Abstract
Objective
Childhood body mass index (BMI) has been related to vascular structure and function. However, little is known about the differing contributions of fat and lean mass to this relationship. Our objectives were to relate the fat and lean mass (bone excluded) components of BMI (fat mass index (FMI) and lean mass index (LMI); mass (kg)/height (m)2) to vascular measures in pre-pubertal children.
Approach and Results
In the UK Southampton Women’s Survey mother-offspring cohort, 983 children had dual x-ray absorptiometry and vascular measurements at 8-9 years. Using linear regression analyses, we found that most vascular measures were related to BMI, but fat and lean mass contributed differently. Systolic blood pressure was positively associated with both FMI (β=0.91 (95% CI: 0.52, 1.30) mm/Hg) and LMI (β=2.16 (1.47, 2.85) mmHg), while pulse rate was positively associated with FMI (β=0.93 (0.48, 1.38) b/min), but negatively associated with LMI (β=-1.79 (-2.59, -0.99) b/min). The positive relation between BMI and carotid intima media thickness was mainly due to a positive association with LMI (β=0.013 (0.008, 0.019) mm). Carotid-femoral pulse wave velocity (PWV), but not carotid-radial PWV, was positively associated with FMI (β=0.06 (0.03, 0.09) m/s). For systolic blood pressure, carotid-femoral PWV and reactive hyperemia significant interactions indicated that the association with fat mass depended on the amount of lean mass.
Conclusions
In pre-pubertal children, differences in vascular structure and function in relation to BMI probably represent combinations of adverse effects of fat mass, adaptive effects of body size and relatively protective effects of lean mass.
Keywords: Body composition, children, vascular measures, blood pressure, intima media thickness, pulse wave velocity, endothelial function
Subject codes: Vascular biology, developmental biology, epidemiology, obesity, pediatrics, blood pressure
Introduction
In adults, measures of vascular structure and function are strong markers for risk of later cardiovascular disease (CVD).1–5 For CVD prevention, it is important to identify vascular damage at the earliest possible stage, and intervene, by reducing cumulative exposure to risk factors. Overweight and obesity are related to increased risk of CVD.6–8 Changes in several vascular CVD risk markers have been observed in children in relation to obesity.9 These include non-specific markers such as increased systolic and diastolic blood pressure (BP) and pulse rate,10–14 but also structural changes in large arteries, such as carotid intima-media thickness (cIMT), carotid distensibility or pulse wave velocity (PWV).15–17 Furthermore, variations in endothelial function measures, including flow-mediated dilatation (FMD) and reactive hyperemia, have also been observed in children and young adults.15, 18 However, there remains uncertainty about whether relations with BMI and obesity in children reflect early pathological changes or mainly represent physiological adaptations to growth and development.15
Body mass index (BMI) is often used as a proxy measure for adiposity, i.e. fat mass, although increased lean mass (the combination of muscle and internal organs) also leads to an increase in BMI. If fat and lean mass are similarly associated with vascular risk markers, then BMI will predict these markers at least as well as these components of body composition separately. However, if fat and lean mass relate to vascular risk markers in different ways, or the impact of fat mass depends on the amount of lean mass, then analyses of BMI in relation to CVD risk in children may be misleading. This could have implications for preventive measures in early life.
Several studies of single or small groups of vascular structure and function measures in relation to BMI and obesity have been conducted in children,9, 10, 12, 15–22 but an extensive range of simultaneous measurements on children before puberty has not been obtained. We therefore made comprehensive measures of vascular structure and function in a well-characterized prospective cohort of 8-9-year old children. We investigated:
-
1)
associations of vascular structure and function with BMI;
-
2)
associations of vascular structure and function with the fat and lean mass components of BMI;
-
3)
whether the associations between fat mass and CVD risk markers depended on the amount of lean mass, i.e. interactions between lean and fat mass.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study population
The Southampton Women’s Survey is an ongoing, prospective cohort study of 12 583, initially non-pregnant, women aged 20–34 years, living in the city of Southampton, UK.23 Assessments of lifestyle, diet and anthropometry were performed at study entry (April 1998–December 2002). Women who subsequently became pregnant were followed through pregnancy and their offspring through infancy and childhood. Here we focus on the follow-up of the children aged 8-9 years.
Exposure variables
At age 8-9 years, height was measured to the nearest 0.1 cm with a portable stadiometer (Leicester height measure; Seca); weight was measured to the nearest 0.1 kg using calibrated digital scales (Seca). Data were used to calculate BMI (weight (kg)/ height (m)2). Body composition was assessed by dual X-ray absorptiometry (DXA) by a bone densitometry technician using a Hologic Discovery instrument (Hologic Inc., Bedford, MA, USA). Fat and lean mass were derived from a whole body scan, using pediatric software. The total radiation dose for each scan was 4.7 microsieverts (pediatric scan mode). Fat mass index (FMI) was calculated as fat mass (kg) / height (m)2 and lean mass index (LMI) was calculated as non-fat mass (minus bone) (kg) / height (m)2. Hence, these two measures represents the fat- and lean mass components of BMI.
Outcome variables
Trained clinical personnel (research nurses, cardiac physiologists and vascular ultrasonographers) performed a wide range of vascular measures. Systolic and diastolic BP and pulse rate, using a Dinamap Critikon 8100 monitor and carotid IMT and distensibility, using a Philips iE33 dedicated vascular ultrasound system (Philips Healthcare, Guilford, UK) were measured once after 10-15 min rest, with the child supine with their head turned 45 degrees to the right of the midline. The left carotid artery was imaged just proximal to the carotid bifurcation, in a longitudinal section, with the vessel horizontally positioned on screen. A video-captured sequence was acquired over 10 seconds, which included at least 4 cardiac cycles. The sequence should show a clearly defined intima on both anterior and posterior aspects of the vessel. Immediately after acquiring the optimum trace, the BP cuff (upper arm, same side) was inflated and systolic and diastolic BP and pulse rate were noted in mmHg and beats/min respectively. To calculate carotid IMT and distensibility the images and traces were analyzed using LIA software at a remote PC. cIMT was measured in mm and was calculated using the frame showing the minimum diastole, where the IMT structure would not be compressed by blood flow in the vessel. The maximum diameter was noted. This process was repeated three times and the software used the mean of these measures in the analysis. Carotid distensibility was determined by measuring the luminal diameter excursion from diastole to systole. The distensibility coefficient (cDC), which reflects intrinsic vascular wall elasticity, was calculated using lumen diameters (D) and blood pressures (P) in the equation ((2xΔDxD)+ ΔD2)/(ΔPxD2) to reflect relative change in cross-sectional area per 10-3 kPa change in BP from systole to diastole.24
Arterial stiffness was recorded transcutaneously as the velocity of the pulse wave between two arterial pulse sites (pulse wave velocity (PWV)), using a Vicorder device (Skidmore Medical Limited, Bristol, UK). In this study PWV was assessed over two different pathways (carotid-femoral and carotid-radial). The child rested supine for 10-15 min, lying on a couch with the upper body tilted in a 30 degree angle. Real-time pulse-wave measures were recorded by placing sensor cuffs (left carotid suprasternal notch, the upper left thigh and left wrist), with the time delay between the two simultaneously measured cardiac cycles measured. Integral software processed the data to calculate the mean time difference between R-wave and pressure wave on a beat-to-beat basis over 10 s, and the PWV was then calculated using the mean time difference and arterial path length between the two recording points. The mean of up to four PWV measurements was calculated.
