Abstract
Background
In contrast to conventional static cold preservation, normothermic machine perfusion (NMP) provides a beneficial alternative preservation of donor livers. However, the liver still suffered cold ischemic injury before attaching to the perfusion device.
Material/Methods
To prevent cold ischemic injury during procurement, we describe a novel procedure called ischemia-free liver procurement (IFLP) under NMP. Two liver grafts were procured from brain death donor under NMP and underwent 2-hour ex vivo NMP followed by 3 and 6 hours of static cold preservation. From procurement to post-transplantation course, evidence was collected to prove that IFLP is safe and benefits recipients.
Results
The post-transplantation course was uneventful, and the liver function tests and histological study revealed minimal hepatocyte and biliary epithelium injury during the preservation.
Conclusions
This preliminary experience demonstrates the clinical feasibility and safety of IFLP under NMP which offering opportunities to increase the number of donor livers and to improve the organ function.
MeSH Keywords: Liver Transplantation, Organ Preservation, Tissue and Organ Procurement
Background
Organ transplantation has been considered one of the medical miracles of the 20th century [1]. Since the first organ transplantation was performed in 1954, organ ischemia reperfusion injury (IRI) has been closely associated with transplantation like body and shadow throughout the history of transplantation. Organ shortage has prompted the use of extended criteria donor organs from older, steatotic, or donation after cardiac death (DCD) donors, as well as organs that have been subjected to prolonged periods of cold storage (CS). However, these “marginal” organs are particularly susceptible to IRI as a result of damage during procurement, preservation, and surgery [2]. Hence, IRI not only contributes to the donor organ shortage (as organs might be harvested and then deemed too damaged for transplantation) but also strongly affects short-term (early graft dysfunction) and long-term graft outcome (graft rejection and chronic graft dysfunction) [3].
Investigators around the world are exploring methods to ameliorate IRI, including pharmacological intervention, use of protective gases, as well as stem cell and gene therapy. These therapies face the challenges of translating animal studies to humans, largely due to the complex factors and mechanisms that contribute to IRI [3]. Recently, normothermic machine perfusion (NMP) device has been invented, which can perfuse a liver with oxygenated blood, nutrients, and medications at normal body temperature to preserve the organ in a functioning status [4]. However, in the current practice, the organs perfused still suffer ischemia and CS injury during the procurement phase because the organs are harvested using conventional procedure [5].
We hypothesized that a better transplantation outcome could be achieved by avoiding ischemia during organ procurement, which could be achieved through surgical technique innovations. In this study, we investigated the feasibility of a novel organ procurement procedure called ischemia-free liver procurement (IFLP). Here we report the first 2 cases of IFLP.
Case Studies
Organ procurement under NMP
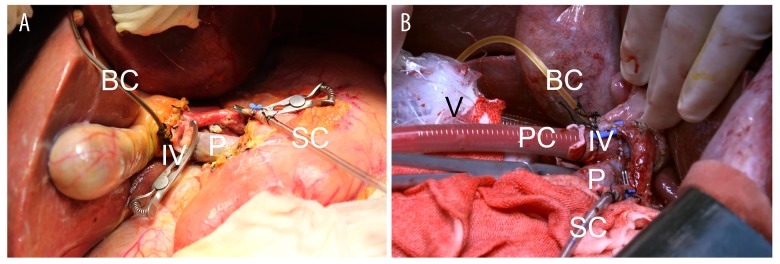
We implemented an “ischemia-free” technique to harvest the donor livers (Figure 1). First, we isolated the donor arteries from the celiac trunk to hepatic artery proper. The hepatic hilum was well dissected to reveal the portal vein and common bile duct, followed by fully mobilization of the liver from the ligaments. A tube was placed in the common bile duct for bile drainage. A short segment of right common iliac vein was procured and then was anastomosed to the upper portal vein in an end-to-side fashion with partial blockage of the portal vein. The infra-hepatic inferior vena cava was intubated by a venous cannula, which was connected to the organ reservoir of the Liver Assist Device (Organ Assist, The Netherland) for venous outflow. The portal vein cannula, which was connected to the portal line of the device, was subsequently inserted to the portal vein through the interposition vein (the right common iliac vein). The supra-hepatic inferior vena cava was clamped, and blood drained to the perfusion device via the tube inside the inferior vena cava. Then an arterial cannula, which was connected to the arterial line of the device, was inserted into the splenic artery without interruption of arterial supply to the liver from the celiac trunk. The in vivo perfusion circuit was established. Finally, the livers were resected from the donor and moved to the organ reservoir under NMP. None of the deceased donors were prisoners sentenced to death.
