Key Points
Question
Can the somatostatin analogue lanreotide slow the rate of decline in kidney function in patients with autosomal dominant polycystic kidney disease (ADPKD)?
Findings
In this randomized clinical trial that included 305 patients with later-stage ADPKD, treatment with lanreotide, 120 mg subcutaneously once every 4 weeks, compared with standard care resulted in a decline of estimated glomerular filtration rate of 3.53 vs 3.46 mL/min/1.73 m2 per year over 2.5 years, a difference that was not statistically significant.
Meaning
Lanreotide was not effective in slowing the decline in kidney function in patients with later-stage ADPKD.
Abstract
Importance
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive cyst formation in both kidneys and loss of renal function, eventually leading to a need for kidney replacement therapy. There are limited therapeutic management options.
Objective
To examine the effect of the somatostatin analogue lanreotide on the rate of kidney function loss in patients with later-stage ADPKD.
Design, Setting, and Participants
An open-label randomized clinical trial with blinded end point assessment that included 309 patients with ADPKD from July 2012 to March 2015 at 4 nephrology outpatient clinics in the Netherlands. Eligible patients were 18 to 60 years of age and had an estimated glomerular filtration rate (eGFR) of 30 to 60 mL/min/1.73 m2. Follow-up of the 2.5-year trial ended in August 2017.
Interventions
Patients were randomized to receive either lanreotide (120 mg subcutaneously once every 4 weeks) in addition to standard care (n = 153) or standard care only (target blood pressure <140/90 mm Hg; n = 152).
Main Outcomes and Measures
Primary outcome was annual change in eGFR assessed as slope through eGFR values during the 2.5-year treatment phase. Secondary outcomes included change in eGFR before vs after treatment, incidence of worsening kidney function (start of dialysis or 30% decrease in eGFR), change in total kidney volume and change in quality of life (range: 1 [not bothered] to 5 [extremely bothered]).
Results
Among the 309 patients who were randomized (mean [SD] age, 48.4 [7.3] years; 53.4% women), 261 (85.6%) completed the trial. Annual rate of eGFR decline for the lanreotide vs the control group was −3.53 vs −3.46 mL/min/1.73 m2 per year (difference, −0.08 [95% CI, −0.71 to 0.56]; P = .81). There were no significant differences for incidence of worsening kidney function (hazard ratio, 0.87 [95% CI, 0.49 to 1.52]; P = .87), change in eGFR (−3.58 vs −3.45; difference, −0.13 mL/min/1.73 m2 per year [95% CI, −1.76 to 1.50]; P = .88), and change in quality of life (0.05 vs 0.07; difference, −0.03 units per year [95% CI, −0.13 to 0.08]; P = .67). The rate of growth in total kidney volume was lower in the lanreotide group than the control group (4.15% vs 5.56%; difference, −1.33% per year [95% CI, −2.41% to −0.24%]; P = .02). Adverse events in the lanreotide vs control group included injection site discomfort (32% vs 0.7%), injection site papule (5.9% vs 0%), loose stools (91% vs 6.6%), abdominal discomfort (79% vs 20%), and hepatic cyst infections (5.2% vs 0%).
Conclusions and Relevance
Among patients with later-stage autosomal dominant polycystic kidney disease, treatment with lanreotide compared with standard care did not slow the decline in kidney function over 2.5 years of follow-up. These findings do not support the use of lanreotide for treatment of later-stage autosomal dominant polycystic kidney disease.
Trial Registration
ClinicalTrials.gov Identifier: NCT01616927
This randomized clinical trial compares the effect of adding the somatostatin analogue lanreotide to standard care vs standard care alone on change in estimated glomerular filtration rate (eGFR) in patients with autosomal dominant polycystic kidney disease (PCKD).
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive cyst formation in both kidneys and loss of renal function.1 A 2017 review of epidemiology literature indicated a disease prevalence of approximately 3 to 4 per 10 000.2 End-stage kidney disease, for which dialysis or kidney transplantation is needed, occurs between the fourth and seventh decade of life in the majority of affected patients.1 Registry data collected between 1991 and 2010 showed that approximately 10% of patients receiving kidney replacement therapy had ADPKD.3
For patients with ADPKD, few treatments are available that can delay the rate of disease progression. Increasing knowledge of the pathophysiology of the disease allowed for the identification of several ADPKD-specific therapeutic targets. It appears that in renal tubular cells affected by polycystic kidney disease, cyclic adenosine monophosphate is increased.4 This second messenger promotes growth of these affected cells and stimulates transepithelial fluid secretion, which are 2 important processes involved with cyst formation and growth.5
Somatostatin is a peptide that is secreted by cells in the pancreas, nervous system, gastrointestinal tract, thyroid gland, and other organs.6 When bound to the somatostatin receptor, it inhibits adenylyl cyclase, the enzyme that produces cyclic adenosine monophosphate in renal tubular cells.7 In models for polycystic kidney disease, somatostatin analogues were shown to be renoprotective.8,9,10,11 Furthermore, clinical trials in patients with ADPKD suggested that somatostatin analogues ameliorated the rate of growth in total kidney volume (TKV).12,13,14,15 These trials, however, included only a limited number of patients, were of short duration, and were partly uncontrolled, making it difficult to draw definitive conclusions on the merits of these agents. For these reasons, the DIPAK-1 study was designed to investigate the renoprotective efficacy and adverse events of the somatostatin analogue lanreotide in patients with later-stage ADPKD.
Methods
Trial Design and Participants
The design and methods of the DIPAK-1 study have been published16 and the full trial protocol is available in Supplement 1. Briefly, an investigator-driven, randomized, open-label clinical trial with blinded end point analysis was performed to test the efficacy of and adverse events associated with lanreotide in patients with later-stage ADPKD. Because lanreotide is a gel, administration of this drug results in temporary injection infiltrates. Manufacturing a placebo that has a similar effect has not been possible from a technical point of view, which precluded execution of this trial as a double-blinded randomized trial. An academic steering committee designed the trial and oversaw its conduct with the assistance of an independent data and safety monitoring committee. The institutional review board at each site approved the protocol. All participants provided written informed consent.
The study included patients aged 18 to 60 years who had later stage ADPKD (diagnosis made in accordance to the modified Ravine criteria17), defined as an estimated glomerular filtration rate (eGFR) of 30 to 60 mL/min/1.73 m2. Main exclusion criteria at the start of the study were bradycardia, a history of gallstones or pancreatitis, and diseases or medication use that could confound end point assessment (eg, diabetes mellitus, use of nonsteroidal anti-inflammatory drugs, use of lithium or tolvaptan).
