Key Points
Question
What is the association of allopurinol use in patients with gout with the risk of developing chronic kidney disease stage 3 or higher?
Findings
In this population-based UK cohort study, the use of allopurinol in patients with gout did not increase the risk of kidney function decline, and was significantly associated with a 13% lower risk at doses of 300 mg or more per day.
Meaning
Allopurinol does not appear to be associated with kidney function decline, and clinicians should consider other potential contributors when faced with kidney function decline in patients with gout.
This cohort study uses data from the UK Health Improvement Network to examine the association of allopurinol use in patients with gout with the risk of developing chronic kidney disease stage 3 or higher.
Abstract
Importance
Clinicians are often cautious about use of allopurinol in patients with gout when renal function declines.
Objective
To assess the association of allopurinol use in gout with the risk of developing chronic kidney disease stage 3 or higher.
Design, Setting, and Participants
A time-stratified propensity score–matched, population-based, prospective cohort study of individuals with newly diagnosed gout who initiated allopurinol (≥300 mg/d) compared with those who did not initiate allopurinol, using the Health Improvement Network (THIN), a United Kingdom general practitioner electronic health records database, was carried out. The data were analyzed using Cox proportional hazards regression. Among adults aged 18 to 89 years with newly diagnosed gout, we propensity score matched 4760 initiators of allopurinol (≥300 mg/d) to the same number of noninitiators of allopurinol, excluding those with chronic kidney disease stage 3 or higher or urate-lowering therapy use before their gout diagnosis.
Exposures
Allopurinol initiation at a dose of 300 mg or more per day.
Main Outcomes and Measures
Development of chronic kidney disease stage 3 or higher.
Results
Of the 4760 allopurinol initiators (3975 men, 785 women) and same number of noninitiators (3971 men, 789 women), 579 and 623, respectively, developed chronic kidney disease stage 3 or higher, with a mean follow-up time of 5 and 4 years, mean age of 57 years, and mean body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 for both groups. Use of allopurinol of at least 300 mg/d was associated with lower risk of developing chronic kidney disease stage 3 or higher compared with nonusers, with a hazard ratio (HR) of 0.87 (95% CI, 0.77-0.97). Allopurinol initiation at less than 300 mg/d was not associated with renal function decline (HR, 1.00; 95% CI, 0.91-1.09).
Conclusions and Relevance
In this large cohort, allopurinol initiation of at least 300 mg/d was associated with a lower risk of renal function deterioration. Because allopurinol does not appear to be associated with renal function decline, clinicians should consider evaluating other potential causes when patients with gout experience renal function decline.
Introduction
Gout is the most common inflammatory arthritis, affecting 3.9% of Americans,1 yet only one-third of patients with gout receive urate-lowering therapy (ULT),2,3 leading to disease progression. This suboptimal management of gout is compounded by the frequently occurring comorbidity of chronic kidney disease (CKD) stage 3 or higher, which occurs in 20% of patients with gout compared with 5% of those without gout,4 making management of gout flares more challenging, and often limiting use of uricosuric agents.5
Allopurinol is the most widely used ULT. Unfortunately, clinicians are often cautious about using allopurinol in CKD, largely owing to concerns about allopurinol hypersensitivity syndrome (AHS). This has led to widespread empirical use of the Hande criteria,6 which guides renal-dosing of allopurinol, though there are no data demonstrating reduction in AHS risk with this approach.7 Instead, renal-dosing of allopurinol compounds the poor management of gout,8 and adds to the perception that allopurinol may be detrimental for renal function. In contrast, recent studies provide support for starting allopurinol at a low dose with gradual dose escalation to serum urate target with close monitoring, even among patients with renal insufficiency, without increased risk of AHS.9,10,11 Further, there is emerging evidence that ULT may be beneficial for kidney dysfunction.12,13,14,15,16,17,18,19,20,21 Thus, there are no clear data to suggest that allopurinol is detrimental to renal function in patients with gout. Despite this, clinicians commonly hold or lower the dose of allopurinol or even discontinue allopurinol entirely when a patient with gout exhibits kidney function decline,22 leading to worse gout outcomes.
Although CKD is common in gout, most people with gout have normal kidney function, particularly early in the course of disease, yet there are limited data regarding renal effects of allopurinol among those with gout and normal kidney function.23,24,25 Importantly, there is no evidence that allopurinol is nephrotoxic. We therefore aimed to assess the relation of allopurinol initiation to the risk of developing CKD stage 3 or higher among people with newly diagnosed gout.
Methods
Study Design
We conducted a time-stratified propensity score–matched cohort study in The Health Improvement Network (THIN), which is a general practitioner (GP) electronic medical records database representative of the UK general population. The institutional review board at Boston University Medical Campus and THIN Review Committee approved the study and written informed consent was waived because all data were deidentified.
