Abstract
Background
Living kidney donors face the risk of renal dysfunction, resulting in end-stage renal disease, cardiovascular disease, or cerebrovascular disease, after donor nephrectomy. Reducing this risk is important to increasing survival of living donors. In this study, we investigated the effect of preoperative distribution of abdominal adipose tissue and nutritional status on postoperative renal function in living donors.
Material/Methods
Seventy-five living donors were enrolled in this retrospective study. Preoperative unenhanced computed tomography images were used to measure abdominal adipose tissue parameters. Prognostic nutritional index (PNI) was used to assess preoperative nutritional status. Donors were divided into 2 groups according to abdominal visceral adipose tissue (VAT) area at the level of the fourth and fifth lumbar vertebrae (<80 or ≥80 cm2). Postoperative renal function was compared in the 2 groups, and prognostic factors for development of chronic kidney disease (CKD) G3b were identified using multivariate analysis.
Results
Donors with a VAT area ≥80 significantly more often had hypertension preoperatively. Although there was no significant difference in preoperative estimated glomerular filtration rate (eGFR) between the 2 groups, postoperative renal function was significantly decreased in donors with a VAT area ≥80 compared to those with a VAT area <80. In multivariate analysis, VAT area ≥80 and PNI <54 were independent factors predicting the development of CKD G3b after 12 months.
Conclusions
Our findings suggest that preoperative VAT and PNI affect postoperative renal function. Further research is required to establish appropriate exercise protocols and nutritional interventions during follow-up to improve outcomes in living donors.
MeSH Keywords: Kidney Function Tests, Kidney Transplantation, Living Donors, Nephrectomy, Nutritional Status
Background
Kidney transplantation is the most successful treatment for end-stage renal disease (ESRD), resulting in improved survival and quality of life. The shortage of donor kidneys is a serious problem in this field [1]. In Japan, the Organ Transplantation Law was revised in 2010. Organ donation is now permitted, with family members’ consent, even if an individual’s intention is unknown, and organ donation by brain-dead donors below 15 years of age is also allowed. Despite this revision of organ donation constraints, the number of deceased donor kidney transplantations has not increased. Hence, approximately 85% of donor organs for kidney transplantation are acquired from living donors, and conditions for living donor kidney donation have been expanded to include not only human leukocyte antigen matching and ABO blood type compatibility, but also donor medical status. The fundamental principle of living kidney donation is to ensure donor safety during the perioperative and postoperative periods.
Living donor nephrectomy itself is associated with some mortality. Perioperative complications occur in approximately 15% of donors. Major complications include bleeding requiring blood transfusion, pulmonary thrombosis requiring transient mechanical ventilation, acute kidney injury requiring transient renal replacement therapy, pulmonary complications, gastrointestinal complications, femoral nerve compression, and wound abnormality. Reportedly, 5-, 10-, 20-, and 30-year survival rates of living kidney donors are 98%, 95%, 86%, and 66%, respectively. The main causes of death after living donor nephrectomy are malignancy, cerebrovascular disease, and cardiovascular disease. Death caused by chronic renal failure was sometimes observed [2–6]. On the other hand, some studies found that mortality and the cause of death of living kidney donors were similar to those in the age-matched general population [7,8]. However, the prevalence of microalbuminuria, resulting in renal impairment, and hypertension was increased after donor nephrectomy. In addition, some donors became either overweight or obese and sometimes developed diabetes. Thus, long-term follow-up of kidney donors is very important [8,9].
Another factor important for improving donor outcomes after donor nephrectomy is preoperative management of conditions such as obesity and hypertension. Obesity is an independent risk factor for the development of chronic kidney disease (CKD) and ESRD and is also associated with hypertension [10,11]. Given the increasing prevalence of obesity, living donors are likely to become obese and develop CKD related to obesity [12]. This emphasizes the crucial role of preoperative management of living donors, which could help maintain the renal function after donor nephrectomy, resulting in good prognosis. Although body mass index (BMI) is generally used to detect obesity, it does not reflect the distribution of abdominal adipose tissue, which has been linked to risk of microalbuminuria, renal dysfunction, and cardiovascular disease by some recent reports [13–17]. Although the Japanese living donor guideline for kidney transplantation states that a BMI less than 30 is appropriate for donors, it does not provide any recommendations with respect to the distribution of abdominal adipose tissue [18]. Therefore, we evaluated the association between living donor postoperative renal function and distribution of abdominal adipose tissue. In addition, we evaluated preoperative nutritional status of living donors by using 2 nutrition indices: the PNI and the Controlling Nutritional Status (CONUT) score. PNI has been proposed for assessing perioperative nutritional status, postoperative complications, and survival of patients with colorectal cancer, whereas CONUT score is also an independent prognostic factor for colorectal cancer [19,20].
The aim of the present study was to evaluate the effects of preoperative distribution of abdominal adipose tissue and nutritional status on renal function of living donors after donor nephrectomy. Realization of the importance of preoperative management in addition to postoperative long-term follow-up could lead to better outcomes in living donors after renal transplantation.
Material and Methods
Patient selection and study design
Figure 1 shows a scheme of the present study. We extracted data on 94 living donors for renal transplantation. They underwent donor nephrectomy at our institution between January 2008 and May 2016. The inclusion criteria were as follows: donor nephrectomy was performed at our institution, preoperative abdominal-to-pelvic unenhanced computed tomography (CT) was performed at our institution, and the follow-up period was at least 12 months. We excluded 19 patients who underwent only MRI as a preoperative imaging modality or underwent CT at another institution. Finally, 75 patients were enrolled in this study, and their clinical information was reviewed retrospectively. These 75 patients were divided into 2 groups according to abdominal visceral adipose tissue (VAT) area at the level of the fourth and fifth lumbar vertebrae (L4–L5; VAT area <80 cm2: n=38 vs. VAT area ≥80 cm2: n=37). We compared renal function 12 months after donor nephrectomy in these 2 groups, and a second comparison was performed at 24 months in patients who were followed for at least that long. In addition, prognostic factors for development of CKD G3b 12 months after donor nephrectomy were investigated. The protocol for this research project was approved by the Institutional Review Board for Clinical Studies (Medical Ethics Committee ID: NMU-1605), which waived the requirement for informed patient consent because of the retrospective nature of the analysis.