Randomization
Patients were referred by physicians at 66 hospitals in the Netherlands to 1 of the 4 study centers (Groningen, Leiden, Nijmegen, and Rotterdam). At a screening visit, eligibility was checked, and, when confirmed, a baseline visit took place. At the baseline visit, patients were randomly assigned (1:1) to the lanreotide group, which received lanreotide and standard care, or to the control group, which received standard care alone. Randomization with a block size of 6 was performed centrally with the use of an interactive voice response system, with stratification according to sex, age (≤45 years or >45 years), and eGFR (≤45 mL/min/1.73 m2 or >45 mL/min/1.73 m2).
Interventions
Patients in the lanreotide group received 120 mg of lanreotide subcutaneously once every 4 weeks, which was down-titrated to 90 mg if eGFR decreased to less than 30 mL/min/1.73 m2 during the trial. Also, if patients did not tolerate 120 mg, lanreotide was down-titrated to 90 mg, 60 mg, or stopped. Lanreotide was injected by trained nurses via a home care service. Standard care was defined as a blood pressure less than 140/90 mm Hg, to be reached with a sodium-restricted diet and, as a first-choice blood pressure–lowering agent, an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. The choice of additional antihypertensive medication and dietary advice was left to the discretion of the treating physician.
Procedures
After the baseline visit at 1 of the 4 study centers, patients were seen at weeks 4, 8, 12, 48, 96, and 120. At the week-120 visit, lanreotide treatment was stopped and patients were seen again 12 weeks later at a posttreatment visit (week 132). In patients who stopped the trial prematurely, early end-of-treatment and posttreatment visits were performed. Every 12 weeks, blood was drawn for local assessment of safety laboratory data and for collection of a blood sample that was shipped to the central laboratory. These blood samples were stored at −80°C until measurement of serum creatinine, with the use of the IDMS-traceable Roche enzymatic method, and cystatin C, using reference material from the International Federation of Clinical Chemistry. All samples of a patient were assessed in 1 run to minimize bias induced by interassay variation. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration formulas for serum creatinine and cystatin C.18 Measurement of creatinine and cystatin C was performed blinded for treatment allocation.
At the baseline (week 0), end of treatment (week 120), and posttreatment (week 132) visits, standardized magnetic resonance (MR) images were obtained without the use of a contrast agent. A quality check was performed within 24 hours, and, in cases of insufficient quality, MR imaging was repeated within 1 week. A detailed description of the methodology, accuracy, and precision of the TKV measurement has been published.19 TKV was assessed with manual tracing planimetry by trained reviewers blinded to patient identity, treatment allocation, and order of study visit and was adjusted for height (htTKV). During these visits, health-related quality of life was also measured using the validated 18-question ADPKD Impact Scale.20 The minimum score of 1 indicates “not bothered at all” and the maximum score of 5 indicates “extremely bothered.” Information on race was determined by the researchers, according to fixed categories, to allow comparison with other studies and assessment of external validity.
Deviations From the Original Protocol
An interim safety analysis revealed 8 instances of hepatic cyst infection in 7 patients using lanreotide. Details of these patients have been published.21 A history of hepatic cyst infection seemed to be a risk factor. This experience led to a protocol amendment that dictated to withdraw all patients from the study who had a history of hepatic cyst infection (3 in the lanreotide group and 0 in the control group) or who experienced a hepatic cyst infection during the study. Thereafter, only 1 additional hepatic cyst infection occurred, also in a patient treated with lanreotide.
Outcomes
The primary outcome was change in kidney function, assessed by the patient’s slope via serial eGFR measurements over time (calculated using the creatinine values measured in 1 run per patient) during the treatment phase. The eGFR measurements from week 12 until the end of the treatment phase were used for analysis of the primary efficacy end point. The baseline and posttreatment eGFR measurements (ie, just before the start of and after stopping lanreotide) were not used because somatostatin analogues can induce acute, reversible renal hemodynamic effects that may compromise an accurate assessment of eGFR slope.22
The key secondary outcomes were (1) change in eGFR between the pretreatment (the mean eGFR from the screening and baseline visits) and posttreatment visits; (2) incidence of worsening kidney function, defined as a sustained (2 consecutive measurements) 30% decrease from pretreatment eGFR or the need for kidney replacement therapy, whichever came first; (3) change in htTKV between the baseline and posttreatment visits; and (4) change in health-related quality of life between the baseline and end-of-treatment visits.
Other secondary outcomes included the patients’ adverse event profile and tolerability of lanreotide. As defined in the statistical analysis plan in Supplement 2, change in total liver volume will be analyzed only in the subgroup of patients with a polycystic liver phenotype (ie, total liver volume >2000 mL). Results on total liver volume will therefore be reported separately.
Statistical Analysis
Enrollment of at least 150 patients per group (300 in total) was needed to show a 30% reduction in slope of eGFR loss on treatment (deemed clinically relevant because it would translate as 3 years of treatment leading to approximately 1 year delayed start of renal replacement therapy, and what exact percentage was also used in other studies23,24,25), assuming a mean (SD) slope in eGFR of −5.1 (4.2) mL/min/1.73 m2 per year in the control group, 80% power to detect this reduction, and a 2-sided ∝ of .05, as well as taking into account 20% protocol violators and/or dropouts.
Continuous data are presented as mean (SD) or as median and interquartile range (IQR), in cases of nonnormal distribution. Categorical data are presented as percentages. For all analyses, we used an intention-to-treat approach, including all randomized patients who had primary efficacy postbaseline data available.
A mixed-model repeated-measures analysis was used to evaluate the primary outcome (ie, slope of eGFR loss during the treatment phase). If kidney replacement therapy was started or death occurred, only eGFR measurements before these events were used for analysis. Within-patient correlations were modeled using an unstructured covariance structure. In order to check model fit, additional models using Toeplitz, autoregressive-1, and compound symmetry structures were performed. The unstructured covariance structure resulted in the best model fit. The following categorical covariates were used in the models as fixed effects: lanreotide treatment (yes/no), time, and lanreotide treatment × time interaction. Because there were no differences in baseline characteristics between the groups for the randomization stratification factors or a center effect, the final models were not adjusted for these covariates.
An exploratory mixed-model repeated-measures analysis of the secondary efficacy end points (change in eGFR, htTKV, and health-related quality of life) was also performed to account for possible differences in follow-up time between the groups. Change in htTKV was compared between the groups using log10-transformed htTKV data, the antilog of the treatment effect, and 95% CIs derived from the mixed-model analysis to provide annual percentage of change of htTKV. Incidence of worsening kidney function was investigated with a time-to-event analysis using a Cox proportional hazards model. The proportionality assumption was tested by calculating Schoenfeld residuals, running a model with the treatment group as a time-dependent covariate, and performing a proportionality test. For the secondary outcomes, missing data were few and therefore not imputed.
