Abstract
Background
Whether slow graft function (SGF) represents an intermediate phenotype between immediate graft function (IGF) and delayed graft function (DGF) in kidney transplant recipients is unknown.
Material/Methods
In a retrospective cohort analysis of 1,222 kidney transplant recipients, we classified patients as having IGF, SGF, and DGF using two different schemas. SGF was defined as serum creatinine (Cr) ≥3.0 mg/dL by postoperative day 5 in Schema 1, and in Schema 2, SGF was defined as Cr >1.5 mg/dL plus a creatinine reduction ratio <20% between postoperative days 1 and 3. A complementary log-log model was used to examine the association of graft function with graft survival and patient survival.
Results
Mean age of study patients was 51.5±13.3 years, 59.9% were male, and 66.7% were white. In Schema 1, SGF and DGF were associated with comparable increases in risk of graft failure compared to IGF (hazard ratio (HR) 1.46, 95% confidence intervals (CI) 1.02–2.10 for SGF and HR 1.56, CI 1.11–2.22 for IGF); estimates were similar for Schema 2 (HR 1.52, CI 1.05–2.20 for SGF and HR 1.54, CI 1.10–2.17 for IGF). However, for mortality, outcomes for SGF were similarly to IGF, both SGF and IGF were associated with lower risk relative to DGF (HR 0.54, CI 0.36–0.80 for SGF in Schema 1; HR 0.58, CI 0.39–0.85 for SGF in Schema 2).
Conclusions
These findings suggest that SGF may be a marker for graft failure but not for mortality, and SGF may therefore represent a phenotype separate from IGF and DGF.
MeSH Keywords: Delayed Graft Function, Graft Survival, Kidney Transplantation, Mortality
Background
The level of function of a transplanted kidney in the immediate postoperative period is correlated with long-term graft and patient survival [1–4]. In roughly a quarter of deceased donor [5,6] and perhaps 5–10% of living donor kidney transplants [7–9], dialysis is required within the first week of transplantation, a situation commonly termed delayed graft function (DGF). DGF is associated with acute rejection, prolonged hospital stays, and healthcare costs [10–13], as well as increased rates of graft failure and mortality [1–4].
The level of postoperative kidney function has implications for bedside clinical care, epidemiological research, outcomes quality reporting, and clinical trials. For example, the development of DGF often influences exposure to calcineurin inhibitors [14–17] and other potential interventions [18,19]. DGF is also a UNOS (United Network for Organ Sharing) reportable outcome [20,21]. Additionally, DGF has also been approved by the U.S. Food and Drug Administration as a surrogate endpoint for trials [20].
However, assessment of post-transplant renal function has historically been relatively crude, with most schemas classify patients merely by the presence or absence of DGF. As a result, many patients may have significant injury but, by default, are considered to have “adequate” graft function if they avoid dialysis [22]. An intermediate phenotype, known as slow graft function (SGF), can be characterized by slower initial postoperative decline in serum creatinine (Cr) compared to immediate graft function (IGF) but without the need for dialysis [23,24]. Schemas proposed to define SGF typically utilize either failure of attainment of an absolute level of Cr by a given postoperative day (POD) (e.g., Cr ≥3 mg/ld. on POD5 [21] or Cr ≥2.5 on POD7 [25]) or an inadequate percentage reduction in Cr over a given period (e.g., a creatinine reduction ratio (CRR) of <30% between POD1 and POD2 [26]).
We investigated the potential utility of risk stratification schemas that categorize patients as having SGF by examining how SGF was associated with long-term patient and graft survival. We employed one schema that classified SGF as occurring when the Cr remains >3.0 mg/dL (in the absence of dialysis) on POD5 [21,22,27,28] and compared it to one schema that defines SGF as occurring when both the Cr remains > 1.5 mg/dL by POD3 and the CRR between POD1 and POD3 is >20% (in the absence of the need for dialysis). We utilized a large, retrospective cohort of patients, from two centers, for whom data on long-term follow-up was available. We hypothesized that SGF would have a strength of association with graft and patient survival as compared to IGF and DGF.