Figure 1.
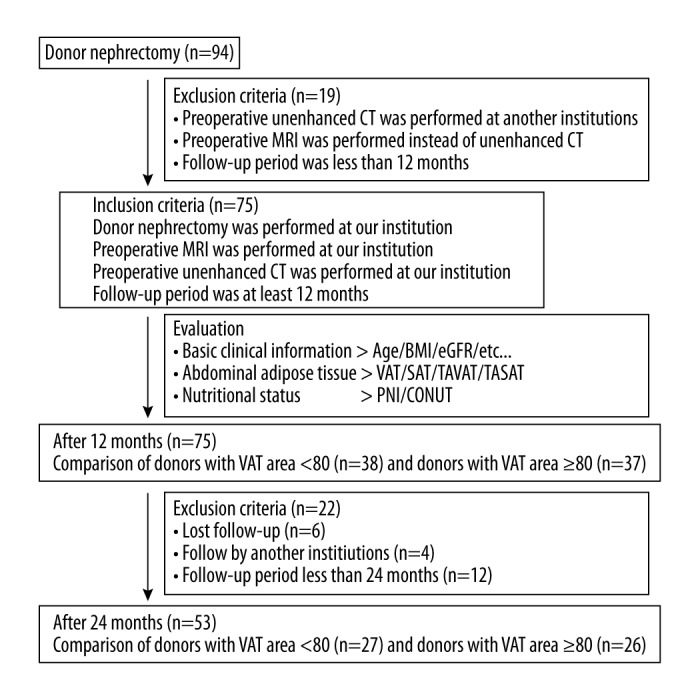
Schematic diagram of the study work flow. Between January 2008 and May 2016, 94 donors underwent donor nephrectomy at our institution. Nineteen donors were excluded because of insufficient radiographic and laboratory data, and the data from 75 remaining donors were included in our retrospective study. We evaluated preoperative basic clinical information, abdominal adipose tissue parameters, and nutritional status, and donors were divided into 2 groups according to VAT area at L4–5 (VAT area <80 and ≥80). Renal function 12 months after donor nephrectomy was compared between the 2 groups, and prognostic factors for development of chronic kidney disease of stage G3b at 12 months after donor nephrectomy were identified by logistic regression analysis. Follow-up data 24 months after donor nephrectomy were available for 53 out of out of the 75 donors, and thus renal function 24 months after donor nephrectomy was also compared between the 2 groups.
Calculation of estimated glomerular filtration rate and classification of CKD stage
Estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) was calculated using the CKD-EPI equation: GFR=144×(serum creatinine/0.7)−0.329×(0.993)Age for women with a serum creatinine level ≤0.7 mg/dL; GFR=144×(serum creatinine/0.7)−1.209× (0.993)Age for women with a serum creatinine level >0.7 mg/dL; GFR=141×(serum creatinine/0.9)−0.411×(0.993)Age for men with a serum creatinine level ≤0.9 mg/dL; GFR=141×(serum creatinine/0.9)−1.209×(0.993)Age for men with a serum creatinine level >0.9 mg/dL [21]. The stage of CKD was classified according to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline published in 2012: CKD G1, ≥90; CKD G2, 60–89.9; CKD G3a, 45–59.9; CKD G3b, 30–44.9; CKD G4, 15–29.9; CKD G5, <15 mL/min/1.73 m2 [22].
Measurement of abdominal adipose tissue parameters by CT
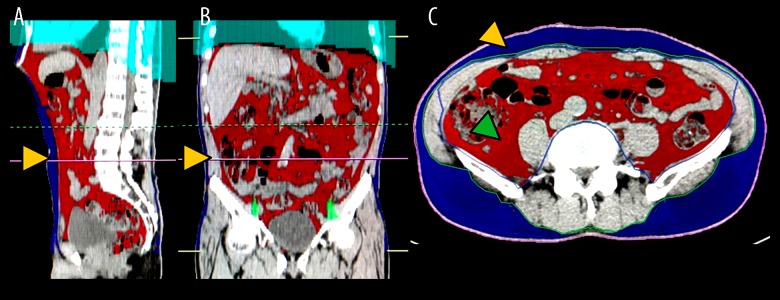
Unenhanced CT images obtained during preoperative screening or examination of vascular structure were analyzed using the Volume Analyzer SYNAPSE VINCENT image analysis system (Fujifilm Medical, Tokyo, Japan) to quantify abdominal adipose tissue area and volume. The following measurements were obtained for analysis: VAT area at the level of L4–L5 (cm2); subcutaneous adipose tissue area at the level of L4–L5 (SAT; cm2); total abdominal visceral adipose tissue volume (TAVAT volume; cm3); and total abdominal subcutaneous adipose tissue volume (TASAT volume; cm3). Representative images used for analyses of abdominal adipose tissue parameters are shown in Figure 2A–2C.
Figure 2.
Representative images used for analyses of abdominal adipose tissue parameters. The Volume Analyzer SYNAPSE VINCENT image analysis system was used to reconstruct three-dimensional (3-D) images as follows: sagittal plane for quantification of the visceral adipose tissue area at L4–5 (red line indicated by yellow arrows) (A); coronal plane for detection of abdominal adipose tissue (red line indicated by yellow arrows) (B); transverse plane for detection of abdominal adipose tissue including visceral adipose tissue (red area indicated by green arrows) and subcutaneous adipose tissue (blue area indicated by yellow arrows) (C).
Nutrition index
Preoperative nutritional status was examined, and its effect on postoperative renal function was investigated. PNI and CONUT scores were used as markers of nutritional status in this study. PNI was calculated using the following formula: 10×serum albumin (g/dL)+0.005×total lymphocyte count (per mm3), and CONUT score was determined from serum albumin level, peripheral lymphocyte count, and total cholesterol level (Table 1). PNI and CONUT score were calculated using baseline blood data obtained preoperatively.
Table 1.