Data Source
The Health Improvement Network contains anonymized data including demographics, diagnoses, prescriptions, laboratory test results, hospital admissions, consultations, and referrals, systematically collected by participating GPs throughout the UK. More than 11 million patients have been registered in THIN and more than 3 million are currently actively enrolled. Diagnoses are recorded as Read codes.26 Prescriptions are recorded using the Multilex codes issued by First Databank.27 The Health Improvement Network has been validated for use in pharmacoepidemiological research.28
Participants
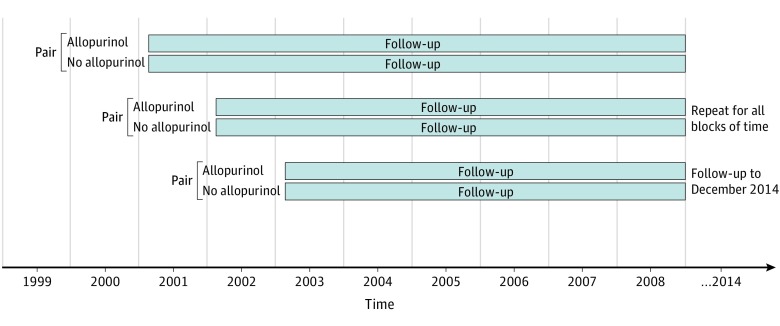
We included participants aged 18 to 89 years (mean, 57 years) with incident (newly diagnosed) gout between January 1, 2000, and December 31, 2014, who had been enrolled with their GP for at least 1 year prior to the gout diagnosis. The diagnosis of gout was based on the first instance of a gout Read code. We excluded individuals with CKD stage 3 or higher prior to gout diagnosis (defined as either glomerular filtration rate [GFR] <60 mL/min on at least 2 occasions more than 90 days apart within 1 year with no intervening GFR ≥75 mL/min or at least 1 Read code for CKD stage 4, CKD stage 5, hemodialysis, peritoneal dialysis, or kidney transplant) and participants with ULT use (allopurinol, febuxostat, probenecid, or sulfinpyrazone) within the year prior to gout diagnosis. From this sample, we identified incident allopurinol users based on the first instance of allopurinol prescription at any time after the gout diagnosis. For our primary analysis, allopurinol initiators were restricted to those who were prescribed a dose of 300 mg or more per day, because this dose is considered to be required for most patients. We created 1-year cohort accrual blocks to account for secular trends (Figure 1). The index date was defined as the date of first allopurinol prescription for the exposed and a randomly assigned date within the 1-year accrual block for each matched unexposed participant. We matched allopurinol initiators to allopurinol nonusers 1:1 using propensity scores to minimize confounding by indication. To calculate the propensity scores, we used logistic regression with incident allopurinol use as the dependent variable and potential confounders that reflect indications for allopurinol use and/or risk of developing CKD (listed below) as the independent variables.
Figure 1. Study Design: 1-Year Cohort Accrual Blocks, With 1:1 Propensity-Score Matching.
Prior to propensity-score matching, we excluded participants with the following within 1 year prior to the index date: (1) use of ULT other than allopurinol; (2) active cancer other than in situ cancers, squamous skin cancer, or basal skin cancer; and (3) no contact with the health care system (ie, no appointment with the GP, no laboratory test, and no prescription). At any time prior to the index date, the following were additional exclusions: (1) other organ or bone marrow transplant; (2) primary kidney disease (including polycystic kidney disease) or systemic vasculitis that affects the kidneys; (3) cirrhosis; and (4) multiple myeloma or renal carcinoma. In addition, we excluded participants whose gout diagnosis occurred on the last day of the study, and individuals lacking data on body mass index (BMI) at any time before the index date or serum urate in the period from 6 months prior to the gout diagnosis through 30 days after the index date.
Outcome Definition
The outcome was defined as either (1) GFR of less than 60 mL per minute on at least 2 occasions more than 90 days apart within 1 year with no intervening GFR of 75 mL or more per minute; (2) hemodialysis or peritoneal dialysis; or (3) kidney transplant.
Fulfillment of the outcome definition based on 2 GFR values was considered to have occurred on the date of the first low GFR value. The second qualifying GFR could occur after study follow-up end (eg, after the ninetieth birthday or after December 31, 2014). The GFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula.29 Analysis of serum creatinine values for computation of GFR in THIN has been previously validated.30
Covariates
Covariates included in the propensity score were (1) gout duration (time between gout diagnosis and index date); (2) baseline serum urate, assessed from 6 months prior to gout diagnosis through 30 days after the index date; (3) baseline kidney function (GFR 60 to <90 mL/min classified as CKD stage 2 vs GFR ≥90 mL/min classified as CKD stage 1); (4) baseline albuminuria status (normal: <3 mg/mmol; microalbuminuria: 3-30mg/mmol; macroalbuminuria: >30 mg/mmol) within 5 years prior to the index date; (5) age at the index date; (6) sex; (7) most recent BMI prior to the index date; (8) comorbidities assessed any time prior to the index date (cardiovascular disease, diabetes mellitus, heart failure, hypertension); (9) hospitalization within 1 year prior to the index date; (10) number of visits to the GP within 1 year prior to the index date; (11) medication use within 1 year prior to the index date (angiotensin-converting-enzyme inhibitors, low-dose aspirin for cardiovascular disease prevention, colchicine, diuretics ([loop, thiazides or thiazide-like], insulin, noninsulin diabetes drugs, losartan, nonlosartan angiotensin II-receptor blockers, nonsteroidal anti-inflammatory drugs [NSAIDs]).
