Key Points
Question
What is the association of institutional availability of multimodal analgesia pathways with adoption and adherence to such pathways, and the frequency of opioid prescriptions at discharge following thyroid and parathyroid surgery?
Findings
In this cohort study of 528 adults who underwent thyroid and parathyroid surgery, availability of institutional multimodal analgesia pathways was associated with progressive increase in adoption and adherence to the multimodal analgesia pathway and with reduced frequency of opioid prescriptions on discharge.
Meaning
Availability of effective nonopioid multimodal analgesia pathways can favorably influence physician prescribing practices, encourage adherence to pathway components, and avoid unnecessary opioid prescriptions following thyroid and parathyroid surgery.
This cohort study assesses the association of institutional availability of multimodal analgesia pathways with adoption and adherence to such pathways, and the frequency of opioid prescriptions at discharge among adults following thyroid and parathyroid surgery.
Abstract
Importance
Prescription opioid use contributes to drug-related adverse effects and risk for dependence and abuse. Multimodal analgesia (MMA) has been shown to be useful in reducing opioid use following orthopedic, gynecologic, and colorectal surgery, but adoption in head and neck surgery has lagged. Recently, we published findings related to the feasibility of MMA protocols in same-day thyroid, parathyroid, and parotid surgery. However, whether such strategies lead to effective and durable reduction in frequency of opioid prescriptions, and affect physician prescribing practices, remains unclear.
Objective
To observe trends in adoption and adherence to institutional MMA protocols following thyroid and parathyroid surgery, and to assess the association of institutional multimodal (nonopioid) analgesia protocols with opioid use and physician prescribing patterns following outpatient thyroid and parathyroid surgery.
Design, Setting, and Participants
Cohort study at a head and neck surgery service at a tertiary care hospital of prescription patterns and retrospective review of patient medical records following implementation of an optional institutional MMA protocol in 2015, based on preoperative administration of acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and gabapentin, and postoperative use of acetaminophen and ibuprofen for analgesia after thyroid and parathyroid surgery. There were 528 adult patients who underwent thyroid and parathyroid surgery between January 1, 2015, and June 30, 2017.
Main Outcomes and Measures
We report on adherence to the MMA protocol over the study period as measure of physician buy-in and adoption of the technique. The frequency of opioid use and physician prescription patterns following thyroid and parathyroid surgery is reported over the study period to study the association of the available MMA pathway with these variables.
Results
A total of 528 patients (mean [SD] age, 53.1 [15.7] years; 80.3% female) underwent outpatient thyroid and parathyroid surgery. The frequency of postoperative opioid prescriptions decreased during the study period (16 of 122 [13.1%] in 2015, 22 of 244 [9.0%] in 2016, 3 of 162 [1.9%] in 2017). Adherence to the MMA protocol increased (0 of 122 cases in 2015, 106 of 244 [43.4%] cases in 2016, 142 of 162 [87.7%] cases in 2017), with reduced likelihood of opioid prescription on discharge (2017 vs 2015 odds ratio, 0.13; 95% CI, 0.04-0.44). Only 1 postoperative hematoma was recorded in the study cohort, and 352 (66.7%) patients achieved same-day discharge, whereas 176 (33.3%) maintained outpatient status but received overnight observation prior to discharge.
Conclusions and Relevance
Adoption and adherence to the MMA protocol increased substantially over the study period for patients undergoing thyroid and parathyroid surgery and was associated with a simultaneous significant decline in prescription of postoperative opioid analgesics. Use of nonopioid multimodal agents, incorporating NSAIDs, was safe and did not lead to increased incidence of bleeding. Availability of effective nonopioid MMA pathways may favorably influence physician prescribing practices and avoid unnecessary opioid prescriptions.
Introduction
Prescription opioids are a substantial contributor to drug-related adverse effects and risk for dependence and abuse.1 Expected adverse effects of opioid medications are well known and frequently include drowsiness, delirium, constipation, and nausea, among others.2 In addition, multiple governing bodies, including the US Food and Drug Administration, Centers for Disease Control and Prevention (CDC), the Surgeon General, the White House, and numerous medical organizations and state boards, have recognized and highlighted the increasing use of opioids, rising rates of opioid addiction, and opioid-associated deaths.3,4,5 In response, several strategies are being developed to decrease adverse effects and risks associated with opioid use.