Scoring system of the CONUT score.
| Variables | Degree of malnutrition | |||
|---|---|---|---|---|
| None | Mild | Moderate | Sever | |
| Serum albumin (g/dL) | ≥3.5 | 3.00–3.49 | 2.50–2.99 | <2.5 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocyte count (/mm3) | ≥1600 | 1200–1599 | 800–1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
CONUT – controlling nutritional status.
Statistical analysis
Statistical analyses were performed and figures were plotted using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). Data are represented using bar charts or box plots, and the t test or the Mann–Whitney U-test was applied for statistical analysis, as appropriate. The interrelationship between VAT area at L4–5 and the other parameters of abdominal adipose tissue or nutrition indices was examined using Spearman’s correlation. To identify prognostic factors for the development of CKD G3b 12 months after donor nephrectomy, univariate and multivariate analyses were performed via logistic regression analysis using IBM SPSS, version 21 (SPSS Inc., Chicago, IL, USA). The cutoff value for development of CKD G3b 12 months after donor nephrectomy was determined by receiver operating characteristics (ROC) curve analysis. Two-sided tests were used in all cases. A P-value <0.05 was considered to indicate a statistically significant difference in all analyses.
Results
Patient characteristics
Table 2 shows the baseline clinical characteristics, preoperative renal function values, abdominal adipose tissue status, nutritional status, and perioperative data for the cohort of 75 donors for living renal transplantation and compares these variables between donors with a VAT area at L4–5 <80 (n=38) and ≥80 (n=37). At donor nephrectomy, the median age in this cohort was 58 years (interquartile range [IQR], 47–64). Twenty-nine men (39%) and 46 women (61%) participated in this study, and there were significantly more women among donors with a VAT area at L4–5 <80 than among those with a VAT area at L4–5 ≥80 (P=0.034). The median preoperative BMI, SAT area at L4–5, TAVAT volume, and TASAT volume in this cohort were 22.5 kg/m2 (IQR, 21.1–24.8), 146.8 cm2 (IQR, 94.6–201.9), 2041.5 cm3 (IQR, 1055.3–3389.8), and 3227.6 cm3 (IQR, 1798.4–4817.7), respectively, with significant differences between donors with a VAT area at L4–5 <80 and ≥80 (P<0.0001, P=0.0004, P<0.0001, and P=0.0010, respectively). Although preoperative serum creatinine level was significantly higher in donors with a VAT area at L4–5 ≥80 than in donors with a VAT area at L4–5 <80 (P=0.0069), there was no significant difference between these 2 groups in median preoperative eGFR (81.9 mL/min/1.73 m2). Donors with a preoperative urinary protein level exceeding 30 mg/dL were not present in this cohort. There were no donors with CKD G4 and G5 throughout the follow-up period. There were significantly more donors with hypertension among those with a VAT area at L4–5 ≥80 than among those with a VAT area at L4–5 <80 (P=0.036). With regard to nutritional status, the median preoperative PNI and CONUT scores in this cohort were 53.0 (IQR, 51.0–55.5) and 0 (IQR, 0–2), respectively, with no significant differences between the 2 groups. As expected, there were no donors with malnutrition preoperatively. The median operative time in this cohort was 247 min (IQR, 156–498), and there was a significant difference between the 2 groups (P=0.011). Donors with a VAT area at L4–5 ≥80 needed a longer operation than donors with a VAT area at L4–5 <80. There were no cases with blood loss requiring transfusion and major perioperative complications greater than grade 2 of the Clavien classification. During the follow-up period, there were no new-onset cases of hypertension or diabetes, and no progression was observed in the 2 cases with preoperative diabetes.
Table 2.
Patients’ background.
| Variables | Number of patients | VAT at L4–5 | P value | ||
|---|---|---|---|---|---|
| <80 | ≥80 | ||||
| Total | 75 | 38 | 37 | ||
| Age at operation (years) | Median (IQR) | 58 (47–64) | 58 (46–64) | 58 (48–64) | 0.51# |
| Gender | 0.034* | ||||
| Male (%) | 29 (39) | 10 (26) | 19 (51) | ||
| Female (%) | 46 (61) | 28 (74) | 18 (49) | ||
| Preoperative BMI (kg/m2) | Median (IQR) | 22.5 (21.1–24.8) | 22.5 (21.1–24.8) | 22.4 (21.0–24.8) | <0.0001# |
| Preoperative serum creatinin (mg/dL) | Median (IQR) | 0.64 (0.58–0.78) | 0.64 (0.58–0.78) | 0.64 (0.58–0.78) | 0.0069# |
| eGFR (mL/min/1.73 m2) | Median (IQR) | 81.9 (74.4–91.8) | 81.9 (74.1–91.9) | 81.9 (73.8–91.5) | 0.092# |
| SAT at L4–5 (cm2) | Median (IQR) | 146.8 (94.6–201.9) | 147.9 (95.6–203.1) | 146.8 (93.8–199.4) | 0.0004# |
| TAVAT (cm3) | Median (IQR) | 2041.5 (1055.3–3389.8) | 1989.3 (1040.0–3337.2) | 2041.5 (1024.7–3431.0) | <0.0001# |
| TASAT (cm3) | Median (IQR) | 3227.6 (1798.4–4817.7) | 3245.8 (1830.9–4830.6) | 3135.3 (1779.7–4646.7) | 0.0010# |
| CCI | 1.00* | ||||
| 0 (%) | 71 (95) | 36 (95) | 35 (95) | ||
| 1 or more (%) | 4 (5) | 2 (5) | 2 (5) | ||
| Hypertension | 0.036* | ||||
| No (%) | 62 (83) | 35 (92) | 27 (73) | ||
| Yes (%) | 13 (17) | 3 (8) | 10 (27) | ||
| Diabetes | 1.00* | ||||
| No (%) | 73 (97) | 37 (97) | 36 (97) | ||
| Yes (%) | 2 (3) | 1 (3) | 1 (3) | ||
| Hyperlipidemia | 0.35* | ||||
| No (%) | 64 (85) | 34 (89) | 30 (81) | ||
| Yes (%) | 11 (15) | 4 (11) | 7 (19) | ||
| Hyperuricemia | 0.36* | ||||
| No (%) | 71 (95) | 37 (97) | 34 (92) | ||
| Yes (%) | 4 (5) | 1 (3) | 3 (8) | ||
| Preoperative nutrition index | |||||
| PNI | Median (IQR) | 53 (51–56) | 53 (51–56) | 53 (51–56) | 0.61# |
| CONUT score | Median (IQR) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0.23# |
| Type of donor nephrectomy | 0.82* | ||||
| Hand assisted (%) | 55 (73) | 27 (71) | 28 (76) | ||
| Laparoscopic (%) | 3 (4) | 2 (5) | 1 (3) | ||
| Open (%) | 17 (23) | 9 (24) | 8 (21) | ||
| Operative time (min) | Median (IQR) | 247 (156–498) | 250 (222–311) | 244 (218–311) | 0.011# |
| Perioperative complications | 1.00* | ||||
| No (%) | 71 (95) | 36 (95) | 35 (95) | ||
| Yes (%) | 4 (5) | 2 (5) | 2 (5) | ||
VAT at L4–5 – visceral adipose tissue at the level of the fourth and fifth lumbar vertebra; IQR – interquartile range; BMI – body mass index; eGFR – estimate glomerular filtration rate; SAT at L4–5 – subcutaneous adipose tissue at the level of the fourth and fifth lumbar vertebra; TAVAT – total abdominal visceral adipose tissue; TASAT – total abdominal subcutaneous adipose tissue; CCI – charlson comorbidity index; PNI – prognostic nutritional index; CONUT – controlling nutritional status;
Chi-square test or Fisher’s exact test;
Mann-Whitney U test.