Participants with missing values for serum creatinine and no Read code for CKD stage 2 were considered to have normal kidney function, thus classified as CKD stage 1 in the propensity score, based on the data that CKD 3 or higher prevalence among gout patients is 19.9%, and is lower in the first years of the disease.4,31
Statistical Analysis
Follow-up time started from the index date and continued until the outcome occurred, death, transfer out of the GP practice, date of last data collection by the GP, when a participant turned 90 years, or end of the study (December 31, 2014).
The relation of incident allopurinol use of at least 300 mg/d to CKD stage 3 or higher among participants with incident gout was assessed using Cox proportional hazards models using an intention-to-treat approach, stratified by 1-year cohort accrual blocks. In a second model, we additionally adjusted for the covariates included in the propensity score. The assessment of covariate balance, evaluation of the proportionality assumption, and multiple imputation of missing data were performed (Supplement). Because participants could stop using allopurinol or have the dose reduced and nonusers could start using the medication, we performed a sensitivity analysis censoring participants at exposure status change. Additional sensitivity analyses included all allopurinol initiators, regardless of initial dose, and restricting to those prescribed a dose less than 300 mg/d. We compared the cumulative incidence of CKD in both groups using Kaplan-Meier curves to assess for the possibility of depletion of susceptibles. To assess the impact of the competing risk of death, we used the Fine and Grey and cause-specific hazard approaches.
All analyses were performed using SAS statistical software (version 9.3, SAS Institute). P values were 2 sided and considered significant if P<.05.
Results
Cohort Selection
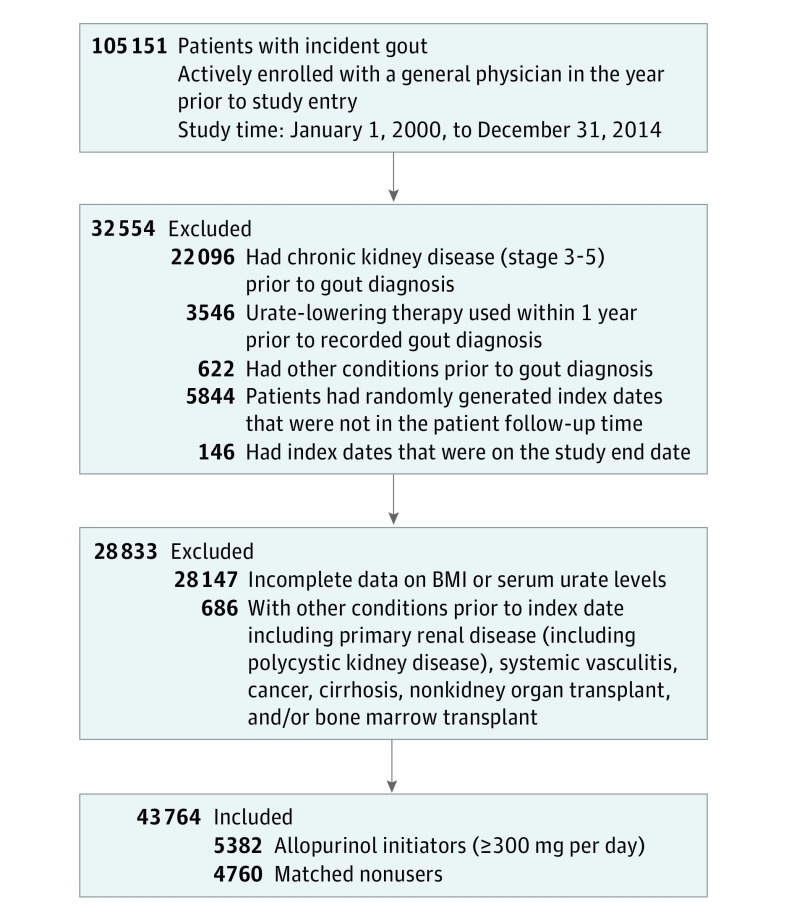
The source population comprised 6 919 613 participants, among which we identified 105 151 with newly diagnosed gout; 22 096 participants were excluded for meeting the outcome definition before the gout diagnosis, and 3531 and 15 participants were excluded owing to using allopurinol and other ULT within 1 year prior to the gout diagnosis, respectively. After applying the additional exclusion criteria and then creating the 1-year cohort accrual blocks, 43 764 participants remained eligible (Figure 2). A total of 17 558 allopurinol initiators were identified, of whom 12 176 were excluded because their initial daily dose was less than 300 mg, leaving 5382 allopurinol initiators using a dose of 300 mg or more per day. Of these, 4760 were propensity-score matched to an equal number of nonusers. Comparison of characteristics of those who were excluded vs included (eTable 1 in the Supplement) indicated no substantial differences between the 2 groups, except for a slightly higher prevalence of CKD stage 2, hypertension, certain medication use, and GP visits among the matched participants, suggesting that those excluded may have been slightly healthier.
