Abstract
Background
Machine cold perfusion is beneficial to the preservation of kidneys for transplantation. At the end of preservation, the perfusion solution contains many proteins. Using a proteomics approach, we searched for useful biomarkers and potential therapeutic targets in the perfusate. Our program is unique in that all transplant kidneys (even living donor kidneys, LKD) are placed on machine cold perfusion prior to transplantation.
Material/Methods
Perfusates from donation after neurological and circulatory determination of death (DNDD and DCDD respectively) and LKD were collected (n=41) and analyzed for LDH, neutrophil gelatinase-associated lipocalin (NGAL), and matrix metalloproteinase-2 (MMP-2) as markers of injury. Perfusate from each kidney was subjected to 2-dimensional gel electrophoresis, then analyzed using software to identify those spots which are significantly different between the 3 groups. Mass spectrometry was used to identify the proteins and their identity was confirmed with Western blot.
Results
The highest levels of MMP-2, LDH, and NGAL were seen for the DCDD kidneys, followed by the DNDD kidneys and then LDK. Peroxiredoxin-2, NGAL, and alpha-1-antitrypsin were identified as significantly different between the different types of donor kidneys, and their role and possible therapeutic strategies are discussed. Collagen fragments, albumin, and immunoglobulin were also identified as possible byproducts of the injury and may be useful is assessing the degree of injury.
Conclusions
Comparison of the perfusates from the different types of kidneys has allowed us to identify proteins that will be useful in future research into reducing injury in transplant kidneys.
MeSH Keywords: Biological Markers, Kidney Transplantation, Organ Preservation
Background
With the ever-increasing shortage of donor kidneys, steps have been taken to increase the donor pool, including the use of expanded criteria donors (ECD) and a return to donation after circulatory determination of death (DCDD). Transplantation of these kidneys has been successful, but it is associated with an increase in the numbers of delayed graft function (DGF) shortly after transplant and a decrease in function at 1 year [1]. Machine cold perfusion has shown reduced incidence of DGF and improved long- and short-term function [2–4] and this benefit may be more pronounced in marginal kidneys and those obtained from DCDD donors [5]. During perfusion of the kidney, there are significant amounts of proteins released into the perfusate; some of these proteins are just beginning to be studied [6].
Our program is unique in that we perform machine cold perfusion on all transplant kidneys obtained from DCDD, donation after neurological determination of death (DNDD), and living donor kidneys (LDK). Each type of donor kidney undergoes some form of injury during the pre-retrieval time, at the time of retrieval, during preservation, and at the time of reperfusion. Injury leads to a decrease in function, shortens graft survival, and is suspected to lead to an increase in rejection due to increased activation of the immune system [7]. The time when a kidney is on a machine perfusion apparatus presents a potential time during which therapeutic interventions to decrease injury could be applied.
We studied perfusates and contrasted the proteins released during preservation of kidneys from each of the types of donation. The similarities and differences revealed novel proteins involved in the injury and offered some insights into interventions geared towards the protection of donor kidneys.
Material and Methods
Sample collection
Samples of kidney perfusate (80 cc) were collected from the cooling bath section of the LifePort apparatus after the kidney had been removed from the apparatus to be transplanted. Samples were spun to remove cellular debris, then the supernatant was stored at −80°C until batch analysis. Ethics approval was obtained from both the University of Saskatchewan Bio Research Ethics Board and the Western University Ethics Review Board.
Delayed Graft Function (DGF) was defined as the need for dialysis in the first 7 days post-transplant. Slow graft function (SGF) was defined as serum creatinine >265 μmol/L on post-transplant day 5 but no need for dialysis by day 5 [8].
Sample preparation
Slurry (0.2 mL) of ceramic hydroxyapatite (CHT) previously equilibrated in 5 mM phosphate buffer with pH 6.8 was added to 0.2 mL of perfusate. After 30-min incubation, the mixture was centrifuged again, and the supernatant was removed. Proteins bound to CHT were released with 1 mL of 0.5 M phosphate buffer with pH 6.8 after 30-min incubation at room temperature. Samples were then cleaned with ReadyPrep 2-D Cleanup Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. Samples were then stored at −80°C. The Bradford protein assay (Bio-Rad, Hercules, USA) was used to assess the protein content of each sample.
