Key Points
Question
What are the frequency and associations of nonsteroidal anti-inflammatory drug (NSAID) use among patients with hypertension, heart failure, or chronic kidney disease?
Findings
Among a retrospective cohort of more than 2.4 million musculoskeletal-related primary care visits by 814 049 older adult patients with hypertension, heart failure, or chronic kidney disease, 9.3% of visits resulted in prescription NSAID use within the following 7 days. Prescription NSAID use was not associated with increased risk of safety-related outcomes at 37 days.
Meaning
Prescription NSAID use was common among high-risk patients, with widespread physician-level variation; however, use had no association with acute safety-related outcomes.
This cohort study uses drug benefit claims data in Ontario, Canada, to estimate the frequency of and variation in prescription nonsteroidal anti-inflammatory drug (NSAID) use among high-risk patients with musculoskeletal disorders and hypertension, heart failure, or chronic kidney disease (CKD) and identifies characteristics associated with prescription NSAID use and its potential association with short-term, safety-related outcomes.
Abstract
Importance
International nephrology societies advise against nonsteroidal anti-inflammatory drug (NSAID) use in patients with hypertension, heart failure, or chronic kidney disease (CKD); however, recent studies have not investigated the frequency or associations of use in these patients.
Objectives
To estimate the frequency of and variation in prescription NSAID use among high-risk patients, to identify characteristics associated with prescription NSAID use, and to investigate whether use is associated with short-term, safety-related outcomes.
Design, Setting, and Participants
In this retrospective cohort study, administrative claims databases were linked to create a cohort of primary care visits for a musculoskeletal disorder involving patients 65 years and older with a history of hypertension, heart failure, or CKD between April 1, 2012, and March 31, 2016, in Ontario, Canada.
Exposure
Prescription NSAID use was defined as at least 1 patient-level Ontario Drug Benefit claim for a prescription NSAID dispensing within 7 days after a visit.
Main Outcomes and Measures
Multiple cardiovascular and renal safety-related outcomes were observed between 8 and 37 days after each visit, including cardiac complications (any emergency department visit or hospitalization for cardiovascular disease), renal complications (any hospitalization for hyperkalemia, acute kidney injury, or dialysis), and death.
Results
The study identified 2 415 291 musculoskeletal-related primary care visits by 814 049 older adults (mean [SD] age, 75.3 [4.0] years; 61.1% female) with hypertension, heart failure, or CKD, of which 224 825 (9.3%) were followed by prescription NSAID use. The median physician-level prescribing rate was 11.0% (interquartile range, 6.7%-16.7%) among 7365 primary care physicians. Within a sample of 35 552 matched patient pairs, each consisting of an exposed and nonexposed patient matched on the logit of their propensity score for prescription NSAID use (exposure), the study found similar rates of cardiac complications (288 [0.8%] vs 279 [0.8%]), renal complications (34 [0.1%] vs 33 [0.1%]), and death (27 [0.1%] vs 30 [0.1%]). For cardiovascular and renal-safety related outcomes, there was no difference between exposed patients (308 [0.9%]) and nonexposed patients (300 [0.8%]) (absolute risk reduction, 0.0003; 95% CI, −0.001 to 0.002; P = .74).
Conclusions and Relevance
While prescription NSAID use in primary care was frequent among high-risk patients, with widespread physician-level variation, use was not associated with increased risk of short-term, safety-related outcomes.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed and effective for the treatment of musculoskeletal pain1,2,3,4,5,6; however, NSAID use is associated with increased risk of cardiovascular and renal complications, including hypertension, edema, myocardial infarction, and impaired renal function, particularly in patients with a history of cardiovascular or renal disease.4,7,8,9,10,11,12 Consequently, international nephrology societies have released recommendations via their respective national Choosing Wisely campaigns against NSAID use in individuals with hypertension, heart failure, or chronic kidney disease (CKD).13,14 While these recommendations offer basic analgesics and nonpharmacological treatments as preferable alternatives,13,14 it is both possible and disconcerting that some physicians might instead prescribe opioids, which typically pose elevated risk of adverse events and dependence vs NSAIDs.15
Despite the above considerations when managing musculoskeletal pain, the frequency of NSAID use and its subsequent clinical association with short-term complications among high-risk patients is not well understood. Although a study16 of large, US commercial claims databases estimated that NSAIDs were used for musculoskeletal pain management in 14.4% to 16.2% of high-risk patients, estimates were limited to the insurance plan level. Therefore, as with prior studies, prescribing patterns among individual physicians, as well as the patient and physician characteristics associated with NSAID use, were not investigated. In addition, although prior research17,18,19,20 has examined cardiac and renal complications in high-risk patients receiving NSAIDs, this association has not been assessed in a real-world population to our knowledge. We see the latter point as critically important given the relative benefit of NSAIDs to manage musculoskeletal pain and the potentially harmful associations of other pain management therapies, such as opioids.
The present study aimed to estimate the frequency of and variation in prescription NSAID use among primary care patients with hypertension, heart failure, or CKD in Ontario, Canada, over time and by physician. Our secondary objectives were to identify patient and physician characteristics associated with prescription NSAID use and to explore its potential association with acute cardiovascular and renal complications.