Figure 2. Flow Diagram of Study Participants.
Participant Characteristics
Covariates were well balanced (eMethods 2 in the Supplement) between the allopurinol initiators and nonusers, with a mean (SD) age of 57 (14) years, mean BMI of 30, and mean GFR was 77 mL/min among both groups; as expected, most were male (7946 [83.5%]) (Table 1). Overall, 6724 (71%) exposed and unexposed participants had CKD stage 2 or eGFR 60 mL/min to 89 mL/min, with the remaining 2796 (29%) having CKD stage 1 or eGFR of 90 mL or more per minute (Table 1). The use of medications was also similar among allopurinol initiators and nonusers, with 2960 (31%) using diuretics and 6960 (73%) using NSAIDs (Table 1). Among those initiating allopurinol at a dose of at least 300 mg/d, 4500 (94.5%) were prescribed a dose of 300 mg/d. The mean duration of allopurinol use was 2.3 years. Additional postbaseline characteristics are provided in eTable 2 in the Supplement.
Table 1. Baseline Characteristics of Participants.
| Demographic | No. (%) | |
|---|---|---|
| Allopurinol Initiators ≥300 mg/d | Noninitiators | |
| Total, No. | 4760 | 4760 |
| Age, mean (SD), y | 57.4 (13.3) | 57.4 (13.9) |
| Male sex | 3975 (83.5) | 3971 (83.4) |
| Body mass index, mean (SD)a | 30.0 (5.4) | 30.1 (5.5) |
| Gout duration, mean (SD), y | 1.2 (2.1) | 1.2 (1.6) |
| Hospitalization in year prior to index date | 516 (10.8) | 510 (10.7) |
| Visits to the general practitioners in year prior to index date | ||
| 0 | 269 (5.7) | 283 (5.9) |
| 1 | 474 (10.0) | 494 (10.4) |
| 2 | 636 (13.4) | 638 (13.4) |
| 3 | 601 (12.6) | 558 (11.7) |
| 4 | 547 (11.5) | 559 (11.7) |
| 5 | 442 (9.3) | 430 (9.0) |
| 6-7 | 675 (14.2) | 667 (14.0) |
| 8-10 | 536 (11.3) | 532 (11.2) |
| ≥11 | 580 (12.2) | 599 (12.6) |
| Mean initiating allopurinol daily dose, mg | ||
| 300 | 4500 (94.5) | NA |
| 400-500 | 176 (3.7) | NA |
| 600 | 80 (1.7) | NA |
| >600 | 4 (0.1) | NA |
| Comorbid conditions | ||
| Chronic kidney disease stage 2 or eGFR 60-89 mL/min per 1.73 m2 | 3354 (70.5) | 3370 (70.8) |
| Hypertension | 2223 (46.7) | 2243 (47.1) |
| Diabetes mellitus | 396 (8.3) | 384 (8.1) |
| Cardiovascular disease | 538 (11.3) | 553 (11.6) |
| Heart failure | 187 (3.9) | 183 (3.8) |
| Concomitant medication use | ||
| Diuretics (loop, thiazide, thiazide-like) | 1472 (30.9) | 1488 (31.3) |
| Angiotensin-converting enzyme inhibitor | 1246 (26.2) | 1264 (26.6) |
| Losartan | 93 (2.0) | 101 (2.1) |
| Other angiotensin II receptor blockers | 359 (7.5) | 367 (7.7) |
| Colchicine | 791 (16.6) | 810 (17.0) |
| Nonsteroidal anti-inflammatory drugs | 3461 (72.7) | 3499 (73.5) |
| Low dose aspirin | 819 (17.2) | 825 (17.3) |
| Insulin | 33 (0.7) | 28 (0.6) |
| Other drugs for diabetes mellitus | 227 (4.8) | 217 (4.6) |
| Laboratory data | ||
| Serum urate level, mean (SD), mg/dL | 8.2 (1.4) | 8.2 (1.4) |
| eGFR, mean (SD), mL/min | 77.0 (17.6) | 77.0 (17.5) |
| Albuminuria | ||
| Missing | 4367 (91.7) | 4365 (91.7) |
| Normal, <3 mg/mmol | 276 (5.8) | 287 (6.0) |
| Moderately increased, 3-30 mg/mmol | 98 (2.1) | 83 (1.7) |
| Severely increased, >30 mg/mmol | 19 (0.4) | 25 (0.5) |
Abbreviations: eGFR, estimated glomerular filtration rate; NA, not applicable.
Body mass index, calculated as weight in kilograms divided by height in meters squared.