Measurement of lactate dehydrogenase (LDH) activity
The activity of LDH was measured with the LDH Activity Assay kit from Sigma-Aldrich (Billerica, MA, US), which quantifies the reduction of NAD to NADH by LDH. One unit of LDH activity is defined as the amount of enzyme that catalyzes the conversion of lactate into pyruvate to generate 1 μmole of NADH per minute at 37°C. The amount of NADH is quantified colorimetrically by measurement of absorbance at 450 nm.
Measurement of NGAL level
NGAL level was measured using an ELISA kit (Abcam, Canada), and expressed as ng of protein per ml of perfusate. Samples and standards are loaded into 96-well plates coated with antibody specific for human NGAL. The wells are washed, and biotinylated anti-NGAL antibody is added. After washing away unbound biotinylated antibody, HRP-conjugated streptavidin is added. The wells are again washed, and a tetramethylbenzidine (TMB) substrate solution is added to the wells and color develops in proportion to the amount of NGAL, which is then quantified by absorbance at 450 nm.
Measurement of MMP activity
Gelatin zymography was performed as previously described [9,10]; 20-microliter samples were applied to 8% polyacrylamide gel copolymerized with 2 mg/mL gelatin for electrophoresis, after which the gels were rinsed 3 times in 2.5% Triton X-100 to remove the SDS. The gels were then washed twice in incubation buffer at room temperature, then incubated at 37°C for 24 h. The gels were stained using 0.05% Coomassie Blue in a mixture of methanol: acetic acid: water (2.5: 1: 6.5, v: v: v) and destained in a solution of 4% methanol and 8% acetic acid. Developed gels were scanned using a GS-800 calibrated densitometer (Bio-Rad, Hercules, CA, USA). Finally, MMP-2 activity was measured using Quantity One® software, version 4.6 (Bio-Rad, Hercules, USA).
Two-dimensional gel electrophoresis (2-DE)
Protein samples for 2-DE were prepared by mixing 0.02 ml of sample with 0.18 ml of rehydration buffer at room temperature.
Two hundred microliters of sample preparation was applied to immobilized pH gradient strips (Bio-Rad, Hercules, CA, USA) and equilibrated for 18 h at 20°C in rehydration buffer. For isoelectric focusing, the Bio-Rad Protein isoelectric focusing cell was used as previously described [11,12]. Next, 2-DE was carried out using precast gradient gels with 8–16% acrylamide (Bio-Rad, Hercules, CA, USA). To minimize variations in resolving proteins, 12 gels were run simultaneously using a Criterion® Dodeca Cell (Bio-Rad, Hercules, CA, USA) and samples were loaded that were common to all the gels in order to help control for differences between gels. The reproducibility of 2-DE and quality of protein loading has been validated [11,13,14] Proteins were next detected with Coomassie Blue (Bio-Rad, Hercules, USA). All the gels were stained in the same bath. In all, 2-DE gels were created for 6 samples from each group. The developed gels that resulted were scanned using a GS-800 densitometer (Bio-Rad, Hercules, USA).
Measurement of spot intensity was done using PDQuest 8.01 software (Bio-Rad, Hercules, USA), and the intensity of separate bands from immunoblotting were analyzed and expressed in arbitrary units with Quantity One® 4.4 software (Bio-Rad, Hercules, USA).