Measurements of flow mediated dilatation (FMD) and reactive hyperemia were obtained from the right brachial artery, 5–10 cm above the antecubital fossa, using high-resolution ultrasound (Acuson Aspen Ultrasound System, Mountain View, CA; USA) with a linear probe, attached to a computer with LIA capture facilities, with the probe held in a stereotactic clamp that allowed micrometer positional adjustment. First, the brachial artery was imaged (baseline). Brachial artery FMD was then induced by a 5 min inflation of a pneumatic cuff to 200 mmHg, around the forearm immediately below the medial epicondyle, followed by rapid deflation using an automatic air regulator device. Brachial artery diameter was measured using edge detection software (Brachial Tools, MIA, IA, USA) from ECG-triggered ultrasound images captured at 3 s intervals throughout the 11 min recording protocol. Flow-mediated dilatation was expressed as the maximum percentage change in vessel diameter from baseline. In parallel, the magnitude of the flow stimulus (Velocity Time Integral (VTI)) and heart rate (HR) were recorded continuously by pulse wave Doppler. Reactive hyperaemia is expressed as percent change in flow, from baseline to maximum flow within 15 s of cuff deflation (RH%), and was calculated as [((VTI peak x HR peak) – (VTI baseline x HR baseline)) / (VTI baseline x HR baseline)] x 100.
Covariates
We used a Directed Acyclic Graph (Supplemental Figure I) to determine confounders to adjust for in the models. These were child sex, age, time spent doing sports activities (hours per week, by maternal interview) and socioeconomic status (as determined by maternal educational level in six categories from none to university degree or above).
Ethics
The SWS study was conducted according to the guidelines laid down in the Declaration of Helsinki and was approved by the Southampton and South West Hampshire Local Research Ethics Committee (08/H0502/95). Written informed consent was obtained from all participating women and by a parent or guardian with parental responsibility on behalf of each child.
Statistical analyses
Statistical analyses were performed using Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.). Descriptive variables were summarized as frequencies with proportions (%), means with standard deviation (SD) or medians with interquartile ranges (IQR), and differences between boys and girls were explored using Chi-Squared tests for categorical variables and t-tests or Mann-Whitney rank sum tests for normally and non-normally distributed continuous variables respectively (Table 1). We assessed associations between BMI and the outcome variables using linear regression models for each outcome separately (Table 2), first univariately and then adjusted for confounders. Last, we replaced BMI in the regression models with FMI and LMI (Table 3), first exploring univariate associations with FMI and LMI (Model 0), then in models just including FMI and confounders (Model 1), in models just including LMI and confounders (Model 2) and finally in models including both FMI and LMI and confounders (Model 3). In supplementary models interactions between the two main exposures (lean mass and fat mass indices) were assessed by adding a multiplicative interaction term. Interactions are illustrated in figure 1-3, showing marginal means from the linear regression model including an interaction term between the continuous variables fat mass index and lean mass index, adjusted for confounders. For illustrative purposes, in the graphs, LMI was fixed at 11.4, 11.9, 12.4 and 13.1 kg/m2 and FMI was fixed at 2.9, 3.7, 4.6 and 5.8 kg/m2. These cutpoints are quintiles (i.e. values that divide the range of data into five equal parts). Interactions between sex and the main exposures were explored both by running the fully-adjusted analyses, including interactions between the exposure and sex, and by running the analyses stratified by sex, describing very similar results in boys and girls (Supplemental Figures II and III). Final regression models were therefore run using the total sample.
Table 1.
Maternal and child characteristics and vascular outcomes at age 8-9 years, by child sex. Numbers are mean (SD), median (IQR) or n (%). Differences between boys and girls are tested by unpaired t-tests, Mann-Whitney rank sum tests or chi-square tests.
| Boys (n=496) |
Girls (n=487) |
p |
|
|---|---|---|---|
| Maternal factors | |||
| Educational level | 0.3 | ||
| None | 8 (2%) | 11 (2%) | |
| CSE | 34 (7%) | 43 (9%) | |
| O-levels | 129 (26%) | 131 (27%) | |
| A-levels | 168 (34%) | 134 (28%) | |
| HND | 32 (6%) | 38 (8%) | |
| Degree | 125 (25%) | 128 (26%) | |
| Primiparous | 264 (53%) | 257 (53%) | 0.9 |
| Pre-pregnant smoking | 112 (23%) | 115 (24%) | 0.7 |
| Pre-pregnant BMI (kg/m2) | 24.4 (22.0, 27.6) | 23.9 (21.8, 27.0) | 0.2 |
| Child explanatory factors | |||
| Age (years) | 9.2 (0.2) | 9.2 (0.3) | 0.5 |
| Time spent doing sports (h/wk) | 2.5 (1.0, 5.0) | 2 (0.5, 4.0) | <0.001 |
| Anthropometric measures | |||
| Height (cm) | 135.8 (5.7) | 135.3 (6.3) | 0.2 |
| Weight (kg) | 29.5 (26.7, 33.5) | 30.5 (27.1, 35.2) | 0.03 |
| BMI (kg/m2) | 16.1 (14.9, 17.6) | 16.5 (15.2, 18.6) | <0.001 |
| BMI z-score (UK-WHO)* | 0.03 (1.16) | 0.12 (1.13) | |
| DXA-measures | |||
| Total fat mass (kg) | 6.3 (4.9, 8.6) | 8.6 (6.8, 11.5) | <0.001 |
| Total lean mass (kg) | 23.2 (3.1) | 22.1 (3.3) | <0.001 |
| Fat mass index (kg/m2) | 3.5 (2.7, 4.6) | 4.8 (3.8, 6.2) | <0.001 |
| Lean mass index (kg/m2) | 12.6 (1.0) | 12.0 (1.2) | <0.001 |
| Child vascular outcomes | |||
| Systolic BP (mmHg) | 105.4 (9.1) | 107.8 (9.6) | <0.001 |
| Diastolic BP (mmHg) | 54.3 (7.4) | 57.6 (7.8) | <0.001 |
| Pulse rate (bpm) | 71.9 (9.6) | 75.4 (10.4) | <0.001 |
| cIMT (mm) | 0.496 (0.067) | 0.486 (0.076) | 0.06 |
| cDC (mm/10-3kPa) | 64.8 (23.3) | 68.2 (24.4) | 0.04 |
| Carotid-femoral PWV (m/s) | 4.55 (0.55) | 4.64 (0.60) | 0.05 |
| Carotid-radial PWV (m/s) | 7.12 (1.02) | 7.10 (0.89) | 0.8 |
| FMD (%) | 6.50 (3.36) | 7.15 (4.09) | 0.02 |
| Reactive hyperemia (%) | 533 (235) | 586 (252) | 0.004 |
Mean BMI z-scores according to the modified UK-WHO BMI reference, adjusted for sex and age.