Methods
Design, Setting, and Participants
We conducted a retrospective cohort study using population-based administrative health care databases linked via unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada. We identified Ontario Health Insurance Plan (OHIP) claims indicating visits to a primary care physician (general practitioner or family physician) for a musculoskeletal disorder (eg, fractures/dislocations, strains/sprains, arthritis, and related conditions), assessed using 3-digit OHIP diagnostic codes (1 per claim), between April 1, 2012, and March 31, 2016, in Ontario, Canada16,21 (eTable 1 in the Supplement). Only visits involving Ontario residents 65 years and older with a valid provincial health card number were eligible to ensure that patients’ prescription drug costs were covered, at least in part, under the Ontario Drug Benefit (ODB) program. Long-term care residents and patients with missing sex or postal code were excluded. Patients were required to have a diagnosis of hypertension, heart failure, or CKD within 365 days before their visit, assessed using International Classification of Diseases and Related Health Problems, Tenth Revision, Canada (ICD-10-CA) codes in National Ambulatory Care Reporting System and Discharge Abstract Database claims, as well as OHIP diagnostic codes contained within OHIP claims16,21,22,23,24,25,26 (eTable 1 in the Supplement). Patients were included in the denominator for all quarters in which they had at least 1 eligible visit.16 If patients had multiple visits per quarter, only their first visit was selected. The use of data in this project was authorized under §45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
Primary Outcome: Prescription NSAID Use
Prescription NSAID use was defined as at least 1 patient-level ODB claim for a prescription NSAID dispensing within 7 days after a visit (eTable 2 and eTable 3 in the Supplement).27 Over-the-counter formulations that are paid for out of pocket are not captured in the ODB claims database and were consequently excluded from our study.27 Aspirin and topical NSAID formulations were excluded because both are basic analgesics (along with acetaminophen and capsaicin) recommended as first-line treatment for pain management over other NSAIDs or opioids.28 Furthermore, aspirin has low nephrotoxicity and is recommended for comorbid cardiovascular disease prevention.4,29
Secondary Outcomes: Cardiovascular and Renal Safety
Multiple binary outcomes that might reflect short-term complications secondary to NSAID use, including exacerbation of preexisting heart and kidney disease, were observed up to 37 days after each visit.13,14,30 Specifically, we captured whether patients (1) had a cardiac complication, defined as a hospitalization or emergency department visit with an ICD-10-CA code for cardiovascular disease (eg, hypertension, heart failure, hypertensive CKD, myocardial infarction, or stroke)31,32,33; (2) had a renal complication, defined as a hospitalization with an ICD-10-CA or Canadian Classification of Health Interventions code indicating hyperkalemia, an acute kidney injury, or acute dialysis23,29,34; or (3) had died, with their death recorded in the Registered Persons Database.35
Covariates
Back pain and arthritis are the most frequently reported types of musculoskeletal pain among older adults and are associated with increased duration of NSAID use. Therefore, we recorded whether an eligible visit was for back pain, arthritis, or another type of musculoskeletal disorder.36,37
Prior NSAID and opioid use (excluding formulations with methadone hydrochloride or naloxone) were each defined as at least 1 ODB claim for a related drug within 90 days before a visit.27,29,30,38,39,40 We independently defined the use of renin-angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) and diuretics30,41,42,43,44,45,46 as (1) current if a related drug was dispensed within 90 days before a visit and the amount supplied was enough to ensure coverage through the visit date plus 7 days (ie, ≥1 day of overlap with the primary outcome if observed); (2) recent if a claim was made in the past 90 days but the amount supplied did not overlap with the visit date plus 7 days; or (3) neither recent nor current if there was no related dispensing within 90 days before a visit.28 We assumed that patients followed the recommended daily dose (ie, the number of days supplied equated with the number of days the drug was taken).
Patient age, sex, and rurality were assessed from the Registered Persons Database.24 Quintiles of the median neighborhood income were used to approximate patients’ socioeconomic status.47 Health region was identified by the Local Health Integrated Network database. Patient history of cancer, liver, or cardiovascular disease (excluding hypertension and heart failure) in the past 3 years was independently assessed via OHIP claims for physician visits, hospital admissions in the Discharge Abstract Database, and emergency department visits in the National Ambulatory Care Reporting System.24,29 Client Agency Program Enrolment tables and OHIP fee codes were cross-referenced to assess if patients were rostered to a family physician.24 Physician sex, international medical graduate status, and years since graduation were ascertained from the Institute for Clinical Evaluative Sciences Physician Database.24 Primary care practice groups (hereafter referred to as practices) were defined as clusters of 3 or more physicians submitting joint billing claims to the Ministry of Health and Long-Term Care for reimbursement, with smaller groups excluded for privacy reasons. We identified each practice’s payment model and size (numbers of physicians and patients) via Client Agency Program Enrolment tables and OHIP claims.48,49
Statistical Analysis
Rates of prescription NSAID use were calculated by quarter and health system level—region (Local Health Integrated Network), practice, and physician—between fiscal years 2012-2013 and 2015-2016. Variation was described via interquartile ranges (IQRs).
Rates of prescription NSAID use over time were analyzed via negative binomial regression with quarter as a continuous independent variable, the aggregate number of visits resulting in prescription NSAID use as the dependent variable, and the log number of visits as an offset term. To account for seasonality, 3 indicator variables were created to represent the quarter in which a visit occurred irrespective of fiscal year.
A mixed-effects logistic regression was used to analyze patients’ odds of prescription NSAID use, while adjusting for all patient-level, physician-level, and practice-level characteristics detailed under the covariates heading above. Practice-specific random effects were included to account for within-practice correlation in prescribing behavior, which enabled calculation of the intracluster correlation coefficient and the median odds ratio. The latter is a measure of heterogeneity in practice-level prescribing behavior that is interpretable on the odds ratio scale.50,51,52 Computational issues with the statistical software prohibited inclusion of random effects to account for within-physician correlation or patient-level repeated measures. Consequently, we limited our regression sample to 1 randomly selected visit per patient—all of which belonged to an identifiable practice and physician—to facilitate convergence without introducing temporal bias.53,54
The association of prescription NSAID use (primary outcome) with experiencing a cardiovascular or renal safety-related outcome within 8 to 37 days after a visit (any secondary outcome) was investigated via a landmark analysis. Patients with any cardiovascular or renal safety-related outcome within 7 days (the landmark date) after their visit were excluded because they would be unlikely or unable to fill a prescription within that week. Patients with an opioid dispensing between 90 days before their visit and 37 days after their visit were excluded to ensure that observed outcomes were not attributable to prescribed opioid use.28 Among the remaining patients, a mixed-effects logistic regression identical to the primary model (excluding the prior opioid use covariate) was used to identify patient-level probability of prescription NSAID use conditional on measured baseline covariates potentially associated with any secondary outcome and/or prescription NSAID use.55 Patients with prescription NSAID use (exposed) were then matched using greedy nearest-neighbor matching to a nonuser (unexposed) on the logit of their propensity score using calipers of width equal to 0.2 of the SD of the logit of the propensity score.55,56,57 The propensity score model was iteratively modified until all comparisons of baseline characteristics between exposed and unexposed individuals had a standardized difference less than 0.10 (absolute value).56 McNemar test for correlated binary proportions was used in the matched sample to assess whether exposed patients were more likely to experience any cardiovascular or renal safety-related outcome than unexposed patients. The association between prescription NSAID use and experience of a safety-related outcome was summarized as the absolute risk difference.56,58
All analyses were performed using statistical software (SAS, version 9.4; SAS Institute Inc). Significance was assessed at 2-sided P ≤ .05.
