Abstract
Background
Postreperfusion syndrome (PRS) is a dreadful and well-documented complication in adult liver transplantation (LT). However, information regarding PRS in pediatric LT is still scarce. We aimed to identify the incidence, risk factors and associated outcomes of pediatric LT in a single-center study.
Material/Methods
The medical records of 75 consecutive pediatric patients who underwent deceased donor liver transplantation (DDLT) from July 2015 to October 2017 were retrospectively reviewed. PRS was determined according to the Peking criteria when significant arrhythmia or refractory hypotension occurred following revascularization of the liver graft. Patients were divided into PRS and non-PRS groups. Preoperative, intraoperative, and postoperative data were collected and compared between the 2 groups. Independent risk factors for PRS were analyzed using binary logistic regression analysis.
Results
PRS occurred in 26 patients (34.7%). Univariate analysis showed that the graft-to-recipient weight ratio (P=0.023), donor warm ischemia time (P<0.001), and the use of an expanded criteria donor (ECD) liver graft (P<0.001) were significant predictors of PRS. Binary logistic regression showed that the use of an ECD liver graft (odds ratio [OR]: 18.668; 95% confidence interval [95% CI]: 4.866–71.622) and lower hematocrit (HCT) level before reperfusion (OR: 0.878; 95% CI: 0.782–0.985) were independent predictors of PRS. PRS was significantly associated with early allograft dysfunction (73.1% vs. 18.4%, P<0.001), primary nonfunction (11.5% vs. 0.0%, P=0.039), and a prolonged hospital stay (median: 30.5 vs. 21.0, P=0.007).
Conclusions
The use of an ECD liver graft and lower HCT level before reperfusion were independent risk factors for PRS in pediatric DDLT. Intraoperative PRS occurrence seems to be associated with poor liver allograft function and worsened patient postoperative outcomes.
MeSH Keywords: Hemodynamics, Intraoperative Complications, Liver Transplantation, Pediatrics, Risk Factors
Background
Postreperfusion syndrome (PRS) is a relatively common but potentially life-threatening intraoperative complication during liver transplantation (LT), with a reported incidence between 3.6% and 81.0% [1–9]. The occurrence of PRS is often associated with poor patient and liver allograft outcomes [3–8]. However, the exact mechanism of PRS is still unclear, but it has been generally attributed to the release of cold, hyperkalemic, acidotic and vasoactive substances from the preservation solution, the donor liver and the recipient’s ischemic intestinal system [10–12]. To date, many studies have investigated PRS in adult LT, while information on PRS in pediatric LT is still scarce. LT in pediatric patients is quite different from that in adult patients with respect to the underlying liver diseases, donor characteristics, surgical techniques, and anesthetic management. In this study, we aimed to identify the risk factors for PRS, as well as its incidence and associated clinical outcomes, in pediatric deceased donor liver transplantation (DDLT).
Material and Methods
Patients
This retrospective study was approved by the Institutional Review Board of Beijing Friendship Hospital (2018-P2-008-01). We included all pediatric patients (<18 years of age) who underwent DDLT at Beijing Friendship Hospital from July 2015 to October 2017. Exclusion criteria were the following: 1) LT from living related or domino donors; 2) LT using the piggyback technique; 3) liver grafts preserved with Celsior or histidine-tryptophan-ketoglutarate (HTK) solution; 4) combined liver-kidney transplantation; and 5) incomplete documentation.
Anesthesia protocol
All the patients were treated under a standardized anesthesia protocol. Anesthesia was induced with midazolam (0.1 mg/kg), propofol (2–3 mg/kg), fentanyl (3–5 μg/kg), and cisatracurium (0.2–0.3 mg/kg) and was maintained using sevoflurane (1.5–2.5%) in an oxygen/air mixture (FiO2 50–60%) combined with remifentanil (0.1–0.3 μg/kg/min) and cisatracurium (1–2 μg/kg/min) infusions. After orotracheal intubation, mechanical ventilation was initiated at a tidal volume of 6–8 mL/kg, a respiratory rate of 15–25 breaths/min, and a positive end-expiratory pressure of 5 cm H2O to maintain an end tidal CO2 partial pressure (PETCO2) of 35–45 mmHg using a pressure-controlled (<15 kg) or volume-controlled (≥15 kg) ventilation mode. Intraoperative monitoring included electrocardiography, pulse oximetry, PETCO2 level, core body temperature, urine output, invasive blood pressure, and central venous pressure (CVP). A triple-lumen central venous catheter was inserted via the internal jugular vein in all the patients for CVP monitoring and for infusion of vasoactive drugs, fluids, and blood products. A pulmonary artery catheter was placed in those who were diagnosed with portopulmonary hypertension preoperatively. All the monitoring data were automatically recorded as previously described [4]. Intravenous fluids (5% albumin and 4% Gelofusine as colloids and 5% dextrose in water as a crystalloid) were used for volume replacement. Packed red blood cells (RBCs) were administered to maintain a hematocrit (HCT) level of 25–30%. Fresh frozen plasma was administered only when significant coagulation disorders were detected by a Sonoclot analyzer (Sienco, Inc., Arvada, CO, USA). Antifibrinolytic therapy with tranexamic acid was administered when there was a significant bleeding tendency due to fibrinolysis. All the patients were protected from hypothermia using a fluid warmer (Astotherm Plus 260; Stihler Electronic, Stuttgart, Germany) and a forced-air warming blanket (Bair Hugger, model 55501/52200; 3M, St. Paul, MN, USA).