Table 2.
Associations between child BMI (exposure) and nine vascular measures (outcomes) at age 8-9 years. Numbers are regression coefficients (β (95% CI)) for the associations between child BMI (kg/m2) and each of the vascular outcomes.
| Univariate | Adj. model* | |||
|---|---|---|---|---|
| β (95% CI) | p | Adj β (95% CI) | p | |
| Systolic BP (mmHg) | 1.41 (1.18, 1.63) | <0.001 | 1.33 (1.11, 1.56) | <0.001 |
| Diastolic BP (mmHg) | 0.46 (0.26, 0.65) | <0.001 | 0.40 (0.20, 0.60) | <0.001 |
| Pulse rate (bpm) | 0.24 (-0.02, 0.50) | 0.08 | 0.14 (-0.12, 0.40) | 0.3 |
| cIMT (mm) | 0.003 (0.001, 0.004) | 0.005 | 0.003 (0.001, 0.005) | 0.003 |
| cDC (mm/10-3kPa) | -0.90 (-1.51, -0.28) | 0.004 | -0.94 (-1.56, 0.31) | 0.003 |
| Carotid-femoral PWV (m/s) | 0.04 (0.02, 0.05) | <0.001 | 0.03 (0.02, 0.05) | <0.001 |
| Carotid-radial PWV (m/s) | -0.03 (-0.06, 0.00) | 0.03 | -0.03 (-0.06, -0.01) | 0.01 |
| FMD (%) | 0.10 (-0.01, 0.21) | 0.07 | 0.09 (-0.02, 0.20) | 0.1 |
| Reactive hyperemia (%) | -3 (-10, 4) | 0.4 | -4 (-11, 3) | 0.3 |
Adjusted for socioeconomic status (maternal educational level) and child sex, age and hours doing sports/week.
Table 3.
Associations between child body composition (fat mass index (FMI) and lean mass index (LMI) (exposures)) and nine CVD risk markers (outcomes) at age 8-9 years. Numbers are regression coefficients (β (95% CI)) for the associations between child FMI and LMI (kg/m2) and each of the vascular outcomes.
| Model 0 * | Model 1 † | Model 2 ‡ | Model 3 § | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | Adj β (95% CI) | p | Adj β (95% CI) | p | Adj β (95% CI) | p | |
| Systolic BP (mmHg)* | ||||||||
| FMI (kg/m2) | 1.64 (1.33, 1.96) | <0.001 | 1.58 (1.25, 1.92) | <0.001 | 0.91 (0.52, 1.30) | <0.001 | ||
| LMI (kg/m2) | 2.56 (2.00, 3.12) | <0.001 | 3.05 (2.46, 3.63) | <0.001 | 2.16 (1.47, 2.85) | <0.001 | ||
| Diastolic BP (mmHg) | ||||||||
| FMI (kg/m2) | 0.73 (0.46, 0.99) | <0.001 | 0.53 (0.25, 0.81) | <0.001 | 0.42 (0.08, 0.75) | 0.02 | ||
| LMI (kg/m2) | 0.29 (-0.19, 0.76) | 0.2 | 0.77 (0.26, 1.27) | 0.003 | 0.36 (-0.24, 0.96) | 0.2 | ||
| Pulse rate (bpm) | ||||||||
| FMI (kg/m2) | 0.70 (0.34, 1.06) | <0.001 | 0.37 (-0.01, 0.75) | 0.06 | 0.93 (0.48, 1.38) | <0.001 | ||
| LMI (kg/m2) | -1.31 (-1.95, -0.68) | <0.001 | -0.88 (-1.56, -0.21) | 0.01 | -1.79 (-2.59, -0.99) | <0.001 | ||
| Carotid IMT (mm) | ||||||||
| FMI (kg/m2) | 0.001 (-0.001, 0.004) | 0.4 | 0.002 (-0.001, 0.004) | 0.2 | -0.002 (-0.005, 0.001) | 0.1 | ||
| LMI (kg/m2) | 0.011 (0.007, 0.016) | <0.001 | 0.011 (0.006, 0.016) | <0.001 | 0.013 (0.008, 0.019) | <0.001 | ||
| Carotid DC (mm/10-3 kPa) | ||||||||
| FMI (kg/m2) | -0.69 (-1.54, 0.16) | 0.1 | -1.02 (-1.93, -0.12) | 0.03 | -0.48 (-1.56, 0.60) | 0.4 | ||
| LMI (kg/m2) | -2.58 (-4.08, -1.09) | 0.001 | -2.22 (-3.83, -0.61) | 0.007 | -1.75 (-3.68, 0.18) | 0.08 | ||
| Carotid-femoral PWV (m/s)* | ||||||||
| FMI (kg/m2) | 0.06 (0.04, 0.08) | <0.001 | 0.06 (0.03, 0.08) | <0.001 | 0.06 (0.03, 0.09) | <0.001 | ||
| LMI (kg/m2) | 0.03 (-0.01, 0.07) | 0.1 | 0.04 (0.00, 0.08) | 0.04 | -0.01 (-0.05, 0.04) | 0.7 | ||
| Carotid-radial PWV (m/s) | ||||||||
| FMI (kg/m2) | -0.01 (-0.05, 0.02) | 0.4 | -0.02 (-0.06, 0.02) | 0.3 | 0.02 (-0.02, 0.07) | 0.3 | ||
| LMI (kg/m2) | -0.10 (-0.16, -0.04) | 0.002 | -0.12 (-0.19, -0.05) | <0.001 | -0.14 (-0.22, 0.06) | <0.001 | ||
| FMD (%) | ||||||||
| FMI (kg/m2) | 0.21 (0.06, 0.36) | 0.008 | 0.19 (0.02, 0.36) | 0.03 | 0.23 (0.03, 0.43) | 0.03 | ||
| LMI (kg/m2) | 0.02 (-0.24, 0.28) | 0.9 | 0.09 (-0.19, 0.36) | 0.5 | -0.12 (-0.45, 0.21) | 0.5 | ||
| Reactive hyperemia (%)* | ||||||||
| FMI (kg/m2) | -3 (-13, 8) | 0.6 | -8 (-19, 3) | 0.2 | -12 (-26, 1) | 0.08 | ||
| LMI (kg/m2) | -5 (-22, 12) | 0.6 | 1 (-17, 19) 0.9 | 12 (-10, 34) | 0.3 | |||
Model 0: Univariate associations between child LMI and FMI and each of the vascular outcomes
Model 1: Associations between child FMI and each of the vascular outcomes, adjusted for maternal education and child sex, age and hours doing sports/week
Model 2: Associations between child LMI and each of the vascular outcomes, adjusted for maternal education and child sex, age and hours doing sports/week
Model 3: Associations between child FMI and LMI and each of the vascular outcomes, adjusted for maternal education and child sex, age and hours doing sports/week, and mutually adjusting for one another.