Material and Methods
Study cohort and data sources
A retrospective cohort analysis of 1,222 recipients of living-donor and deceased-donor kidney transplants from two centers was performed. The cohort consisted of all 996 consecutive transplantations at Hennepin County Medical Center between January 1, 2000 and December 31, 2012 plus all 226 consecutive transplantations at the University of Kansas Medical Center between May 1, 2007 and December 31, 2009. Information was drawn from the respective electronic medical records at both centers and combined with data from DonorNet, the electronic organ placement system operated by UNOS.
From these sources, donor and recipient risk factors commonly associated with outcomes were collected. Recipient factors included age, gender, race, body mass index (BMI), history of diabetes, history of previous transplantation, panel reactive antibody (PRA) level, duration of maintenance dialysis (in which 0 years indicated a preemptive transplant) and initial type of calcineurin inhibitor prescribed. Donor factors included age and gender, donation source (living versus deceased), donor criteria category [standard criteria donation (SCD), extended criteria donation (ECD), or donation after circulatory death (DCD], donor cause of death in deceased donors, cold ischemia time (CIT), and level of human leukocyte antigen (HLA) mismatch.
Graft function classification schemas
We selected two graft function classification schemas for purposes of comparison, one drawn from the literature [21,26] and one that we developed. For both schemas, patients who required dialysis within the first week post-transplantation were classified as DGF. Where the schemas varied was in how they distinguished between IGF and SGF. Classification Schema 1, initially proposed by Humar et al. [21], defined IGF as Cr <3.0 mg/dL by POD5 and SGF as Cr ≥3.0 mg/dL. Schema 2 defined IGF as either Cr ≤1.5 mg/dL or CRR ≥20% between POD1 and POD3, and SGF as Cr >1.5 mg/dL and CRR <20% between POD1 and POD3.
Outcomes
Primary outcomes were death-censored graft failure and all-cause mortality. Additionally, estimated glomerular filtration (eGFR), calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [29] was assessed at one month, one year, three years, and five years.
Statistical analysis
To examine the associations between three levels of graft function (IGF, SGF, and DGF) in each definition and the time-to-event outcomes (death-censored graft failure and all-cause mortality), a two-step approach was used given the restricted size of the sample and resultant concerns about power. In step one, a univariable analyses (i.e., single explanatory measure) was conducted for each of the 15 covariates listed in Table 1. Using a standard model-building approach, the covariates which demonstrated statistical significance with a p-value <0.05 in the univariable analyses were included in the multivariable model, while those covariates with p-value ≥0.05 were not. Additionally, two potentially key factors (recipient diabetes and type of calcineurin inhibitor prescribed) were forced into the multivariable model for death-censored graft failure to be consistent with the model for all-cause mortality.
Table 1.
Participant demographic information.
| N=1,222 | |
|---|---|
| Recipient factors* | |
| Age (years) | 51.1±13.3 |
| Sex (% male) | 59.9% |
| Race (% black) | 15.9% |
| BMI (kg/m2) | 28.2 |
| Diabetes (%) | 39.9% |
| Prior transplantation (%) | 12.4% |
| PRA ≥20% (%) | 19.6% |
| Dialysis duration, years | 1.3 (0.2, 3.4) |
| Use of CNI | |
| Tacrolimus | 18.5% |
| Cyclosporine | 81.5% |
| Donor factors | |
| Age (years) | 46±14.6 |
| Sex (% male) | 53.7% |
| Donation type n, (%) | |
| Living donation | 609 (49.8%) |
| SCD | 503 (41.2%) |
| ECD | 27 (2.2%) |
| DCD | 83 (6.8%) |
| Cause of Death (deceased donors) | |
| Trauma | 49% |
| CVA | 32.1% |
| Anoxia | 17.3% |
| Other | 1.6% |
| Other factors | |
| Cold ischemic time (hours) | 16.2±8.4 |
| Number of HLA mismatch | 3.2±1.8 |
Continuous variables are shown as mean ± one standard deviation with the exception of dialysis duration, which is shown as median and 25th/75th percentiles.