Subgroup analyses were a priori defined and performed for the primary and secondary outcomes, with treatment group (yes/no) and subgroup variable as independent variables and their interaction term (treatment group × subgroup variable). If this interaction term was significant, the subgroup variable was considered as a moderator for treatment effect.
The safety analysis population included all randomized patients. After early discontinuation of treatment in a patient, efforts were made to collect adverse event data during the rest of the planned study period. No adverse event data were collected after a patient started kidney replacement therapy (a study end point).
Mixed models were checked for presence of outliers; results were validated by testing measures of kidney function other than creatinine-based eGFR; and comparisons were made for change in htTKV, not only between baseline and posttreatment visits, but also as post hoc analysis between baseline and end-of-treatment visits.
All analyses were performed with the statistical software SAS version 9.4 (SAS Institute Inc). A 2-sided P value less than .05 indicated statistical significance. No adjustment of significance threshold for multiple comparisons was made for the analyses of secondary endpoints, which should therefore be interpreted as exploratory.
Results
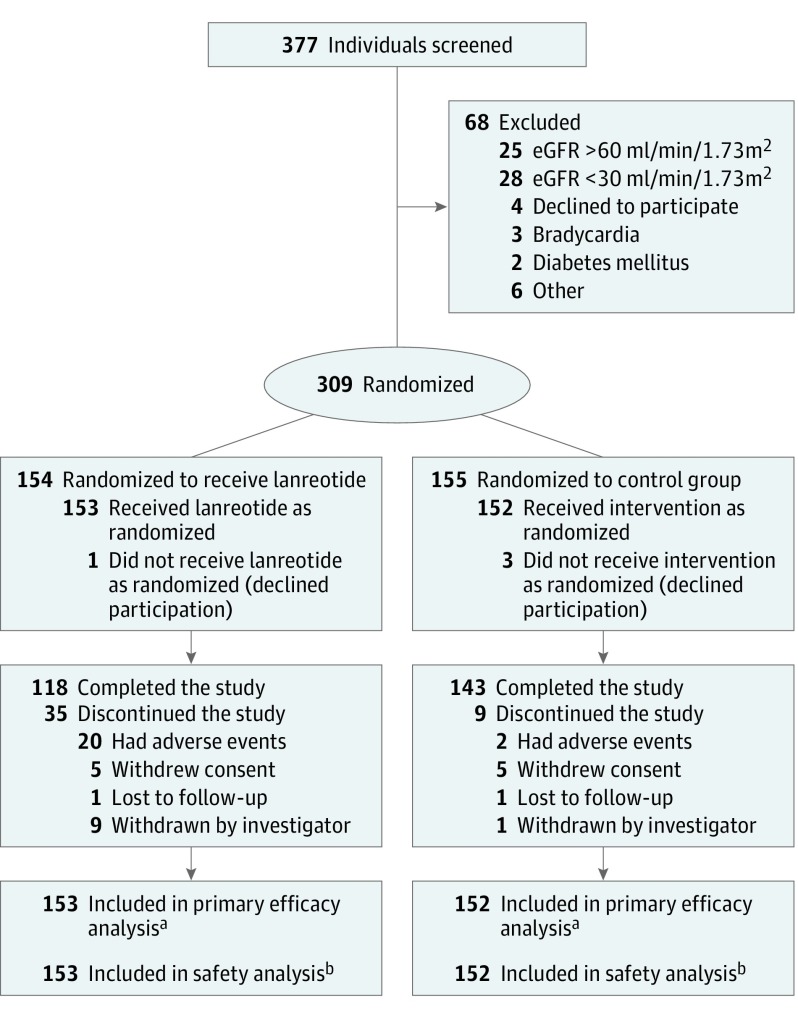
A flowchart describing patient selection and follow-up is presented in Figure 1. Of the 377 patients assessed for eligibility, 309 were enrolled in the study from July 2012 to February 2014. Follow-up was completed in August 2017. Of these 309 patients, 154 were randomized to the lanreotide group and 155 to the control group. Four patients withdrew immediately after randomization without having efficacy or adverse event data collected (1 in the lanreotide and 3 in the control group). Therefore, the primary efficacy as well as the safety analysis includes 153 patients in the lanreotide group and 152 in the control group.
Figure 1. Patient Enrollment and Flow Through the Study of Lanreotide for Polycystic Kidney Disease.
Enrollment occurred from July 2012 to February 2014, and follow-up of the 2.5-year trial was completed in August 2017. Supplement 3 contains additional information regarding specific reasons for exclusion and withdrawal.
aIf patients did not complete the study, eGFR data for the primary efficacy analysis (slope of eGFR decline on treatment) was based on all available data points as long as medication was given.
bFor the safety analysis, all information was used until patients withdrew consent or were lost to follow-up.
Demographic and clinical characteristics at baseline were balanced between both study groups (Table 1). Thirty-five patients withdrew from the lanreotide group and 9 from the control group. No differences in baseline characteristics were noted per randomization group between patients who completed and patients who withdrew during the study (eTable 1 in Supplement 3). Mean duration of the treatment phase was 104 weeks (95% CI, 98-109) for the lanreotide group and 117 weeks (95% CI, 114-119) for the control group. Lanreotide was down-titrated in 26 patients (to 90 mg, subcutaneously, once every 4 weeks in 21 patients and to 60 mg, subcutaneously, once every 4 weeks in 5 patients). In 14 patients, this titration was done per protocol because they reached an eGFR less than 30 mL/min/1.73 m2. Thirty-five patients (23%) stopped lanreotide early, of whom 20 (13%) did so because of adverse events. In the patients who continued receiving lanreotide, the mean dose that was given at the end of the treatment phase was 112 mg.