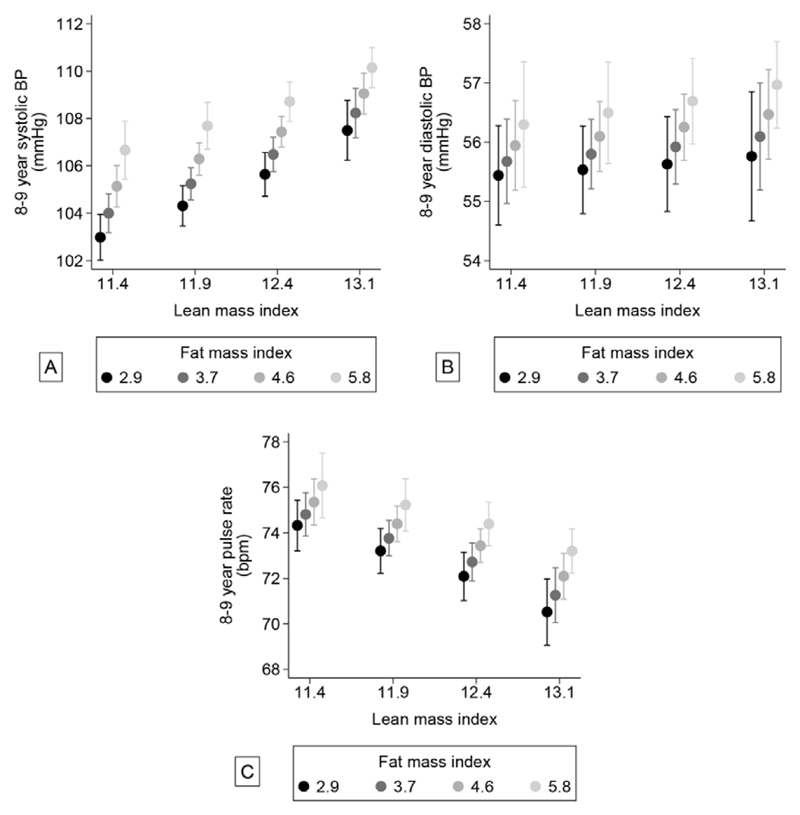
Figure 1. Associations between lean- and fat mass index and systolic BP (A), diastolic BP (B) and pulse rate (C).
Values are marginal means from a linear regression model including an interaction term between fat mass index and lean mass index, adjusted for confounders. Lean mass index was fixed at 11.4, 11.9, 12.4 and 13.1 kg/m2 and fat mass index was fixed at 2.9, 3.7, 4.6 and 5.8. These cut-off points are quintiles (i.e. values that divide the range of data into five equal parts).
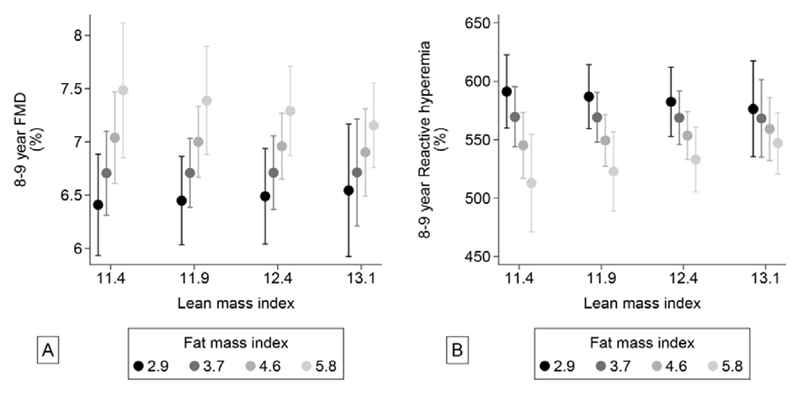
Figure 3. Associations between lean- and fat mass index and brachial FMD (A) and brachial reactive hyperemia (B).
Values are marginal means from a linear regression model including an interaction term between fat mass index and lean mass index, adjusted for confounders. Lean mass index was fixed at 11.4, 11.9, 12.4 and 13.1 kg/m2 and fat mass index was fixed at 2.9, 3.7, 4.6 and 5.8. These cut-off points are quintiles (i.e. values that divide the range of data into five equal parts).