Results
Cohort Characteristics
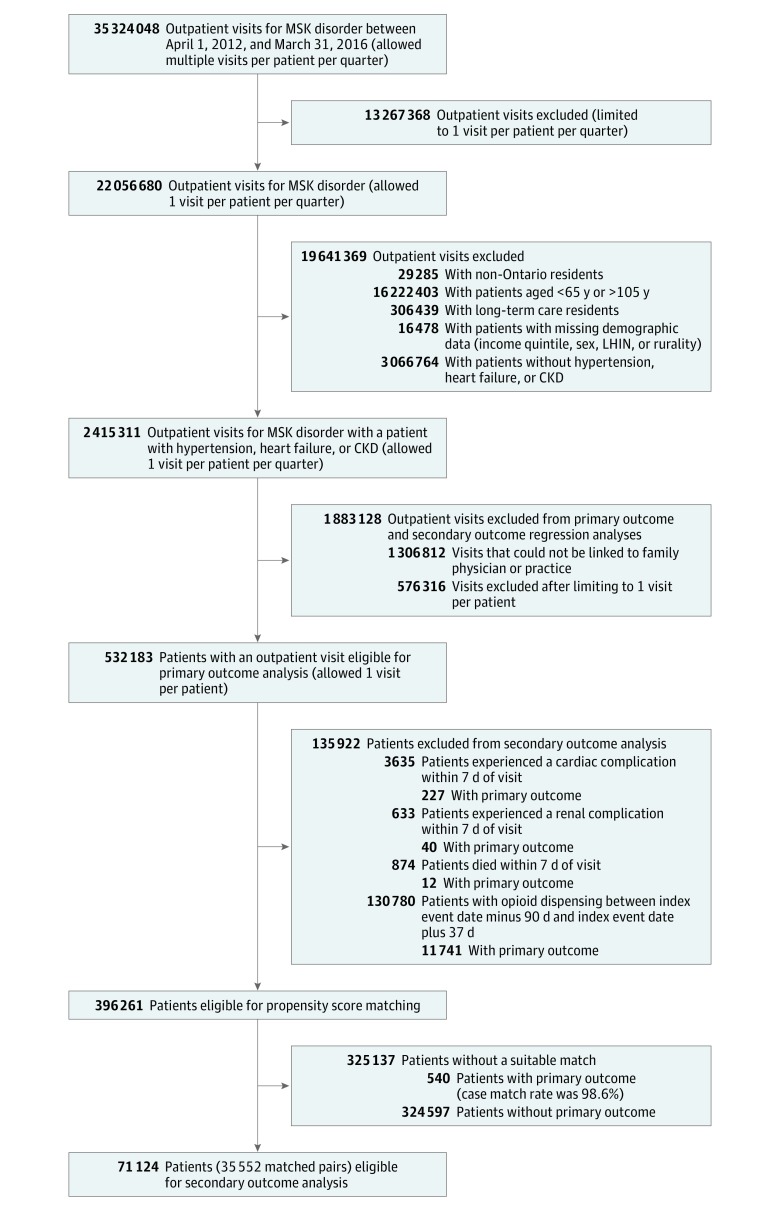
We identified 2 415 291 primary care visits for musculoskeletal disorders involving 814 049 older adult patients (mean [SD] age, 75.3 [4.0] years; 61.1% female) with hypertension, heart failure, or CKD (Figure 1). Prescription NSAID use was observed after 224 825 visits (9.3%), with 79.6% of dispensing claims for traditional vs selective NSAIDs.
Figure 1. Cohort Creation.
CKD indicates chronic kidney disease; LHIN, Local Health Integrated Network; and MSK, musculoskeletal.
Table 1 summarizes patient-level and physician-level characteristics by prescription NSAID use status. Most visits (92.7%) involved patients with hypertension. Visits followed by prescription NSAID use involved a greater proportion of patients with hypertension alone (90.8% vs 81.9%) or prior NSAID use (33.6% vs 13.4%). Prior opioid use was less prevalent among those with (18.0%) prescription NSAID use vs those without (21.0%).
Table 1. Patient-Level, Physician-Level, and Practice-Level Characteristics Associated With Prescription NSAID Use After a Primary Care Visit for a Musculoskeletal Disorder Among 2 415 291 Visitsa.
| Characteristic | Prescription NSAID Use | |
|---|---|---|
| Yes | No | |
| Patient Level | (n = 224 825 Visits) | (n = 2 190 466 Visits) |
| Diagnosis in past year, No. (%) | ||
| Hypertension | 216 282 (96.2) | 2 022 597 (92.3) |
| Heart failure | 11 344 (5.0) | 213 047 (9.7) |
| CKD | 10 467 (4.7) | 223 174 (10.2) |
| Qualifying diagnoses, No. (%) | ||
| Hypertension | 204 202 (90.8) | 1 794 724 (81.9) |
| Heart failure | 4641 (2.1) | 79 368 (3.6) |
| CKD | 3560 (1.6) | 75 796 (3.5) |
| Hypertension and heart failure | 5515 (2.5) | 93 200 (4.3) |
| Hypertension and CKD | 5719 (2.5) | 106 899 (4.9) |
| Heart failure and CKD | 342 (0.2) | 12 705 (0.6) |
| Hypertension, heart failure, and CKD | 846 (0.4) | 27 774 (1.3) |
| Reason for visit, No. (%) | ||
| Back pain | 40 034 (17.8) | 308 553 (14.1) |
| Arthritis | 88 603 (39.4) | 776 629 (35.5) |
| Prior NSAID use, No. (%) | 75 544 (33.6) | 292 534 (13.4) |
| Prior opioid use, No. (%) | 40 550 (18.0) | 460 536 (21.0) |
| Renin-angiotensin inhibitor use, No. (%)b | ||
| Current | 127 073 (56.5) | 1 108 404 (50.6) |
| Recent | 28 557 (12.7) | 211 126 (9.6) |
| None | 69 195 (30.8) | 870 936 (39.8) |
| Diuretic use, No. (%) | ||
| Current | 45 391 (20.2) | 533 335 (24.3) |
| Recent | 13 554 (6.0) | 135 750 (6.2) |
| None | 165 880 (73.8) | 1 521 381 (69.5) |
| Proton pump inhibitor use, No. (%) | ||
| Current | 63 032 (28.0) | 538 034 (24.6) |
| Recent | 22 443 (10.0) | 170 287 (7.8) |
| None | 139 350 (62.0) | 1 482 145 (67.7) |
| Age, mean (SD), y | 74.2 (6.0) | 75.5 (7.6) |
| Sex, No. (%) | ||
| Male | 92 259 (41.0) | 848 146 (38.7) |
| Female | 132 566 (59.0) | 1 342 320 (61.3) |
| Rurality, No. (%) | ||
| Rural | 17 379 (7.7) | 209 665 (9.6) |
| Urban | 207 446 (92.3) | 1 980 801 (90.4) |
| Neighborhood income quintile, No. (%) | ||
| 1, Lowest | 48 412 (21.5) | 400 485 (18.3) |
| 2 | 50 037 (22.3) | 451 885 (20.6) |
| 3 | 46 673 (20.8) | 443 697 (20.3) |
| 4 | 43 456 (19.3) | 457 160 (20.9) |
| 5, Highest | 36 247 (16.1) | 437 239 (20.0) |
| Diagnosis or event, No. (%) | ||
| Cancer | 36 420 (16.2) | 450 693 (20.6) |
| Liver disease | 4104 (1.8) | 50 262 (2.3) |
| Other cardiovascular | 54 943 (24.4) | 702 893 (32.1) |
| Hospitalization in past year, No. (%) | 58 868 (26.2) | 838 984 (38.3) |
| Charlson Comorbidity Index, mean (SD) | 0.3 (1.2) | 0.5 (0.4) |
| Rostered to a family physician, No. (%) | 224 418 (99.8) | 2 183 311 (99.7) |
| Physician Levelc | (n = 220 146) | (n = 2 141 807) |
| Sex, No. (%) | ||
| Male | 162 034 (73.6) | 1 482 739 (69.2) |
| Female | 58 112 (26.4) | 659 068 (30.8) |
| IMG, No. (%) | 74 243 (33.7) | 544 117 (25.4) |
| Years since graduation, mean (SD) | 29.0 (12.0) | 28.2 (11.2) |
| Practice Levelc | (n = 220 146) | (n = 2 141 807) |
| Primary care practice model, No. (%)d | ||
| FFS | 29 688 (13.5) | 255 685 (11.9) |
| FHG | 90 279 (41.0) | 713 614 (33.3) |
| FHN | 290 (0.1) | 2870 (0.1) |
| FHO | 52 840 (24.0) | 594 332 (27.7) |
| FHT | 34 762 (15.8) | 473 535 (22.1) |
| Other | 12 287 (5.6) | 101 771 (4.8) |
Abbreviations: CKD, chronic kidney disease; FFS, fee-for-service; FHG, family health group; FHN, family health network; FHO, family health organization; FHT, family health team; IMG, international medical graduate; NSAID, nonsteroidal anti-inflammatory drug.