BMI – body mass index; PRA – panel reactive antibody; SCD – standard criteria donors; ECD – extended criteria donors; DCD – donation after cardiac death; CVA – cardiovascular accident; HLA – human leukocyte antigen.
For multivariable modeling (step two), a complementary log-log model was used. Briefly, a complementary log-log model is a discrete analog of the continuous proportional hazards model and is advantageous over the Cox model for use when the time-to event outcomes are interval-censored. The inter-relationship between, and appropriate use of, a Cox proportional hazards model for time-continuous data and a complementary log-log model for time-interval data has been discussed by Allison [30]. Using a standard approach, adjusted hazard ratios (HR) with 95% confidence intervals (CI) were calculated by exponentiating the parameter estimates of the various covariates obtained from our model. (HR interpretations are analogous to those from Cox proportional hazards regression models, and were suitable to the censoring pattern our data presented, which differed from the standard right-censored data commonly analyzed with Cox models). Predicted survival curves were generated for the three graft function categories after adjusting for the effect of the other covariates in the model. Since the outcome was interval censored, survival predictions were made using the complementary log-log model at the aforementioned timepoints and a smooth predicted survival curve was generated by means of polynomial fit. All analyses were performed using SAS at the 5% level of significance.
Protection of human research participants
The research protocol was approved by the Human Research Participants Committees (Institutional Review Boards) at Hennepin County Medical Center and University of Kansas Medical Center.
Results
Characteristics of the cohort
A total of 1,222 kidney transplant recipients were included, with a median (25th/75th percentile) follow-up duration of 87.3 months (62.1/117.1 months). The demographic and clinical characteristics of the cohort, divided into recipient factors, donor factors, and other factors, are shown in Table 1. The mean age was 51.1±13.3 years, 59.9% of recipients were male, 15.9% of recipients were black, and 19.6% of recipients had a PRA titer >20%; 49.8% of recipients received kidneys from living donors. Graft failure occurred in 26 recipients by one-year post-transplantation, in 96 recipients by three years post-transplantation, in 154 recipients by five years post-transplantation, and in 216 recipients by 10 years post-transplantation. Corresponding totals for recipient deaths by post-transplantation years (one, three, five, and ten years) were 49, 114, 174, and 285 deaths, respectively.
Distribution of graft function category by schema
The distribution of recipients classified as having IGF, SGF, and DGF varied, as expected, based on the schema utilized (Figure 1). While the percentage of patients classified as having DGF was, by definition, constant (21.5%), the percent classified as having SGF varied, at 14.6% for Schema 1 and 12.8% for Schema 2.
Figure 1.

Distribution of DGF, SGF, and IGF.
Graft failure
The association between postoperative graft function classification and long-term graft failure, is shown in Table 2, which shows the multivariable analysis including the risk factors with statistical significance in the univariable model. For Schema 1, SGF was associated with a higher risk for graft failure compared to IGF (HR 1.46, 95% CI 1.02–2.10), but there was no difference when SGF was compared to DGF; IGF was associated with lower risk of graft failure compared to DGF (HR 0.64, 95% CI 0.45–0.90). Findings for Schema 2 were similar to those for Schema 1: SGF was associated with higher risk for graft failure than IGF (HR 1.52, 95% CI 1.05–2.20), but there were no differences between SGF and DGF; IGF was associated with lower risk for graft failure than DGF (HR 0.65, 95% CI 0.46–0.91).
Table 2.