Surgical technique
All the patients underwent LT using the surgical technique described previously [4]. Liver grafts were procured from donation after brain death (DBD) or donation after circulatory death (DCD) donors and were preserved with University of Wisconsin solution. Anastomosis of the graft was performed using the conventional technique without venovenous bypass. Just before anastomosis of the portal vein (PV), the liver grafts were flushed via the PV using a room-temperature 5% albumin solution with a flush volume of 1 mL per g of the liver graft. Finally, revascularization of the liver graft was initiated when the PV clamp was removed.
Prophylaxis, management, and evaluation of PRS
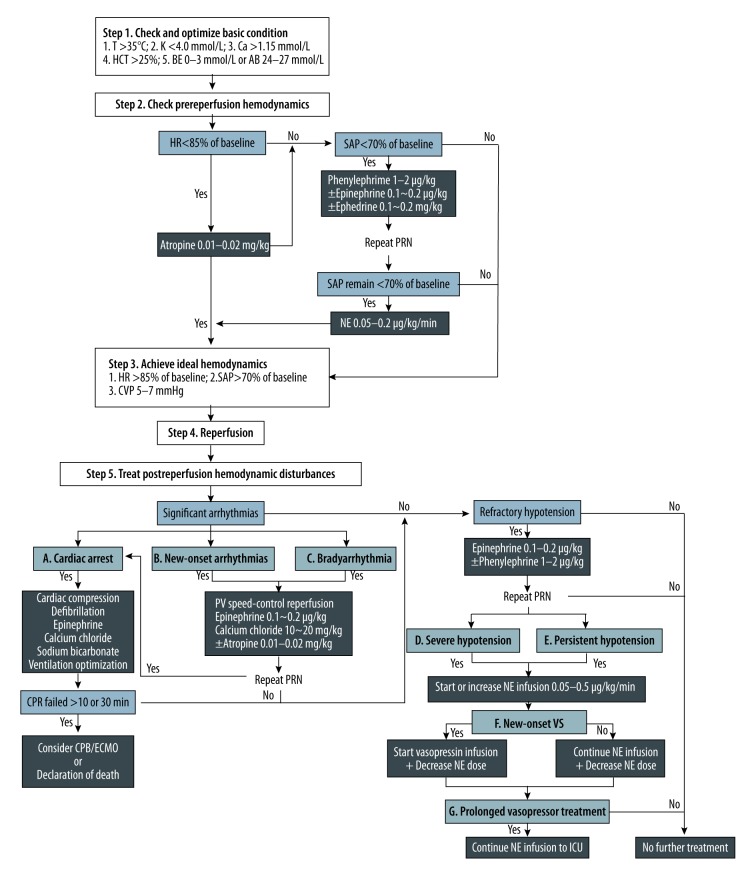
All the patients underwent the same protocol for prophylaxis and management of PRS; this protocol was almost the same as that used for adult patients in our institution [4]. PRS was diagnosed according to the Peking criteria when one or more of the 7 fatal or non-fatal postreperfusion cardiovascular complications were present (for details, see Table 1 and Figure 1). Finally, the patients were divided into PRS and non-PRS groups based on intraoperative data obtained from electronic anesthesia records.
Table 1.
Peking criteria for PRS in pediatric liver transplantation.
| Complication | Definition | Timing |
|---|---|---|
| Significant arrhythmias | ||
| • Bradyarrhythmia | A decrease in HR ≥15% of the pre-reperfusion level | Immediate reperfusion period |
| • New-onset arrhythmias | Hemodynamically significant arrhythmias (hyperkalemia-related and/or others) | Immediate reperfusion period |
| • Cardiac arrest | Loss of spontaneous heart beat and requires cardiac massage | Immediate reperfusion period |
| Refractory hypotension | ||
| • Severe hypotension | A drop in SAP unresponsive to an accumulated bolus of 1 μg/kg epinephrine | Immediate reperfusion period |
| • Persistent hypotension | A drop in SAP ≥30% of the pre-reperfusion level and lasting ≥5 min | Immediate reperfusion period |
| • New-onset vasoplegic syndrome | NE ≥0.5 μg/kg/min, SAP <30–50% of baseline, high CO, and low SVR | Late reperfusion period |
| • Prolonged vasopressor treatment | Refractory hypotension requiring prolonged NE infusion to ICU | At the end of surgery |
Presence of one or more of the 7 factors indicates PRS. CO – cardiac output; HR – heart rate; NE – norepinephrine; ICU – intensive care unit; PRS – postreperfusion syndrome; SAP – systolic arterial pressure; SVR – systemic vascular resistance.
Figure 1.
Suggested prevention, diagnosis, and treatment algorithm for PRS in pediatric DDLT: the Beijing Friendship Hospital (BFH) experience. AB – actual bicarbonate; BE – base excess; Ca – ionized calcium concentration; CPB – cardiopulmonary bypass; CPR – cardiopulmonary resuscitation; CVP – central venous pressure; DDLT – deceased donor liver transplantation; ECMO – extracorporeal membrane oxygenation; HCT – hematocrit; HR – heart rate; ICU – intensive care unit; K – serum potassium concentration; NE – norepinephrine; PRN – as necessary; PRS – postreperfusion syndrome; PV – portal vein; SAP – systolic arterial pressure; T – body temperature; VS – vasoplegic syndrome.