Mass spectrometry (MS)
Proteins from 2-DE were excised from the gel. The gel pieces containing protein of interest were processed using the ProGest automated system (Genomic Solutions, Huntingdon, UK). Samples were reduced using 100 uL of 10 mM dithiothreitol (DTT), alkylated with 100 uL of 55 mM iodoacetamide and finally digested with trypsin. The peptides were extracted from the gel pieces by three 20-min incubations with a solution (30 μL) containing acetonitrile (50%) and formic acid (5%) in LC–MS-grade water with gentle agitation. The extracts were pooled and dried using a vacuum concentrator (Speed Vac Concentrator, SPD 111 V-230, Thermo Electron Corp, Gormley, Canada) and finally resuspended in LC–MS-grade water (15 μL) containing acetonitrile (3%) and formic acid (0.5%). LC-MS/MS was performed using a nano flow liquid chromatography system (Ultimate3000RSLCnanno, ThermoScientific, Toronto, Canada) interfaced to a hybrid ion trap-orbitrap high-resolution tandem mass spectrometer (VelosPro, ThermoScientific, Toronto, Canada) operated in data-dependent acquisition (DDA) mode. One μL of each sample was injected onto a capillary column (50 cm×75 um PicoTip/PicoFrit Self packed column with Jupiter C18 4u chromatographic media, Phenomenex, Torrance, CA, USA) at a flow rate of 300 nl min−1. Samples were electro-sprayed at 1.2 kV using a dynamic nanospray probe. Chromatographic separation was carried out using 90-min linear gradients (mobile phase A: 0.1% formic acid in MS-grade water, mobile phase B: 0.1% formic acid in MS-grade acetonitrile,) from 3% B to 35% B over 60 min, then increasing to 95% B over 5 min. MS/MS spectra were acquired using both collision-induced dissociation (CID) and higher-energy collisional dissociation (HCD) for the top 15 peaks in the survey 30 000 resolution MS scan. The raw files were acquired (Xcalibur, ThermoFisher) and exported to Proteome Discoverer 2.0 (ThermoFisher, Toronto, Canada) software for peptide and protein identification against the NCBInr and Swiss-Prot databases for Homo sapiens (human). We used the MASCOT (www.matrixscience.com) search engine to identify each protein. For the MS/MS ions search, we assumed: 1) incomplete protein digestion by trypsin (1 missed cleavage level). Also, thresholds of analysis ±50 ppm (fraction expressed as parts per million) and ±200 mmu (absolute milli-mass units) error windows on experimental peptide mass and for fragment mass tolerance were used. Mandatory alkylation and reduction of cysteines with iodoacetamide, and variable oxidation of methionine were assumed. The Mowse scoring algorithm was used to justify the accuracy of protein identification, which is incorporated into the MASCOT search engine algorithm. The Mowse score is then the probability that the observed match is a random event, and this is presented as −10×log10(P) where P is the absolute probability.
Immunoblot analysis
Protein (30 μg) from perfusate was separated using 12% SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, Hercules, USA). Protein level of alpha-1-antitrypsin (A1AT) was measured using mouse monoclonal antibody (Abcam, Toronto, Canada). Protein level of peroxyredoxin-2 and fatty acid binding protein were measured using rabbit polyclonal antibody (Abcam, Toronto, Canada). DJ1 was measured using goat polyclonal antibody (Abcam, Toronto, Canada). Band densities were measured using Versa Doc 5000 and Quantity One 4.6 software (Bio-Rad, Hercules, USA). Equal protein loading was additionally verified by measurement of actin level with mouse monoclonal anti-actin antibody (Abcam, Toronto, Canada).
Statistical analysis
Patient characteristics and protein spot levels on 2-DE and immunoblot were compared using ANOVA (which is incorporated into the PDQuest software), followed by the t test with Bonferroni correction for pairwise comparisons. The chi-squared test was used to compare counts (proportions) between the 3 groups. P-value of <0.05 was considered significant in all cases, and p-value <0.0166 was considered significant for 3 comparisons between 3 groups (Bonferroni correction). Data are expressed as the mean ±SEM.
Results
Clinical characteristics
The characteristics of the 3 types of donors are compared in Table 1. The main difference to be noted between the 3 groups is the machine cold perfusion time and the cold ischemic time (CIT), for which the time is the longest for DNDD kidneys and the shortest by far for LDK.
Table 1.
Donor kidney characteristics.