For all characteristics, P < .001. P values are not adjusted for correlation within practices or patients.
Includes angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Characteristics are only provided for those primary care visits that involved a patient rostered to an identifiable physician and practice (n = 2 361 953).
Represents the patient enrollment model, which informs practice organization and remuneration.
Temporal Trends
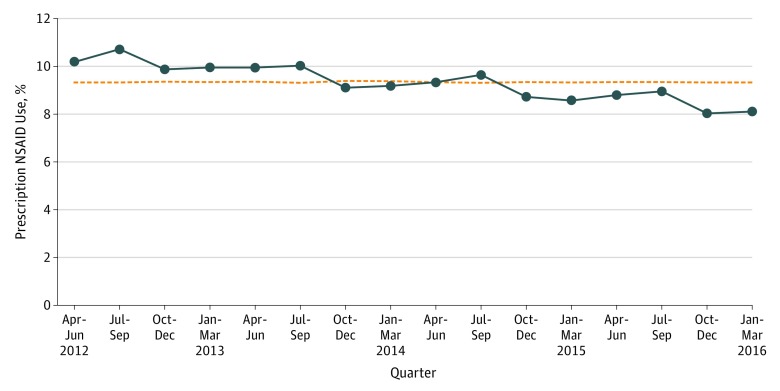
Based on 2 415 291 visits, Figure 2 shows a declining trend in prescription NSAID use over time, with an absolute reduction of 2.1% between the first quarter (April to June 2012 [10.2%]) and last quarter (January to March 2016 [8.1%]). On average, the provincewide prescribing rate decreased by 2.0% per quarter (rate ratio, 0.98; 95% CI, 0.98-0.99).
Figure 2. Prescription Nonsteroidal Anti-inflammatory Drug (NSAID) Use Over Time (by Quarter) in Ontario, Fiscal Year 2012-2013 to Fiscal Year 2015-2016.
The horizontal hatched line represents the mean rate.
Variation by Health System Level
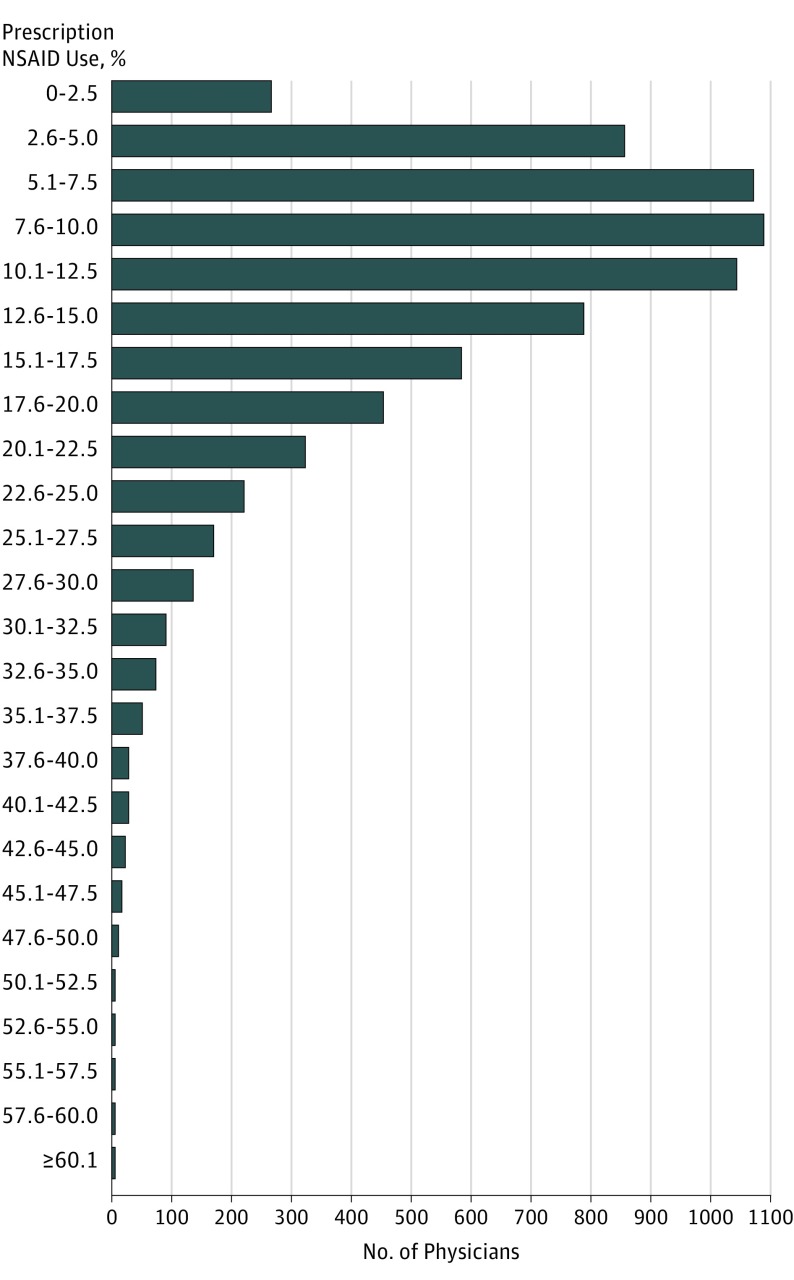
Prescription NSAID use ranged from 6.7% to 14.4% across 14 health regions (median, 8.4%; IQR, 7.3%-9.6%) and from 0.9% to 60.3% among 688 primary care practices (median, 10.1%; IQR, 6.3%-17.0%) (eFigure 1 and eFigure 2 in the Supplement). Figure 3 shows substantial variation in use among 7365 primary care physicians (range, 0.9%-69.2%; median, 11.0%; IQR, 6.7%-16.7%).