Data collection
Preoperative recipient variables, liver graft factors, intraoperative details, and postoperative outcomes, including age, gender, height, weight, indications for LT, Child-Turcotte-Pugh (CTP) score, Pediatric End-stage Liver Disease (PELD) score, donor warm ischemia time (WIT), graft weight, graft-to-recipient weight ratio (GRWR), graft cold ischemia time (CIT), graft WIT, presence of an expanded criteria donor (ECD) liver graft, hemodynamic and blood gas parameters around the reperfusion period, vasopressor administration after reperfusion, intraoperative blood loss, transfusion requirements, mechanical ventilation time, intensive care unit (ICU) stay time, hospital stay duration, and occurrence of early allograft dysfunction (EAD), primary nonfunction (PNF) and acute kidney injury (AKI), were collected and compared between the 2 groups. The ECD criteria were defined as follows: a DCD liver graft, donor age >60 years, liver macrosteatosis >30%, donor serum sodium level >155 mmol/L, graft CIT >12 hours, and a reduced-size or split liver graft. EAD, PNF and AKI were assessed according to Olthoff’s criteria [13], Ploeg’s definition [14] and the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [15], respectively.
Statistical analyses
Continuous variables are presented as the mean ± standard deviation, median (range), or median (interquartile range) and were compared using independent t-tests or Mann-Whitney U tests. Categorical variables are presented as the number and proportion and were compared using a χ2 test or Fisher’s exact test. To identify the independent risk factors associated with the presence of PRS, potentially significant variables with P values <0.10 in the univariate analysis were further analyzed by stepwise binary logistic regression. Statistical analyses were performed using SPSS software Version 17.0 (SPSS, Inc., Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
Baseline characteristics
From July 2015 to October 2017, 227 consecutive pediatric patients underwent LT at our institution; of these patients, 152 were excluded for the following reasons: presence of a living related LT (n=147) or domino donor LT (n=2), preservation of liver grafts in HTK solution (n=1), performance of LT using the piggy-back technique (n=1), and incomplete data from clinical records (n=1). Based on these exclusion criteria, a total of 75 patients were included in the final analysis. The most common indications for pediatric DDLT in this study were biliary atresia (50.7%) and acute liver failure (14.7%). The median (range) age of the patients (39 males and 36 females) was 2.3 (range, 0.4–11.2) years. The median (range) height and weight were 87 (range, 60–145) cm and 12.0 (range, 5.5–35.0) kg, respectively. The median (range) CTP score was 8 (range 5–14), and the median (range) PELD score was 13 (range, −11–45). The number of patients who required preoperative vasopressor support, dialysis, and mechanical ventilation was 3, 2, and 2, respectively. The other baseline characteristics are summarized in Table 2.
Table 2.
Patient characteristics.
| Variables | Patients (n=75) |
|---|---|
| Age (y)a | 2.3 (0.4–11.2) |
| Male gender (%) | 39 (52.0) |
| Height (cm)a | 87 (60–145) |
| Weight (kg)a | 12.0 (5.5–35.0) |
| CTP scorea | 8 (5–14) |
| PELD scorea | 6 (−11–45) |
| Indication for LT (%) | |
| Biliary atresia | 38 (50.7) |
| Irreversible graft failureb | 11 (14.7) |
| UCDsc | 7 (9.3) |
| Hepatoblastoma | 3 (4.0) |
| MMA | 3 (4.0) |
| PFIC | 3 (4.0) |
| Othersd | 10 (13.3) |
Data are mean (range).
Irreversible graft failure may result from primary nonfunction or vascular or biliary complications after liver transplantation.
Ornithine transcarbamylase deficiency (OTCD) 3 cases, hyperornithinemia-hyperammonemia-homocitrullinuria (3H) syndrome 2 cases, argininosuccinic aciduria (ASA) 1 case, and argininemia 1 case.
Caroli disease 2cases, Wilson’s disease 1 case, maple syrup urine disease (MSUD) 1 case, familial hypercholesterolemia 1 case, Alagille syndrome 1 case, congenital hepatic fibrosis 1 case, cryptogenic cirrhosis 1 case, choledochal cyst 1 case, and fulminant hepatic failure 1 case.
CTP – Child-Turcotte-Pugh; MMA – methylmalonic academia; PELD – Pediatric End-stage Liver Disease; PFIC – progressive familial intrahepatic cholestasis; UCD – urea cycle disorder.
Incidence of PRS and postreperfusion complications
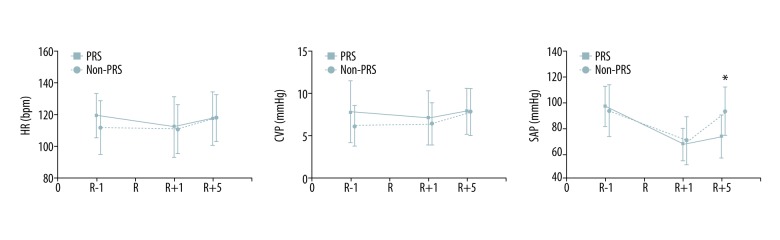
Sixty-nine patients (92.0%) met the criteria for PRS according to Aggarwal’s definition [16]. However, 26 patients developed at least one manifestation of PRS after employing the Peking criteria for PRS, and 12 (46.2%) of them were diagnosed with PRS based on a single manifestation of PRS. Persistent hypotension, either alone or accompanied by other findings, was the most common cause of PRS (65.4%), and other common findings included new-onset vasoplegic syndrome (VS) (34.6%), severe hypotension (30.8%), and bradyarrhythmia (26.9%) (for details, see Figure 2). Additionally, one patient died during the operation due to postreperfusion cardiac arrest (PRCA) associated with severe PRS. Thus, the incidence of PRS was 34.7%, and the intraoperative PRS-related mortality was 1.3%. During the immediate reperfusion period, the PRS group required a larger amount of epinephrine than did the non-PRS group (median: 0.74 vs. 0.17, P<0.001). At 1 min after reperfusion, there were no significant differences found between the 2 groups with respect to heart rate (HR), systolic arterial pressure (SAP), and CVP. At 5 min after reperfusion, the SAP level was significantly lower and the serum potassium concentration was significantly higher among the PRS patients (72.7±17.2 vs. 92.1±19.0, P<0.001; and 4.0±0.7 vs. 3.2±0.8, P<0.001, respectively) (Figure 3 and Table 4). During the late reperfusion period, the PRS group required more frequent and larger amounts of norepinephrine (NE) (median: 0.3 vs. 0.0, P<0.001; and 80.0% vs. 10.2%, P<0.001, respectively), and VS was significantly more frequent in the PRS group than in the non-PRS group (36.0% vs. 0.0%; P<0.001). Following surgery, 2 patients in the PRS group who manifested with VS required continuation of NE infusion in the ICU despite treatment with vasopressin. There were no significant differences between the PRS and non-PRS groups with respect to anesthesia time, operative time, blood loss, transfusions, and other parameters (for details, see Table 3).