A sub-sample of children also had DXA-measures of fat- and lean mass at 4 years and 6-7 years of age. In order to create z-scores for growth, linear associations between age and each of fat mass, lean mass and height were adjusted for to create fat mass, lean mass and height measures independent of age for each of these three follow-up points. From these, age-adjusted fat mass and lean mass indices were calculated. Residual growth in fat mass index for child j is defined as the difference between observed fat mass index at time p (FMIpj) and predicted FMI at time is obtained by regressing FMIpj on all previous FMI measurements:
Residual growth in fat mass index is therefore the residual error (êj) and is the conditional growth relative to that predicted from all previous fat mass index measurements. The residual growth measurements are orthogonal to all preceding fat mass index measurements. The same procedure was used to calculate residual growth in lean mass index. In this sample we were therefore able to explore whether the vascular outcomes at age 8-9 were associated with FMI and LMI at 4 years, and with conditional changes in FMI and LMI from 4 to 6-7 years and from 6-7 to 8-9 years.
Results
Study population and characteristics
Among the 12,583 women recruited to the SWS, 3,158 women became pregnant and had a live singleton birth, constituting the total sample for the SWS cohort (Supplemental Figure IV, Flow chart). This cohort has been followed up several times since birth.23 Of these a sub-sample of 1,216 children participated in the 8-9-year follow-up. Due to logistical challenges and the time-consuming nature of measurements, not all measures could be taken for all participants. Most had only one or two missing measures and 76% had seven complete measures or more, and at least one vascular measure was taken on 1,166 children. Of these, 983 children also had valid DXA-measurements of fat and lean mass, constituting the final sample for the present study. Of these, a sub-sample (n=398) of children also had DXA measures at 4 and 6-7 years of age.
Maternal and child characteristics and child anthropometry, and DXA- and vascular measures are presented, for boys and girls separately in Table 1. Girls had less lean mass, but more fat mass than boys, and for several vascular outcomes there were significant sex differences. However, the relationships between LMI and FMI and the vascular outcomes (i.e. the increase or decrease in each of the vascular measures per unit change in LMI or FMI) were similar in boys and girls (Supplemental Figures II and III), and our main analyses were therefore performed in and results presented for the total sample. Boys and girls had a mean BMI z-score according to the UK-WHO BMI reference of 0.03 and 0.12 respectively, indicating that the sample is representative of a general UK child population.
Traditional CVD-risk factors; blood pressure and pulse rate
Systolic BP was positively associated with BMI (Table 2), FMI and LMI, but most strongly with LMI (Table 3). The highest systolic BPs were observed in children with both high fat and lean mass. However, there was a significant negative interaction (p=0.03) between lean and fat mass, suggesting that the impact of having more fat mass decreased if the child had more lean mass (Figure 1a).
Diastolic BP was also positively associated with BMI (Table 2) and FMI, but not with LMI after adjusting for FMI (Table 3). Hence, the highest diastolic BP was found in children with high fat mass, irrespective of their lean mass (Figure 1b).
Pulse rate was not associated with BMI (Table 2). However, in the models including both FMI and LMI (Table 3, Model 3), we observed a strong positive association with FMI but a strong negative association with LMI. Consequently, the highest pulse rate was observed in children with low lean mass but high fat mass (Figure 1c).
Measures of arterial structure and stiffness
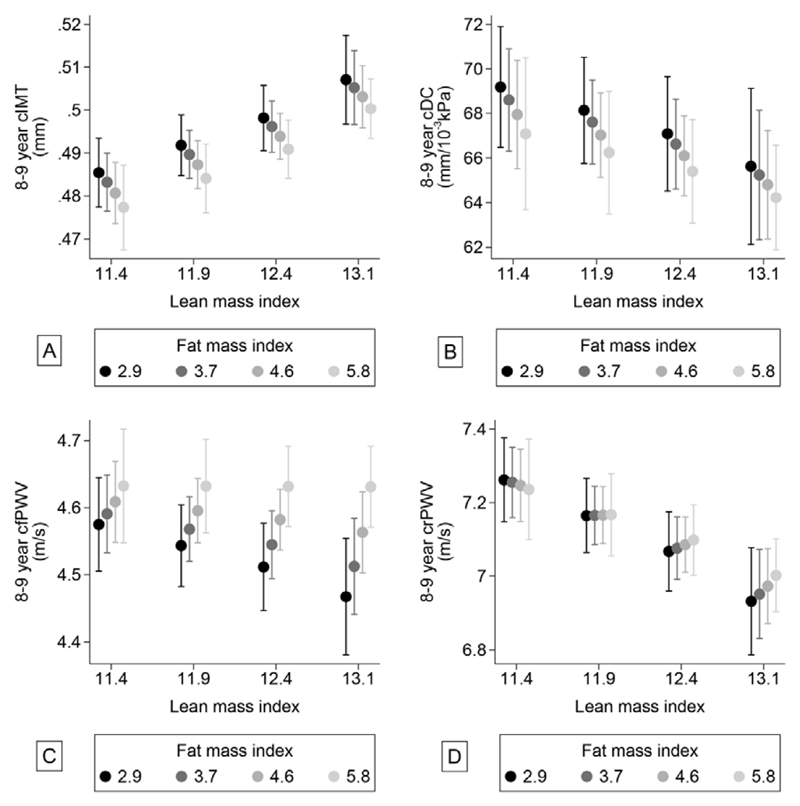
Carotid IMT was strongly positively associated with BMI (Table 2), but not with FMI (Table 3). Hence, the association with BMI was mainly due to a strong positive relation with LMI (Table 3 and Figure 2a). Children with higher BMI also had less distensible carotid walls (lower cDC), due to a negative relation with both LMI and FMI. However, neither of these associations were significant when adjusting for each other and other covariates (Table 3 and Figure 2b).
Figure 2. Associations between lean- and fat mass index and carotid IMT (cIMT) (A) and carotid distensibility coefficient (cDC) (B), carotid-femoral PWV (C) and carotid-radial PWV (D).
Values are marginal means from a linear regression model including an interaction term between fat mass index and lean mass index, adjusted for confounders. Lean mass index was fixed at 11.4, 11.9, 12.4 and 13.1 kg/m2 and fat mass index was fixed at 2.9, 3.7, 4.6 and 5.8. These cut-off points are quintiles (i.e. values that divide the range of data into five equal parts).
Carotid-femoral PWV was positively associated with BMI (Table 2) and FMI (Table 3), but not with LMI. However, there was a strong positive interaction between LMI and FMI (p=0.001). Consequently, the lowest carotid-femoral PWVs were observed in children with high lean mass and low fat mass (Figure 2c). Carotid-radial PWV, on the other hand, was negatively associated with BMI (Table 2), due to a negative association with LMI, but was not significantly associated with FMI (Table 3). The lowest values were found in children with high lean mass (Figure 2d). Also, for this outcome, there tended to be a positive interaction between lean and fat mass, but this was of borderline significance (p=0.06).