Multimodal analgesia (MMA) has been shown to be effective to control postoperative pain in patients undergoing orthopedic, colorectal, and gynecologic oncologic surgery.6,7,8 However, there has been a reluctance in instituting MMA protocols in head and neck surgery, owing to concerns over use of nonsteroidal anti-inflammatory drugs (NSAIDs) and a perceived fear of bleeding or hematoma, and skepticism about the ability of nonopioid medications to adequately control postoperative pain. The juxtaposition of these concerns with potential benefits of enhanced recovery and MMA have encouraged interest from clinicians investigating the safety, feasibility, and value of such strategies.9,10
Our institution started offering the option of a nonopioid MMA pathway to interested patients and surgical professionals in 2015. The incorporation of such a pathway in clinical care remains voluntary and based on the clinical judgment of surgical care professionals and shared decision making with the patient. Previously, we conducted a pilot study of prospectively collected data on 64 patients undergoing outpatient thyroid, parathyroid, and parotid surgery, which showed that the MMA protocol–based analgesia strategy was safe. Moreover, it was associated with high patient satisfaction for MMA strategy compared with opioid use.10 However, whether institution of MMA protocols had an effect on the frequency of opioid prescription, adoption of such protocols, and, ultimately, physician prescription patterns on discharge remained unclear. In this study, we examine institutional trends and the learning curve related to the adoption of, and adherence to, MMA protocols, and the influence such protocols on frequency of opioid prescription among patients undergoing thyroid and parathyroid surgery.
Methods
This was a retrospective study of patients who underwent outpatient thyroid and parathyroid surgery at a tertiary care hospital from January 1, 2015, to June 30, 2017. The study was approved by the Nebraska Methodist Hospital institutional review board. Owing to the minimal risk to the patient population and retrospective nature of data collection, a consent waiver was granted by the review board. Adults who underwent elective outpatient thyroid lobectomy (with or without isthmusectomy) or total thyroidectomy, with or without central neck dissection, and parathyroid exploration were included to assess for frequency of MMA protocol adherence, and trends in opioid prescription on discharge over the study duration. Patients with additional surgical sites or significant deviation from surgical plan were excluded.
At our institution, as part of established enhanced recovery pathways, patients planning to undergo elective outpatient head and neck surgery are counseled by the surgery and anesthesia teams about anticipated perioperative and postoperative pain management strategy. On availability of the MMA protocol, patients were offered the possible use of an analgesia plan using acetaminophen, NSAIDs, and gabapentin as adjuncts. Clinic-based counseling was provided about postoperative pain management using acetaminophen and NSAIDs, but patients remained aware of opportunities for escalation of analgesic choice to opioid agents, if needed for rescue. When medically appropriate, or when patients or physicians expressed discomfort with the new strategy, an opioid-based analgesia strategy was permitted based on shared decision making between the patient and their treating health care professional. Clinicians and nursing teams were provided education through departmental grand rounds, and physician champions remained available to address concerns and advise clinical teams and patients who chose to opt in to use the MMA strategy. The researchers had no input in selection of analgesia or other clinical decisions specific to individual perioperative care events.
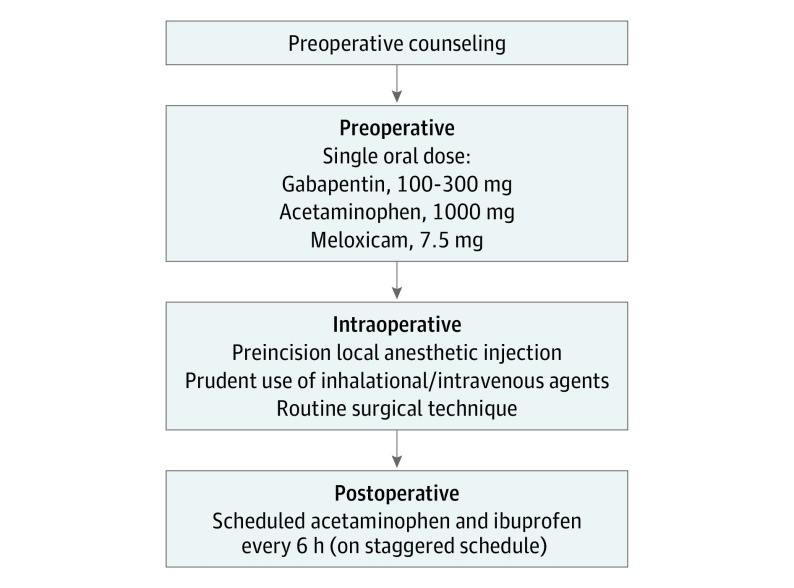