Figure 3. Variation in Prescription Nonsteroidal Anti-inflammatory Drug (NSAID) Use by 7536 Primary Care Physicians in Ontario, Fiscal Year 2012-2013 to Fiscal Year 2015-2016.
Characteristics Associated With Prescription NSAID Use
Table 2 summarizes our primary mixed-effects regression results based on 532 163 patients (Figure 1). Prescription NSAID use was observed in 9.0% of patients, with traditional NSAIDs (82.7%) more commonly dispensed than selective cyclooxygenase 2 inhibitors (18.3%). Histories of hypertension, back pain, arthritis, prior NSAID use, and renin-angiotensin inhibitor use were associated with increased odds of prescription NSAID use. Conversely, patients with heart failure, CKD, or prior opioid use had reduced odds of prescription NSAID use. Physicians who were male, international medical graduates, or further removed from graduation had increased odds of prescribing an NSAID.
Table 2. Patient-Level, Physician-Level, and Practice-Level Characteristics Associated With Prescription NSAID Use After a Primary Care Visit for a Musculoskeletal Disorder Among 532 163 Patientsa.
| Characteristic | Odds Ratio (95% CI)b | P Value |
|---|---|---|
| Fixed Effects | ||
| Patient Level | ||
| Diagnosis in past year, yes vs no | ||
| Hypertension | 1.06 (1.01-1.13) | .47 |
| Heart failure | 0.72 (0.69-0.76) | <.001 |
| CKD | 0.57 (0.55-0.60) | <.001 |
| Reason for visit, yes vs no | ||
| Back pain | 1.49 (1.45-1.53) | <.001 |
| Arthritis | 1.64 (1.61-1.68) | <.001 |
| Prior NSAID use, yes vs no | 2.79 (2.73-2.86) | <.001 |
| Prior opioid use, yes vs no | 0.78 (0.76-0.80) | <.001 |
| Renin-angiotensin inhibitor usec | ||
| Current vs neither | 1.47 (1.44-1.51) | <.001 |
| Recent vs neither | 1.55 (1.49-1.60) | <.001 |
| Diuretic use | ||
| Current vs neither | 0.88 (0.86-0.91) | <.001 |
| Recent vs neither | 1.16 (1.11-1.22) | <.001 |
| Age, y | ||
| 70-74 vs 65-69 | 0.94 (0.91-0.96) | <.001 |
| 75-79 vs 65-69 | 0.85 (0.83-0.88) | <.001 |
| 80-84 vs 65-69 | 0.75 (0.72-0.77) | <.001 |
| ≥85 vs 65-69 | 0.63 (0.61-0.66) | <.001 |
| Male vs female | 1.14 (1.12-1.17) | <.001 |
| Rural vs urban | 0.90 (0.86-0.93) | <.001 |
| Neighborhood income quintile | ||
| 2 vs 1, Lowest | 0.94 (0.91-0.97) | <.001 |
| 3 vs 1 | 0.91 (0.88-0.93) | <.001 |
| 4 vs 1 | 0.86 (0.83-0.88) | <.001 |
| 5 vs 1 | 0.80 (0.78-0.83) | <.001 |
| Diagnosis or event, yes vs no | ||
| Cancer | 0.89 (0.87-0.92) | <.001 |
| Liver disease | 0.86 (0.80-0.93) | <.001 |
| Other cardiovascular | 0.80 (0.78-0.82) | <.001 |
| Hospitalization in past year, yes vs no | 0.76 (0.74-0.78) | <.001 |
| Physician Level | ||
| Male vs female | 1.15 (1.12-1.18) | <.001 |
| IMG vs non-IMG | 1.21 (1.19-1.24) | <.001 |
| Years since graduation | ||
| 21-30 vs 0-20 | 1.05 (1.02-1.08) | <.001 |
| >30 vs 0-20 | 1.05 (1.03-1.08) | <.001 |
| Practice Level | ||
| Primary care practice modeld | ||
| FHG vs FFS | 1.10 (1.06-1.14) | <.001 |
| FHN vs FFS | 0.92 (0.67-1.26) | .60 |
| FHO vs FFS | 0.91 (0.88-0.95) | <.001 |
| FHT vs FFS | 0.82 (0.79-0.86) | <.001 |
| Other vs FFS | 1.12 (1.06-1.18) | <.001 |
| Random Effects | ||
| Practice-level variance (SE) | 0.61 (0.04) | NA |
| Practice, median odds ratio (95% CI)b | 2.11 (2.04-2.18) | <.001 |
| Practice, ICC (%)e | 15.7 | NA |
Abbreviations: CKD, chronic kidney disease; FFS, fee-for-service; FHG, family health group; FHN, family health network; FHO, family health organization; FHT, family health team; ICC, intracluster correlation coefficient; IMG, international medical graduate; NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug.
All reported values based on SAS PROC GLIMMIX output; model estimation method was residual log pseudo-likelihood; denominator df estimation method is between and within (bw); covariance structure was standard variance (vc).
Adjusted for all other factors present in the model/table, as well as quarter and season in which a visit occurred.
Includes angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Represents the primary care patient enrollment model, which informs practice organization and remuneration.
Calculated using the latent response formulation where within-practice variance is 3.29.
The magnitude of the median odds ratio of 2.11 in Table 2 suggests that the unexplained heterogeneity between practices is of greater relevance than any measured characteristic except prior NSAID use (odds ratio, 2.79) for understanding an individual patient’s odds of prescription NSAID use. The intracluster correlation coefficient indicates that 15.7% of the total variation in prescription NSAID use was attributable to practice-level clustering.
Association of Prescription NSAID Use With Safety-Related Outcomes
Our sample of 35 552 matched patient pairs, each consisting of an exposed and nonexposed patient matched on the logit of their propensity score for prescription NSAID use (exposure), is summarized in eTable 4 in the Supplement. Only 16.8% of exposed patients used selective NSAIDs. Patients with and without prescription NSAID use had similar rates of cardiac complications (288 [0.8%] vs 279 [0.8%]), renal complications (34 [0.1%] vs 33 [0.1%]), and death (27 [0.1%] vs 30 [0.1%]). Exposed patients (308 [0.9%]) were as likely to experience any cardiovascular or renal safety-related outcome as nonexposed patients (300 [0.8%]) (absolute risk difference, 0.0003; 95% CI, −0.001 to 0.002; P = .74) (eTable 5 in the Supplement).