Risk of CKD Related to Allopurinol Use
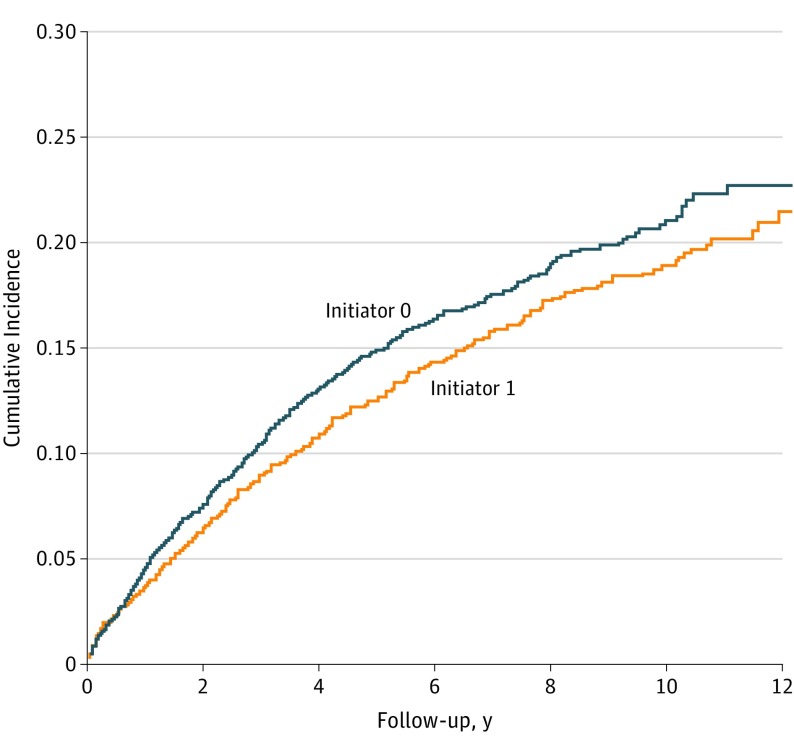
Of the 4760 participants in each group, 579 allopurinol initiators and 623 nonusers developed CKD stage 3 or higher during a mean follow-up time of 5 and 4 years, respectively (Table 2). The propensity score–matched hazard ratio (HR) was 0.87 (95% CI, 0.77-0.97). When additionally adjusted for the propensity score covariates, the effect estimate remained virtually unchanged (HR, 0.88; 95% CI, 0.79-0.99). Allopurinol initiators had lower cumulative incidence of CKD stage 3 or higher during the entire follow-up time compared with nonusers (Figure 3).
Table 2. Risk of Developing Chronic Kidney Disease (Stage ≥3) Among Patients With Incident Gout and Incident Allopurinol Use (at Least 300 mg/d).
| Main Results | Incident Allopurinol User | Non-Allopurinol User |
|---|---|---|
| Total, No. | 4760 | 4760 |
| Incident CKD stage ≥3, No. (%) | 579 (12.2) | 623 (13.1) |
| Death, No. (%) | 254 (5.3) | 240 (5.0) |
| Mean follow-up time, y | 4.9 | 4.5 |
| Crude incidence rate (CKD stage ≥3) per 1000 person-years | 24.9 | 29.4 |
| Propensity score–matched hazards ratio (95% CI) | 0.87 (0.77-0.97) | |
| Adjusted hazard ratio (95% CI)a | 0.88 (0.79-0.99) | |
Abbreviation: CKD, chronic kidney disease.
Variables included in the propensity-score model and included in the adjusted hazard ratio model: (1) gout duration; (2) baseline serum urate; (3) baseline kidney function and albuminuria; (4) general (age, sex, body mass index [calculated as weight in kilograms divided by height in meters squared]); (5) comorbidities (cardiovascular disease, diabetes mellitus, heart failure, hypertension); (6) hospitalization; (7) number of visits to the general practitioner; and (8) medication use (angiotensin-converting-enzyme inhibitor, aspirin, colchicine, diuretics, insulin, other drugs for diabetes mellitus, losartan, other angiotensin II receptor blockers, nonsteroidal anti-inflammatory drugs).
Figure 3. Kaplan-Meier Curve of Risk of Chronic Kidney Disease (Stage ≥3) by Allopurinol Initiation (≥300 mg/d) vs Noninitiators.
Six allopurinol initiators and 4 nonusers progressed to dialysis or kidney transplant, with 7 of them having met the GFR definition prior to dialysis and/or transplant. Allopurinol initiators visited their GP more often than nonusers (mean [SD] number of visits: 20.5 [23.2] vs 18.3 [22.4], respectively) and had their GFR assessed more frequently (mean [SD] GFR assessments: 4.7 [5.6] vs 4.0 [5.3], respectively) during the follow-up period.
Censoring allopurinol initiators when they stopped using allopurinol or reduced the dose and nonusers when they started using allopurinol resulted in similar findings, with an adjusted propensity score–matched HR of 0.83 (95% CI, 0.72-0.95). For the sample as a whole, regardless of allopurinol dose at initiation, the propensity score–matched HR was 1.00 (95% CI, 0.93-1.08). When restricting to allopurinol initiators who were prescribed a dose of less than 300 mg/d, no effect was found, with an adjusted HR of 1.02 (95% CI, 0.93-1.12). Imputation of missing data resulted in similar findings (HR, 0.92; 95% CI, 0.84-1.00).