While the institutional MMA pathway has been described in detail in our previous study,10 a brief description is provided herein (Figure 1). Patients were administered a single dose of orally administered acetaminophen (1000 mg), gabapentin (100-300 mg, depending on age and creatinine clearance), and meloxicam (7.5 mg) with a sip of water approximately 1 hour before procedure initiation. The MMA protocol at our institution does not include intravenous NSAIDs, such as ketorolac; however, it allows for intravenous acetaminophen when oral administration is not considered appropriate. Patients received standard-of-care intraoperative anesthesia care, and intravenous dexamethasone and ondansetron for antiemetic effect. Planned incisions were subcutaneously infiltrated with lidocaine, 1%, with 1:100 000 epinephrine or bupivacaine, 0.25%, with 1:200 000 epinephrine solution, according to the surgeon’s choice, prior to making the incision. The procedures were performed by 6 surgeons as per their usual technique. No specific changes in technique, postoperative care, or discharge patterns were requested for patients receiving MMA. Postoperatively, patients were moved to an observation unit where oral intake of fluids and nutrition was resumed. Following resumption of oral intake, patients were encouraged to use oral ibuprofen, 600 mg, and acetaminophen, 500 mg, every 6 hours, on an alternate staggered schedule for the initial 48 hours and subsequently were allowed to use these medications as needed for discomfort. Patients were discharged home on this analgesia regimen when they met discharge criteria, typically 2 to 4 hours after procedure completion.
Figure 1. Outline of Institutional Multimodal Analgesia Protocol.
On retrospective review, cases in which the MMA protocol was used were identified, and adherence to the MMA protocol over the study period as measure of physician buy-in and adoption of the technique was recorded. Use of individual analgesia adjuncts (acetaminophen, NSAIDs, and gabapentin) and adherence to the use of a combination of all 3 agents as intended in the institutional pathway was assessed. The frequency of postdischarge opioid use and trend in physician prescription patterns were identified over the study period to study the association of the available MMA pathway with these variables. In addition, a review of clinical records was performed to identify analgesia-related adverse events, including incidence of readmission, unplanned return to emergency department, bleeding complications, renal insufficiency, or other complications directly related to the choice of analgesia strategy.
Deidentified data were collected and stored in a password-protected file on a secure drive. All analyses were performed using SAS statistical software (version 9.3; SAS Institute). Mean (SD) values are reported for normally distributed data, and median values (with range) are presented where data were not distributed normally. The Pearson χ2 test was used to assess for differences in case distribution by year. The Mantel-Haenszel χ2 test was used to identify differences in demographic characteristics, benign or malignant indication for surgery, and prescribing patterns by year of prescription. The level of significance was assigned at P < .05. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals for trends in frequency of opioid prescription on discharge.
Results
During the study period, a total of 528 patients underwent outpatient thyroid and parathyroid surgery. Their mean (SD) age was 53.1 (15.7) years, and women constituted most of the cohort (424 [80.3%]). Within the cohort, 220 patients (41.7%) were recorded as undergoing surgery for a diagnosis of malignant neoplasm. This included 157 patients (29.7%) undergoing thyroid lobectomies, 162 (30.7%) with total thyroidectomies, 63 (11.9%) undergoing total thyroidectomy with central neck dissection, 35 (6.6%) with completion thyroidectomy, 24 (4.5%) with transcervical excision of a substernal goiter, and 87 (16.5%) with parathyroid explorations. In a minority of patients in whom a central neck dissection was performed at the time of thyroidectomy, the median numbers of lymph nodes excised were 9 (range, 1-18), 11 (3-26), and 14 (4-40) for the years 2015, 2016, and 2017, respectively. There were no statistical differences in age, sex, or surgical indication (malignant vs nonmalignant disease), and procedural milieu over the study period (Table 1).