Chronological changes of the renal function after donor nephrectomy
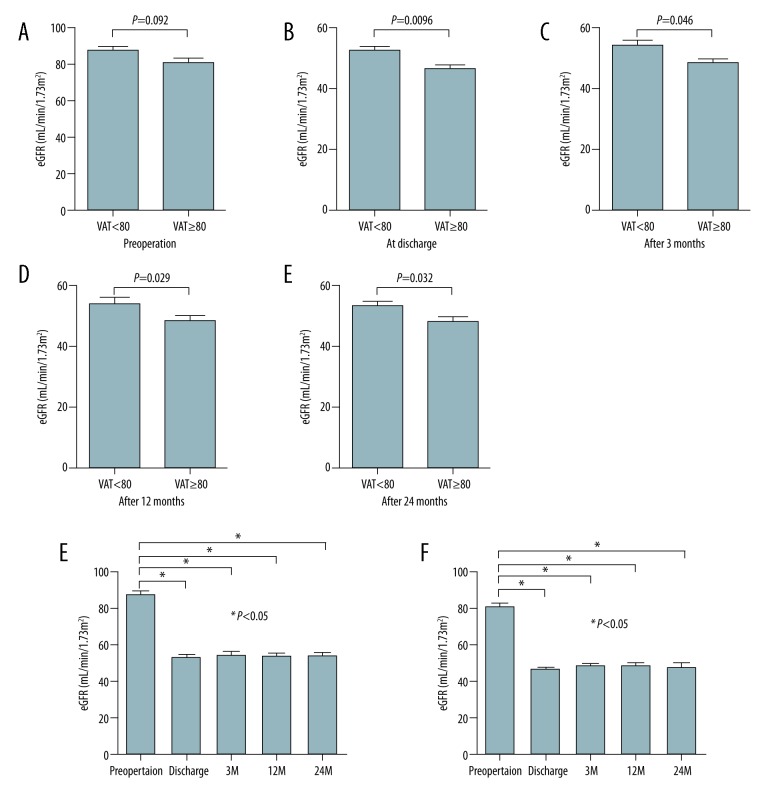
Preoperative eGFR tended to be lower in donors with a VAT area at L4–5 ≥80 than in those with a VAT area at L4–5 <80, but the difference did not reach the level of statistical significance (Figure 3A; P=0.092). In contrast, eGFR of donors with a VAT area at L4–5 ≥80 preoperatively was significantly lower at the time of discharge, as well as at 3 and 12 months after donor nephrectomy, than that of donors with a VAT area at L4–5 <80 (Figure 3B; P=0.0096, Figure 3C; P=0.046, and Figure 3D; P=0.029, respectively). These results suggest that preoperative VAT area at L4–5 can predict renal function 12 months after donor nephrectomy. We evaluated renal function 24 months after donor nephrectomy in 53 donors who were observed for 24 months (Figure 1; VAT area at L4–5 <80: n=27, VAT area at L4–5 ≥80: n=26). eGFR of donors with a VAT area at L4–5 ≥80 preoperatively significantly decreased 24 months after donor nephrectomy compared to that of donors with a VAT area at L4–5 <80 (Figure 3E; P=0.032). Although postoperative renal function was significantly lower than preoperative renal function in both groups, there were no significant differences in postoperative renal function at any time point in either group (Figure 3F, 3G). A slight improvement in postoperative renal function from 3 months after donor nephrectomy was observed in both groups (VAT area at L4–5 <80; P=0.89, VAT area at L4–5 ≥80; P=0.32, respectively).
Figure 3.
Comparison of renal function of living donors according to VAT area at L4–5 using estimated glomerular filtration rate. There was no significant difference in preoperative renal function between donors with a VAT area at L4–5 ≥80 and <80 (A). Postoperative renal function was significantly lower in donors with a VAT area at L4–5 ≥80 than in those with a VAT area <80 at discharge (B) and 3 months (C), 12 months (D), and 24 months (E) after donor nephrectomy. Chronological changes in renal function were evaluated in donors with a VAT area at L4–5 <80 (F) and ≥80 (G). Neither group showed a significant improvement of renal function.