Figure 1.
Photo of machine perfusion in situ. IV – interposition vein portal vein; P – portal vein; PC – portal vein cannula; SC – splenic arterial cannula; BC – bile duct cannula; V – venous cannula.
Ex vivo perfusion and assessment of donor liver
The perfusate was prepared according to the previous report [6]. The final hemoglobin concentration was 8.5 g per deciliter (hematocrit, 0.25). The perfusate was warmed up to 37°C beforehand, and the oxygenator was supplied with a mixture of O2 and air (30% O2). The viability of the graft was assessed by blood gas analysis and liver function tests of the perfusate, as well as bile production according to reported literature [7].
Organ implantation and post-transplantation management
The liver implantation was performed using a modified piggyback technique as we described elsewhere. An immunosuppressive regimen with anti-IL-2 receptor antibody (basiliximab) induction, tacrolimus and mycophenolate mofetil maintenance, was used in these patients. We obtained biopsies of the donor livers before procurement, after machine perfusion, as well as after revascularization. Liver function tests and coagulation function analysis were performed daily. Post-transplantation complications, such as vascular, biliary, and infectious complications, were also monitored. The study protocol was approved by the Ethical Committee of The First Affiliated Hospital of Sun Yat-sen University, and informed consent was obtained from the participant.
Demographics and patient characteristics
In Case 1, the patient was a 45-year-old male with decompensated liver cirrhosis secondary to hepatitis B virus (HBV) infection (Table 1). The calculated model for end-stage liver disease (MELD) score was 17. The donor was a 43-year-old male with confirmed diagnosis of brain death caused by hypertensive intracerebral hemorrhage. Routine laboratory panel revealed normal liver function tests.
Table 1.
Characteristics of the donors and recipients.
| Case 1 | Case 2 | |
|---|---|---|
| Donor characteristics | ||
| Age (year) | 43 | 32 |
| Sex | Male | Female |
| Height (cm) | 170 | 165 |
| Weight (Kg) | 65 | 45 |
| Blood type (Rh) | A(+) | O(+) |
| Causes of brain death | Hypertensive intracerebral hemorrhage | Hypertensive intracerebral hemorrhage |
| Normothermic perfusion time (min) | 120 | 120 |
| Cold ischemic preservation time (hours) | 6 | 3 |
| Macrovesicular steatosis (%) | <5 | >50 |
| Recipient characteristics | ||
| Age (year) | 45 | 56 |
| Sex | Male | Male |
| Blood type (Rh) | A(+) | O(+) |
| Primary diagnosis | HBV-related decompensated liver cirrhosis | HBV-related decompensated liver cirrhosis |
| MELD | 17 | 15 |
| Time of anhepatic phase (min) | 57 | 50 |
| Blood loss of surgery (mL) | 3500 | 400 |
| ICU stay | 4 days | 20 hours |
UW – University of Wisconsin; MELD – model for end-stage liver disease; ICU – intensive care unit.
In Case 2, a 56-year-old male with decompensated liver cirrhosis secondary to hepatitis B (Table 1). The calculated MELD score was 15. The donor was a 32-year-old female with a history of hypertension, who suffered brain death secondary to intracranial hemorrhage. The last aspartate transaminase (AST), alanine transaminase (ALT), and total bilirubin (Tbil) levels before procurement were 110 U/L, 160 U/L, and 64.5 μmol/L respectively.