Table 1. Baseline Demographic and Clinical Characteristics.
| Characteristic | Lanreotide (n = 153) |
Control (n = 152) |
|---|---|---|
| Men, No. (%) | 71 (46.4) | 71 (46.7) |
| Women, No. (%) | 82 (53.6) | 81 (53.3) |
| Age, mean (SD), y | 48.2 (7.4) | 48.5 (7.2) |
| Race, No. (%)a | ||
| White | 147 (96.1) | 148 (97.4) |
| Asian | 2 (1.3) | 3 (2.0) |
| Missing | 4 (2.6) | 1 (0.7) |
| PKD genotypeb, No. (%) | ||
| PKD1 truncating | 68 (44.4) | 70 (46.1) |
| PKD1 nontruncating | 37 (24.2) | 41 (27.0) |
| PKD2 | 37 (24.2) | 27 (17.8) |
| No mutation detected | 6 (3.9) | 9 (5.9) |
| Missing | 5 (3.3) | 5 (3.3) |
| Height, mean (SD), m | 1.77 (0.1) | 1.76 (0.1) |
| Weight, mean (SD), kg | 84.5 (16.5) | 83.6 (17.3) |
| BMI, mean (SD) | 26.9 (4.5) | 27.1 (4.8) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 132.3 (12.6) | 133.4 (14.0) |
| Diastolic | 82.3 (9.0) | 82.1 (10.0) |
| Antihypertensive medication, No. (%) | 134 (87.6) | 136 (89.5) |
| RAAS blocker, No. (%) | 124 (81.1) | 126 (82.9) |
| Serum creatinine, mean (SD), mg/dL | 1.46 (0.3) | 1.45 (0.3) |
| eGFRc, mean (SD), mL/min/1.73 m2 | 51.0 (11.5) | 51.4 (11.2) |
| CKD stagesd, No. (%) | ||
| 2 (mild CKD) | 41 (26.8) | 41 (27.0) |
| 3a (mild to moderate CKD) | 55 (35.9) | 62 (40.8) |
| 3b (moderate to severe CKD) | 57 (37.3) | 47 (30.9) |
| 4 (severe CKD) | 0 | 2 (1.3) |
| TKV, mL | 2046 (1383-2964) | 1874 (1245-2868) |
| htTKV, median (IQR), mL/m | 1138 (790-1670) | 1029 (723-1668) |
| ADPKD class, No. (%)e | ||
| 1A/1B (low-risk disease) | 24 (15.7) | 25 (16.4) |
| 1C/1D/1E (high-risk disease) | 119 (77.8) | 120 (78.9) |
| 2 (atypical disease) | 6 (3.9) | 5 (3.3) |
Abbreviations: BMI, body mass index calculated as weight in kilograms divided by height in meters squared; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; htTKV, height-adjusted total kidney volume; PKD, polycystic kidney disease; RAAS, renin angiotensin aldosterone system; TKV, total kidney volume.
As determined by the researcher.
Mutation analysis was done by Sanger sequencing and multiplex ligation-dependent probe amplification. No GANAB, HNF1-β, or PKHD1 mutations were detected.
eGFR inclusion criterion for the trial was calculated with creatinine at the screening visit and the modification of diet in renal disease equation, whereas by protocol amendment eGFR results for the trial are calculated for all time points with the CKD-EPI equation.18
Higher CKD stage indicates more impaired kidney function.
Mayo ADPKD classification predicts prognosis, and is based on total kidney volume indexed for height and age. Classes 1C, 1D, and 1E indicate a worse prognosis than classes 1A and 1B. Class 2 is atypical disease, where no prognosis can be assessed.
Primary Outcome
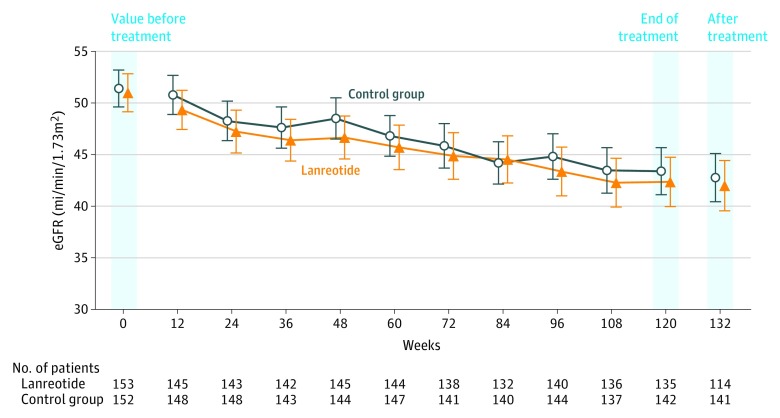
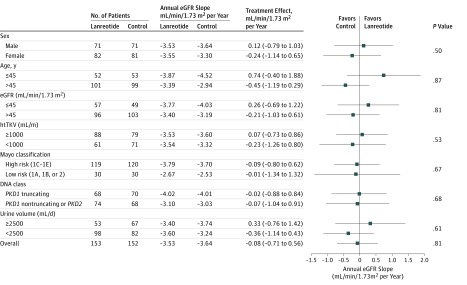
During the first 12 weeks of treatment, a slight, statistically significant eGFR decline of −1.6 mL/min/1.73 m2 (95% CI, −2.40 to −0.78) was observed in the lanreotide group compared with −0.6 mL/min/1.73 m2 (95% CI, −1.20 to 0.10) in the control group. During the 2.5-year treatment period, the slope of eGFR decline was −3.53 mL/min/1.73 m2 per year (95% CI, −4.00 to −3.07) in the lanreotide group vs −3.46 mL/min/1.73 m2 per year (95% CI, −3.89 to −3.02) in the control group (Figure 2). The mean difference in slope of eGFR decline between both groups was −0.08 mL/min/1.73 m2 per year and was not significant (95% CI, −0.71 to 0.56; P = .81). A prespecified subgroup analysis did not provide evidence that lanreotide improved the primary outcome in any of the subgroups studied, including patients with more rapidly progressive disease, such as patients with class 1C, 1D, or 1E Mayo-classified ADPKD (Figure 3).
Figure 2. Effect of Lanreotide and Standard Care Compared With Standard Care Only on Change in Kidney Function in Patients With Autosomal Dominant Polycystic Kidney Disease.
Kidney function over time, depicting mean and 95% CIs for estimated glomerular filtration rate (eGFR).
Figure 3. Effect of Lanreotide and Standard Care Compared With Standard Care Only on Estimated Glomerular Filtration Rate (eGFR) in Patients With Autosomal Dominant Polycystic Kidney Disease.
The effect of lanreotide on slope of eGFR decline during the treatment phase according to prespecified baseline subgroups. htTKV indicates height-adjusted total kidney volume.