The association of VAT area with other abdominal adipose tissue parameters and nutrition indices
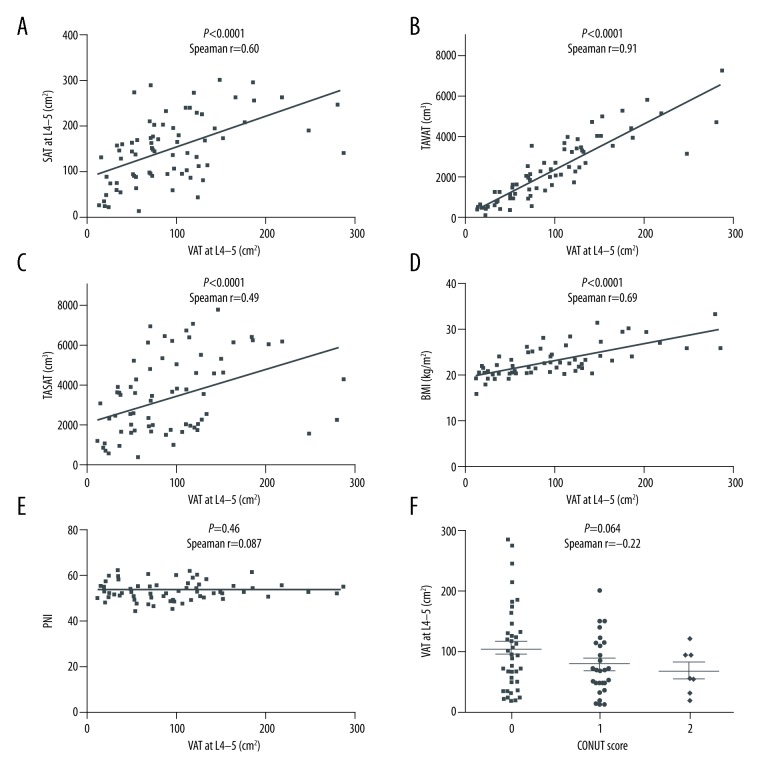
We evaluated the correlation between VAT area at L4–5 and other abdominal adipose tissue parameters and nutrition indices. VAT area at L4–5 was significantly correlated with SAT area at L4–5, TAVAT volume, and TASAT volume (Figure 4A; P<0.0001, Figure 4B; P<0.0001, Figure 4C; P<0.0001, respectively). These results suggest that abdominal VAT area is closely related to abdominal SAT area, and VAT area at L4–5 could be a surrogate marker for TAVAT volume and TASAT volume. VAT area at L4–5 was also significantly correlated with BMI (Figure 4D; P<0.0001). Thus, VAT area at L4–5 could also be a surrogate marker for BMI. In contrast, VAT area at L4–5 was not correlated with PNI and CONUT scores (Figure 4E; P=0.46, Figure 4F; P=0.064, respectively).
Figure 4.
The correlation between preoperative VAT area at L4–5 and other abdominal adipose tissue parameters, including BMI and nutrition indices. VAT area at L4–5 was significantly correlated with SAT area at L4–5 (A), TAVAT volume (B), TASAT volume (C), and BMI (D). In contrast, there was no significant correlation between VAT area at L4–5 and nutrition indices (E, PNI; F, CONUT score).
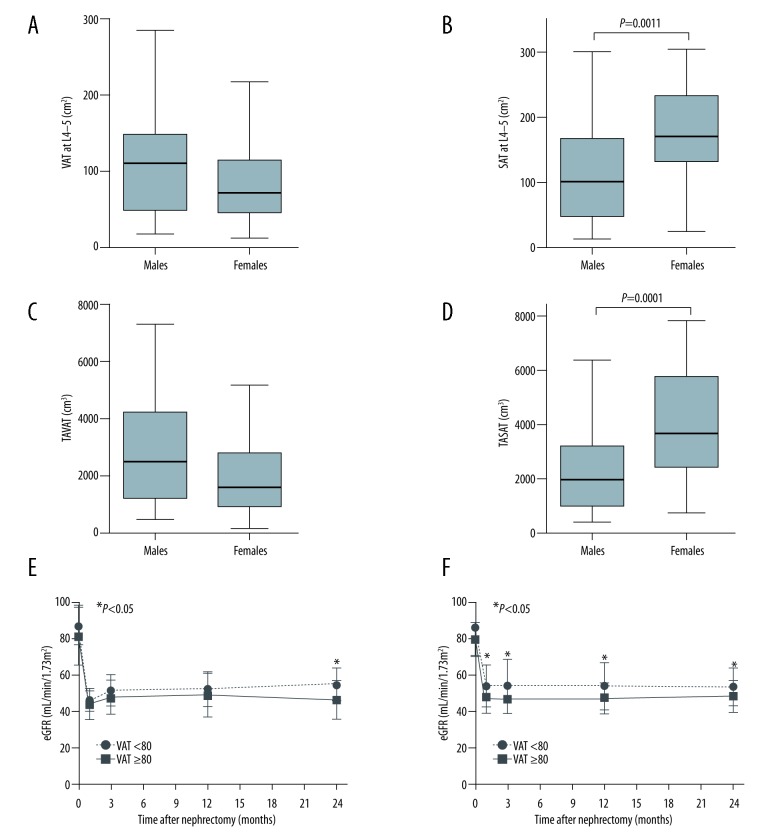
Sex difference in the distribution of abdominal adipose tissue and renal function
We evaluated the difference of the abdominal adipose tissue between males and females. VAT area at L4–5 and TAVAT volume tended to increase in males compared with females but the difference did not reach to statistical significance (Figure 5A; P=0.068, Figure 5C; P=0.085, respectively). On the other hand, SAT area at L4–5 and TASAT volume were significantly increased in females compared with males (Figure 5B; P=0.0011, Figure 5D; P=0.0001, respectively). With regard to renal function, there were no significant differences in preoperative renal function between donors with a VAT area at L4–5 <80 and those with a VAT area at L4–5 ≥80 in both males and females. In males, postoperative renal function of donors with a VAT area at L4–5 ≥80 significantly decreased compared with that of donors with a VAT area at L4–5 <80 at 24 months after donor nephrectomy (Figure 5E; P=0.045). In females, postoperative renal function of donors with a VAT area at L4–5 ≥80 also significantly decreased compared with that of donors with a VAT area at L4–5 <80 at discharge, 3, 12, and 24 months after donor nephrectomy (Figure 5F; P=0.043, P=0.046, P=0.037, and P=0.048, respectively). Although postoperative renal function was affected by preoperative VAT area at L4–5 in both males and females, postrenal function in females was strongly affected by VAT.