Factors associated with death-censored graft failure.
| Factors* | Schema 1 | Schema 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| SGF vs. IGF | 1.46 | 1.02–2.10 | 0.038 | 1.52 | 1.05–2.20 | 0.029 |
| SGF vs. DGF | 0.93 | 0.63–1.38 | 0.73 | 0.99 | 0.66–1.49 | 0.96 |
| IGF vs. DGF | 0.64 | 0.45–0.90 | 0.011 | 0.65 | 0.46–0.91 | 0.013 |
| Recipient age | 0.96 | 0.95–0.97 | <0.001 | 0.96 | 0.95–0.97 | <0.001 |
| Recipient race (black) | 2.08 | 1.54–2.82 | <0.001 | 2.06 | 1.52–2.80 | <0.001 |
| Recipient DM | 1.46 | 1.09–1.96 | 0.017 | 1.46 | 1.09–1.96 | 0.011 |
| Recipient dialysis duration | 1.01 | 0.95–1.06 | 0.71 | 1.01 | 0.96–1.06 | 0.73 |
| Donor age | 1.02 | 1.01–1.03 | 0.002 | 1.02 | 1.01–1.03 | 0.002 |
| CNI (Tacrolimus) | 1.34 | 0.93–1.93 | 0.12 | 1.33 | 0.92–1.93 | 0.13 |
| HLA mismatch | 1.09 | 1.01–1.18 | 0.03 | 1.10 | 1.01–1.19 | 0.02 |
Only covariates which demonstrated statistical significance with a p-value <0.05 in the univariate analyses were included.
SGF – slow graft function; IGF – immediate graft function; DGF – delayed graft function; DM – diabetes mellitus; CNI – calcineurin inhibitor; HLA – human leukocyte antigen.
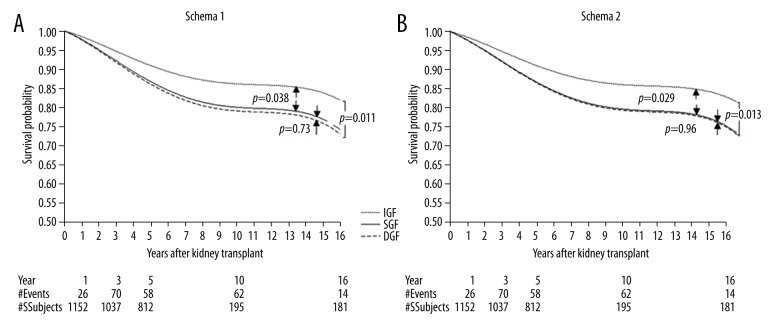
Graft survival of recipients with IGF, SGF, and DGF based on the two classification schemas are shown in Figure 2. In both Schemas 1 and Schema 2, graft survival in patients with SGF and DGF were similar, but were significantly worse than patients with IGF (p=0.038 and p=0.029 between IGF and SGF for Schemas 1 and 2, respectively).
Figure 2.
Death censored graft failure. (A) Graft survival with Schema 1. (B) Graft survival with Schema 2.
Mortality
The relationship between postoperative graft function classification and mortality is shown, analogously, in Table 3, which shows multivariable analysis including only the risk factors with statistical significance in the univariable model. In distinction to our findings for graft failure, the HRs for death were no different for both schemas between SGF and IGF (HR 0.85, 95% CI 0.58–1.25 for Schema 1 and HR 0.93, 95% CI 0.64–1.36 for Schema 2). SGF was associated with lower mortality compared to DGF (HR 0.51, 95% CI 0.34–0.75 for Schema 1 and HR 0.54, 95% CI 0.37–0.80 for Schema 2).
Table 3.
Factors associated with all-cause mortality.
| Factors* | Schema 1 | Schema 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| SGF vs. IGF | 0.92 | 0.62–1.34 | 0.66 | 1.01 | 0.69–1.47 | 0.97 |
| SGF vs. DGF | 0.54 | 0.36–0.80 | 0.002 | 0.58 | 0.39–0.85 | 0.006 |
| IGF vs. DGF | 0.59 | 0.45–0.77 | <0.001 | 0.57 | 0.44–0.76 | <0.001 |
| Recipient age | 1.05 | 1.04–1.06 | <0.001 | 1.05 | 1.04–1.06 | <0.001 |
| Recipient race | 1.24 | 0.89–1.74 | 0.20 | 1.24 | 0.89–1.73 | 0.20 |
| Recipient DM | 1.59 | 1.25–2.02 | <0.001 | 1.58 | 1.25–2.01 | <0.001 |
| Recipient dialysis duration | 1.07 | 1.03–1.12 | 0.002 | 1.08 | 1.02–1.12 | 0.003 |
| Donor age | 1.01 | 0.99–1.02 | 0.13 | 1.01 | 0.99–1.02 | 0.13 |
| CNI (Tacrolimus) | 0.56 | 0.38–0.82 | 0.003 | 0.55 | 0.38–0.81 | 0.002 |
Only covariates which demonstrated statistical significance with a p-value <0.05 in the univariate analyses were included.