Measures of endothelial function
FMD was neither associated with BMI (Table 2), nor with LMI (Table 3). However, there was a weak, but significant, positive association with FMI. Hence, the highest FMD was observed in children with high fat mass (Table 3, Figure 3a). Reactive hyperemia was also not associated with BMI (Table 2) or with LMI, but borderline negatively associated with FMI (Table 3). Moreover, there was a significant interaction (p=0.01) indicating that the adverse impact of increasing fat mass was larger, and highly significant, if the child had low lean mass (Figure 3b). The lowest (“most adverse”) reactive hyperemia were observed in children with low lean mass and high fat mass.
Sub-sample analyses
In our sub-sample of children with DXA measured at 4- and 6-7 years of age we found that both fat- and lean mass at age 4, and relative increases from 4 to 8-9 years of age were positively associated with increased systolic BP at age 8-9 (Supplemental Table I and II). Furthermore, increases in FMI between 4 and 6-7 years of age were associated with higher carotid-femoral PWV (Supplemental Table II). LMI at age 4 was negatively associated with carotid-femoral- and carotid-radial PWV, and relative increases in LMI between 6-7 and 8-9 years of age also tended to be associated with lower carotid-radial PWV.
Discussion
In 8-9-year-old children, we found that high FMI was associated with higher systolic and diastolic BP, pulse rate and carotid-femoral PWV, all of which are considered to be CVD risk factors, but also with higher childhood brachial FMD, which is generally thought to be advantageous. On the other hand, for the vascular outcomes systolic BP, carotid IMT, pulse rate and carotid-radial PWV the lean mass component of BMI seemed to play the more important role, and high LMI was associated with higher systolic BP and carotid IMT, but lower pulse rate and carotid-radial PWV. Moreover, for systolic BP, carotid-femoral PWV and reactive hyperemia, the association with fat mass depended on the amount of lean mass. Together, the findings suggest a relatively protective effect of lean mass.
Adiposity as a CVD risk factor
Our results confirm previous findings showing a relation between increased BMI and fat mass and CVD risk markers, including in young children.15, 17, 18, 25–30 The mechanisms underlying this effect of adiposity are still not fully understood, but could include increased cardiac output, blood pressure, sympathetic nervous activation, inflammation and insulin resistance.9, 31 In contrast, for FMD we observed a positive association with increasing fat mass, even after adjusting for sex, which is surprising given the strong relation between adiposity and lower FMD in adults,3 and also in some studies comparing obese and normal-weight children.9 It is however in agreement with results from children in another large UK-cohort (the ALSPAC study).15 In that study the authors suggested that such improved endothelial dependent dilatation in obese children may represent an adaptive response despite deleterious effects of obesity.
Lean mass as a moderator of CVD risk
BMI is often used as a proxy measure of fat mass, and associations between BMI and CVD risk markers are interpreted as indicating an adverse effect of adiposity. However, we found that for some vascular outcomes, such as systolic BP and carotid IMT, the positive associations with BMI were mainly related to the lean mass component. This is consistent with some studies in adults.32 It has been suggested that a positive association between lean mass and systolic BP and carotid IMT may be explained by a strong relation with stroke volume and hence cardiac output, possibly representing adaptations of the cardiovascular system to a larger body size.33 The increasing blood pressure will lead to increases in carotid IMT, resulting in remodeling of the medial layer in an attempt to preserve wall stress. In contrast to these findings, we observed that for other vascular measures, such as pulse rate, carotid-femoral PWV and carotid-radial PWV, the lowest and most advantageous values were found in children with high lean mass. Moreover, for some outcomes, namely systolic BP, carotid-radial PWV and reactive hyperemia, we found that the deleterious effect of increasing fat mass depended on the amount of lean mass. Together, these results may indicate a relatively protective effect of lean mass. The underlying mechanisms remain speculative, but could reflect higher lean mass representing more muscle and cardiac mass, better metabolic function in the periphery, or greater cardiovascular fitness and hence also a higher capacity to cope with increasing adiposity levels. They may potentially also represent better arterial function due to increased cardiac output and sustained hyperemia (and therefore shear stress) present with increased tissue mass. High lean mass could be an innate trait, but could also be related to greater physical activity. Whatever the mechanistic explanation, these findings clearly suggest that, as a risk factor for CVD, adiposity should not be studied in isolation, and that lean mass also needs to be taken into account. These findings could also be in line with literature demonstrating that low lean mass (“thinness”) is associated with an increased risk of long-term cardio-metabolic disease and poorer prognosis, particularly if combined with fat gain.34–36 Further research is needed on whether interventions to promote cardiovascular and muscular fitness in children, in contrast to merely reducing adiposity, will be beneficial in reducing later risk of CVD.
Pulse wave velocities over different pathways
In our study, PWV was measured over two different pathways: carotid-femoral (aortic) and carotid-radial (mainly brachial) arteries. Several studies have shown a positive association between increased BMI/fat mass and carotid-femoral PWV in children.9, 37, 38 However, we observed that when including lean mass and the interaction between lean and fat mass in the model, there was a negative relation between lean mass and carotid-femoral PWV, although a positive relation with fat mass. The ALSPAC study observed that BMI and adiposity measures were negatively associated with carotid-radial PWV.15, 19 Children with obesity are also more likely to have more lean mass. Our study shows that the observed negative relationship between BMI/fat mass and carotid-radial PWV is mainly related to the negative association with lean mass, which will only be apparent if lean mass is taken into account. As increased PWV is a measure of vascular stiffness and CVD risk, for both carotid-femoral and carotid-radial PWV there seems to be a protective effect of having greater lean mass. Further, a significant adverse effect of fat mass was only observed for carotid-femoral PWV. As carotid-femoral PWV is generally considered to represent arterial stiffening in the elastic aorta, whereas carotid-radial PWV predominantly represents changes in the more muscular brachial artery, this could reflect that in children the influence of fat on arterial stiffness may differ somewhat between elastic and muscular arteries.
It could be argued that some of the effect of FMI and LMI on PWV is mediated through accompanying changes in mean arterial pressure (MAP), as maintaining arterial patency and distensibility requires pressure. Although the aim of our study was to assess the total effects of FMI and LMI, adjusted for confounders, and not mediating effects, we have tried adjusting for MAP in our models. This produced only marginal changes to the results. This suggests that changes in MAP are not the main explanation for the observed associations.