Table 1. Baseline Characteristics of Patients Undergoing Thyroid and Parathyroid Surgery.
| Characteristic | Overall | Year | ||
|---|---|---|---|---|
| 2015 | 2016 | 2017 | ||
| Age, mean (SD), y | 53.1 (15.7) | 55.6 (15.8) | 52.9 (16.4) | 51.5 (14.4) |
| Female sex, No. (%) | 424 (80.3) | 97 (79.5) | 195 (79.9) | 132 (81.5) |
| Surgical indication, malignant disease, No. (%) | 220 (41.7) | 47 (38.5) | 107 (43.9) | 66 (40.7) |
| Total No. of procedures | 528 | 122 | 244 | 162 |
| Thyroid lobectomy | 157 (29.7) | 32 (26.2) | 72 (29.5) | 53 (32.7) |
| Total thyroidectomy | 162 (30.7) | 39 (32.0) | 72 (29.5) | 51 (31.5) |
| Thyroidectomy with central neck dissection | 63 (11.9) | 16 (13.1) | 27 (11.1) | 20 (12.4) |
| Completion thyroidectomy | 35 (6.6) | 8 (6.6) | 20 (8.2) | 7 (4.3) |
| Excision of substernal goiter, transcervical approach | 24 (4.5) | 9 (7.4) | 6 (2.5) | 9 (5.6) |
| Parathyroid exploration | 87 (16.5) | 18 (14.8) | 47 (19.3) | 22 (13.6) |
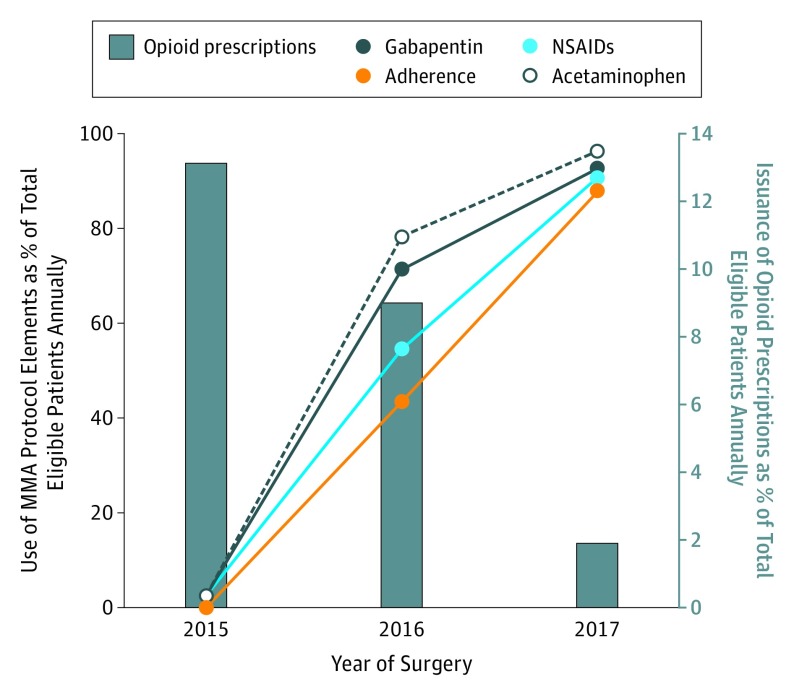
In 2015, when the MMA protocol was first offered, a total of 122 patients could qualify for the pathway. Reflecting initial reticence of patients and health care professionals, only 3 of 122 patients (2.5%) preoperatively received an NSAID, 3 (2.5%) received gabapentin, and 3 (2.5%) others received acetaminophen. None of the patients received a combination of all 3 adjunctive MMA agents that year, suggestive of lack of initial buy-in by patients and clinicians and poor adherence to the protocol. In this subgroup, 16 patients (13.1%) received an opioid prescription on discharge.
Encouragingly in 2016, 106 of 244 patients (43.4%) who qualified for the MMA protocol received the 3-agent combination preoperative MMA. Moreover, 133 patients (54.5%) received NSAIDs as part of their regimen, 174 (71.3%) received gabapentin, and 191 (78.3%) received acetaminophen. Overall, 22 patients (9.0%) were discharged with a prescription for an opioid.
During the 6 months of study period in the year 2017, a total of 162 patients met inclusion criteria. With a consistent and progressive uptick in adoption and adherence to the MMA protocol, 142 of 162 patients (87.7%) received all 3 preoperative MMA agents. In this subgroup, 147 of 162 (90.7%) received NSAIDs, 150 (92.6%) received gabapentin, and 156 (96.3%) received acetaminophen as part of their regimen. Only 3 patients (1.9%) received an opioid prescription on discharge (Table 2 and Figure 2).