Discussion
Among a large, Ontario-based cohort of primary care visits involving older adults with a musculoskeletal disorder and recent history of hypertension, heart failure, or CKD, we found that almost 10% resulted in prescription NSAID use despite patients’ high risk for cardiovascular and renal complications. We observed widespread prescribing variation, up to 77-fold, among primary care practices and physicians and identified several patient, physician, and practice characteristics associated with NSAID use. We found that prescription NSAID use was not associated with significantly higher (or lower) risk of cardiovascular or renal safety-related outcomes.
It has been previously established that prescription and over-the-counter NSAID use is common among patients with preexisting heart or kidney disease despite evidence-based recommendations against this practice.13,14 One study16 found that prescription NSAID use for musculoskeletal pain management ranged from 14.4% to 16.2% in patients with hypertension, heart failure, or CKD. A study59 of patients with preexisting cardiovascular disease found that self-reported use of both prescription and over-the-counter NSAIDs was common, particularly among patients with angina or myocardial infarction. Another study28 found that 5.7% of patients with moderate to severe CKD reported NSAID use; however, use was primarily driven by over-the-counter medications, and many of the patients were unaware of their condition. Our study adds to these prior investigations by demonstrating frequent prescription NSAID use among older adults at high risk for complications secondary to NSAID use.
In addition to summarizing prescribing frequency and variation among both primary care practices and individual physicians, our study is also the first to date to identify some of the patient and physician characteristics associated with prescription NSAID use. Patients with hypertension, prior NSAID users, and younger patients had greater odds of prescription NSAID use. Conversely, patients with heart failure, CKD, prior opioid use, and hospitalization in the past year had reduced odds of prescription NSAID use. Our findings suggest that prescription NSAID users were generally healthier and had less severe disease than nonusers. For healthy patients with a history of hypertension, NSAIDs may be an appropriate treatment if followed closely. Consistent with other studies24,53 of low-value care, we found that physicians who were male, international medical graduates, or further removed from medical school had greater odds of prescribing an NSAID. Physicians with these common risk factors for overuse could be the subject of future studies to understand what drives their frequent prescribing despite existing recommendations.53,60
Rosenberg and colleagues16 found relative stability among several low-value care services, even after release of corresponding Choosing Wisely US recommendations. In fact, they observed an increase in NSAID use of 2.0% per quarter between 2011 and 2013. Applying similar methods to our Canadian cohort, we observed a significant decline in prescription NSAID use of 2.0% per quarter between 2012-2013 and 2015-2016. While prescription use declined further after the October 2014 release of Choosing Wisely Canada’s NSAID recommendation, the minimal number of postrelease observations precluded more robust analysis to assess whether this observation was simply the result of secular trends.
Last, we found that rates of acute cardiovascular or renal safety-related outcomes were low and statistically equivalent between patients with and without prescription NSAID use. In contrast, prior research has demonstrated that NSAID use significantly elevates a patient’s risk of myocardial infarction, stroke, or other cardiac complications.17,18,19 Prior large-scale studies demonstrating increased risk of cardiovascular events with NSAID use,35 including Adenomatous Polyp Prevention on Vioxx (APPROVe)18 and Vioxx in Colorectal Cancer Therapy: Definition of Optimal Regime (VICTOR)19 trials, have focused on long-term NSAID use (from 18 months to 5 years) and more distal outcomes, whereas our study examined use at 7 days and its potential association with short-term safety. Our inability to demonstrate increased risk among older adults with prescription NSAID use is consistent with a prior study20 by Mamdani and colleagues that focused on the association of short-term NSAID use with myocardial infarction at 30 days. The similarity in risk between users and nonusers, each group primarily consisting of patients with hypertension, suggests that the short-term association of NSAIDs in high-risk patients with musculoskeletal pain may not be as dangerous as initially thought. Considering present concerns regarding opioid use for noncancer pain, the ability of physicians to prescribe NSAIDs to manage musculoskeletal pain in the short term could be an important clinical option in this patient population. Moreover, these new data may prompt revisiting select Choosing Wisely recommendations, particularly those recommending against short-term NSAID use.13,14
Limitations
This study has several limitations worth acknowledging. First, administrative data often lack important clinical information, such as presence of symptoms.24,53,60 We can only assume that patients with a musculoskeletal disorder were initially seen with related reports of pain at their primary care visit. Furthermore, the potential absence of functional or symptom-level information prevents us from making firm conclusions about the balance between benefits in terms of function or symptomatic relief and risks of harm for those patients receiving NSAIDs. Our estimates of prescription NSAID use are likely underestimates of overall use because we excluded aspirin and topical NSAID prescriptions, and the ODB does not capture the use of nonprescription, over-the-counter formulations of basic analgesics (aspirin, topical NSAIDs, acetaminophen, and capsaicin)4,16,27,28 or nonpharmacological alternatives (eg, physical exercise and therapy); however, our primary interest was the use of medications with high risk profiles.27,40 Propensity score matching does not account for unmeasured confounders, such as over-the-counter medication use or disease severity, which may have biased the association of prescription NSAID use with safety-related outcomes toward the null.55,56,57 Last, our cohort only consisted of patients 65 years and older covered under the ODB program who were predominantly hypertensive, limiting the generalizability of our findings to younger patients, those without ODB coverage, and nonhypertensive, high-risk patients.
Despite these limitations, we were able to estimate prescribing rates across Ontario and by region, practice, and individual practitioner, showing substantial variability in NSAID prescribing at all levels of the health care system. This observed variation, along with the identification of patient and physician characteristics associated with NSAID use, presents an opportunity for quality improvement, with some potential targets for any resulting interventions.