| LDK (n=16) | DNDD (n=16) | DCDD (n=9) | p-Value | |
|---|---|---|---|---|
| Donor age (yrs ±SEM) | 44.3±3.5 | 51.3±3.5 | 40.4±3.1 | 0.172* |
| Donor gender (M: F) | 7: 9 | 5: 11 | 7: 2 | 0.187# |
| Donor kidney side (L: R) | 15: 1 | 10: 5 | 5: 4 | 0.2# |
| Donor baseline serum creatinine (μmol/L ±SEM) | 71.0±2.7 | 86.6±5.5 | 81.2±6.3 | 0.034* |
| Donor hypertension | 0 | 2 | 0 | – |
| Machine cold perfusion (hours ±SEM) | 3.2±0.3 | 15.9±1.3 | 10.3±1.8 | <0.0001* |
| Cold ischemic time (hours ±SEM) | 3.6±0.4 | 17.6±1.4 | 11.6±1.3 | <0.0001* |
| Warm ischemic time (minutes ±SEM) | 46±5 | 38±7 | 37±8 | 0.08* |
| Slow graft function | 0 | 1 | 8 | <0.0001# |
| Delayed graft function | 0 | 1 | 4 | 0.003# |
Calculated using ANOVA;
calculated using chi-squared test.
SEM – standard error of the mean.
Markers of kidney injury (LDH, NGAL, and MMPs) in perfusate
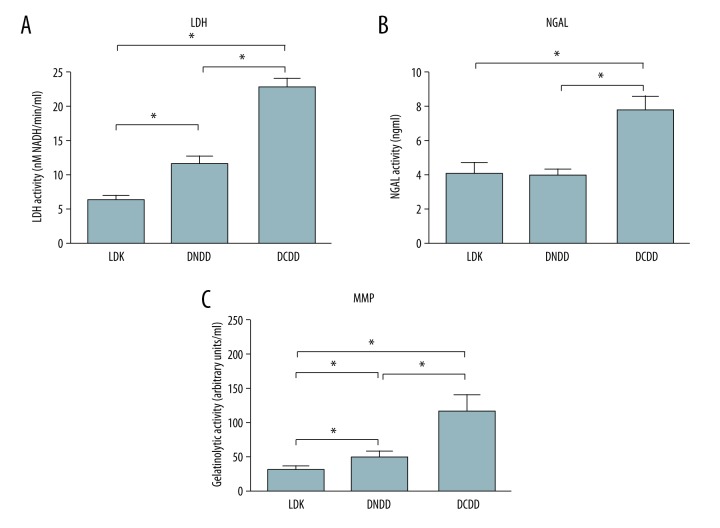
Significant levels of all measured markers were observed in the perfusate from each perfused kidney (Figure 1). Markedly higher levels of LDH and NGAL level were observed in kidney perfusate from DCDD kidneys versus DNDD kidneys or LDK (Figure 1A, 1B), suggesting a greater amount of injury in the case of DCDD kidneys compared to the other 2 types and this in spite of the fact that DCDD kidneys have a shorter preservation time than DNDD kidneys. [LDH: 23.30±2.70 (DCDD n=9) vs. 11.61±1.09 (DNDD n=16) vs. 6.38±0.70 nM NADH/mL (LDK n=16), p<0.0001] and [NGAL: 7.87±0.78 (DCDD n=9) vs. 3.95±0.45 (DNDD n=16) vs. 4.09±0.68 nM NADH/mL (LDK n=16), p<0.0003]. Recently, we showed that MMPs are involved in kidney injury that occurs during machine cold perfusion (1). Gelatinolytic activity, represented by levels of MMP-2 and MMP-9 (Figure 1C), was more than 3-fold higher in DCDD donors vs. DNDD or LDK [111.6±12.2 (DCDD n=9) vs. 38.5±4.4 (DNDD n=16) vs. 28.5±2.43 arbitrary units (LDK n=16), p<0.0001], which parallels the injury markers for the 3 types of kidneys.
Figure 1.
Levels of markers of kidney injury, (A) lactate dehydrogenase (LDH); (B) neutrophil gelatinase-associated lipocalin (NGAL), and (C) Gelatinolytic activity for LDK (n=16), DNDD (n=16), and DCDD (n=9) transplant kidneys. * Represents p<0.05 using ANOVA. LDK – living donor kidney; DNDD – donation after neurologic determination of death; DCDD – donation after circulatory determination of death; MMP – matrix metalloproteinase; NGAL – neutrophil gelatinase associated lipocalin.
Total protein level and 2-dimensional electrophoresis of perfusates proteins
Sample preparations from kidney perfusate were analyzed for total protein and by 2-dimensional electrophoresis (2-DE) (Figure 2). Total protein in the perfusate from DCDD donors was significantly higher than that in the perfusate from DNDD and LDK donors (Figure 2A). Total protein in the perfusate from LDK was significantly lower than from DNDD and DCDD kidneys (Figure 2A).