Figure 2.
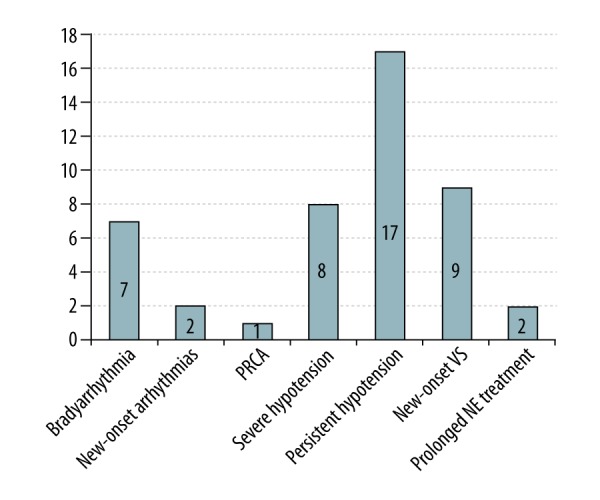
Types of manifestations of PRS in the 26 pediatric DDLT patients. DDLT – deceased donor liver transplantation; NE – norepinephrine; PRCA – postreperfusion cardiac arrest; PRS – postreperfusion syndrome; VS – vasoplegic syndrome.
Figure 3.
Hemodynamic changes during the reperfusion period in pediatric DDLT. CVP – central venous pressure; DDLT – deceased donor liver transplantation; HR – heart rate; PRS – postreperfusion syndrome; R – reperfusion; SAP – systolic arterial pressure. * P<0.05.
Table 4.
Univariate analysis of risk factors for PRS during pediatric DDLT.
| Variables | PRS group (n=26) | Non-PRS group (n=49) | P-value |
|---|---|---|---|
| Age (y) | 2.7 (0.9–4.6) | 2.2 (0.8–6.3) | 0.854 |
| Female gender (%) | 12 (46.2%) | 24 (49.0%) | 0.816 |
| Height (cm) | 90 (71–105) | 84 (68–117) | 0.738 |
| Weight (kg) | 12.0 (7.9–16.4) | 12.0 (7.5–20.0) | 0.902 |
| CTP score | 8 (6–10) | 8 (6–11) | 0.991 |
| PELD score | 6.5 (−2.3–18.3) | 6.0 (−4.5–18.0) | 0.969 |
| Graft weight (g) | 410 (337–627) | 412 (308–500) | 0.308 |
| GRWR (%) | 4.12 (3.55–4.52) | 3.43 (2.30–4.23) | 0.023 |
| Donor WIT (min) | 13 (5–15) | 5 (3–6) | <0.001 |
| Graft CIT (min) | 645 (508–661) | 580 (471–660) | 0.237 |
| Graft WIT (min) | 51±11 | 49±13 | 0.489 |
| ECD liver graft (%) | 18 (69.2%) | 5 (10.2%) | <0.001 |
| Metabolic data before reperfusion | |||
| K (mmol/L) | 3.9±0.6 | 3.7±0.6 | 0.076 |
| CA (mmol/L) | 1.16 (1.06–1.36) | 1.10 (1.01–1.25) | 0.206 |
| GLU (mmol/L) | 6.6±3.0 | 7.0±2.7 | 0.422 |
| LAC (mmol/L) | 2.9 (1.9–4.0) | 2.7 (2.2–3.6) | 0.570 |
| HCT (%) | 24 (22–30) | 27 (24–32) | 0.057 |
| Temperature (°C) | 35.5 (35.0–36.4) | 35.9 (34.9–36.3) | 0.718 |
CA – serum calcium concentration; CIT – cold ischemia time; CTP – Child-Turcotte-Pugh; DDLT – deceased donor liver transplantation; ECD – expanded criteria donor; GLU – serum glucose concentration; GRWR – graft-to-recipient weight ratio; HCT – hematocrit; K – serum potassium concentration; LAC – serum lactate concentration; PELD – Pediatric End-stage Liver Disease; PRS – postreperfusion syndrome; WIT – warm ischemia time.
Table 3.