Table 2. Frequency of Adherence to Multimodal Analgesia (MMA) Protocol and Opioid Prescriptions on Discharge.
| Outcome Variable | Year | ||
|---|---|---|---|
| 2015 | 2016 | 2017 | |
| Adherence (to all 3 components of MMA), No. (%) | 0 | 106 (43.4) | 142 (87.7) |
| Opioid prescription on discharge, No. (%) | 16 (13.1) | 22 (9.0) | 3 (1.9) |
| Likelihood of opioid prescription on discharge, OR (95% CI)a | 1 [Reference] | 0.66 (0.33-1.30) | 0.13 (0.04-0.44) |
Abbreviation: OR, odds ratio.
Logistic regression model.
Figure 2. Annual Frequency of Adherence to Multimodal Analgesia (MMA) Protocol, Use of Individual Components, and Issuance of Opioid Prescriptions on Discharge.
NSAIDs indicates nonsteroidal anti-inflammatory drugs.
The statistically significant increase in adoption and adherence to the MMA protocol over the study period was mirrored in the notable trends toward reduced frequency of opioid prescriptions at discharge. The odds ratio for receipt of postdischarge prescription opioids for cases performed in the year 2016 compared with those in 2015 was 0.66 (95% CI, 0.33-1.30). In 2017, with high adherence to the MMA protocol, the odds of receiving postdischarge prescription opioids were 0.13 (95% CI, 0.04-0.44) (Table 2).
During the study period, 352 of 528 patients (66.7%) were able to achieve same-day discharge, while 176 (33.3%) were assigned an observation status and spent a night in a short-stay unit. On review of 30-day complications of care, we identified 1 postoperative hematoma requiring return to the operating room. Of note, on close review of the medical records, it was found that this patient had experienced a high-speed motor vehicle collision as an unrestrained passenger prior to returning to the emergency department with a neck hematoma. No other bleeding events were identified in the patient cohort. No patients experienced renal injury, allergic reactions, or other adverse events in relation to the analgesia adjuncts used as part of the MMA protocol. Overall, 8 patients required unplanned return to the emergency department. Two patients reported dizziness on postdischarge days 4 and 13, respectively. One patient reported palpitations on postdischarge day 18 and underwent a cardiac workup with negative results. Another patient presented with altered mental status attributed to suspected overdose of antianxiety medications on postdischarge day 4. Other reasons for unplanned emergency department visits included a fall resulting in rib fracture, pyelonephritis, and paresthesia for 1 patient each. No other readmissions to the hospital were recorded in relation to the altered analgesia strategy.
Discussion
This study found that the availability of an optional nonopioid institutional MMA protocol was associated with substantial buy-in and adherence to the pathway for patients undergoing thyroid and parathyroid surgery. As expected, there was a learning curve among health care professionals who delivered perioperative care and used the MMA protocol during the study period. Increase in adoption and adherence to the components of the MMA protocol over the study duration paralleled a progressive decline in opioid prescriptions offered on discharge following thyroid and parathyroid surgery.
The use of opioid medications has traditionally been the mainstay for treatment of acute and chronic pain. Specifically, the reliance on opioids for postoperative pain management is linked to a variety of factors.11 These include societal and cultural perception of what constitutes ideal pain management, and variables linked to patient anxiety and expectations related to their postsurgical experience. In addition, health care professionals’ perspectives on analgesia, and its relation to perceived patient satisfaction and outcomes, may influence their preference in issuing opioid prescriptions. This strategy, however, is hobbled by the many adverse effects of opioid medications that have been well recognized, including pruritus, nausea, vomiting, constipation, and others.2 A reactionary approach to counter these adverse effects compels physicians to use various other medications, such as antiemetics, stool softeners, and laxatives, and contributes to increased cost and duration of hospitalization.12
The agonizing statistics related to a nationwide crisis stemming from opioid addiction and abuse have highlighted the role of prescription opioids and the unintended consequences of often well-meaning but misguided pain management strategies heavily dependent on opioid agents. The CDC data suggest that opioid-related deaths continue to increase.3 In 2015, of the 52 000 drug-related deaths in the United States, it is estimated that 33 000 deaths (63.4%) were related to opioids. Alarmingly, more than 15 000 of these deaths may have been related to prescription opioid use. These grim statistics should lead to introspection by stakeholders in a health care economy that has overseen a quadrupling of prescription opioid–related deaths since 1999, paralleling a similar increase in prescription sales.3 Although belatedly, the alarm bells are ringing loud and have drawn attention from lay public, patients, physicians, health policy advisors, health institutions, media, and lawmakers.4,5 A wide variety of policy prescriptions have been offered, including public and physician education, issuance of warnings by professional organizations and state medical boards, and in some cases, provisions that restrict opioid prescribing. In addition, some states have developed sophisticated tools that track opioid prescriptions to individual patients and generate alerts when patterns that raise concern for abuse emerge.