SGF – slow graft function; IGF – immediate graft function; DGF – delayed graft function; DM – diabetes mellitus; CNI – calcineurin inhibitor.
Patient survival, by classification schema, is shown in Figure 3. In both schemas, patient survival was similar between patients with SGF and IGF, but significantly better than patients with DGF (p=0.002 and p=0.006 between SGF and DGF in Schemas 1 and 2, respectively).
Figure 3.
All-cause patient mortality. (A) Patient survival with Schema 1. (B) Patient survival with Schema 2.
Graft function
Five-year change in eGFR by IGF, SGF, and DGF status is shown in Figure 4 by schema. For both schemas, the eGFR values in patients with SGF tended to be similar to those in patients with DGF over time; however, eGFR values were lower than in patients with IGF. For example, for Schema 1, the eGFR values at five years were 56.0, 51.2, and 50.6 mL/min/1.73 m2 for patients with IGF, SGF, and DGF, respectively. Analogous values were 55.7, 52.1, and 50.6 mL/min/1.73 m2 in Schema 2.
Figure 4.
Five-year eGFR distribution. (A) 5-year eGFR distribution with Schema 1. (B) Five-year eGFR distribution with Schema 2.
Discussion
In this large, retrospective cohort study of kidney transplant recipients in which the median follow-up period exceeded seven years, we found that SGF appeared to characterize a group of patients who had a risk of long-term graft failure comparable to those with DGF, but all-cause mortality was comparable to those with IGF. This suggests that the need for dialysis alone (the traditional defining tenet of DGF) may not capture all the risks associated with poorer graft survival. However, suboptimal kidney function in the immediate post-transplantation period appears to confer no additional mortality risk compared to IGF. SGF might therefore be considered an “intermediate” phenotype in which the graft-function implications are similar to DGF, but the mortality implications are akin to IGF. SGF appears to be a phenotype that is “concealed” inside IGF when traditional schemas (i.e., those that characterize patients merely by the presence or absence of DGF) are used.
Incorporation of the concept of SGF into post-transplantation care might provide important clinical benefits. Appreciation of SGF as a distinct phenotype permits a finer degree of risk stratification for long-term graft and patient outcomes than is available when using cruder classification schemas. In addition, and perhaps more practically, SGF might influence early therapeutic decisions such as optimizing volume status, reducing CNI exposure, avoiding nephrotoxins, and using calcium channel blockers, all strategies that have been previously suggested [31].
It is for these reasons that SGF has been the subject of study for several decades. An early proponent of the concept of SGF were Humar et al. [21], whose classification system formed the basis of Schema 1. Two decades ago, this group showed that patients with SGF had rates of graft and patient survival similar to those of patients with DGF patients, but worse than patients with IGF. Nonetheless, use of the same schema in the intervening years demonstrated varying findings. A follow-up study in 2002 found that SGF appeared to represent a true intermediate phenotype in which five-year graft survival in patients with SGF was distinguishable from patients with IGF and DGF; mortality was not examined [32]. These studies did not explicitly utilize extensive risk factor adjustment. To improve analytic rigor, we adjusted for a host of factors associated with both graft and patient survival. Further, we utilized a substantially larger cohort, followed over a longer time period than previously reported in studies, to more fully explore the potential utility of this schema. Our findings suggest that the schema as originally envisioned by Humar et al. confers utility for risk stratification in that it identifies SGF patients as at risk for graft failure similar to patients with DGF, but at no increased risk for all-cause mortality compared to patients with IGF.