Postreperfusion complications and postoperative outcomes.
| Variables | PRS group (n=26) | Non-PRS group (n=49) | P-value |
|---|---|---|---|
| During the immediate reperfusion period | |||
| Dose of epinephrine (μg/kg) | 0.74 (0.65–1.16) | 0.17 (0.08–0.28) | <0.001 |
| K at 5 min of reperfusion (mmol/L) | 4.0±0.7 | 3.2±0.8 | <0.001 |
| GLU at 5 min of reperfusion (mmol/L) | 10.9±3.9 | 11.6±3.5 | 0.422 |
| LAC at 5 min of reperfusion (mmol/L) | 5.4±2.4 | 4.4±1.4 | 0.056 |
| During the late reperfusion period | |||
| Number requiring NE infusion (%)a | 20 (80.0%) | 5 (10.2%) | <0.001 |
| Dose of NE (μg/kg/min)a | 0.30 (0.20–0.45) | 0.00 (0.00–0.00) | <0.001 |
| New-onset VS (%)a | 9 (36.0%) | 0 (0.0%) | <0.001 |
| At the end of surgery | |||
| Number requiring NE infusion (%)a | 2 (8.0%) | 0 (0.0%) | 0.111 |
| Duration of anesthesia (min)a | 468±89 | 453±90 | 0.509 |
| Duration of surgery (min)a | 375 (330–435) | 360 (330–405) | 0.586 |
| Blood loss (ml/kg)a | 32.9 (18.2–64.1) | 21.3 (12.0–40.9) | 0.078 |
| RBC transfusion (ml/kg)a | 34.8 (21.9–66.5) | 26.7 (13.3–51.0) | 0.097 |
| FFP transfusion (ml/kg)a | 0 (0–20) | 0 (0–25) | 0.786 |
| During the postoperative period | |||
| Peak ALT (IU/L)a | 1487 (848–2210) | 497 (287–735) | <0.001 |
| Peak AST (IU/L)a | 4179 (2828–5818) | 1167 (715–1674) | <0.001 |
| Peak LDH (IU/L)a | 3826 (2836–5946) | 1446 (1073–2189) | <0.001 |
| Peak GGT (IU/L)a | 200 (103–353) | 249 (133–372) | 0.560 |
| Peak TB (umol/L)a | 118.9±95.3 | 122.8±79.8 | 0.853 |
| EAD (%) | 19 (73.1%) | 9 (18.4%) | <0.001 |
| PNF (%) | 3 (11.5%) | 0 (0.0%) | 0.039 |
| Graft loss within 1 month (%) | 4 (15.4%) | 0 (0.0%) | 0.012 |
| AKI (%)a | 4 (16.0%) | 4 (6.1%) | 0.217 |
| Ventilation time (hours)a | 2.4 (1.8–6.3) | 2.5 (1.6–3.9) | 0.513 |
| ICU stay (days)a | 4.3 (2.9–5.8) | 3.5 (2.8–4.5) | 0.205 |
| Hospital stay (days)a | 30.5 (22.0–49.8) | 21.0 (17.5–25.5) | 0.007 |
| In-hospital death (%)a | 1 (4.0%) | 0 (0.0%) | 0.338 |
The patient died during the operation was excluded from the final analysis in the PRS group.
AKI – acute kidney injury; ALT – alanine aminotransferase; AST – aspartate aminotransferase; EAD – early allograft dysfunction; FFP – fresh frozen plasma; GGT – gamma-glutamyl transpeptidase; GLU – serum glucose concentration; ICU – intensive care unit; K – serum potassium concentration; LAC – serum lactate concentration; LDH – lactic dehydrogenase; NE – norepinephrine; PNF – primary nonfunction; PRS – postreperfusion syndrome; RBC – red blood cell; TB – total bilirubin; VS – vasoplegic syndrome.
Independent risk factors for PRS
Univariate analysis showed that the risk factors for PRS in pediatric DDLT included donor WIT (median: 13 vs. 5, P<0.001), GRWR (median: 4.12 vs. 3.43, P=0.023), and use of an ECD liver graft (69.2% vs. 10.2%, P<0.001) (Table 4). Multivariate logistic regression analysis revealed that the use of an ECD donor graft (odds ratio [OR]: 18.668; 95% confidence interval [95% CI]: 4.866–71.622) and a lower HCT level before reperfusion (OR: 0.878; 95% CI: 0.782–0.985) were found to be independent predictors of PRS (Table 5). Additionally, the most common forms of ECDs in this study were DCD (42.9%) and partial (split or reduced-size) liver grafts (37.1%), and the other forms of ECDs are shown in Figure 4.
Table 5.
Multivariate analysis of risk factors associated with PRS during pediatric DDLT.
| Risk factors | OR | 95% CI | P-value |
|---|---|---|---|
| ECD liver graft | 18.668 | 4.866–71.622 | <0.001 |
| HCT before reperfusion | 0.878 | 0.782–0.985 | 0.027 |
CI – confidence interval; DDLT – deceased donor liver transplantation; ECD – expanded criteria donor; HCT – hematocrit; OR – odds ratio.
Figure 4.
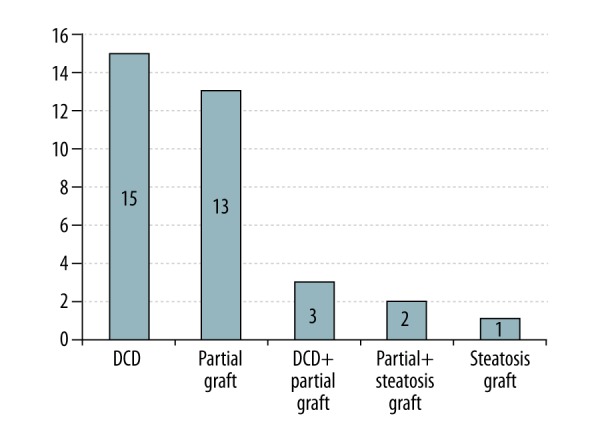
Forms of expanded criteria donor liver grafts in this study. DCD – donation after cardiac death.