Figure 5.
Comparison of abdominal adipose tissue distribution and renal function between males and females. Male donors were tended to increase in a VAT area at L4–5 (A), but female donors significantly increased in a SAT area at L4–5 (B). Similar to the relationship between VAT and SAT area, male donors tended to increase in TAVAT volume (C) and female donors significantly increased in TASAT volume (D). With regard to renal function, postrenal function in male donors with a VAT area at L4–5 ≥80 was significantly decreased at 12 months compared with male donors with a VAT area at L4–5 <80 (E) and postrenal function in female donors with a VAT area at L4–5 ≥80 was significantly decreased at discharge, 3, 12, and 24 months compared with female donors with a VAT area at L4–5 <80 (F).
Univariate and multivariate analysis of prognostic factors for the development of CKD G3b 12 months after donor nephrectomy
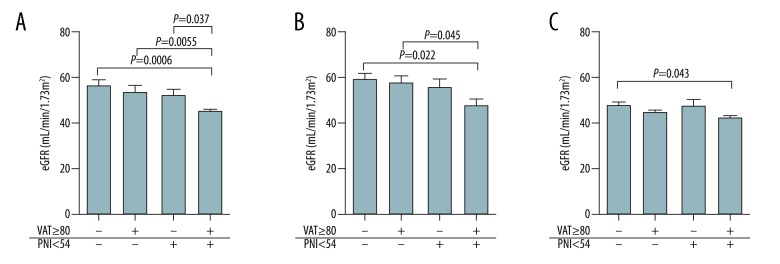
To explore the prognostic impact of preoperative VAT area at L4–5 on renal function, we performed univariate analysis followed by multivariate analysis (Table 3). An event was defined as development of CKD G3b 12 months after donor nephrectomy, and TAVAT volume and TASAT volume were omitted from the candidate factors because these 2 factors were closely correlated with VAT area at L4–5, as described above. The univariate analysis revealed that age greater than 60 years and VAT area at L4–5 ≥80 predicted poor prognosis in terms of development of CKD G3b (odds ratio [OR]=3.2, 95% confidence interval [CI]=1.2–8.5; P=0.028; OR=3.1, 95%CI=1.1–8.2; P=0.032, respectively). BMI ≥22 and SAT area at L4–5 ≥170 were not statistically significant prognostic factors, but tended to predict poor prognosis. Hypertension was not a significant prognostic factor. In contrast, preoperative eGFR ≥80 and PNI ≥54 were positive prognostic factors (OR=0.2, 95%CI=0.08–0.6; P=0.0033; OR=0.3, 95%CI=0.09–0.8; P=0.015, respectively). The multivariate analysis also revealed that a VAT area at L4–5 ≥80 was an independent negative prognostic factor (OR=3.8, 95%CI=1.2–11.6; P=0.021). In addition, a preoperative eGFR ≥80 and PNI ≥54 were independent positive prognostic factors (OR=0.2, 95%CI=0.08–0.7; P=0.011; OR=0.3, 95%CI=0.08–0.8; P=0.025, respectively). The postoperative eGFR of donors with a VAT area at L4–5 ≥80 and PNI <54 was significantly lower than those of donors with a VAT area at L4–5 <80 and/or PNI ≥54, suggesting that it is important to achieve at least a VAT area at L4–5 <80 or a PNI ≥54 preoperatively (Figure 6A). In subgroup analysis, postoperative eGFR of donors with a VAT area at L4–5 ≥80 and a PNI <54 was also significantly lower than that of donors with a VAT area at L4–5 <80 and/or a PNI ≥54 in subgroups with both preoperative eGFR ≥80 and <80 (Figure 6B, 6C).
Table 3.
Logistic regression analysis of prognostic factors for the development of CKD G3b 12 months after donor nephrectomy.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age | <60 | 1 | 1 | ||||
| ≥60 | 3.2 | 1.2–8.5 | 0.028 | 2.1 | 0.6–6.6 | 0.22 | |
| Gender | Male | 1 | |||||
| Female | 0.4 | 0.2–1.1 | 0.09 | ||||
| BMI | <22 | 1 | |||||
| ≥22 | 2.6 | 0.9–7.4 | 0.086 | ||||
| VAT at L4–5 | <80 | 1 | 1 | ||||
| ≥80 | 3.1 | 1.1–8.2 | 0.032 | 3.8 | 1.2–11.6 | 0.021 | |
| SAT at L4–5 | <170 | 1 | |||||
| ≥170 | 2.8 | 1.0–7.3 | 0.051 | ||||
| Hypertension | No | 1 | |||||
| Yes | 2.5 | 0.7–8.2 | 0.2 | ||||
| Preoperative eGFR | <80 | 1 | 1 | ||||
| ≥80 | 0.2 | 0.08–0.6 | 0.0033 | 0.2 | 0.08–0.7 | 0.011 | |
| PNI | <54 | 1 | 1 | ||||
| ≥54 | 0.3 | 0.09–0.8 | 0.015 | 0.3 | 0.08–0.8 | 0.025 | |
| CONUT | 0 | 1 | |||||
| ≥1 | 1.2 | 0.5–3.1 | 0.81 | ||||
OR – odds ratio; CI – confidence interval; BMI – body mass index; VAT – visceral adipose tissue; SAT – subcutaneous adipose tissue; eGFR – estimate glomerular filtration rate; PNI – prognostic nutritional index; CONUT – controlling nutritional status; Logistic regression analysis.
Figure 6.
Comparison of renal function at 12 months according to risk classification. Postoperative renal function of donors with a VAT area at L4–5 ≥80 and a PNI <54 was significantly lower than that of donors with a VAT area at L4–5 <80 and a PNI ≥54, a VAT area at L4–5 ≥80 and a PNI ≥54, and a VAT area at L4–5 <80 and a PNI ≥54 (A). In donors with a preoperative eGFR ≥80, postoperative renal function of those with a VAT area at L4–5 ≥80 and a PNI <54 was significantly lower than postoperative renal function of those with a VAT area at L4–5 <80 and a PNI ≥54 (B). The same result was observed in donors with a preoperative eGFR <80 (C).