However, we hypothesized that a novel schema, which combined an element of relative Cr change with absolute Cr level might help more finely risk stratify patients for key outcomes. Because absolute Cr levels, which vary by age, muscle mass/activity, diet, and inflammatory status [33], trends in creatinine change during the first three postoperative days might provide a dynamic measure of kidney function. Three days was chosen since it likely captures the initial period of the host immune response effect on graft function. Despite our rationale, we found our proposed schema performed very similar to Schema 1 in identifying a group of patients at increased risk for graft dysfunction but not all-cause mortality.
That SGF appears to be associated with poor graft survival but not patient survival may be because SGF is a signal for renal-limited injury. Essentially all renal allografts sustain acute injury during procurement, preservation, and reperfusion. Severe injury, which can manifest as DGF, may be a systemic phenomenon in which DGF-related ischemia reperfusion injury is associated with systemic upregulation of cytokines and adhesion molecules and increased oxidative stress, presumably eliciting inflammatory responses beyond kidney. It is conceivable that such injury, if severe, could have long-term implications for patient survival [34,35]. In contrast, a less severe inflammatory cascade, presumably manifesting as SGF, may be associated with adverse outcomes that, while important, are relatively confined to the kidney. Evidence for this is strengthened by the finding that the five-year eGFR trends we observed were broadly concordant with the findings for graft survival: patients with SGF had an eGFR trend similar to those with DGF (and distinct from that of IGF) for both schemas, which is consistent with a recent study [36]. However, although the idea that the degree of renal dysfunction may be related to extra-renal outcomes is appealing, this hypothesis should be considered speculative at present.
Strengths of our study included the size of the cohort study, which was one of the largest reported in the literature, our very long duration of follow-up, and our generalizability. We deliberately chose to include both living and deceased kidney transplants, as has done in previous studies [22,37–39], because the central pathophysiological process of DGF, namely ischemia-reperfusion injury, is common to both living and deceased kidney transplants and DGF becomes more common with the growth of paired kidney exchange programs. Realizing that donor source impacts outcomes, we explicitly adjust for this in our models.
Our study had several important limitations. First, our study was retrospective in nature, and so, as with all observational studies, causality cannot be inferred. Second, our models did not adjust for all potential covariates which might influence graft and patient survival, such as, recipient socioeconomic status, or KDPI, neither of which were available in our database. Third, we did not specifically study whether the definition of DGF itself is informative: DGF is, by nature, a retrospective diagnosis based on the need for dialysis within seven days following transplantation, and the provision of dialysis varies by center and even by treating physician. Future work might investigate whether a definition of DGF based solely on the need for dialysis is itself an informative concept.
Conclusions
In summary, our study suggests that the concept of SGF provides useful risk stratification information for important outcomes, at least if defined using approaches used in our study such as Schema 1 and Schema 2 that account for evolving renal function beyond the second postoperative day. Patients with SGF appear to represent an intermediate phenotype for whom the risk of graft loss is similar to patients with DGF, while the risk of death is no worse than for patients with IGF. Graft and patient outcomes may, therefore, not be coupled, depending on the level of renal dysfunction in the week after transplantation. As such, important information may be lost if patients are classified using traditional systems that merely classify patients by the presence or absence of DGF. Future work should be undertaken to investigate how information from events in the immediate post-transplantation period can be used to improve the prediction of key clinical outcomes.
Footnotes
Conflicts of interests
None.