Figure 2.
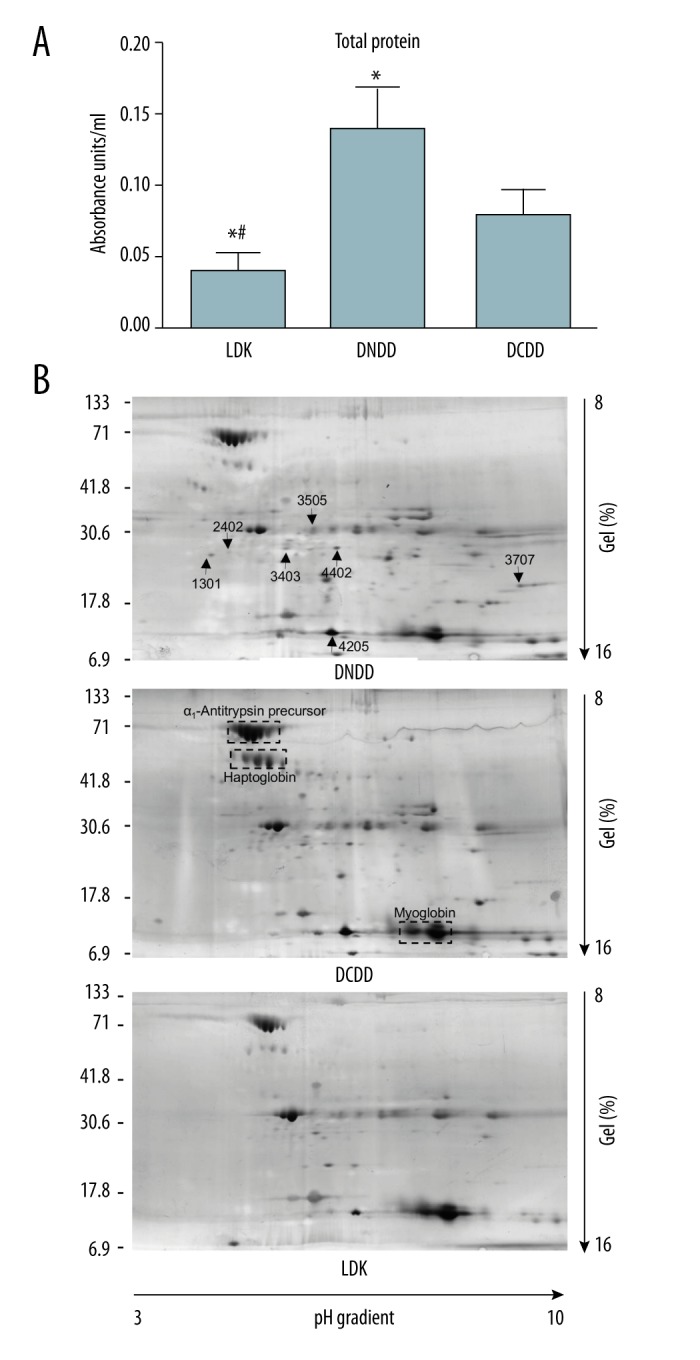
A) Total protein in perfusate for LDK (n=16), DNDD (n=16), and DCDD (n=9) transplant kidneys. (B) Two-dimensional electrophoresis (2-DE) of perfusates. Representative 2-DE gel from each analyzed group of perfusate is shown. The arrows on the upper gel indicate protein spots that differ between groups. The boxes on the middle gel highlight 3 common protein clusters. * Represents p<0.05 vs. DNDD group using ANOVA followed by t test with Bonferroni correction. # Represents p<0.05 vs. DCDD group using ANOVA followed by t test with Bonferroni correction. LDK – living donor kidney; DNDD – donation after neurologic determination of death; DCDD – donation after circulatory determination of death.