Secondary Outcomes
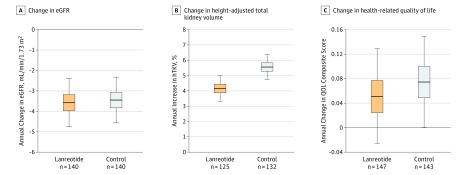
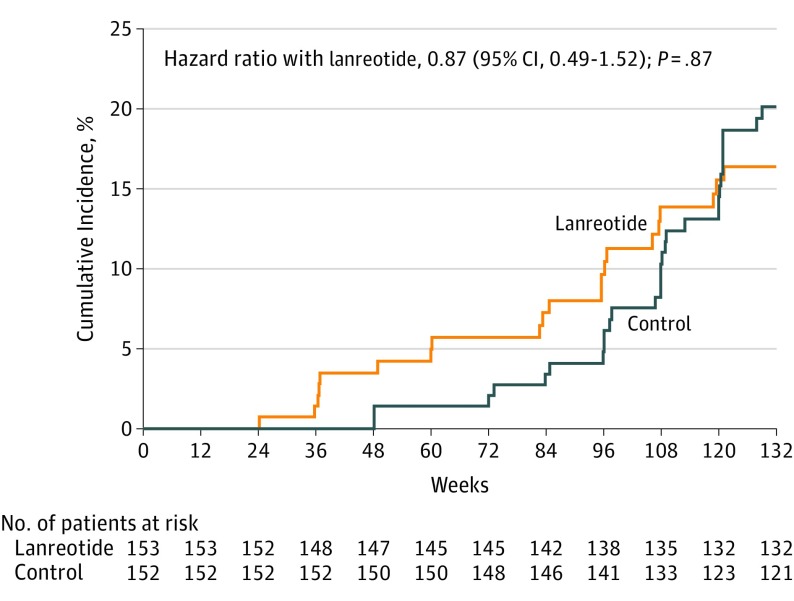
Four secondary outcomes were analyzed. The change in eGFR between pretreatment and posttreatment visits was −3.58 mL/min/1.73 m2 per year in the lanreotide group vs −3.45 mL/min/1.73 m2 in the control group. The mean difference between the groups was not significant (−0.13 mL/min/1.73 m2 per year [95% CI, −1.76 to 1.50]; P = .88) (Figure 4A). The incidence of worsening kidney function was also not significantly different between groups, with 21 patients in the lanreotide group and 29 in the control group reaching this outcome (including 3 patients in the lanreotide group and 2 in the control group who started kidney replacement therapy), resulting in a hazard ratio of 0.87 (95% CI, 0.49-1.52; P = .87) with lanreotide (Figure 5). The rate of change in htTKV between the pretreatment and posttreatment visits was significantly lower in the lanreotide group, with 4.15% per year in the lanreotide group and 5.56% per year in the control group (Figure 4B) (difference, −1.33% per year [95% CI, −2.41 to −0.24]; P = .02), corresponding with a 24% reduction in htTKV growth rate. Beneficial effects of lanreotide on increase in htTKV were observed in all subgroups tested (eFigure 3 in Supplement 3).
Figure 4. Effect of Lanreotide and Standard Care Compared With Standard Care Only on Secondary Outcomes.
A, Change in kidney function, calculated as change in estimated glomerular filtration rate (eGFR) measured 12 weeks after the end of treatment visit (ie, at the posttreatment visit) compared with the pretreatment value (difference, −0.13 mL/min/1.73 m2 per year [95% CI, −1.76 to 1.50]; P = .88). B, Change in height-adjusted total kidney volume (htTKV; difference, −1.33% per year [95% CI, −2.41 to −0.24]; P = .02). C, Change in health-related quality of life (QOL; difference −0.03 units per year [95% CI, −0.13 to 0.08]; P = .67). QOL is measured on a scale ranging from 1 (not bothered) to 5 (extremely bothered). For all panels, boxplots show predicted mean and 25th and 75th percentile, and lower and upper ends of the error bars show predicted 2.5th and 97.5th percentile, respectively, as derived from the mixed model analyses.
Figure 5. Effect of Lanreotide and Standard Care Compared With Standard Care Only on the Secondary Outcome of Worsening Kidney Function .
The cumulative incidence of worsening kidney function (30% decrease in estimated glomerular filtration rate or start of dialysis). Mean duration of the treatment phase was 104 and 117 weeks for the lanreotide and control group, respectively.
A post hoc model assessment indicated that 1 patient in the lanreotide group was an extreme outlier (high leverage) and influential to the model fit. This patient had a very steep increase in htTKV of 61% per year due to the rare event of a pathogenic truncating mutation in the PKD1 gene as well as a pathogenic truncating mutation in the PKD2 gene (Supplement 3). When this patient was removed from the analyses, the difference in htTKV growth rate between the lanreotide and control groups increased (3.84% and 5.56% per year, respectively; difference, −1.62% per year [95% CI, −2.59 to −0.64]; P = .001).
Detailed information on the change in htTKV can be found in eTables 2A and 2B in Supplement 3. These tables also show that, after stopping lanreotide, there was a modest rebound effect resulting in an increase in htTKV that was not observed in the control group. Quality of life was not affected in the lanreotide or control group, as measured by composite score (Figure 4C) (0.05 vs 0.07, respectively; difference, −0.03 units per year [95% CI, −0.13 to 0.08]; P = .67) or any of the 3 quality of life domains (ie, physical, emotional, fatigue). Prespecified subgroup analyses for the secondary outcomes did not show a consistent pattern suggesting benefit from lanreotide in any of the subgroups studied (eFigures 1-4 in Supplement 3).
Post Hoc Analyses
Additional post hoc analyses were performed. First, creatinine was measured in a central laboratory after completion of the trial. To verify that taking measurements after completion of the study had not affected the results, the primary outcome of the study was also calculated using creatinine values that were measured during the trial in the laboratories of the 4 participating centers. Similar results were obtained, with a slope of eGFR decline in the lanreotide group of −3.45 and in the control group of −3.50 mL/min/1.73 m2 per year (difference, −0.05 mL/min/1.73 m2 per year [95% CI, −1.85 to 1.95]; P = .96). Second, some of the endpoints are based on GFR estimated with creatinine instead of GFR measured with exogenous tracers. We checked, therefore, whether this procedure may have influenced the results. No differences in 24-hour urinary creatinine excretion, 24-hour urinary urea excretion, serum urea, or plasma cystatin C were observed between the groups at any time during the study (eTable 3 in Supplement 3). No significant difference between the lanreotide and control groups was found when the primary outcome was assessed as a slope using 24-hour creatinine clearance (−4.66 vs −5.50, respectively; difference, 0.87 mL/min/1.73 m2 per year [95% CI, −1.44 to 3.18]; P = .46) or eGFR cystatin C (−3.67 vs −3.34, respectively; difference, −0.34 mL/min/1.73 m2 per year [95% CI, −1.98 to 1.30]; P = .69) as measures for kidney function. Therefore, the primary data seem robust. Third, change in htTKV was also assessed using data from the MR images obtained at week 120 at the end-of-treatment visit instead of the MR images obtained at week 132 at the posttreatment visit (eTable 2 in Supplement 3). The difference between the lanreotide and control groups in the htTKV growth rate at week 120 was stronger (3.55 vs 5.81, respectively; difference, −2.14% per year [95% CI, −3.14% to −1.12%]; P < .001). Fourth, changes in eGFR from baseline to the end-of-treatment visit vs changes in htTKV from baseline to the end-of-treatment visit were correlated in the control group, but not in the lanreotide group (r = −0.26, P = .002, and r = 0.07, P = .45, respectively). In addition, blood pressure was not different between the groups throughout the trial, with difference in systolic blood pressure ranging from −1.1 to 1.9 mm Hg (eTable 6 in Supplement 3).