Machine perfusion
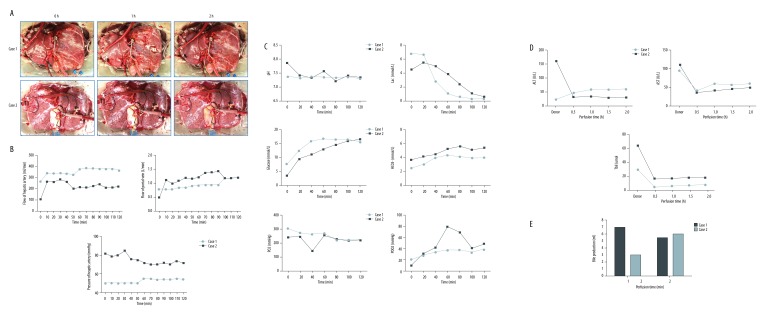
In Case 1, the donor liver underwent NMP for 120 minutes then flushed with 2 L cold University of Wisconsin (UW) solution. The liver was cold stored for 6 hours. The liver was well perfused with pink color (Figure 2A). The flow of hepatic artery is between 280–350 mL/min (Figure 2B). The portal vein flow was maintained at 0.75–1.0 L/min. The pH value was stable within the normal range and the lactic acid levels dropped quickly from 6.2 mmol/L to lower than 0.3 mmol/L (Figure 2C). The AST, ALT, and Tbil levels were maintained stable and within a low range (Figure 2D). After 2-hour perfusion, the bile production increased to 6 mL/hour (Figure 2E). These results suggested the liver quality was good and fulfilled the transplantation criteria.
Figure 2.
Records of liver and functional parameters during NMP. (A) Photos of liver during NMP. (B) Perfusion characteristics of 2 cases. (C, D) Blood gas analysis and liver function analysis of the perfusate. (E) Bile production. NMP, normothermic machine perfusion.
The liver of Case 2 was also procured with ischemia-free technique. The NMP lasted for 120 minutes then was shifted to 3-hour CS (Figure 2A). The flow of hepatic artery and portal vein were around 200 mL/min and 1.0 L/min, respectively (Figure 2B). The lactic acid reached 4.8 mmol/L and then decreased step by step (Figure 2C). The pH, AST, ALT, and Tbil levels were maintained stable in the normal range (Figure 2C, 2D). The bile production progressively increased from 3 mL/hour to 6 mL/hour (Figure 2E). These results together indicated excellent quality of the liver for transplantation.
Post-transplantation course
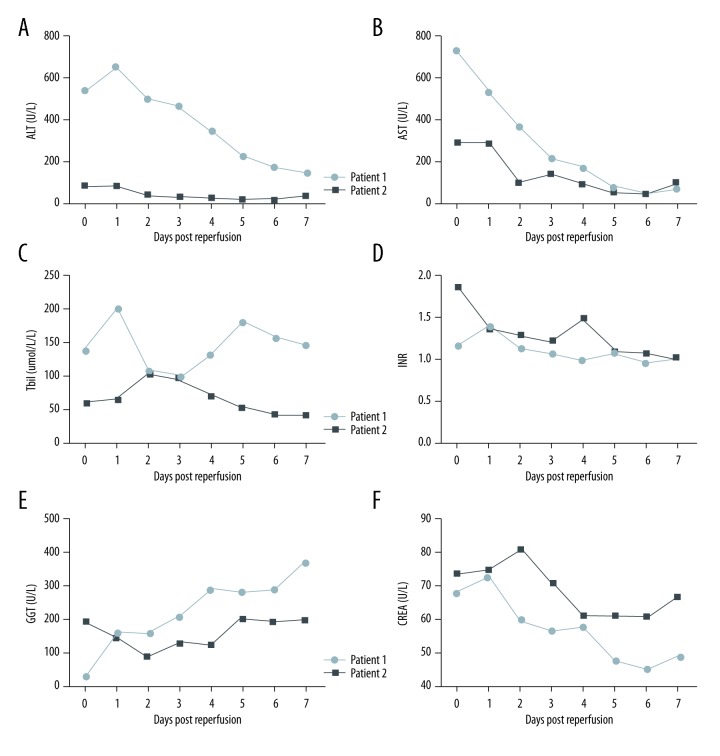
The Case 1 patient was extubated at 36-hours post-transplantation and the intensive care unit stay was 4 days (Table 1). Figure 3 shows that the post-transplantation peak levels of AST, ALT, and Tbil reached 538 U/L, 649 U/L, and 202 μmol/L in the first 24 hours, respectively (Figure 3A–3C). The international normalized ratio (INR) remained below 1.4 after surgery (Figure 3D). The peak of gamma-glutamyl transpeptidase (GGT) was 375 U/L on post-transplantation day (POD) 7 (Figure 3E). Creatinine stayed at normal levels within the first week (Figure 3F). There was no biliary or vascular complication observed after transplantation. The patient was discharged from the hospital on POD 25.