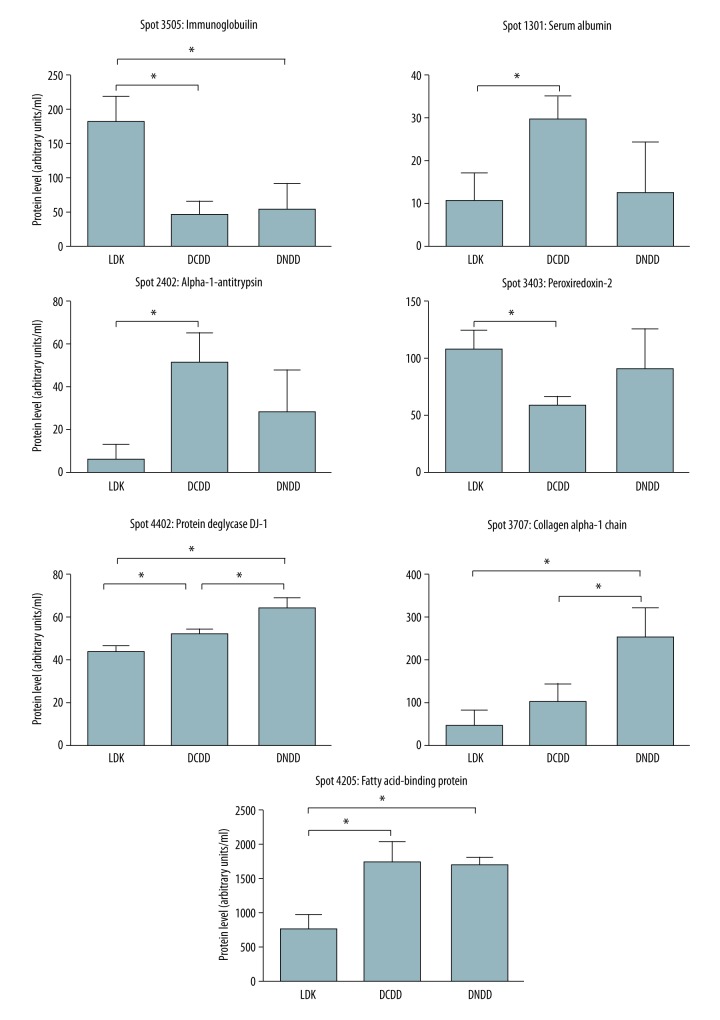
Silver staining of the 2-DE gels revealed several proteins released to KPS-1 during cold perfusion (Figure 2B). The 3 abundant protein clusters A1AT precursors, haptoglobin, and myoglobin served as reference points (Figure 2B middle panel) and did not differ between groups. Quantitative analysis of protein spots revealed that 7 protein spots were significantly different between the 3 groups (Figure 3). Using mass spectrometry analysis, these spots were identified as serum albumin, A1AT, peroxiredoxin-2, heavy chain of immunoglobulin, fragment of collagen 1, fatty acid binding protein (FABP), and protein deglycase (DJ-1) (Table 2).
Figure 3.
Results of densitometric analysis of protein spots from 2-DE gels from LDK, DCDD, and DNDD perfusates (n=6 gels per kidney type). * Represents p<0.05 between the 2 groups in the brackets, using ANOVA followed by t test with Bonferroni correction. LDK – living donor kidney; DNDD – donation after neurologic determination of death; DCDD – donation after circulatory determination of death; FABP – fatty acid binding protein.
Table 2.
Identification of protein spots.
| Protein spot No. | Mowse score* | Queries matched | Sequence coverage (%) | pI(Exp#)/MW(Exp) (kDa) | Identified protein (UniProtKB/Swiss-Prot ID) | Protein role |
|---|---|---|---|---|---|---|
| 3707 | 88 | 2 | 1 | 5.6 (9.2)/139.883 (19.5) | Collagen alpha-1(I) chain (P02452) | Cytoskeletal |
| 3505 | 821 | 28 | 22 | 9.08 (4.9)/23.391 (32.0) | Immunoglobulin (Q8NHL6) | Immune |
| 2402 | 368 | 10 | 13 | 5.37 (4.4)/46.878 (25.5) | Alpha-1-antitrypsin (P01009) | Metabolic |
| 3403 | 579 | 20 | 17 | 5.66 (5.4)/22.049 (23.5) | Peroxiredoxin-2 (P32119) | Metabolic |
| 4402 | 487 | 18 | 23 | 6.33 (6.2)/20.050 (23.5) | Protein deglycase DJ-1 (Q99497) | Metabolic |
| 4205 | 269 | 8 | 12 | 6.59 (6.1)/14.824 (12.5) | Fatty acid-binding protein (P05413) | Transport |
| 1301 | 366 | 10 | 5 | 5.92 (4.1)/71.317 (23.5) | Serum albumin (P02768) | Transport |
Ions score is −10×Log(P), where P is the probability that the observed match is a random event. Individual ions scores >19 indicate identity or extensive homology (p<0.05);
Exp – experimental.