The Fine and Grey and the cause-specific hazard competing risk of death regression models yielded effect estimates virtually identical to our primary results, with an HR of 0.87 (95% CI, 0.78-0.98) and 0.87 (95% CI, 0.78-0.97), respectively.
Discussion
This study is one of few that have evaluated the relation of allopurinol to renal function among patients with gout and normal or near-normal kidney function at baseline. Allopurinol use, initiated at a dose of at least 300 mg/d, was associated with a 13% reduction in the risk of developing CKD stage 3 or higher. In contrast, initiation of allopurinol at a dose of less than 300 mg/d had no association with developing CKD stage 3 or higher, consistent with current thinking that most patients need doses higher than 300 mg/d to achieve clinically meaningful outcomes.8,32,33,34,35,36 Nonetheless, at minimum, allopurinol does not seem to have a detrimental effect on renal function in individuals with gout.
Most studies to date evaluating the effects of urate-lowering on renal function have been conducted in nongout populations with varying degrees of baseline kidney function, often CKD stage 3.12,13,14,15,16,17,19,37,38,39,40,41,42,43,44,45,46 Most of these studies have demonstrated either a beneficial or no significant association with renal function. Importantly, no study has identified a harmful association with allopurinol. Nonetheless, because people with gout have intrinsic differences compared with those with asymptomatic hyperuricemia, including higher mortality, more comorbidities, and more NSAID use, these studies’ results are not directly applicable to gout patients.
In one of the first intervention studies23 evaluating renal function effects of urate-lowering in 59 patients with gout whose initial mean (SD) GFR was 94 (24) mL per minute randomized to allopurinol and colchicine or to colchicine alone, there was a significant difference in kidney function favoring allopurinol over a 2-year period. In a prospective observational study,24 xanthine oxidase inhibitor use over 6 months in patients with gout and normal kidney function was associated with a significant increase in GFR compared with healthy participants. In another study25 of 179 participants with gout whose mean baseline GFR was approximately 70 mL per minute, high-dose febuxostat (120 mg/d) was associated with significant improvement in GFR compared with placebo over a 4-week period, whereas lower doses and allopurinol, 300 mg/d, was not, and increases in GFR were significantly associated with reductions in serum urate. In an administrative database, gout control related to allopurinol adherence was associated with significantly decreased risk of end-stage renal disease in a sample with hypertension.47
The decline of kidney function in patients with gout is multifactorial. Beyond the natural decline that occurs with aging, there are the postulated deleterious effects of hyperuricemia, the frequent use of NSAIDs, highly prevalent comorbidities, such as hypertension and diabetes mellitus, and common use of other medications, such as diuretics.4,48 The potential beneficial effect of allopurinol may occur through serum urate reduction and reduction of oxidative stress through xanthine oxidase inhibition itself.49 In addition, patients who achieve the serum urate target experience reduction in flares, making renally-harmful NSAID use less frequent. Of note, achievement of target serum urate in gout management often requires doses higher than 300 mg, in line with our findings.
Strengths and Limitations
We recognize some limitations of our study. First, as with any observational study, there is potential for residual confounding despite our use of propensity score matching. However, for unmeasured confounding to change our results such that allopurinol’s true effect is harmful would be unlikely (E value, 2.84).50 Second, there is potential misclassification of allopurinol status because allopurinol use was based on prescription data, without ability to evaluate adherence in these data. Third, consistent with the known suboptimal management and monitoring of gout therapy, only few patients had subsequent measurements of serum urate after treatment initiation, limiting our ability to evaluate the effect of serum urate on GFR. Nonetheless, our research question was specifically about the effects of allopurinol on renal function, regardless of mechanism. Fourth, there was possible detection or surveillance bias unfavorable to allopurinol use because patients recieving allopurinol visited their GP more frequently, had their GFR assessed more often, and had slightly longer follow-up. Thus, allopurinol users could be more readily diagnosed with CKD simply on the basis of having laboratory tests assessed more frequently and over a longer period, making our findings conservative.
We also recognize that our primary focus on those who initiated allopurinol receiving at least 300 mg/d is not the current recommended starting dose of allopurinol (100 mg/d or 50 mg/d for CKD stage 4 or worse) with monitored dose escalation until serum urate target is achieved to reduce risk of AHS.51 Because our study spanned the years 2000 to 2014, prior to more recent treatment guidelines, when allopurinol dosing had traditionally been started at 300 mg/d without dose escalation, we opted to study what would be more likely to be biologically relevant, in line with the literature indicating that doses less than 300 mg/d typically do not enable patients to achieve their target serum urate.36 Nonetheless, our study findings suggest that starting at recommended lower doses with monitored dose escalation should not negatively impact renal function.
Our study also has a number of strengths. The large population-based sample offered an opportunity to detect even small detrimental effects if there were any to be noted. The new-user design, as standard in pharmacoepidemiological studies, with the additional restriction to newly diagnosed gout to ensure that allopurinol use was truly incident, and the use of propensity scores to account for confounding by indication were important strategies to minimizing bias.