Relationship between PRS and early postoperative outcomes
Postoperative peak alanine aminotransferase, aspartate aminotransferase, and lactic dehydrogenase levels during the first week were significantly higher among PRS patients than among non-PRS patients (median: 1487 vs. 497, P<0.001; median: 4179 vs. 1167, P<0.001; and median: 3826 vs. 1446, P<0.001, respectively), while the peak total bilirubin and gamma-glutamyl transpeptidase levels were comparable between the 2 groups. Moreover, rates of EAD, PNF, and early graft loss were significantly greater in the PRS group (73.1% vs. 18.4%, P<0.001; 11.5% vs. 0.0%, P=0.039; and 15.4% vs. 0.0%, P=0.012, respectively). Three patients in the PRS group were retransplanted for the following reasons: 2 due to PNF at 3 and 5 days, and 1 due to hepatic artery thrombosis within the first month. Hospital stay duration was longer in the PRS group than in the non-PRS group (median: 30.5 vs. 21.0, respectively, P=0.007). Other postoperative outcomes, including rates of AKI, renal replacement therapy, duration of mechanical ventilation, re-intubation, length of ICU stay and in-hospital death, did not differ significantly between the 2 groups (Table 3).
Discussion
The performance of graft reperfusion during LT is often accompanied by severe hemodynamic alterations, which presents a particular challenge for anesthesiologists. Hemodynamic events that occur following graft reperfusion are generally known as PRS. In our study, we found that the use of ECD liver grafts as well as a lower HCT at reperfusion were independent predictors of PRS in pediatric DDLT. Additionally, we confirmed that occurrence of PRS was associated with worsened liver graft and patient outcomes. To our knowledge, this is the first study to investigate PRS in pediatric patients. In our study, the PRS occurrence rate in pediatric DDLT was 34.7%, which is relatively lower than that in an adult population in our institution using similar diagnostic criteria [4].
Generally, the most important difference in PRS occurrence is the inconsistency in the definition of PRS. The classic definition of PRS denoted by Aggarwal et al. in 1987 is that of a cardiovascular collapse following liver graft reperfusion, and the criteria included a >30% decrease in the mean arterial pressure (MAP) from baseline that lasted for at least 1 min within the first 5 min of the reperfusion period [16]. Although this aforementioned definition has been widely recognized, it may also be regarded as a narrow definition of PRS, as it involves only immediate hemodynamic alterations. Therefore, a broader definition of PRS has been proposed to describe the systemic events following reperfusion of an ischemic tissue or organ, including drastic disturbances in hemodynamics, coagulation, electrolytes, acid-base balance, and metabolic function [17]. Notably, the classic criteria for PRS seem somewhat unserviceable and disadvantageous when employed for DDLT, especially when an ECD liver graft is implanted. If a patient was at great risk for severe arrhythmias or even cardiac arrest, it would be impractical to wait such a long time before making a diagnosis. Consequently, the development of diagnostic criteria for PRS based on its severity would be more practical in preventing and treating PRS in DDLT.
PRS was classified by Hilmi et al. as being either mild or severe, with severe PRS including persistent severe hypotension, significant arrhythmias or asystole, prolonged vasopressor support, or the occurrence of prolonged or recurrent fibrinolysis [5]. The Peking criteria for PRS also address only the more serious form of PRS, but fibrinolysis is removed as a criterion, and additional modifications are made to Hilmi’s classification. First, MAP is replaced by SAP as an indicator of postreperfusion hypotension since SAP is more sensitive than MAP when blood pressure drops suddenly in children. Second, the magnitude of the HR reduction is changed from 30% to 15% because a rapid slowdown of HR is more accurate than a temporary drop in SAP in predicting PRCA. Finally, the usage of epinephrine is individually measured by body weight rather than a constant dose. In fact, both the Peking and Hilmi’s criteria consider a long timeframe and would inevitably include PRCA and VS, with PRCA being the most severe form of arrhythmias, and VS being the most severe form of hypotension following liver graft reperfusion.
There are 2 major aspects of the diagnosis of PRS according to the Peking criteria. First, the diagnosis time covers from immediately after reperfusion to the end of operation and includes 3 important diagnostic timings. Second, 2 main aspects of clinical manifestations are significant arrhythmias and persistent severe hypotension. According to our experience, almost all the severe arrhythmias occurred during the immediate reperfusion period and might further lead to the occurrence of PRCA, while postreperfusion hypotension occurred with various degrees of hypotension and might persist from the immediate reperfusion period to the postoperative period. As a consequence, it is critical to treat PRS based on its clinical manifestations during different phases after reperfusion. During the immediate reperfusion period, treating new-onset arrhythmias and avoiding cardiac arrest are top priorities, and calcium chloride together with epinephrine should be considered as the first-choice drug for treatment of postreperfusion new-onset arrhythmias. During the late reperfusion period, patients with manifestations of vasoplegia or VS should be treated with NE and vasopressin infusions.