Figure 3.
(A–F) Liver function and biochemistry tests after transplantation.
The Case 2 patient was extubated at 10-hours post-transplantation. The post-transplantation course was unremarkable with excellent graft function: The AST and ALT levels peaked at 89 U/L and 292 U/L respectively on POD 1 followed by a rapid decline to normal values on POD 2 (Figure 3A, 3B). Similarly, Tbil peaked at 104.5 μmol/dL on POD 2 and declined daily (Figure 3C) while the GGT gradually increased from POD2 and reached 203 U/L on POD5 (Figure 3E). The INR and creatinine levels remained normal after transplantation (Figure 3D). There was no biliary or vascular complication observed. The patient was discharged from the hospital on POD 26.
Ischemia reperfusion injury data
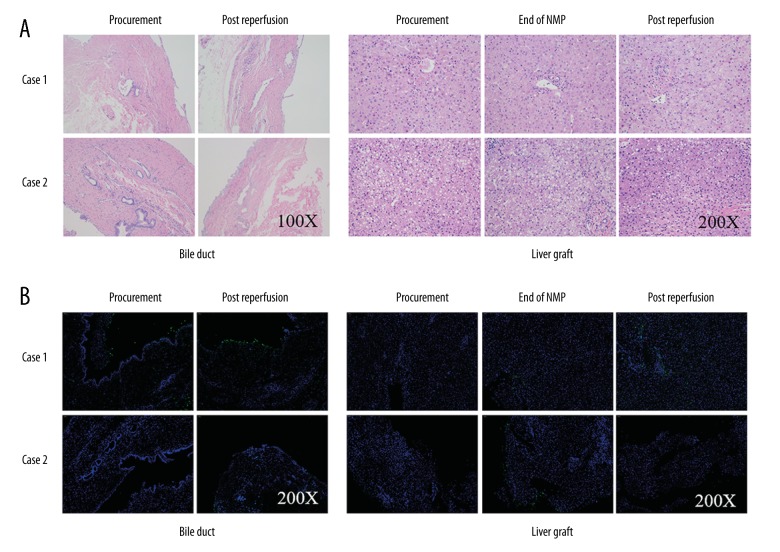
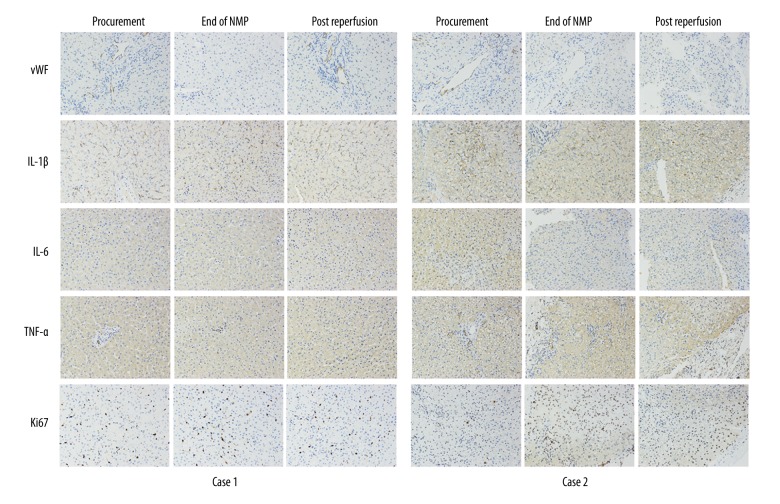
There was no obvious histological change in bile duct and liver allograft by hematoxylin and eosin (H&E) staining at the end of NMP and post reperfusion (Figure 4). However, there was hydropic degeneration of hepatocytes and mild inflammatory cell infiltration in the portal area, as well as minimal sinusoidal congestion at 2-hours post allograft re-vascularization, which could be referred to as a minor IRI of the liver. Notably, H&E staining of the donor liver tissue biopsy before procurement showed over 30% macro-vesicular steatosis (Figure 4A). The TdT-mediated dUTP nick end labeling (TUNEL) staining of biliary epithelium and liver showed slight apoptosis at the end of NMP and after re-vascularization. Similar results were obtained in von Willebrand factor (vWF) staining, a marker of endothelial injury (Figure 5). The positive level of IL-1β, IL-6, and TNF-α staining declined slightly at the end of NMP as well as after re-vascularization. There was a slight hydropic degeneration of hepatocytes at 2-hours post allograft re-vascularization. Moreover, the enhancement of Ki67 staining, an indicator of cell proliferation, was documented after NMP (Figure 5).