Sub-sample analyses showed that both fat and lean mass at age 4 and relative increases in fat- and lean mass from 4 years of age and onwards were associated with several vascular outcomes at age 8-9 years. This could indicate that some vascular changes and adaptations in relation to body size happen very early in life. There are studies suggesting that associations between body size and measures of vascular structure and function may even represent adaptations happening during fetal development.21 However, this has not been extensively investigated.
Sex-differences
In our study girls had higher, i.e. more adverse, systolic and diastolic BP, pulse rate and carotid-femoral PWV, while FMD and reactive hyperemia were higher, and hence more advantageous, than in boys. Sex-differences in BP and reactive hyperemia remained significant even when differences in body composition, and other confounders were taken into account (data not shown), while differences in carotid-femoral PVW and FMD disappeared. Some of these results are slightly different from those observed in some other studies in children.15, 19 and could be due to differences in age and pubertal status between studies, which may modify the relationship between body composition and vascular measures. Moreover, in our main analyses we did not find any interactions with sex, and supplemental stratified analyses showed that the relationships between FMI and LMI and the vascular outcomes are similar in boys and girls.
Strengths
This study has several strengths, the most important being an integrated and comprehensive set of cardiovascular outcomes, representing different aspects of cardiovascular structure and function, and ultimately of CVD risk. In addition, PWV was measured in two different arterial segments, revealing differences between elastic and muscular vessels which may reflect the differing effects of central versus peripheral vascular function in relation to body composition. A major strength of the study was the ability to distinguish between the effects of fat and lean mass components of childhood BMI.
Limitations
Limitations of the study include insufficient statistical power for exploring more subtle relationships and interactions, even though the data were derived from a large, well-characterized cohort. Further work is required to understand the mechanistic basis of the observed relationships between body composition and CVD risk factors in children, and to dissect out those effects that are adaptive versus those that suggest early pathology. We adjusted for sports participation in our models, but except for pulse rate, this had very little effect on the results. However, child physical activity levels reported by a parent are known to be relatively weakly related to “real” physical activity level. Furthermore, as this variable primarily includes organized sports it will probably be only a crude measure of total and real physical activity level. It should also be noted that, although nested in a large cohort study, these findings are mainly from one follow-up period and so are cross-sectional; hence causal relationships cannot be inferred. We did however have a sub-sample of children with DXA-measures at 4 and 6-7 years of age, enabling us to assess longitudinal effects, but as this sub-sample was relatively small, we had limited statistical power to draw strong conclusions from these analyses.
Conclusions
We found that most measures of vascular structure and function in 8-9-year old children were related to their BMI and fat mass. However, for some of these vascular outcomes the relations with BMI were mainly explained by the lean mass component. Moreover, whether the relations with fat- or lean mass were positive or negative differed between vascular outcomes. These results suggests that in pre-pubertal children, differences in structure and cardiovascular function in relation to BMI and adiposity do not only represent adverse effects of fat mass, but also combinations of adaptive effects of body size and protective effects of lean mass.
Supplementary Material
Highlights.
In 8-9-year-old children, the fat mass component of BMI was associated with higher systolic and diastolic BP, pulse rate and carotid-femoral PWV.
However, for some vascular outcomes the lean mass component of BMI seemed to play the more important role, and high lean mass was associated with higher systolic BP and carotid IMT, but lower pulse rate and carotid-radial PWV.
Moreover, for systolic BP, carotid-femoral PWV and reactive hyperemia, the association with fat mass depended on the amount of lean mass, suggesting a relatively protective effect of lean mass.
Acknowledgements
We are most grateful to the late Ann Donald who contributed greatly to the development of the cardiac structure and function measures in the first part of the project before her untimely death. We acknowledge the work of Corinne Nisbet who performed the FMD measures, the research nurses under the supervision of Julia Hammond who performed the PWVs and the bone density technicians under the supervision of Pat Taylor who performed the DXAs. Finally, we thank the many participants in the Southampton Women’s Survey who gave their time and support to the study over many years.
Sources of Funding:
Research leading to these results was funded by the UK Medical Research Council (MC_UU_12011/4), University of Southampton; British Heart Foundation Program Grant RG/07/009/23120; UK Food Standards Agency (contracts N05049 and N05071); the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and a NIHR Senior Investigator award to KMG (NF-SI-0515-10042); European Union’s Seventh Framework Program (project Early Nutrition under grant agreement nº289346).
The South-Eastern Norway Regional Health Authority funded a one-year travel award (HSO-2016126) for LS to be a visiting researcher at the MRC Life Course Epidemiology Unit, University of Southampton, UK.
Abbreviations
- BMI
Body mass index
- CVD
Cardiovascular disease
- FMI
Fat mass index
- LMI
Lean mass index
- BP
Blood pressure
- cDC
Carotid distensibility coefficient
- cIMT
Carotid intima media thickness
- PWV
Pulse wave velocity
- FMD
Flow-mediated dilatation
- DXA
Dual x-ray absorptiometry
Footnotes
Disclosures:
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and is part of an academic consortium that has received research funding from Abbott Nutrition and Danone. Members of HMI’s team have received funds from Nestec, Abbott Nutrition and Danone. The other authors have no relationships relevant to the contents of this paper to disclose.