Although the exact pathophysiological mechanisms of PRS are not fully understood, many risk factors associated with PRS, including factors related to the donor liver, recipient, preservation solution, surgical technique, and anesthesia management, have been reported in the adult population. We found that the use of an ECD liver graft was a predictor of PRS in pediatric DDLT. The criteria for ECDs [18] commonly include older donor age, higher degree of steatosis, DCD liver graft, prolonged CIT, and a reduced-size or split liver graft. Steatotic liver grafts are more susceptible to ischemia-reperfusion injury (IRI) and are associated with more pronounced hemodynamic derangement [19–21]. Previously, Chung et al. [19] and Chui et al. [20] identified that the severity of graft steatosis was associated with PRS in adult LT. A recent single-center study in China also found that macrosteatosis on a DCD liver graft biopsy was an independent risk factor for postreperfusion hyperkalemia and PRS in adult DDLT [21]. DCD liver grafts are also prone to severe IRI. Compared with adult patients who underwent LT from DBD liver grafts, adult patients who underwent LT from DCD grafts experienced higher rates of PRS [4,22,23]. A prolonged CIT was the most frequently cited risk factor for PRS in several previous studies [3,6,8,20]. Although the donor CIT in the PRS group was longer than that in the non-PRS group, the difference was not significant in our study, which may have been due to the donor CIT being well-controlled in both groups. The age of the donor presents a risk factor for PRS in adult LT [9]. However, the majority of the pediatric patients in this study were allocated and implanted with a donor liver from a pediatric patient. Thus, we were unable to determine the influence of donor age on PRS. Despite the observed relationship between ECD liver grafts and PRS, the exact mechanism remains to be elucidated. Nevertheless, reperfusion of ECD liver grafts with the release of cytokines, free radicals, nitric oxide, and intracellular potassium ions due to IRI may play an important role [4,24]. Theoretically, optimization of the quality of grafts prior to implantation may minimize PRS related to ECD liver grafts. Recent studies reported that ECD liver grafts preserved under normothermic machine perfusion (NMP) were associated with less or even no PRS after reperfusion [25,26]. Further studies of NMP would yield new insights into the fundamental prevention and treatment of PRS.
Among recipient and anesthesia factors, we found that a lower HCT level was another risk factor for PRS. There has been 1 previous study that associated the occurrence of PRS with preoperative hemoglobin level in adult LT [19]. Generally, most transfusion guidelines [27–29] in pediatric LT have recommended that the intraoperative HCT level should be maintained between 25% and 30% to minimize risks for HAT and transfusion-related complications. Our findings are important and cautionary because no previous studies have focused on the adverse effects of a restricted RBC transfusion on PRS. Therefore, it is worth reconsidering whether it would be more appropriate to adjust the transfusion strategy during the anhepatic phase in pediatric LT. In our study, a statistically significant difference in GRWR was observed in the PRS group, but this made no difference in the incidence of PRS, as shown by the multivariate regression analysis. However, another previous study found that the mismatch in size between the recipient and liver graft, described by the body surface area index, represents a risk factor for the incidence and severity of PRS [9]. Cirrhotic cardiomyopathy, diastolic dysfunction and hyperdynamic circulation associated with advanced liver diseases may play an important role in the development of postreperfusion hemodynamic instability. Both MELD score and CTP score were indicated as risk factors for PRS in adult LT in many previous studies [4,19,30]. Surprisingly, the authors failed to demonstrate a relationship between severity of the recipient’s liver diseases and PRS. This finding may be attributed to a very low PELD score in both groups.
Another significant finding of our study is that occurrence of PRS was associated with poor liver graft and patient outcomes postoperatively, and such an association has already been reported repeatedly in adult DDLT recipients [3–8]. Notably, it is unclear whether PRS is a problem itself or whether it solely indicates a problem related to graft conditions. To our knowledge, the intraoperative occurrence of PRS and poor postoperative liver allograft function may both be related to the quality of the donor liver grafts. Therefore, some quantifiable parameters for characterizing donor graft profiles and severity of liver IRI may prompt the early prediction and prevention of PRS in LT from cadaveric donors. Bezinover and colleagues [24] found that the concentration of TNF-α obtained from flushed blood at the beginning of reperfusion was a predictor of postreperfusion hemodynamic instability. More recently, a study by Zhang et al. [4] demonstrated that there was a significant correlation between flushed fluid potassium concentration measured at the end of PV flushing and development of severe PRS in adult DDLT.
There are several limitations in our study. First, this is a single-institution, retrospective study, and the relatively small sample size limits the results of multivariate regression analysis for use in identifying factors associated with PRS. Second, prophylactic medication before reperfusion was not standardized in this retrospective study, resulting in an underestimation of the overall prevalence of PRS. Future studies are warranted to exclude the influence of prophylactic medication and to more precisely estimate the prevalence of PRS. Lastly, this study is limited by its lack of long-term survival analysis. Future investigations should be focused on assessing the impact of graft quality on PRS and, possibly, its role in long-term outcomes.
Conclusions
The present study identified 2 independent risk factors for PRS in pediatric LT: HCT level before reperfusion and use of an ECD liver graft. Moreover, occurrence of PRS was significantly associated with post-transplant liver allograft dysfunction, which may be a consequence of poor liver graft quality. Further efforts to optimize the quality of liver grafts prior to implantation would prove advantageous in PRS prophylaxis.
Footnotes
Conflicts of interest
None.