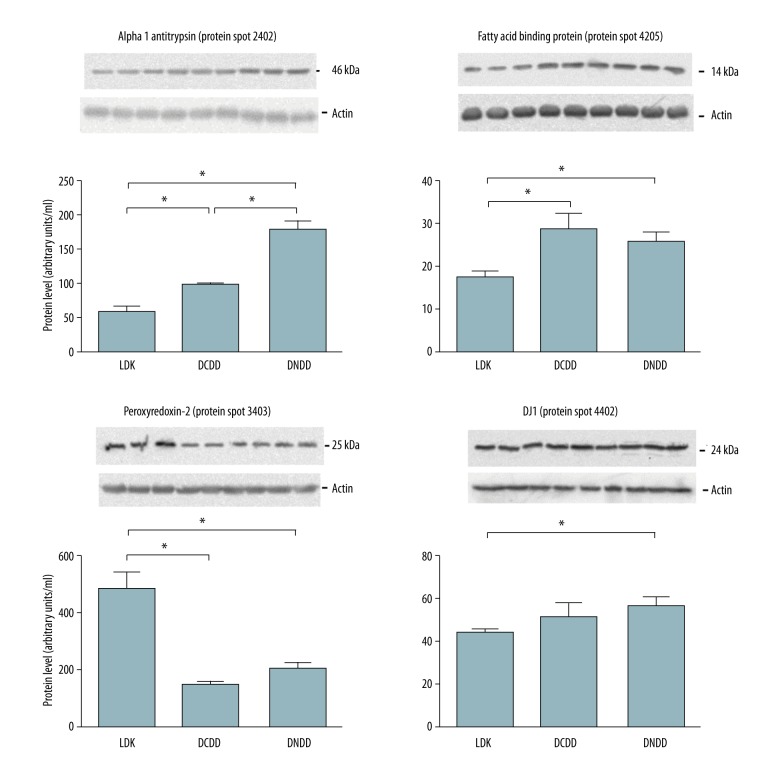
Western blot analysis of identified proteins
Immunoblotting was performed to confirm results from 2-DE and mass spectrometry analysis (Figure 4).
Figure 4.
Results of Western blot analysis of protein spots with commercially available monoclonal antibodies from LDK, DCDD, and DNDD perfusates (n=3 per group). * p<0.05 vs. control group. LDK – living donor kidney; DNDD – donation after neurologic determination of death; DCDD – donation after circulatory determination of death; FABP – fatty acid binding protein.
Discussion
Our work and the work of others has suggested that significant injury occurs at the time of kidney donation, including from LDK donors, DNDD donors, and DCDD donors [16,17]. Our study is unique in that we are one of the few transplant programs to use machine cold perfusion for living donor kidneys [18]. This allows us to make comparisons between the proteins released into the machine cold perfusate for kidneys obtained from LDK donors, DNDD donors, and DCDD donors. Proteomic analysis of the perfusate revealed that 7 protein spots were significantly different between the analyzed groups. Mass spectrometry analysis and confirmation with Western blot identified these proteins as peroxiredoxin-2, fatty acid binding protein (FABP), A1AT, heavy chain of immunoglobulin, serum albumin, fragment of collagen 1, and protein deglycase (DJ-1). In all cases, immunoblot confirmed the protein as identified by MS and the relative amounts of protein correlated well between MS and immunoblot, with the exception of A1AT. This may be due to post-translational modifications, for which the antibody specificity is difficult to predict. In such cases, the amount of protein detected in 2-DE is favored to be more accurate [19].