Conclusions
Among a sizable cohort of primary care visits by high-risk older adults for musculoskeletal disorders, almost 10% were followed by prescription NSAID use. No significant difference in acute safety-related outcomes was found between NSAID users and nonusers. Future studies on the optimal strategies to manage musculoskeletal pain in this patient population should be undertaken.
eTable 1. Inclusion Criteria and Index Event Definitions
eTable 2. Primary and Secondary Outcome Definitions
eTable 3. Preliminary Analysis of Distribution of NSAIDs Prescribed Within 30 Days of Index Visit by Number of Days Since Index Visit
eTable 4. Distribution of Baseline Characteristics by Primary Outcome for Unmatched (n = 396,248) and Matched Samples (n = 71,104)
eTable 5. 2x2 Tables for McNemar’s Test of Correlated Binary Proportions for Overall Matched Sample and Sample Stratified by History of Hypertension
eFigure 1. Prescription Nonsteroidal Anti-inflammatory Drug (NSAID) Use by Health Region (LHIN), 2010/11 to 2015/16
eFigure 2. Prescription Nonsteroidal Anti-inflammatory Drug (NSAID) Use by Primary Care Practice Group, 2010/11 to 2015/16
References
- 1.Brattwall M, Turan I, Jakobsson J. Musculoskeletal pain: prescription of NSAID and weak opioid by primary health care physicians in Sweden 2004-2008: a retrospective patient record review. J Pain Res. 2010;3:131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasselström J, Liu-Palmgren J, Rasjö-Wrååk G. Prevalence of pain in general practice. Eur J Pain. 2002;6(5):375-385. doi: 10.1016/S1090-3801(02)00025-3 [DOI] [PubMed] [Google Scholar]
- 3.Lawry GV II, Schuldt SS, Kreiter CD, Densen P, Albanese MA. Teaching a screening musculoskeletal examination: a randomized, controlled trial of different instructional methods. Acad Med. 1999;74(2):199-201. doi: 10.1097/00001888-199902000-00020 [DOI] [PubMed] [Google Scholar]
- 4.Jin J. Nonsteroidal anti-inflammatory drugs. JAMA. 2015;314(10):1084. doi: 10.1001/jama.2015.9936 [DOI] [PubMed] [Google Scholar]
- 5.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986-1000. doi: 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5(1):19-34. doi: 10.3121/cmr.2007.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knights KM, Mangoni AA, Miners JO. Non-selective nonsteroidal anti-inflammatory drugs and cardiovascular events: is aldosterone the silent partner in crime? Br J Clin Pharmacol. 2006;61(6):738-740. doi: 10.1111/j.1365-2125.2006.02678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hörl WH. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals (Basel). 2010;3(7):2291-2321. doi: 10.3390/ph3072291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day RO, Graham GG. Non-steroidal anti-inflammatory drugs (NSAIDs). BMJ. 2013;346:f3195. [DOI] [PubMed] [Google Scholar]
- 10.Huerta C, Castellsague J, Varas-Lorenzo C, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531-539. doi: 10.1053/j.ajkd.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 11.Gooch K, Culleton BF, Manns BJ, et al. . NSAID use and progression of chronic kidney disease. Am J Med. 2007;120(3):280.e1-280.e7. doi: 10.1016/j.amjmed.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1984;310(9):563-572. doi: 10.1056/NEJM198403013100905 [DOI] [PubMed] [Google Scholar]
- 13.Canadian Society of Nephrology Five things patients and physicians should question. https://choosingwiselycanada.org/nephrology. Updated June 2017. Accessed July 15, 2017.
- 14.American Society of Nephrology Five things patients and physicians should question. http://www.choosingwisely.org/societies/american-society-of-nephrology. Released April 4, 2012. Accessed July 15, 2017.
- 15.Krebs EE, Gravely A, Nugent S, et al. . Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872-882. doi: 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg A, Agiro A, Gottlieb M, et al. . Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. doi: 10.1001/jamainternmed.2015.5441 [DOI] [PubMed] [Google Scholar]
- 17.Farkouh ME, Greenberg JD, Jeger RV, et al. . Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66(6):764-770. doi: 10.1136/ard.2006.066001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bresalier RS, Sandler RS, Quan H, et al. ; Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators . Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial [published correction appears in N Engl J Med. 2006;355(2):221]. N Engl J Med. 2005;352(11):1092-1102. doi: 10.1056/NEJMoa050493 [DOI] [PubMed] [Google Scholar]
- 19.Kerr DJ, Dunn JA, Langman MJ, et al. ; VICTOR Trial Group . Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med. 2007;357(4):360-369. doi: 10.1056/NEJMoa071841 [DOI] [PubMed] [Google Scholar]
- 20.Mamdani M, Rochon P, Juurlink DN, et al. . Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med. 2003;163(4):481-486. doi: 10.1001/archinte.163.4.481 [DOI] [PubMed] [Google Scholar]
- 21.Gunz AC, Canizares M, Mackay C, Badley EM. Magnitude of impact and healthcare use for musculoskeletal disorders in the paediatric: a population-based study. BMC Musculoskelet Disord. 2012;13:98. doi: 10.1186/1471-2474-13-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18-e26. [PMC free article] [PubMed] [Google Scholar]
- 23.Beckman KD. How to document and code for hypertensive diseases in ICD-10. Fam Pract Manag. 2014;21(2):5-9. [PubMed] [Google Scholar]
- 24.Bhatia RS, Bouck Z, Ivers NM, et al. . Electrocardiograms in low-risk patients undergoing an annual health examination. JAMA Intern Med. 2017;177(9):1326-1333. doi: 10.1001/jamainternmed.2017.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleet JL, Dixon SN, Shariff SZ, et al. . Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. 2013;14:81. doi: 10.1186/1471-2369-14-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canadian Institute for Health Information Canadian coding standards for version 2015 ICD-10-CA and CCI. https://secure.cihi.ca/free_products/Coding%20standard_EN_web.pdf. Accessed July 22, 2017.
- 27.Government of Ontario. Ontario Drug Benefit Formulary/Comparative Drug Index. 28:08:04 Analgesics nonsteroidal anti-inflammatory agents. https://www.formulary.health.gov.on.ca/formulary/results.xhtml?class=280804000. Accessed January 2, 2018.
- 28.Ndlovu M, Bedson J, Jones PW, Jordan KP. Pain medication management of musculoskeletal conditions at first presentation in primary care: analysis of routinely collected medical record data. BMC Musculoskelet Disord. 2014;15:418. doi: 10.1186/1471-2474-15-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plantinga L, Grubbs V, Sarkar U, et al. ; CDC CKD Surveillance Team . Nonsteroidal anti-inflammatory drug use among persons with chronic kidney disease in the United States. Ann Fam Med. 2011;9(5):423-430. doi: 10.1370/afm.1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525. doi: 10.1136/bmj.e8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sud M, Wang X, Austin PC, et al. . Presentation blood glucose and death, hospitalization, and future diabetes risk in patients with acute heart failure syndromes. Eur Heart J. 2015;36(15):924-931. doi: 10.1093/eurheartj/ehu462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braga JR, Tu JV, Austin PC, et al. . Outcomes and care of patients with acute heart failure syndromes and cardiac troponin elevation. Circ Heart Fail. 2013;6(2):193-202. doi: 10.1161/CIRCHEARTFAILURE.112.000075 [DOI] [PubMed] [Google Scholar]
- 33.Helin-Salmivaara A, Virtanen A, Vesalainen R, et al. . NSAID use and the risk of hospitalization for first myocardial infarction in the general population: a nationwide case-control study from Finland. Eur Heart J. 2006;27(14):1657-1663. doi: 10.1093/eurheartj/ehl053 [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui NF, Coca SG, Devereaux PJ, et al. . Secular trends in acute dialysis after elective major surgery: 1995 to 2009. CMAJ. 2012;184(11):1237-1245. doi: 10.1503/cmaj.110895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham DJ, Campen D, Hui R, et al. . Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365(9458):475-481. doi: 10.1016/S0140-6736(05)70270-1 [DOI] [PubMed] [Google Scholar]
- 36.Podichetty VK, Mazanec DJ, Biscup RS. Chronic non-malignant musculoskeletal pain in older adults: clinical issues and opioid intervention. Postgrad Med J. 2003;79(937):627-633. doi: 10.1136/pmj.79.937.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Shidhani A, Al-Rawahi N, Al-Rawahi A, Murthi S. Nonsteroidal anti-inflammatory drugs (NSAIDs) use in primary health care centres in A’Seeb, Muscat: a clinical audit. Oman Med J. 2015;30(5):366-371. doi: 10.5001/omj.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Government of Ontario. Ontario Drug Benefit Formulary/Comparative Drug Index. 28:08:08 Analgesics opiate agonists. https://www.formulary.health.gov.on.ca/formulary/results.xhtml?class=280808000. Accessed January 3, 2018.