Source of support: The study was supported by Capital Special Program for Health Research and Development (No.2016-1-2021) and Scientific Research Key Program of Beijing Municipal Commission of Education (NO.KZ201510025026)
References
- 1.Daniela K, Michael Z, Florian I, et al. Influence of retrograde flushing via the caval vein on the post-reperfusion syndrome in liver transplantation. Clin Transplant. 2004;18(6):638–41. doi: 10.1111/j.1399-0012.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 2.Ryu HG, Jung CW, Lee CS, et al. Nafamostat mesilate attenuates postreperfusion syndrome during liver transplantation. Am J Transplant. 2011;11(5):977–83. doi: 10.1111/j.1600-6143.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 3.Bukowicka B, Akar RA, Olszewska A, et al. The occurrence of postreperfusion syndrome in orthotopic liver transplantation and its significance in terms of complications and short-term surval. Ann Transplant. 2011;16(2):26–30. doi: 10.12659/aot.881861. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Tian M, Sun L, et al. Association between flushed fluid potassium concentration and severe postreperfusion syndrome in deceased donor liver transplantation. Med Sci Monit. 2017;23:5158–67. doi: 10.12659/MSM.907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14(4):504–8. doi: 10.1002/lt.21381. [DOI] [PubMed] [Google Scholar]
- 6.Paugam-Burtz C, Kavafyan J, Merckx P, et al. Postreperfusion syndrome during liver transplantation for cirrhosis: outcome and predictors. Liver Transpl. 2009;15(5):522–29. doi: 10.1002/lt.21730. [DOI] [PubMed] [Google Scholar]
- 7.Khosravi MB, Sattari H, Ghaffaripour S, et al. Post-reperfusion syndrome and outcome variables after orthotopic liver transplantation. Int J Organ Transplant Med. 2010;1(3):115–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Xu ZD, Xu HT, Yuan HB, et al. Postreperfusion syndrome during orthotopic liver transplantation: A single-center experience. Hepatobiliary Pancreat Dis Int. 2012;11(1):34–39. doi: 10.1016/s1499-3872(11)60123-9. [DOI] [PubMed] [Google Scholar]
- 9.Fukazawa K, Yamada Y, Gologorsky E, et al. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J Cardiothorac Vasc Anesth. 2014;28(4):994–1002. doi: 10.1053/j.jvca.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Siniscalchi A, Gamberini L, Laici C, et al. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J Gastroenterol. 2016;22(4):1551–69. doi: 10.3748/wjg.v22.i4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong SM. Postreperfusion syndrome during liver transplantation. Korean J Anesthesiol. 2015;68(6):527–39. doi: 10.4097/kjae.2015.68.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsay M. The reperfusion syndrome: Have we made any progress? Liver Transpl. 2008;14(4):412–14. doi: 10.1002/lt.21418. [DOI] [PubMed] [Google Scholar]
- 13.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transp. 2010;16(8):943–49. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 14.Ploeg RJ, D’Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation – a multivariate analysis. Transplantation. 1993;55(4):807–13. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2(Suppl 1):1–138. [Google Scholar]
- 16.Aggarwal S, Kang Y, Freeman JA, et al. Postreperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19(4 Suppl 3):54–55. [PubMed] [Google Scholar]
- 17.Kodakat SK, Ginsburg R, Gopal PB, et al. A case of post-reperfusion syndrome following surgery for liver trauma. Br J Anaesth. 2006;96(1):31–35. doi: 10.1093/bja/aei278. [DOI] [PubMed] [Google Scholar]
- 18.Vodkin I, Kuo A. Extended criteria donors in liver transplantation. Clin Liver Dis. 2017;21(2):289–301. doi: 10.1016/j.cld.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Chung IS, Kim HY, Shin YH, et al. Incidence and predictors of post-reperfusion syndrome in living donor liver transplantation. Clin Transplant. 2012;26(4):539–43. doi: 10.1111/j.1399-0012.2011.01568.x. [DOI] [PubMed] [Google Scholar]
- 20.Chui AK, Shi L, Tanaka K, et al. Postreperfusion syndrome in orthotopic liver transplantation. Transplant Proc. 2000;32(7):2116–17. doi: 10.1016/s0041-1345(00)01595-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhang WJ, Xia WL, Pan HY, et al. Postreperfusion hyperkalemia in liver transplantation using donation after cardiac death grafts with pathological changes. Hepatobiliary Pancreat Dis Int. 2016;15(5):487–92. doi: 10.1016/s1499-3872(16)60116-9. [DOI] [PubMed] [Google Scholar]
- 22.Blasi A, Hessheimer AJ, Beltrán J, et al. Liver transplant from unexpected donation after circulatory determination of death donors: A challenge in perioperative management. Am J Transplant. 2016;16(6):1901–8. doi: 10.1111/ajt.13621. [DOI] [PubMed] [Google Scholar]
- 23.Pan X, Apinyachon W, Xia W, et al. Perioperative complications in liver transplantation using donation after cardiac death grafts: A propensity-matched study. Liver Transpl. 2014;20(7):823–30. doi: 10.1002/lt.23888. [DOI] [PubMed] [Google Scholar]
- 24.Bezinover D, Kadry Z, McCullough P, et al. Release of cytokines and hemodynamic instability during the reperfusion of a liver graft. Liver Transpl. 2011;17(3):324–30. doi: 10.1002/lt.22227. [DOI] [PubMed] [Google Scholar]
- 25.Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: Hyperoxia and vasoplegia-important lessons from the first 12 cases. Transplantation. 2017;101(5):1084–98. doi: 10.1097/TP.0000000000001661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Guo Z, Zhao Q, et al. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am J Transplant. 2018;18(3):737–44. doi: 10.1111/ajt.14583. [DOI] [PubMed] [Google Scholar]
- 27.Alper I, Ulukaya S. Anesthetic management in pediatric liver transplantation: A comparison of deceased or live donor liver transplantations. J Anesth. 2010;24(3):399–406. doi: 10.1007/s00540-010-0928-z. [DOI] [PubMed] [Google Scholar]
- 28.Nacoti M, Cazzaniga S, Lorusso F, et al. The impact of perioperative transfusion of blood products on survival after pediatric liver transplantation. Pediatr Transplant. 2012;16(4):357–66. doi: 10.1111/j.1399-3046.2012.01674.x. [DOI] [PubMed] [Google Scholar]
- 29.Ulukaya S, Acar L, Ayanoglu HO. Transfusion requirements during cadaveric and living donor pediatric liver transplantation. Pediatr Transplant. 2005;9(3):332–37. doi: 10.1111/j.1399-3046.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 30.Siniscalchi A, Dante A, Spedicato S, et al. Hyperdynamic circulation in acute liver failure: Reperfusion syndrome and outcome following liver transplantation. Transplant Proc. 2010;42(4):1197–99. doi: 10.1016/j.transproceed.2010.03.097. [DOI] [PubMed] [Google Scholar]