Source of support: Departmental sources
References
- 1.Butala NM, Reese PP, Doshi MD, Parikh CR. Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation. 2013;95:1008–14. doi: 10.1097/TP.0b013e3182855544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–74. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Perez Fontan M, Rodriquez-Carmona A, Bouza P, et al. Outcome of grafts with long-lasting delayed function after renal transplantation. Transplantation. 1996;62:42–47. doi: 10.1097/00007890-199607150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi N, McBride MA, Hariharan S. Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis. Kidney Int. 2004;65:1906–13. doi: 10.1111/j.1523-1755.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 5.Irish WD, McCollum DA, Tesi RJ, et al. Nomogram for predicting the likelihood of delayed graft function in adult cadaveric renal transplant recipients. J Am Soc Nephrol. 2003;14:2967–74. doi: 10.1097/01.asn.0000093254.31868.85. [DOI] [PubMed] [Google Scholar]
- 6.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279–96. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senel FM, Karakayali H, Moray G, Haberal M. Delayed graft function: Predictive factors and impact on outcome in living-related kidney transplantations. Ren Fail. 1998;20:589–95. doi: 10.3109/08860229809045151. [DOI] [PubMed] [Google Scholar]
- 8.Park HS, Hong YA, Kim HG, et al. Delayed graft function in living-donor renal transplantation: 10-year experience. Transplant Proc. 2012;44:43–46. doi: 10.1016/j.transproceed.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 9.Simpkins CE, Montgomery RA, Hawxby AM, et al. Cold ischemia time and allograft outcomes in live donor renal transplantation: Is live donor organ transport feasible? Am J Transplant. 2007;7:99–107. doi: 10.1111/j.1600-6143.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- 10.Lu CY, Penfield JG, Kielar ML, et al. Hypothesis: Is renal allograft rejection initiated by the response to injury sustained during the transplant process? Kidney Int. 1999;55:2157–68. doi: 10.1046/j.1523-1755.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 11.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66:1697–701. doi: 10.1097/00007890-199812270-00022. [DOI] [PubMed] [Google Scholar]
- 12.Almond PS, Troppmann C, Escobar F, Frey DJ, Matas AJ. Economic impact of delayed graft function. Transplant Proc. 1991;23:1304. [PubMed] [Google Scholar]
- 13.Rosenthal JT, Danovitch GM, Wilkinson A, Ettenger RB. The high cost of delayed graft function in cadaveric renal transplantation. Transplantation. 1991;51:1115–18. [PubMed] [Google Scholar]
- 14.McTaggart RA, Gottlieb D, Brooks J, et al. Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am J Transplant. 2003;3:416–23. doi: 10.1034/j.1600-6143.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith KD, Wrenshall LE, Nicosia RF, et al. Delayed graft function and cast nephropathy associated with tacrolimus plus rapamycin use. J Am Soc Nephrol. 2003;14:1037–45. doi: 10.1097/01.asn.0000057542.86377.5a. [DOI] [PubMed] [Google Scholar]
- 16.Shaffer D, Langone A, Nylander WA, et al. A pilot protocol of a calcineurin-inhibitor free regimen for kidney transplant recipients of marginal donor kidneys or with delayed graft function. Clin Transplant. 2003;17(Suppl 9):31–34. doi: 10.1034/j.1399-0012.17.s9.5.x. [DOI] [PubMed] [Google Scholar]
- 17.Gonwa TA, Mai ML, Smith LB, et al. Immunosuppression for delayed or slow graft function in primary cadaveric renal transplantation: use of low dose tacrolimus therapy with postoperative administration of anti-CD25 monoclonal antibody. Clin Transplant. 2002;16:144–49. doi: 10.1034/j.1399-0012.2002.1o078.x. [DOI] [PubMed] [Google Scholar]
- 18.Schroppel B, Legendre C. Delayed kidney graft function: From mechanism to translation. Kidney Int. 2014;86:251–58. doi: 10.1038/ki.2014.18. [DOI] [PubMed] [Google Scholar]
- 19.Nashan B, Abbud-Filho M, Citterio F. Prediction, prevention, and management of delayed graft function: Where are we now? Clin Transplant. 2016;30:1198–208. doi: 10.1111/ctr.12832. [DOI] [PubMed] [Google Scholar]