Figure 4.
(A) Hematoxylin and eosin staining showed well preservation of bile duct and liver at the end of NMP and post reperfusion. (B) The TdT-mediated dUTP nick end labeling (TUNEL) staining revealed slight low proportion of apoptosis in Case 1 both in bile duct and liver. Case 2 showed no sign of apoptosis during the whole process.
Figure 5.
Immunohistochemistry of vWF, IL-1β, IL-6, and TNF-α demonstrated an improvement in liver graft both Case 1 and Case 2 at the end of NMP and post reperfusion. Ki67 staining showed promotion of viability in Case 2 after machine perfusion. NMP – normothermic machine perfusion; vWF – von Willebrand factor
Discussion
IRI is still a major obstacle for short-term and long-term transplantation outcomes, especially in the current era of a growing number of the marginal organ donations. The principle of NMP is to avoid cold ischemia and maintain normal metabolism throughout the preservation period and provide oxygen and essential substrates via dual perfusion. However, cold flush is still required before and after NMP, when the organ once again suffers ischemia, CS, and reperfusion injury [8]. Therefore, in the current practice of NMP, the organ suffers IRI twice during the whole procedure, which is considered to diminish the benefits of NMP [9]. For the same reason, NMP is usually started at the place of organ procurement and continued during organ transport. However, transporting a “warm” organ faces the imminent risk of graft loss due to warm ischemia.
In this study, we described a novel organ procurement procedure under NMP. These are also the first 2 clinical cases of liver transplantation using organs preserved by NMP in China. To our knowledge, the liver function tests (AST, ALT, and bilirubin) in the perfusate during NMP were the lowest in the literature. Notably, these markers did not increase after the start of NMP when compared to donor results. Moreover, the pathological changes and release of inflammatory cytokines were minimal at the end of NMP. Together, these results suggest the novel procurement procedure is able to largely avoid the “first” IRI of NMP.
For the safety of patients, we confirmed the viability of grafts after 2-hour NMP using reported criteria before we started the recipient hepatectomy [7]. We therefore implanted the liver after 3-hour CS post-NMP in Case 2 while after 6-hour CS post-NMP in Case 1 (due to difficult hepatectomy). The post-transplantation allograft function was much better in Case 2 than in Case 1. This suggests that the duration of CS after NMP might affect the post-transplantation allograft function. The transplantation outcome might be even better if the NMP was done throughout the recipient hepatectomy.
It has been reported that NMP is able to resuscitate marginal livers which are sensitive to IRI [10]. In Case 2, there was over 30% macro-vesicular steatosis of the donor liver. However, the liver function tests and pathological analysis revealed mild liver injury using the novel procedure. NMP has been shown to allow for real-time assessment of viability of marginal grafts by analyzing objective data about liver function [7]. Therefore, by using ischemia-free liver procurement; (IFLP), NMP is able to further minimize the organ utilization and optimize transplantation outcomes. Since the NMP technique is available in the preservation of lung, heart, and kidney [11–13], this novel procedure can be equally implemented in these organ transplantations.
