illustrated by the similarity of the Charlson Comorbidity Index of 1.6 for fish oil and statin cohort and 1.4 in statin monotherapy cohort.
Strengths
A strength of this study was its adherence rates. Adherence rates were high in both cohorts (Table 6). Fish oil and statin cohort did have slightly lower adherence compared with that of statin monotherapy. This may demonstrate extra pill burden influencing adherence. Overall demographics, laboratory values, disease, and adherence rates were clinically similar in both cohorts, thus reducing the potential for confounders.
Limitations
Limitations of this study include its retrospective chart review design. This design is susceptible to incorrect recording of events. The primary outcome, aggregate adverse cardiovascular events, may have been incorrectly recorded in the medical record as other diseases, such as coronary artery disease or heart disease, and therefore not captured by ICD 9 code retrieval. Also, important information, such as laboratory data, disease, and medication adherence, may not have been documented for all patients. Of note 1 patient in the fish oil and statin combination cohort did not have any recorded laboratory data, disease, or adherence data.
Another limitation is lack of access to medical notes from non-VA providers, which can result in missed data collection. To reduce this limitation, the study excluded veterans that received non-VA fish oil, statins, or other hyperlipidemia medications for > 1 year. Veterans were included only if they used VA-provided fish oil or statins. This inclusion and exclusion criteria reduced the chance of missing data from other facilities because it favored inclusion of only subjects that received care exclusively through the VA.
Last, on study initiation it was not realized that fish oil was not provided by the health care system until about the year 2004. This resulted in less risk days for the fish oil and statin cohort. However, Kaplan Meier analysis lessens this issue from being a confounder. Time to event rates for both the primary and secondary outcomes were similar and most likely would have continued to trend together with the same therapy duration.
CONCLUSION
Fish oil and statin combination therapy when compared with statin monotherapy failed to show that a statistically significant difference exists in the rates of MI, stroke, transient ischemic attack, coronary artery bypass graft, and percutaneous intervention. The clinical difference of fish oil and statin combination therapy vs statin monotherapy is most likely small or nonexistent. From our literature search, this is the only study concerning the use of fish oil and statin combination therapy in the veteran population. It is most likely that fish oil and statin combination therapy and statin monotherapy are similar for the reduction of time to aggregate adverse cardiovascular events and all-cause mortality in the veteran population.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Fargo VA Healthcare System.
Footnotes
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
- 1.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. [Accessed July 26, 2018]. https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_04.pdf. Published June 30, 2016.
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;2014;63(25 pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 4.Macchia A, Romero M, D’Ettorre A, Tognoni G, Mariani J. Exploratory analysis on the use of statins with or without n-3 PUFA and major events in patients discharged for acute myocardial infarction: an observational retrospective study. PLoS One. 2013;8(5):e62772. doi: 10.1371/journal.pone.0062772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ORIGIN Trial Investigators. Bosch J, Gerstein HC, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 6.The Risk and Prevention Study Collaborative Group. Roncaglioni MC, Tombesi M, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 7.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]