Conclusions
In this large, newly diagnosed cohort of patients with gout who had normal or near-normal kidney function, the initiation of allopurinol of at least 300 mg/d was associated with lower risk of developing CKD stage 3 or higher. These findings in the context of the existing body of literature, including the recent demonstration of safe allopurinol dose escalation in patients with gout and CKD,11 indicate that allopurinol was not associated with poor renal function in patients with gout. Because allopurinol did not appear to be associated with renal function decline, clinicians should consider evaluating other factors when faced with renal function decline in their patients with gout rather than lowering the dose of or discontinuing allopurinol, a strategy that has contributed to the ongoing suboptimal treatment of gout.
eMethods.
eTable 1. Comparison of characteristics of excluded versus included subjects
eTable 2. Post-baseline characteristics of allopurinol initiators at ≥300mg/d versus non-initiators
eTable 3. Sensitivity analysis: Baseline characteristics of allopurinol initiators at <300mg/d versus non-initiators
eTable 4. Sensitivity analysis: Risk of developing CKD ≥3 among subjects with incident gout and incident allopurinol use of <300mg/d
eTable 5. Comparisons of baseline characteristics of participants for allopurinol initiators at ≥300mg/d versus <300mg/d
References
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141. doi: 10.1002/art.30520 [DOI] [PubMed] [Google Scholar]
- 2.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Eligibility for and prescription of urate-lowering treatment in patients with incident gout in England. JAMA. 2014;312(24):2684-2686. doi: 10.1001/jama.2014.14484 [DOI] [PubMed] [Google Scholar]
- 3.Juraschek SP, Kovell LC, Miller ER III, Gelber AC. Gout, urate-lowering therapy, and uric acid levels among adults in the United States. Arthritis Care Res (Hoboken). 2015;67(4):588-592. doi: 10.1002/acr.22469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2012;125(7):679-687 e671. [DOI] [PubMed] [Google Scholar]
- 5.Vargas-Santos AB, Neogi T. Management of gout and hyperuricemia in CKD. Am J Kidney Dis. 2017;70(3):422-439. doi: 10.1053/j.ajkd.2017.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76(1):47-56. doi: 10.1016/0002-9343(84)90743-5 [DOI] [PubMed] [Google Scholar]
- 7.Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: walking the tightrope between adequate urate lowering and adverse events. Semin Dial. 2007;20(5):391-395. doi: 10.1111/j.1525-139X.2007.00270.x [DOI] [PubMed] [Google Scholar]
- 8.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006;33(8):1646-1650. [PubMed] [Google Scholar]
- 9.Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64(8):2529-2536. doi: 10.1002/art.34488 [DOI] [PubMed] [Google Scholar]
- 10.Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. doi: 10.1002/art.30119 [DOI] [PubMed] [Google Scholar]
- 11.Stamp LK, Chapman PT, Barclay ML, et al. A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout. Ann Rheum Dis. 2017;76(9):1522-1528. doi: 10.1136/annrheumdis-2016-210872 [DOI] [PubMed] [Google Scholar]
- 12.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51-59. doi: 10.1053/j.ajkd.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 13.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128-132. [PubMed] [Google Scholar]
- 14.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388-1393. doi: 10.2215/CJN.01580210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65(4):543-549. doi: 10.1053/j.ajkd.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 16.Pai BH, Swarnalatha G, Ram R, Dakshinamurty KV. Allopurinol for prevention of progression of kidney disease with hyperuricemia. Indian J Nephrol. 2013;23(4):280-286. doi: 10.4103/0971-4065.114499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol. 2014;41(5):955-962. doi: 10.3899/jrheum.131159 [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Nakayama M, Kanno M, et al. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: a parallel-group, randomized, controlled trial. Clin Exp Nephrol. 2015;19(6):1044-1053. doi: 10.1007/s10157-015-1095-1 [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Shin S, Kim K, Choi S, Lee K. Effect of urate lowering therapy on renal disease progression in hyperuricemic patients with chronic kidney disease. J Rheumatol. 2015;42(11):2143-2148. doi: 10.3899/jrheum.150067 [DOI] [PubMed] [Google Scholar]
- 20.Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L. Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol. 2016;68(8):2035-2043. doi: 10.1002/art.39654 [DOI] [PubMed] [Google Scholar]
- 21.Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66(6):945-950. doi: 10.1053/j.ajkd.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 22.Melton BL, Zillich AJ, Russell SA, et al. Reducing prescribing errors through creatinine clearance alert redesign. Am J Med. 2015;128(10):1117-1125. doi: 10.1016/j.amjmed.2015.05.033 [DOI] [PubMed] [Google Scholar]
- 23.Gibson T, Rodgers V, Potter C, Simmonds HA. Allopurinol treatment and its effect on renal function in gout: a controlled study. Ann Rheum Dis. 1982;41(1):59-65. doi: 10.1136/ard.41.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L, Wei L, Chen H, et al. Influence of urate-lowering therapies on renal handling of uric acid. Clin Rheumatol. 2016;35(1):133-141. doi: 10.1007/s10067-014-2806-9 [DOI] [PubMed] [Google Scholar]
- 25.Kim HA, Seo YI, Song YW. Four-week effects of allopurinol and febuxostat treatments on blood pressure and serum creatinine level in gouty men. J Korean Med Sci. 2014;29(8):1077-1081. doi: 10.3346/jkms.2014.29.8.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart-Buttle CD, Read JD, Sanderson HF, Sutton YM. A language of health in action: Read Codes, classifications and groupings. Proc AMIA Annu Fall Symp. 1996;75-79. [PMC free article] [PubMed] [Google Scholar]
- 27.First Databank Multilex for primary care. http://www.fdbhealth.co.uk/multilex-overview/. Accessed August 05, 2017.