Discussion
The present study shows that preoperative VAT area at L4–5 and PNI can predict postoperative renal function and may be prognostic factors for CKD G3b at 12 months after donor nephrectomy. Preoperative VAT area at L4–5 correlates with other abdominal adipose tissue parameters, such as SAT area at L4–5, TAVAT volume, and TASAT volume. Preoperative VAT area at L4–5 also correlates with BMI, and donors with a preoperative VAT area at L4–5 ≥80 tend to have a high BMI. Although BMI is generally recognized as the marker for obesity, VAT area at L4–5 can also be a surrogate marker for obesity. In the present study, VAT area at L4–5 was a stronger prognostic marker for postoperative renal function than BMI. In addition, donors with preoperative hypertension tended to have a VAT area at L4–5 ≥80, suggesting that obesity is a risk factor for hypertension. Excessive abdominal adipose tissue makes it difficult to perform donor nephrectomy, resulting in a long operative time.
Calculating from the approximate curve of the association between VAT area at L4–5 and BMI, VAT >80 means BMI >23. Body mass is composed of various elements, including fat, muscle, bone, visceral tissues, and water. We speculate that visceral adipose tissue itself has a role in damaging residual kidney function. Therefore, we should pay attention not only to BMI, but also to visceral adipose tissue when performing donor selection.
It is well known that obesity, which is a factor of metabolic syndrome, is closely correlated with hypertension, diabetes, and glomerular hyperfiltration, resulting in the development of CKD. VAT plays a key role in the development of these diseases [10–12,23]. Although BMI is commonly used to assess the degree of obesity, Rankinen et al. provided evidence indicating that prediction of distribution of abdominal adipose tissue based on BMI is inaccurate [13]. In the present study, we measured various adipose tissue parameters using the Volume Analyzer SYNAPSE VINCENT image analysis system and investigated their associations with postoperative renal function. The adipose tissue parameters were obtained from preoperative CT scans acquired during screening or examination of vascular structure, without additional inconvenience to the patient. Preoperative renal function tended to be lower in donors with a VAT area at L4–5 ≥80, but the difference did not reach the level of statistical significance. After donor nephrectomy, renal function of donors with a VAT area at L4–5 ≥80 was significantly lower than that of donors with a VAT area at L4–5 <80, and this difference remained significant for at least 2 years. In addition, although postrenal function of female donors with a VAT area at L4–5 ≥80 was significantly lower than that of female donors with a VAT area at L4–5 <80, the difference did not persist over time. On the other hand, renal function of male donors with a VAT area at L4–5 ≥80 was gradually decreased and a significant difference was observed at 24 months after donor nephrectomy. This result suggests that preoperative VAT area could be a prognostic factor for postoperative renal function. This association may reflect a direct effect, since VAT, which is not resected, with an exception of the flank pad, during donor nephrectomy, may influence the remaining contralateral residual kidney via inflammatory cytokines and adipokines such as interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), leptin, and adiponectin or via insulin resistance [24,25]. Males tended to increase in abdominal visceral adipose tissue and this may strongly affect the long-term difference in renal function. Importantly, our results revealed that VAT area could potentially be a better prognostic factor for CKD than BMI. Thus, medical personnel involved in renal transplantation should educate donors regarding the importance of preoperative diet or exercise aimed at reducing VAT in addition to BMI. This could lead to an improvement in donor’s renal function postoperatively, resulting in a better prognosis.
With regard to nutritional status, this study suggests that PNI could be a predictor of postoperative renal function. Originally, PNI was proposed as an index to determine the feasibility of resection and anastomosis of the gastrointestinal tract. This procedure is considered safe in patients with a PNI of 45 or more, can be risky in patients with a PNI of 40–45, and is contraindicated to patients with a PNI <40. In addition, PNI could be a prognostic factor of survival in cancer [19,24,26]. Generally, living donors are healthy and have good nutritional status. The lowest PNI of 45 and the highest CONUT score of 2 in this study reflect this fact. Our results suggest that a better nutritional status, which corresponds to a higher PNI, has a positive effect on postoperative renal function. Since PNI was calculated based on serum albumin concentration and peripheral blood lymphocyte count, it also reflects the immune status [19]. Although the current study does not provide an exact mechanism, a high concentration of serum albumin could suppress the action of cytokines or adipokines, allowing the immune systems to maintain healthy homeostasis. There was no correlation between PNI and abdominal adipose tissue parameters in this study. It is therefore likely that a good preoperative immune status leads to an acceptable renal function after donor nephrectomy. A good immune status might play an important role in protecting residual renal function through suppression of adipocytes or other immune-related cells, which negatively impact renal function at high concentrations.
This study identified VAT area at L4–5 and PNI as new predictors of postoperative renal function in living donors. Although these 2 factors are not correlated with each other, postoperative renal function of donors with a VAT area at L4–5 <80 and PNI ≥54 tended to be better than that of donors with a VAT area at L4–5 <80 alone or PNI ≥54 alone. Obesity in living donors results in a high risk of hypertension development and reduction of eGFR after donor nephrectomy [27]. Generally, individuals with a BMI >30 are rare in Japan. Thus, VAT area could be a new surrogate marker of obesity in Japan, and, more broadly, in Asia. Obesity and poor nutritional status could be a consequence of eating habits, and donors tend to maintain their eating habits after the surgery [28]. This emphasizes the need to establish and promote population-specific nutritional regimens after donor nephrectomy to improve renal protection in living donors. Moreover, a specific physical activity regimen consisting of moderate-intensity exercise, which is easy to use both preoperatively and postoperatively, should also be established for living donors. These recommendations, if implemented, could improve prognosis through protection of renal function and suppression of cardiovascular and cerebrovascular diseases in living donors.
This study has some limitations. The patient data were obtained from a single institution, and the sample size was small. The study had a retrospective design, resulting in potential selection bias, and the follow-up period was relatively short. The optimal cutoffs need to be validated using an independent multiinstitutional sample to establish a novel risk assessment tool specific to living donors. In addition, some experiments, including evaluation of cytokines/adipokines levels, are needed in order to elucidate the scientific basis of these results.