Adverse Events
Adverse events in the lanreotide group were predominantly related to the injection site (eg, pain, nodule, papule) or gastrointestinal (eg, feces abnormalities, abdominal discomfort, nausea) (Table 2 and eTable 4 in Supplement 3). A total of 62 patients had 84 serious adverse events, of which 55 occurred in the lanreotide group and 29 in the control group (Table 2 and eTable 5 in Supplement 3). Hepatic cyst infections occurred more frequently in the lanreotide group (9 instances in 8 patients). These hepatic cyst infections were managed with antibiotics and resolved without sequelae. Other serious adverse events potentially related to lanreotide treatment occurred as often in the control group, or were rare in the lanreotide group (Table 2). The physical examination and additional laboratory tests did not provide additional safety signal besides slight, significant increases in serum γ glutamyltransferase, glucose, and glycosylated hemoglobin (eTables 6 and 7 in Supplement 3). One patient died during the study. In this patient, lanreotide treatment was stopped after 24 weeks when a squamous cell lung carcinoma was discovered, which was the cause of death 6 months later.
Table 2. Common Adverse Events and Serious Adverse Eventsa,b.
| Patients Who Had Adverse Event, No. (%) | ||
|---|---|---|
| Lanreotide Group (n = 153) |
Control Group (n = 152) |
|
| Adverse events | ||
| Any adverse event | 153 (100) | 151 (99) |
| Adverse event leading to withdrawal | 16 (10) | 0 |
| Specific adverse events | ||
| Abnormal feces | 139 (91) | 10 (6.6) |
| Abdominal discomfort | 121 (79) | 30 (20) |
| Fatigue | 64 (42) | 32 (21) |
| Injection site discomfort | 49 (32) | 1 (0.7) |
| Nausea | 45 (29) | 7 (4.6) |
| Dizziness | 31 (20) | 12 (7.9) |
| Flatulence | 27 (18) | 0 |
| Bradycardia | 23 (15) | 9 (5.9) |
| Alopecia | 16 (10) | 0 |
| Chest pain | 12 (7.8) | 2 (1.3) |
| Decreased appetite | 11 (7.2) | 1 (0.7) |
| Injection papule | 9 (5.9) | 0 |
| Glycated hemoglobin increased | 8 (5.2) | 1 (0.7) |
| Influenza-like illness | 31 (20) | 46 (30) |
| Nasopharyngitis | 19 (12) | 37 (24) |
| Serious adverse events | ||
| Any serious adverse event | 43 (28) | 19 (12.5) |
| Serious adverse event leading to withdrawal | 4 (2.6) | 2 (1.3) |
| Specific serious adverse events | ||
| Hepatic cyst infectionc | 8 (5.2) | 0 |
| Renal cyst infection | 3 (2) | 3 (2) |
| Pyelonephritis | 2 (1.3) | 1 (0.7) |
| Epigastric pain | 2 (1.3) | 0 |
| Fever | 2 (1.3) | 0 |
| Urinary tract infection | 1 (0.7) | 2 (1.3) |
| Pancreatitis | 1 (0.7) | 0 |
| Cholelithiasis | 1 (0.7) | 0 |
Listed are all adverse events with an incidence >5% that occurred significantly more often in the lanreotide or control group, and serious adverse events with an incidence >2% or that were at least possibly related to lanreotide treatment.
Adverse events were collected by spontaneous report. A full list of adverse events as well as of serious adverse events is provided as eTables 4 and 5 in Supplement 3. Adverse events were categorized according to the preferred terms of the Medical Dictionary for Regulatory Activities.
There were 8 patients with 9 instances of hepatic cyst infections. In 2 of these patients, this event led to treatment withdrawal. In the other 6 patients, treatment was withdrawn later by the investigator because of a protocol amendment.
Discussion
Among patients with later-stage ADPKD, treatment with lanreotide compared with standard care did not slow the decline in kidney function over 2.5 years of follow-up.
When this trial was started in 2011, limited clinical data were available on the efficacy of somatostatin analogues to preserve kidney function in patients with ADPKD (eTable 8 in Supplement 3). These studies suggested a beneficial effect, especially on the rate of TKV growth, in patients with ADPKD.12,13,14,15 During the trial the results of the ALADIN study became available. This study investigated the effects of 3 years of treatment with the somatostatin analogue octreotide in 75 patients with ADPKD with an eGFR greater than 40 mL/min/1.73 m2.26 For the prespecified efficacy outcomes, no statistically significant effect was observed. However, a positive effect of octreotide was seen in post hoc analyses for slope in GFR decline on treatment and for change in TKV. Differences in baseline characteristics, which favored the octreotide group, did not allow firm conclusions.26 In the present larger trial, the characteristics of both study groups were balanced. No effect was found on the prespecified primary outcome slope of eGFR decline on treatment or on any other eGFR-related outcome. The exploratory secondary outcome data do, however, suggest an association between lanreotide and rate of htTKV growth, as in the ALADIN study.
It has been hypothesized that in patients with ADPKD, drug effects on htTKV can be used as surrogate for possible effects on kidney function.27 The present data suggest that the effects of lanreotide on eGFR and TKV are divergent and therefore may seem unrelated. However, it could also be that lanreotide has an intrinsic nephrotoxic effect that offsets any potential benefit that could be obtained from its effect on htTKV. Such a nephrotoxic effect is, however, not known from literature in patients without ADPKD. Other potential explanations could be that the association with TKV growth was not large enough to translate into a functional benefit during the duration of the clinical trial, that lanreotide was given in too low of a dose to have an effect on eGFR decline, or that the absence of a correlation may be because patients were included with later stage ADPKD, in whom growth in TKV may have a less dominant role in causing eGFR decline than in patients with earlier-stage ADPKD.27
It remains uncertain whether the present results are specific for lanreotide or are class related. There are differences between somatostatin analogues with respect to their affinity for the various somatostatin receptor subtypes that can be found along the renal tubule,6,28,29,30,31 as well as differences in efficacy and adverse event profile.9,11,32,33 In addition, it has been suggested that renoprotective drugs may be less efficacious in later-stage ADPKD.27 Subgroup analysis, however, did not reveal an interaction between treatment efficacy and disease stage.