- 28.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393-401. doi: 10.1002/pds.1335 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461-470. doi: 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 30.Denburg MR, Haynes K, Shults J, Lewis JD, Leonard MB. Validation of The Health Improvement Network (THIN) database for epidemiologic studies of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2011;20(11):1138-1149. doi: 10.1002/pds.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández-Cuevas CB, Roque LH, Huerta-Sil G, et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009;15(2):65-67. doi: 10.1097/RHU.0b013e31819c0dba [DOI] [PubMed] [Google Scholar]
- 32.Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63. doi: 10.1186/ar2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinders MK, van Roon EN, Jansen TL, et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis. 2009;68(1):51-56. doi: 10.1136/ard.2007.083071 [DOI] [PubMed] [Google Scholar]
- 34.Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/d versus benzbromarone 100-200 mg/d in patients with gout. Ann Rheum Dis. 2009;68(6):892-897. doi: 10.1136/ard.2008.091462 [DOI] [PubMed] [Google Scholar]
- 35.Rees F, Hui M, Doherty M. Optimizing current treatment of gout. Nat Rev Rheumatol. 2014;10(5):271-283. doi: 10.1038/nrrheum.2014.32 [DOI] [PubMed] [Google Scholar]
- 36.Rees F, Jenkins W, Doherty M. Patients with gout adhere to curative treatment if informed appropriately: proof-of-concept observational study. Ann Rheum Dis. 2013;72(6):826-830. doi: 10.1136/annrheumdis-2012-201676 [DOI] [PubMed] [Google Scholar]
- 37.Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? a Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2017;76(1):133-139. doi: 10.1136/annrheumdis-2015-209046 [DOI] [PubMed] [Google Scholar]
- 38.Krishnamurthy A, Lazaro D, Stefanov DG, Blumenthal D, Gerber D, Patel S. The effect of allopurinol on renal function. J Clin Rheumatol. 2017;23(1):1-5. doi: 10.1097/RHU.0000000000000480 [DOI] [PubMed] [Google Scholar]
- 39.Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39(4):1227-1233. doi: 10.1007/s11255-007-9253-3 [DOI] [PubMed] [Google Scholar]
- 40.Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887-1894. doi: 10.2215/CJN.11451210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao MP, Ang DS, Gandy SJ, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1382-1389. doi: 10.1681/ASN.2010111185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y, Chen W, Jalal D, et al. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35(3):153-160. doi: 10.1159/000331453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P, Chen Y, Wang B, Zhang F, Wang D, Wang Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin Endocrinol (Oxf). 2015;83(4):475-482. doi: 10.1111/cen.12673 [DOI] [PubMed] [Google Scholar]
- 44.Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU-FLASH trial for CKD). J Cardiol. 2015;66(4):298-303. doi: 10.1016/j.jjcc.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 45.Bose B, Badve SV, Hiremath SS, et al. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2014;29(2):406-413. doi: 10.1093/ndt/gft378 [DOI] [PubMed] [Google Scholar]
- 46.Kanji T, Gandhi M, Clase CM, Yang R. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2015;16:58. doi: 10.1186/s12882-015-0047-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perreault S, Nuevo J, Baumgartner S, Morlock R. Any link of gout disease control among hypertensive patients and onset of end-stage renal disease? Results from a population-based study. World J Nephrol. 2017;6(3):132-142. doi: 10.5527/wjn.v6.i3.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson RJ. Why focus on uric acid? Curr Med Res Opin. 2015;31(suppl 2):3-7. doi: 10.1185/03007995.2015.1087979 [DOI] [PubMed] [Google Scholar]
- 49.Neogi T, George J, Rekhraj S, Struthers AD, Choi H, Terkeltaub RA. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. 2012;64(2):327-338. doi: 10.1002/art.33369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 51.Khanna D, Fitzgerald JD, Khanna PP, et al. ; American College of Rheumatology . 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446. doi: 10.1002/acr.21772 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Comparison of characteristics of excluded versus included subjects
eTable 2. Post-baseline characteristics of allopurinol initiators at ≥300mg/d versus non-initiators
eTable 3. Sensitivity analysis: Baseline characteristics of allopurinol initiators at <300mg/d versus non-initiators
eTable 4. Sensitivity analysis: Risk of developing CKD ≥3 among subjects with incident gout and incident allopurinol use of <300mg/d
eTable 5. Comparisons of baseline characteristics of participants for allopurinol initiators at ≥300mg/d versus <300mg/d