Conclusions
We reveal that preoperative distribution of abdominal adipose tissue and nutritional status could be predictors of postoperative renal function in living donors. Therefore, management of preoperative VAT and nutritional status could lead to better outcomes in living donors. Further research is needed to develop appropriate exercise protocols and nutritional interventions to improve the prognosis of living, originally healthy donors.
Acknowledgments
The authors would like to thank the patients for their important contribution to this study. We also thank Mariko Yoshimura (Department of Urology, Nara Medical University, Nara, Japan) for invaluable help with collecting and summarizing the data.
Abbreviations
- ESRD
end-stage renal disease
- CKD
chronic kidney disease
- BMI
body mass index
- PNI
prognostic nutritional index
- CONUT
controlling nutritional status
- CT
computed tomography
- VAT
visceral adipose tissue
- L4–L5
at the level of the fourth and fifth lumbar vertebra
- eGFR
estimated glomerular filtration rate
- KDIGO
the Kidney Disease: Improving Global Outcomes
- SAT
subcutaneous adipose tissue
- TAVAT
total abdominal visceral adipose tissue
- TASAT
total abdominal subcutaneous adipose tissue
- IQR
interquartile range
- OR
odds ratio
- CI
confidence interval
- IL
interleukin
- TNF-α
tumor necrosis factor alpha
Footnotes
Source of support: Departmental sources
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Lentine KL, Lam NN, Axelrod D, et al. Perioperative complications after living kidney donation: A national study. Am J Transplant. 2016;16(6):1848–57. doi: 10.1111/ajt.13687. [DOI] [PubMed] [Google Scholar]
- 3.Johnson EM, Remucal MJ, Gillingham KJ, et al. Complications and risks of living donor nephrectomy. Transplantation. 1997;64(8):1124–28. doi: 10.1097/00007890-199710270-00007. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto M, Akioka K, Nobori S, et al. Short-and long-term donor outcomes after kidney donation: Analysis of 601 cases over a 35-year period at Japanese single center. Transplantation. 2009;87:419–23. doi: 10.1097/TP.0b013e318192dc95. [DOI] [PubMed] [Google Scholar]
- 5.Yasumura T, Nakai I, Oka T, et al. Experience with 247 living related donor nephrectomy cases at a single institution in Japan. Jpn J Surg. 1988;18:252–58. doi: 10.1007/BF02471441. [DOI] [PubMed] [Google Scholar]
- 6.Imai N, Shibagaki Y, Yazawa M, et al. Follow-up rates of living kidney donor in Japan: A single center study. Indian J Nephrol. 2016;26(6):423–26. doi: 10.4103/0971-4065.172229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehrman-Ekholm I, Elinder CG, Stenbeck M, et al. Kidney donors live longer. Transplantation. 1997;64(7):976–78. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Saran R, Marshall SM, Madsen R, et al. Long-term follow-up of kidney donors: A longitudinal study. Nephrol Dial Transplant. 1997;12(8):1615–21. doi: 10.1093/ndt/12.8.1615. [DOI] [PubMed] [Google Scholar]
- 9.El-Agroudy AE, Sabry AA, Wafa EW, et al. Long-term follow-up of living kidney donors: A longitudinal study. BJU Int. 2007;100(6):1351–55. doi: 10.1111/j.1464-410X.2007.07054.x. [DOI] [PubMed] [Google Scholar]
- 10.Stenvinkel P, Zoccali C, Ikizler TA. Obesity in CKD – what should nephrologists know? J Am Soc Nephrol. 2013;24(11):1727–36. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu CY, Iribarren C, McCulloch CE, et al. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–50. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwahara K, Uehara A, Yamamoto M, et al. Current status of health among workers in Japan: Results from the Japan Epidemiology Collaboration on Occupational Health Study. Ind Health. 2016;54(6):505–14. doi: 10.2486/indhealth.2016-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rankinen T, Kim SY, Pérusse L, et al. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23(8):801–9. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366(9497):1640–49. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 15.Chandie Shaw PK, Berger SP, Mallat M, et al. Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care. 2007;30(7):1840–44. doi: 10.2337/dc07-0028. [DOI] [PubMed] [Google Scholar]
- 16.Pinto-Sietsma SJ, Navis G, Janssen WM, et al. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41(4):733–41. doi: 10.1016/s0272-6386(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morozumi K, Ichimaru N, Katayama A, et al. Japanese living donor guideline for kidney transplantation. The Japan Society for Transplantation. 2014. Available at: http://www.asas.or.jp/jst/pdf/manual/008.pdf.
- 19.Mohri Y, Inoue Y, Tanaka K, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688–92. doi: 10.1007/s00268-013-2156-9. [DOI] [PubMed] [Google Scholar]
- 20.Iseki Y, Shibutani M, Maeda K, et al. Impact of the preoperative Controlling Nutritional Status (CONUT) Score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(7):e0132488. doi: 10.1371/journal.pone.0132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 23.Bosma RJ, van der Heide JJ, Oosterop EJ, et al. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int. 2004;65(1):259–65. doi: 10.1111/j.1523-1755.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 24.Miyake M, Morizawa Y, Hori S, et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer. 2017;17(1):237. doi: 10.1186/s12885-017-3231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyun YY, Lee KB, Oh KH, et al. Serum adiponectin and protein-energy wasting in predialysis chronic kidney disease. Nutrition. 2017;33:254–60. doi: 10.1016/j.nut.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5. [PubMed] [Google Scholar]
- 27.Nogueira JM, Weir MR, Jacobs S, et al. A study of renal outcomes in obese living kidney donors. Transplantation. 2010;90(9):993–99. doi: 10.1097/TP.0b013e3181f6a058. [DOI] [PubMed] [Google Scholar]
- 28.Phillips S, DeMello S. Nutrition and the kidney donor. J Ren Nutr. 2014;24(2):e15–17. doi: 10.1053/j.jrn.2013.12.001. [DOI] [PubMed] [Google Scholar]