- 39.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. doi: 10.1371/journal.pmed.1002396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehrlich GE. Back pain. J Rheumatol Suppl. 2003;67:26-31. [PubMed] [Google Scholar]
- 41.Camin RM, Cols M, Chevarria JL, et al. . Acute kidney injury secondary to a combination of renin-angiotensin system inhibitors, diuretics and NSAIDS: “the triple whammy.” Nefrologia. 2015;35(2):197-206. doi: 10.1016/j.nefro.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 42.Loboz KK, Shenfield GM. Drug combinations and impaired renal function: the “triple whammy.” Br J Clin Pharmacol. 2005;59(2):239-243. doi: 10.1111/j.0306-5251.2004.2188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fournier JP, Sommet A, Durrieu G, Poutrain JC, Lapeyre-Mestre M, Montastruc JL; French Network of Regional Pharmacovigilance Centres . Drug interactions between antihypertensive drugs and non-steroidal anti-inflammatory agents: a descriptive study using the French Pharmacovigilance database. Fundam Clin Pharmacol. 2014;28(2):230-235. doi: 10.1111/fcp.12014 [DOI] [PubMed] [Google Scholar]
- 44.Nitsch D, Tomlinson LA. Safety of coprescribing NSAIDs with multiple antihypertensive agents: triple drug combinations are associated with increased hospital admission for acute kidney injury, but questions remain. BMJ. 2013;346:e8713. doi: 10.1136/bmj.e8713 [DOI] [PubMed] [Google Scholar]
- 45.Adhiyaman V, Asghar M, Oke A, White AD, Shah IU. Nephrotoxicity in the elderly due to co-prescription of angiotensin converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs. J R Soc Med. 2001;94(10):512-514. doi: 10.1177/014107680109401005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas MC. Diuretics, ACE inhibitors and NSAIDs: the triple whammy. Med J Aust. 2000;172(4):184-185. [DOI] [PubMed] [Google Scholar]
- 47.Statistics Canada Postal CodeOM Conversion File (PCCF), reference guide. https://www150.statcan.gc.ca/n1/pub/92-154-g/92-154-g2017001-eng.htm. Published June 2017. Accessed August 24, 2018.
- 48.Kiran T, Kopp A, Moineddin R, Glazier RH. Longitudinal evaluation of physician payment reform and team-based care for chronic disease management and prevention. CMAJ. 2015;187(17):E494-E502. doi: 10.1503/cmaj.150579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glazier RH, Hutchinson B, Kopp A Comparison of family health teams to other primary care models, 2004/05 to 2011/12. https://www.ices.on.ca/Publications/Atlases-and-Reports/2015/Comparison-of-Family-Health-Teams. Published November 2015. Accessed July 23, 2017.
- 50.Merlo J, Chaix B, Ohlsson H, et al. . A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290-297. doi: 10.1136/jech.2004.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81-88. doi: 10.1093/aje/kwi017 [DOI] [PubMed] [Google Scholar]
- 52.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- 53.Bouck Z, Mecredy G, Ivers NM, et al. . Routine use of chest x-ray for low-risk patients undergoing a periodic health examination: a retrospective cohort study. CMAJ Open. 2018;6(3):E322-E329. doi: 10.9778/cmajo.20170138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiernan K, Tao J, Gibbs P Tips and strategies for mixed modeling with SAS/STAT procedures. Paper 332-2012. http://support.sas.com/resources/papers/proceedings12/332-2012.pdf. Accessed June 23, 2017.
- 55.Austin PC. An introduction to propensity score methods for reduction of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res. 2011;46(1):119-151. doi: 10.1080/00273171.2011.540480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan T, Yung YF, Stokes M Propensity score methods for causal inference with the PSMATCH procedure. Paper SAS332-2017. https://support.sas.com/resources/papers/proceedings17/SAS0332-2017.pdf. Accessed August 24, 2018.
- 58.SAS Usage note 46997: estimating the risk (proportion) difference for matched pairs data with binary response. http://support.sas.com/kb/46/997.html. Accessed January 18, 2018.
- 59.Castelli G, Petrone A, Xiang J, Shrader C, King D. Rates of nonsteroidal anti-inflammatory drug use in patients with established cardiovascular disease: a retrospective, cross-sectional study from NHANES 2009-2010. Am J Cardiovasc Drugs. 2017;17(3):243-249. doi: 10.1007/s40256-016-0212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pendrith C, Bhatia M, Ivers NM, et al. . Frequency of and variation in low-value care in primary care: a retrospective cohort study. CMAJ Open. 2017;5(1):E45-E51. doi: 10.9778/cmajo.20160095 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Inclusion Criteria and Index Event Definitions
eTable 2. Primary and Secondary Outcome Definitions
eTable 3. Preliminary Analysis of Distribution of NSAIDs Prescribed Within 30 Days of Index Visit by Number of Days Since Index Visit
eTable 4. Distribution of Baseline Characteristics by Primary Outcome for Unmatched (n = 396,248) and Matched Samples (n = 71,104)
eTable 5. 2x2 Tables for McNemar’s Test of Correlated Binary Proportions for Overall Matched Sample and Sample Stratified by History of Hypertension
eFigure 1. Prescription Nonsteroidal Anti-inflammatory Drug (NSAID) Use by Health Region (LHIN), 2010/11 to 2015/16
eFigure 2. Prescription Nonsteroidal Anti-inflammatory Drug (NSAID) Use by Primary Care Practice Group, 2010/11 to 2015/16