Lanreotide-related adverse events were, in general, to be expected from the known adverse event profile of this drug, and were predominantly injection-site–related or gastrointestinal. Most of the gastrointestinal symptoms occurred in the first months of treatment, were mild to moderate in intensity, and resolved spontaneously. With respect to serious adverse events, several hepatic cyst infections were observed with lanreotide treatment, especially in patients with a history of liver cyst infections. This may be a disease-specific adverse event of lanreotide, but, with other somatostatin analogues, hepatic cyst infections have also been observed in patients with ADPKD.34,35
The percentage of patients who stopped lanreotide, including those who did so because of adverse events, is similar to the percentage of patients with ADPKD who stopped treatment with the vasopressin V2 receptor antagonist tolvaptan in the TEMPO 3:4 study (23% and 15%, respectively).36 This latter study showed that tolvaptan treatment decreased the rate of growth in TKV during treatment, similarly as the present study with lanreotide. In contrast to lanreotide, tolvaptan also improved the rate of decline in kidney function.36,37 Both drugs are supposed to lower intracellular cyclic AMP by inhibiting adenylyl cyclase at the basolateral membrane of renal tubular cells,5 tolvaptan by blocking the vasopressin V2 receptor and lanreotide via stimulation of the somatostatin type 2 receptor.5 Additional research is needed to explain the difference in renoprotective efficacy between these drugs. Somatostatin analogues and tolvaptan have been suggested to inhibit different isoforms of adenylyl cyclase38 via different mechanisms,6 and that the 2 G protein-coupled receptors may interact with different downstream proteins.39
The dropout rate was higher in the lanreotide group than in the control group (23% vs 6%). This rate is not expected to have had a major effect on the results because the primary outcome, slope through serial eGFR values on treatment only, is not affected by early stopping of treatment. In addition, the secondary endpoint, change in eGFR before treatment vs after treatment, does incorporate information on patients that stopped treatment early. Both analyses, although different in design, lead to the same conclusion that lanreotide did not improve the rate of eGFR decline.
Limitations
This study has several limitations. First, it had an open-label design. To minimize bias, the primary and most secondary endpoints were chosen to be based on objectively measured variables, which were assessed centrally by personnel blinded for treatment allocation. Second, the study population consisted predominantly of white patients. Whether the results hold true for other races requires additional study. Third, the rate of eGFR decline in the control group was less than expected in the power analysis (−3.46 vs −5.1 mL/min/1.73 m2 per year), but similar to recent literature (−3.5, −3.70, and −3.61 mL/min/1.73 m2 per year in the Everolimus, TEMPO 3:4, and REPRISE trials, respectively).36,37,40 The slower rate of eGFR decline in the control group did, moreover, not affect the power because the SD of the rate of eGFR decline in the control group also was lower than expected (2.72 instead of 4.2).
Conclusions
Among patients with later-stage ADPKD, treatment with lanreotide compared with standard care did not slow the decline in kidney function over 2.5 years of follow-up. These findings do not support the use of lanreotide to preserve kidney function in later-stage ADPKD.
Trial Protocol
Statistical Analysis Plan
eFigure 1: Subgroup analysis for the secondary outcome change in kidney function pre- versus post-treatment
eFigure 2: Subgroup analysis for the secondary outcome incidence of worsening kidney function
eFigure 3. Subgroup analysis for the secondary outcome change in height adjusted total kidney volume pre- versus post-treatment
eFigure 4.Subgroup analysis for the secondary outcome impact of lanreotide on quality of life
eTable 1. Demographic and clinical characteristics of the patients at baseline stratified according to randomization group, and per randomization group to whether patients completed or discontinued to study early
eTable 2. Change in htTKV in- and excluding the patient with very rapid disease progression
eTable 3. Summary of Key Laboratory Efficacy Parameters
eTable 4. Most common Adverse Events
eTable 5. All Serious Adverse Events
eTable 6. Summary of Key Physical Parameters
eTable 7. Summary of Key Laboratory Safety Parameters
eTable 8. Summary of studies performed with somatostatin analogues in ADPKD
Data Sharing Statement
References
- 1.Ong AC, Devuyst O, Knebelmann B, Walz G, ERA-EDTA Working Group for Inherited Kidney Diseases . Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385(9981):1993-2002. doi: 10.1016/S0140-6736(15)60907-2 [DOI] [PubMed] [Google Scholar]
- 2.Willey CJ, Blais JD, Hall AK, Krasa HB, Makin AJ, Czerwiec FS. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. 2017;32(8):1356-1363.27325254 [Google Scholar]
- 3.Spithoven EM, Kramer A, Meijer E, et al. . Renal replacement therapy for ADPKD in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(suppl 4):iv15-iv25. doi: 10.1093/ndt/gfu017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith LA, Bukanov NO, Husson H, et al. . Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol. 2006;17(10):2821-2831. doi: 10.1681/ASN.2006020136 [DOI] [PubMed] [Google Scholar]
- 5.Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol. 2014;25(1):18-32. doi: 10.1681/ASN.2013040398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20(3):157-198. doi: 10.1006/frne.1999.0183 [DOI] [PubMed] [Google Scholar]
- 7.Friedlander G, Amiel C. Somatostatin and alpha 2-adrenergic agonists selectively inhibit vasopressin-induced cyclic AMP accumulation in MDCK cells. FEBS Lett. 1986;198(1):38-42. doi: 10.1016/0014-5793(86)81180-2 [DOI] [PubMed] [Google Scholar]
- 8.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132(3):1104-1116. doi: 10.1053/j.gastro.2006.12.039 [DOI] [PubMed] [Google Scholar]
- 9.Masyuk TV, Radtke BN, Stroope AJ, et al. . Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58(1):409-421. doi: 10.1002/hep.26140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopp K, Hommerding CJ, Wang X, Ye H, Harris PC, Torres VE. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol. 2015;26(1):39-47. doi: 10.1681/ASN.2013121312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kugita M, Nishii K, Yamaguchi T, et al. . Beneficial effect of combined treatment with octreotide and pasireotide in PCK rats, an orthologous model of human autosomal recessive polycystic kidney disease. PLoS One. 2017;12(5):e0177934. doi: 10.1371/journal.pone.0177934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Remuzzi A, Ondei P, et al. . Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68(1):206-216. doi: 10.1111/j.1523-1755.2005.00395.x [DOI] [PubMed] [Google Scholar]