- 20.Orandi BJ, James NT, Hall EC, et al. Center-level variation in the development of delayed graft function after deceased donor kidney transplantation. Transplantation. 2015;99:997–1002. doi: 10.1097/TP.0000000000000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humar A, Johnson EM, Payne WD, et al. Effect of initial slow graft function on renal allograft rejection and survival. Clin Transplant. 1997;11:623–27. [PubMed] [Google Scholar]
- 22.Akkina SK, Connaire JJ, Israni AK, et al. Similar outcomes with different rates of delayed graft function may reflect center practice, not center performance. Am J Transplant. 2009;9:1460–66. doi: 10.1111/j.1600-6143.2009.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boom H, Mallat MJ, de Fijter JW, et al. Delayed graft function influences renal function, but not survival. Kidney Int. 2000;58:859–66. doi: 10.1046/j.1523-1755.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 24.Moore J, Shabir S, Chand S, et al. Assessing and comparing rival definitions of delayed renal allograft function for predicting subsequent graft failure. Transplantation. 2010;90:1113–16. doi: 10.1097/TP.0b013e3181f86966. [DOI] [PubMed] [Google Scholar]
- 25.Zeraati AA, Naghibi M, Kianoush S, Ashraf H. Impact of slow and delayed graft function on kidney graft survival between various subgroups among renal transplant patients. Transplant Proc. 2009;41:2777–80. doi: 10.1016/j.transproceed.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigo E, Ruiz JC, Pinera C, et al. Creatinine reduction ratio on post-transplant day two as criterion in defining delayed graft function. Am J Transplant. 2004;4:1163–69. doi: 10.1111/j.1600-6143.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigo E, Fernandez-Fresnedo G, Ruiz JC, et al. Similar impact of slow and delayed graft function on renal allograft outcome and function. Transplant Proc. 2005;37:1431–32. doi: 10.1016/j.transproceed.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 28.Wang CJ, Shafique S, McCullagh J, et al. Implications of donor disseminated intravascular coagulation on kidney allograft recipients. Clin J Am Soc Nephrol. 2011;6:1160–67. doi: 10.2215/CJN.07280810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allison PL. Survival Analysis Using SAS - A Practical Guide Chapter 7. Cary, North Carolina: SAS Institute; 2012. [Google Scholar]
- 31.Peeters P, Vanholder R. Therapeutic interventions favorably influencing delayed and slow graft function in kidney transplantation: mission impossible? Transplantation. 2008;85:S31–37. doi: 10.1097/TP.0b013e318169c548. [DOI] [PubMed] [Google Scholar]
- 32.Humar A, Ramcharan T, Kandaswamy R, et al. Risk factors for slow graft function after kidney transplants: A multivariate analysis. Clin Transplant. 2002;16:425–29. doi: 10.1034/j.1399-0012.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- 33.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]
- 34.Tapiawala SN, Tinckam KJ, Cardella CJ, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21:153–61. doi: 10.1681/ASN.2009040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–27. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 36.Shin JH, Koo EH, Ha SH, et al. The impact of slow graft function on graft outcome is comparable to delayed graft function in deceased donor kidney transplantation. Int Urol Nephrol. 2016;48:431–39. doi: 10.1007/s11255-015-1163-1. [DOI] [PubMed] [Google Scholar]
- 37.Vilar E, Varagunam M, Yaqoob MM, Raftery M, Thuraisingham R. Creatinine reduction ratio: A useful marker to identify medium and high-risk renal transplants. Transplantation. 2010;89:97–103. doi: 10.1097/TP.0b013e3181be3dd1. [DOI] [PubMed] [Google Scholar]
- 38.Nel D, Vogel J, Muller E, et al. Slow early graft function: A neglected entity after renal transplantation. Nephron Clin Pract. 2012;120:c200–4. doi: 10.1159/000340032. [DOI] [PubMed] [Google Scholar]
- 39.Govani MV, Kwon O, Batiuk TD, et al. Creatinine reduction ratio and 24-hour creatinine excretion on posttransplant day two: Simple and objective tools to define graft function. J Am Soc Nephrol. 2002;13:1645–49. doi: 10.1097/01.asn.0000014253.40506.f6. [DOI] [PubMed] [Google Scholar]