However, there are some issues we still need to study. One is that the procurement technique implies the necessary dissection of the vascular pedicles before procurement, which was demonstrated to be deleterious for the liver compared with the rapid technique with perfusion before pedicles dissection, for cold perfusion. Another issue is that the lateral clamping of the portal vein reduces the portal inflow during the suture of the iliac vein fragment. The results so far support that both issues limit damage to the liver. But we still need to monitor the liver function more frequently to find out the level of harm to the liver allograft during the procurement.
Conclusions
In this study, we have proved the feasibility of IFLP, although more cases are needed to confirm its potential benefits. Importantly, the success of IFLP strongly suggests that ischemia-free liver implantation (IFLI) is achievable because the in situ NMP circuit in IFLI is the same as in IFLP.
Abbreviations
- NMP
normothermic machine perfusion
- IRI
ischemia reperfusion injury
- IFLP
ischemia-free liver procurement
- UW
University of Wisconsin solution
- POD
post-transplantation day
- MELD
model for end-stage liver disease
- CS
cold storage
- AST
aspartate transaminase
- ALT
alanine transaminase
- Tbil
total bilirubin
- GGT
gamma-glutamyl transpeptidase
- INR
International Normalized Ratio
- H&E
hematoxylin and eosin
- TUNEL
TdT-mediated dUTP nick end labeling
- IFLI
ischemia-free liver implantation.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (81373156 and 81471583), the Special Fund for Science Research by Ministry of Health (201302009), the Key Clinical Specialty Construction Project of National Health and Family Planning Commission of the People’s Republic of China, the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007), Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002), Guangdong Provincial Natural Science Funds for Major Basic Science Culture Project (2015A030308010), Guangdong Provincial Natural Science Funds for Distinguished Young Scholars (2015A030306025), Guangdong Provincial Natural Science Funds (2016A030310141), Special support program for training high level talents in Guangdong Province (2015TQ01R168), and Pearl River Nova Program of Guangzhou (201506010014)
Conflict of interests
None.
References
- 1.Morris PJ. Transplantation – a medical miracle of the 20th century. N Engl J Med. 2004;351(26):2678–80. doi: 10.1056/NEJMp048256. [DOI] [PubMed] [Google Scholar]
- 2.Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology”. J Hepatol. 2014;61(2):418–31. doi: 10.1016/j.jhep.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation – from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10(2):79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pezzati D, Liu Q, Hassan A, et al. Normothermic machine perfusion: A new world deserving careful exploration. Am J Transplant. 2017;17(7):1956–57. doi: 10.1111/ajt.14249. [DOI] [PubMed] [Google Scholar]
- 5.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: A phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16(6):1779–87. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 6.Watson CJ, Randle LV, Kosmoliaptsis V, et al. 26-hour storage of a declined liver before successful transplantation using ex vivo normothermic perfusion. Ann Surg. 2017;265(1):e1–e2. doi: 10.1097/SLA.0000000000001834. [DOI] [PubMed] [Google Scholar]
- 7.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16(11):3235–45. doi: 10.1111/ajt.13875. [DOI] [PubMed] [Google Scholar]
- 8.Marecki H, Bozorgzadeh A, Porte RJ, et al. Liver ex situ machine perfusion preservation: A review of the methodology and results of large animal studies and clinical trials. Liver Transpl. 2017;23(5):679–95. doi: 10.1002/lt.24751. [DOI] [PubMed] [Google Scholar]
- 9.Selten J, Schlegel A, de Jonge J, Dutkowski P. Hypo- and normothermic perfusion of the liver: Which way to go? Best Pract Res Clin Gastroenterol. 2017;31(2):171–79. doi: 10.1016/j.bpg.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Perera T, Mergental H, Stephenson B, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl. 2016;22(1):120–24. doi: 10.1002/lt.24369. [DOI] [PubMed] [Google Scholar]
- 11.Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. The J Heart Lung Transplant. 2017;36(7):744–53. doi: 10.1016/j.healun.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: A case series. Lancet. 2015;385(9987):2585–91. doi: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 13.Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16(11):3282–85. doi: 10.1111/ajt.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]