- 13.van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, de Man RA, Drenth JP. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137(5):1661-1668. [DOI] [PubMed] [Google Scholar]
- 14.Hogan MC, Masyuk TV, Page LJ, et al. . Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052-1061. doi: 10.1681/ASN.2009121291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Wetzels JF, Drenth JP. Effect of lanreotide on polycystic liver and kidneys in autosomal dominant polycystic kidney disease: an observational trial. Liver Int. 2015;35(5):1607-1614. doi: 10.1111/liv.12726 [DOI] [PubMed] [Google Scholar]
- 16.Meijer E, Drenth JP, D’Agnolo H, et al. . Rationale and design of the DIPAK 1 study: a randomized controlled clinical trial assessing the efficacy of lanreotide to Halt disease progression in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2014;63(3):446-455. doi: 10.1053/j.ajkd.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei Y, Obaji J, Dupuis A, et al. . Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205-212. doi: 10.1681/ASN.2008050507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spithoven EM, van Gastel MD, Messchendorp AL, et al. . Estimation of total kidney volume in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2015;66(5):792-801. doi: 10.1053/j.ajkd.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 20.Oberdhan D, Cole JC, Krasa HB, et al. . Development of the autosomal dominant polycystic kidney disease impact scale: a new health-related quality-of-life instrument. Am J Kidney Dis. 2018;71(2):225-235. doi: 10.1053/j.ajkd.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 21.Lantinga MA, D’Agnolo HM, Casteleijn NF, et al. . Hepatic cyst infection during use of the somatostatin analog lanreotide in autosomal dominant polycystic kidney disease: an interim analysis of the randomized open-label multicenter DIPAK-1 study. Drug Saf. 2017;40(2):153-167. doi: 10.1007/s40264-016-0486-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt A, Pleiner J, Schaller G, et al. . Renal hemodynamic effects of somatostatin are not related to inhibition of endogenous insulin release. Kidney Int. 2002;61(5):1788-1793. doi: 10.1046/j.1523-1755.2002.00320.x [DOI] [PubMed] [Google Scholar]
- 23.Ihle BU, Becker GJ, Whitworth JA, Charlwood RA, Kincaid-Smith PS. The effect of protein restriction on the progression of renal insufficiency. N Engl J Med. 1989;321(26):1773-1777. doi: 10.1056/NEJM198912283212601 [DOI] [PubMed] [Google Scholar]
- 24.Beck GJ, Berg RL, Coggins CH, et al. . Design and statistical issues of the modification of diet in renal disease trial. Control Clin Trials. 1991;12(5):566-586. doi: 10.1016/0197-2456(91)90069-X [DOI] [PubMed] [Google Scholar]
- 25.Wright JT Jr, Bakris G, Greene T, et al. . Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421-2431. doi: 10.1001/jama.288.19.2421 [DOI] [PubMed] [Google Scholar]
- 26.Caroli A, Perico N, Perna A, et al. . Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382(9903):1485-1495. doi: 10.1016/S0140-6736(13)61407-5 [DOI] [PubMed] [Google Scholar]
- 27.Grantham JJ, Torres VE. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol. 2016;12(11):667-677. doi: 10.1038/nrneph.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofland LJ, Lamberts SWJ. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24(1):28-47. doi: 10.1210/er.2000-0001 [DOI] [PubMed] [Google Scholar]
- 29.Bhandari S, Watson N, Long E, et al. . Expression of somatostatin and somatostatin receptor subtypes 1-5 in human normal and diseased kidney. J Histochem Cytochem. 2008;56(8):733-743. doi: 10.1369/jhc.2008.950998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates CM, Kegg H, Grady S. Expression of somatostatin receptors 1 and 2 in the adult mouse kidney. Regul Pept. 2004;119(1-2):11-20. doi: 10.1016/j.regpep.2003.12.015 [DOI] [PubMed] [Google Scholar]
- 31.Bates CM, Kegg H, Petrevski C, Grady S. Expression of somatostatin receptors 3, 4, and 5 in mouse kidney proximal tubules. Kidney Int. 2003;63(1):53-63. doi: 10.1046/j.1523-1755.2003.00716.x [DOI] [PubMed] [Google Scholar]
- 32.Gadelha MR, Bronstein MD, Brue T, et al. . Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875-884. doi: 10.1016/S2213-8587(14)70169-X [DOI] [PubMed] [Google Scholar]
- 33.McKeage K. Pasireotide in acromegaly: a review. Drugs. 2015;75(9):1039-1048. doi: 10.1007/s40265-015-0413-y [DOI] [PubMed] [Google Scholar]
- 34.Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. J Hepatol. 2013;59(1):153-159. doi: 10.1016/j.jhep.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 35.Hogan MC, Masyuk TV, Page L, et al. . Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27(9):3532-3539. doi: 10.1093/ndt/gfs152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres VE, Chapman AB, Devuyst O, et al. . Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407-2418. doi: 10.1056/NEJMoa1205511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres VE, Chapman AB, Devuyst O, et al. . Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377(20):1930-1942. doi: 10.1056/NEJMoa1710030 [DOI] [PubMed] [Google Scholar]
- 38.Rieg T, Kohan DE. Regulation of nephron water and electrolyte transport by adenylyl cyclases. Am J Physiol Renal Physiol. 2014;306(7):F701-F709. doi: 10.1152/ajprenal.00656.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moeller HB, Fenton RA. Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch. 2012;464(2):133-144. doi: 10.1007/s00424-012-1129-4 [DOI] [PubMed] [Google Scholar]
- 40.Walz G, Budde K, Mannaa M, et al. . Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):830-840. doi: 10.1056/NEJMoa1003491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1: Subgroup analysis for the secondary outcome change in kidney function pre- versus post-treatment
eFigure 2: Subgroup analysis for the secondary outcome incidence of worsening kidney function
eFigure 3. Subgroup analysis for the secondary outcome change in height adjusted total kidney volume pre- versus post-treatment
eFigure 4.Subgroup analysis for the secondary outcome impact of lanreotide on quality of life
eTable 1. Demographic and clinical characteristics of the patients at baseline stratified according to randomization group, and per randomization group to whether patients completed or discontinued to study early
eTable 2. Change in htTKV in- and excluding the patient with very rapid disease progression
eTable 3. Summary of Key Laboratory Efficacy Parameters
eTable 4. Most common Adverse Events
eTable 5. All Serious Adverse Events
eTable 6. Summary of Key Physical Parameters
eTable 7. Summary of Key Laboratory Safety Parameters
eTable 8. Summary of studies performed with somatostatin analogues in ADPKD
Data Sharing Statement