In specifically considering postoperative pain management, while it is still uncertain whether a short course of opioids may contribute to problems related to dependence, addiction, and abuse, it is clear that opioid prescribing behaviors significantly contribute to the opioid epidemic by adding to a pool of available medications in the community. Surveys of nonmedical opioid users suggest that pills that were either given free, stolen, or bought from friends or family who were the original prescription recipients were a substantial source of the abused opioids.3
These are some of the many reasons why health care professionals must look for alternatives that offer the promise of effective postoperative analgesia and favorable outcomes for patients, feasibility and cost-effectiveness for health care systems, and reduced potential for abuse. With these goals in mind, MMA protocols have generated considerable interest in a variety of surgical specialties, including orthopedic, colorectal, and gynecologic oncologic surgery.6,7,8 However, there have been limited data on application of such pathways in head and neck surgery. Previously, we reported10 findings from a prospective pilot study assessing the impact of nonopioid MMA strategy on pain control and patient satisfaction following outpatient thyroid, parathyroid, and parotid surgery. In that study, more than 6 in 10 patients could be discharged without opioid prescriptions, and 87.5% of patients reported “high” or “very high” satisfaction with nonopioid MMA strategy.
These encouraging findings prompted us to investigate whether the favorable patient experience with the institutional MMA strategy translated to improved physician confidence in this optional nonopioid analgesia pathway, and whether it led to improved adherence to the components of the MMA protocol and reduced frequency of opioid prescription on discharge.
We found that following establishment of the MMA protocol in 2015, health care teams caring for patients undergoing thyroid and parathyroid surgery were initially reticent in their willingness to opt in to the alternative analgesia pathway. However, adoption and adherence to the protocol components improved progressively over the course of the study duration to 87.7% of patients operated on in the first half of the year 2017. This trend was associated with a statistically significant decline in prescription of opioids on discharge over this period. This gradual change likely reflects the clinicians’ learning curve, educational efforts centered around the availability of the MMA protocol, and a pool of participants characterized by a variety of clinical backgrounds, personal experiences, varied levels of risk tolerance, and a mix of early and late adopters. The optional nature of the MMA pathway at our institution and the gradual but favorable trends in adoption, however, reflect a real-world scenario in which the pathway is not obligatory but encourages reduction in postdischarge opioid prescriptions by offering effective alternatives and yet permits shared clinical decision making based on clinical needs discussed by the patient and their treating team.
In addition, our experience suggests that, earlier in the course of the study, clinicians were especially reluctant to incorporate preoperative NSAIDs when considering MMA strategy for patients being considered for outpatient thyroid and parathyroid surgery. Specific concerns pertained to perceived risk of hematoma or postoperative bleeding and its sequelae in a vessel-rich field close to the airway. In this study, we identified 1 postoperative hematoma in the entire patient cohort. In a previously reported smaller cohort, we observed no postoperative bleeding events with use of NSAIDs as part of MMA protocol. Our experience suggests that combined with meticulous intraoperative hemostasis, perioperative NSAID use may not adversely affect frequency of postoperative bleeding events. This is congruent with findings by other investigators who found comparable incidence of bleeding, with or without perioperative NSAID use, following thyroid and tonsil surgery, respectively.13,14 Others have reported safe use of perioperative NSAIDs in procedures involving carotid endarterectomy and head and neck cutaneous surgery.15,16 While clinicians and patients may consider factors such as baseline patient health, available surgical expertise, institutional support, and the availability of rescue mechanisms, these data may help to clarify and assuage anxiety about the risk of bleeding and encourage inclusion of NSAIDs as part of MMA pathways.