References
- 1.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. International journal of cardiology. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 4.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circulation research. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 7.Dale C, Fatemifar G, Palmer T, et al. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes and type 2 diabetes: A mendelian randomization analysis. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 9.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. Journal of the American College of Cardiology. 2013;62:1309–1319. doi: 10.1016/j.jacc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Brion MA, Ness AR, Davey Smith G, Leary SD. Association between body composition and blood pressure in a contemporary cohort of 9-year-old children. J Hum Hypertens. 2007;21:283–290. doi: 10.1038/sj.jhh.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hof MH, Vrijkotte TG, de Hoog ML, van Eijsden M, Zwinderman AH. Association between infancy bmi peak and body composition and blood pressure at age 5-6 years. PLoS One. 2013;8:e80517. doi: 10.1371/journal.pone.0080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang RC, Burrows S, Mori TA, Oddy WH, Beilin LJ. Lifecourse adiposity and blood pressure between birth and 17 years old. American journal of hypertension. 2015;28:1056–1063. doi: 10.1093/ajh/hpu266. [DOI] [PubMed] [Google Scholar]
- 13.Prasad VK, Drenowatz C, Hand GA, Lavie CJ, Sui X, Demello M, Blair SN. Relation of body's lean mass, fat mass, and body mass index with submaximal systolic blood pressure in young adult men. The American journal of cardiology. 2016;117:394–398. doi: 10.1016/j.amjcard.2015.10.060. [DOI] [PubMed] [Google Scholar]
- 14.Tesfaye F, Nawi NG, Van Minh H, Byass P, Berhane Y, Bonita R, Wall S. Association between body mass index and blood pressure across three populations in africa and asia. J Hum Hypertens. 2007;21:28–37. doi: 10.1038/sj.jhh.1002104. [DOI] [PubMed] [Google Scholar]
- 15.Charakida M, Jones A, Falaschetti E, Khan T, Finer N, Sattar N, Hingorani A, Lawlor DA, Smith GD, Deanfield JE. Childhood obesity and vascular phenotypes: A population study. Journal of the American College of Cardiology. 2012;60:2643–2650. doi: 10.1016/j.jacc.2012.08.1017. [DOI] [PubMed] [Google Scholar]
- 16.Doyon A, Kracht D, Bayazit AK, et al. Carotid artery intima-media thickness and distensibility in children and adolescents: Reference values and role of body dimensions. Hypertension. 2013;62:550–556. doi: 10.1161/HYPERTENSIONAHA.113.01297. [DOI] [PubMed] [Google Scholar]
- 17.Melo X, Santa-Clara H, Pimenta NM, Carrolo M, Martins SS, Minderico CS, Fernhall B, Sardinha LB. Body composition phenotypes and carotid intima-media thickness in 11-13-year-old children. European journal of pediatrics. 2014;173:345–352. doi: 10.1007/s00431-013-2164-7. [DOI] [PubMed] [Google Scholar]
- 18.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: The role of body composition. Obesity. 2012;20:165–171. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donald AE, Charakida M, Falaschetti E, Lawlor DA, Halcox JP, Golding J, Hingorani AD, Smith GD, Deanfield JE. Determinants of vascular phenotype in a large childhood population: The avon longitudinal study of parents and children (alspac) European heart journal. 2010;31:1502–1510. doi: 10.1093/eurheartj/ehq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpoff L, Vinet A, Schuster I, Oudot C, Goret L, Dauzat M, Obert P, Perez-Martin A. Abnormal vascular reactivity at rest and exercise in obese boys. European journal of clinical investigation. 2009;39:94–102. doi: 10.1111/j.1365-2362.2008.02068.x. [DOI] [PubMed] [Google Scholar]
- 21.Toemen L, de Jonge LL, Gishti O, van Osch-Gevers L, Taal HR, Steegers EA, Hofman A, Helbing WA, Jaddoe VW. Longitudinal growth during fetal life and infancy and cardiovascular outcomes at school-age. J Hypertens. 2016;34:1396–1406. doi: 10.1097/HJH.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 22.Nowson CA, Crozier SR, Robinson SM, Godfrey KM, Lawrence WT, Law CM, Cooper C, Inskip HM, Southampton Women's Survey Study G Association of early childhood abdominal circumference and weight gain with blood pressure at 36 months of age: Secondary analysis of data from a prospective cohort study. BMJ Open. 2014;4:e005412. doi: 10.1136/bmjopen-2014-005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C, Group SWSS Cohort profile: The southampton women's survey. Int J Epidemiol. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, task force iii: Recommendations for user procedures. American journal of hypertension. 2002;15:445–452. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- 25.Brandon LJ, Fillingim J. Body composition and blood pressure in children based on age, race, and sex. Am J Prev Med. 1993;9:34–38. [PubMed] [Google Scholar]
- 26.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. Journal of the American College of Cardiology. 2009;54:2396–2406. doi: 10.1016/j.jacc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Imhof K, Zahner L, Schmidt-Trucksass A, Hanssen H. Association of body composition and blood pressure categories with retinal vessel diameters in primary school children. Hypertens Res. 2016;39:423–429. doi: 10.1038/hr.2015.159. [DOI] [PubMed] [Google Scholar]
- 28.Weber DR, Leonard MB, Shults J, Zemel BS. A comparison of fat and lean body mass index to bmi for the identification of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2014;99:3208–3216. doi: 10.1210/jc.2014-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaillard R, Steegers EA, Tiemeier H, Hofman A, Jaddoe VW. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: The generation r study. Circulation. 2013;128:2202–2210. doi: 10.1161/CIRCULATIONAHA.113.003881. [DOI] [PubMed] [Google Scholar]
- 30.Wiklund P, Tormakangas T, Shi Y, Wu N, Vainionpaa A, Alen M, Cheng S. Normal-weight obesity and cardiometabolic risk: A 7-year longitudinal study in girls from prepuberty to early adulthood. Obesity. 2017;25:1077–1082. doi: 10.1002/oby.21838. [DOI] [PubMed] [Google Scholar]
- 31.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of the obesity society and the american society of hypertension. J Clin Hypertens (Greenwich) 2013;15:14–33. doi: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno M, Puig J, Moreno-Navarrete JM, Xifra G, Ortega F, Ricart W, Fernandez-Real JM. Lean mass, and not fat mass, is an independent determinant of carotid intima media thickness in obese subjects. Atherosclerosis. 2015;243:493–498. doi: 10.1016/j.atherosclerosis.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the rotterdam study. Stroke; a journal of cerebral circulation. 1997;28:2442–2447. doi: 10.1161/01.str.28.12.2442. [DOI] [PubMed] [Google Scholar]
- 34.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. The New England journal of medicine. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 35.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: Impact of lean mass index and body fat in the "obesity paradox". Journal of the American College of Cardiology. 2012;60:1374–1380. doi: 10.1016/j.jacc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 36.Arabshahi S, Busingye D, Subasinghe AK, Evans RG, Riddell MA, Thrift AG. Adiposity has a greater impact on hypertension in lean than not-lean populations: A systematic review and meta-analysis. Eur J Epidemiol. 2014;29:311–324. doi: 10.1007/s10654-014-9911-6. [DOI] [PubMed] [Google Scholar]
- 37.Correia-Costa A, Correia-Costa L, Caldas Afonso A, Schaefer F, Guerra A, Moura C, Mota C, Barros H, Areias JC, Azevedo A. Determinants of carotid-femoral pulse wave velocity in prepubertal children. International journal of cardiology. 2016;218:37–42. doi: 10.1016/j.ijcard.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 38.Cote AT, Phillips AA, Harris KC, Sandor GG, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: Systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35:1038–1044. doi: 10.1161/ATVBAHA.114.305062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.