While patient satisfaction measures are not the focus of this retrospective observational study, we have previously reported favorable findings related to feasibility, safety, and patient satisfaction associated with the MMA strategy in a separate publication.10 Clearly, the retrospective nature of the study and optional nature of the key intervention limit our ability to draw inferences about the motivating factors that may have influenced adoption of the MMA pathway. For example, clinicians may have been more inclined to opt in owing to the educational efforts surrounding the institutional MMA protocol, mentorship by physician champions, or other factors, such as an increasing awareness of the opioid abuse epidemic in the United States, scrutiny by regulators, increasing administrative burden related to opioid prescription tracking, and a sense of responsibility in curbing easy availability of prescription opioids in the community. Nevertheless, the availability of an alternative to conventional opioid-based pain management allowed clinicians to grow comfortable with the optional MMA protocol and progressively decrease the frequency of opioid prescriptions while preserving their ability to care for patients based on their clinical judgment and perceived patient needs. Our observations suggest that the availability of actionable, effective, and safe analgesia alternatives, such as the MMA protocol, is critical to bridging the gap between the desire and the ability to effect nonopioid based analgesia.
Limitations
We acknowledge the limitations of this study, including its retrospective nature, the lack of a true control group for the intervention, and a study cohort based at a single institution. In addition, these findings pertain to patients undergoing thyroid and parathyroid surgery, and additional investigations may be warranted to identify the role of MMA in other major head and neck procedures.
Conclusions
Introduction of an optional institutional nonopioid MMA pathway was associated with a learning curve. Adoption and adherence to the MMA protocol increased substantially over the study period for patients undergoing thyroid and parathyroid surgery. Progressive adoption of the MMA protocol was associated with a simultaneous significant decline in prescription of postoperative opioid analgesics. Availability of effective nonopioid MMA pathways may favorably influence physician prescribing practices and avoid unnecessary opioid prescriptions.
References
- 1.Baker DW. History of the Joint Commission’s pain standards: lessons for today’s prescription opioid epidemic. JAMA. 2017;317(11):1117-1118. doi: 10.1001/jama.2017.0935 [DOI] [PubMed] [Google Scholar]
- 2.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2)(suppl):S105-S120. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Opioid overdose. Updated February 9, 2017. https://www.cdc.gov/drugoverdose/index.html. Accessed April 15, 2017.
- 4.FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [news release]. Silver Spring, MD: US Food and Drug Administration; August 31, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Accessed February 24, 2018. [DOI] [PubMed]
- 5.Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. https://addiction.surgeongeneral.gov/. Accessed February 24, 2018. [PubMed]
- 6.Feldheiser A, Aziz O, Baldini G, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60(3):289-334. doi: 10.1111/aas.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi: 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 8.Page AJ, Gani F, Crowley KT, et al. Patient outcomes and provider perceptions following implementation of a standardized perioperative care pathway for open liver resection. Br J Surg. 2016;103(5):564-571. doi: 10.1002/bjs.10087 [DOI] [PubMed] [Google Scholar]
- 9.Dort JC, Farwell DG, Findlay M, et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: a consensus review and recommendations from the Enhanced Recovery After Surgery Society. JAMA Otolaryngol Head Neck Surg. 2017;143(3):292-303. doi: 10.1001/jamaoto.2016.2981 [DOI] [PubMed] [Google Scholar]
- 10.Oltman J, Militsakh O, D’Agostino M, et al. Multimodal analgesia in outpatient head and neck surgery: a feasibility and safety study. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1207-1212. doi: 10.1001/jamaoto.2017.1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. doi: 10.1136/bmj.g1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400-406. doi: 10.1345/aph.1H386 [DOI] [PubMed] [Google Scholar]
- 13.Chin CJ, Franklin JH, Turner B, Sowerby L, Fung K, Yoo JH. Ketorolac in thyroid surgery: quantifying the risk of hematoma. J Otolaryngol Head Neck Surg. 2011;40(3):196-199. [PubMed] [Google Scholar]
- 14.Mudd PA, Thottathil P, Giordano T, et al. Association between ibuprofen use and severity of surgically managed posttonsillectomy hemorrhage. JAMA Otolaryngol Head Neck Surg. 2017;143(7):712-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichhorn W, Kluwe L, Heiland M, Gröbe A. Lack of evidence for increased risk of postoperative bleeding after cutaneous surgery in the head and neck in patients taking aspirin. Br J Oral Maxillofac Surg. 2014;52(6):527-529. doi: 10.1016/j.bjoms.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 16.Batchelder A, Hunter J, Cairns V, Sandford R, Munshi A, Naylor AR. Dual antiplatelet therapy prior to expedited carotid surgery reduces recurrent events prior to surgery without significantly increasing peri-operative bleeding complications. Eur J Vasc Endovasc Surg. 2015;50(4):412-419. doi: 10.1016/j.ejvs.2015.07.019 [DOI] [PubMed] [Google Scholar]