Peroxiredoxin is an antioxidant protein that is present in many cell types, including kidney cells, whose function includes the protection of cells from oxidative damage related to H2O2. Peroxiredoxin-2 has been shown to be upregulated during warm ischemia, such as is seen in DCDD [20], and has been shown to be protective against ischemia-reperfusion injury in a mouse kidney model [21].
In our study, it is interesting that higher levels of peroxiredoxin were found in the perfusate of living kidney donors compared to the deceased donors; an explanation should be sought. Although living donor kidneys are said to be the “optimal” kidneys for transplantation, they are not without injury at the time of procurement. In the time from the stapling of the artery, through to the stapling of the vein, to the removal of the kidney from the donor’s abdominal cavity, to the ice bath on the back table and cold flush, the kidney is ischemic, and even worse, it is ischemic at a temperature of 37°C (normothermic ischemia). In the case of DNDD, the amount of time that the kidney is subjected to normothermic ischemia is in fact quite minimal at the time of procurement. All 3 types of donation, therefore, have a stressor capable of inducing injury to the transplant kidney [19]. One explanation for the highest levels of peroxiredoxin being found in the perfusate for living donor kidneys is that the other 2 types of donation lead to a depletion of peroxiredoxin due to the relatively long periods of time for injury to occur during which gradual hypotension (in the case of DCDD) or longer exposure to cytokines (in the case of DNDD) occurs. Given its documented protective function and the difference between the 3 groups in terms of this particular protein, this is a protein that requires more study in the area of organ preservation.
Fatty acid binding protein (FABP) has previously been documented to be a useful marker of kidney injury, both in the transplant setting [22] and in the non-transplant setting [23]. FABP is an intra-cytoplasmic protein involved in fatty acid trafficking, but also has been shown to reduce oxidative stress in ischemia-reperfusion [24]. FABP is reported to have its highest levels in the cells of the distal tubules, so FABP is often considered to be primarily a marker of injury to these distal tubules when used to monitor kidney injury. Levels of FABP in perfusate have been shown to correlate with high vascular resistance and early graft dysfunction [6], but are not sufficiently sensitive nor specific to use in deciding on graft discard. That FABP was found in the highest levels in DCDD donors is not surprising. However, the considerable expression of FABP in the perfusate for LDK was somewhat unexpected. This supports the earlier assertion that there is significant injury that occurs even in the setting of LDK, likely related to the period of ischemia at 37°C at the time of graft extraction until cooling on the back table.
A1AT has been studied extensively due to its role in diseases that occur in cases of congenital A1AT deficiency. It is a serine protease inhibitor, an acute-phase reactant produced primarily by the liver. A1AT has anti-inflammatory and cell protection properties documented extensively in the setting of myocardial infarction models [25], but has also been documented to play a role in protection against renal injury in an ischemia-reperfusion model [26]. This protein is part of one of the large clusters found in our analysis of kidney transplant perfusate. However, the A1AT spot identified was a fragment of A1AT; various fragments have been shown to have various anti-inflammatory properties in sepsis and other inflammatory states [27]. A recent study documented the activation of alpha-one-antitrypsin genes in the renal proximal tubules in the setting of ischemic stress [28], a process which was termed ‘hepatization’ by the authors. The group further documented that alpha-one-antitrypsin protects renal tubular cells by blocking the accumulation of neutrophil elastase activity; alpha-one-antitrypsin is then both a marker of renal injury as well as an enzyme that protects against further injury. In that sense, it is not surprising that the lowest levels were found in our study in the perfusate of living donor kidneys, while the highest levels were found in DCDD kidneys.
It is interesting to note that blocking alpha-one-antitrypsin afforded myocardial protection in a model of cardiac ischemia, suggesting that this serine protease should be studied more in the renal setting. The role of alpha-one-antitrypsin in not only in cellular injury, but also in inflammation, particularly given the importance of inflammation in transplantation, and it deserves further study. The fact that a clinically available form (Aralast® [Baxter, USA], Zemeira® [CSL Behring, USA)) of alpha-one-antitrypsin is available will be helpful in studying its role both in animal models and in future clinical trials.
Other proteins identified in the study as being significantly different between the 3 groups included protein deglycase DJ-1, albumin, and collagen alpha-1. Protein deglycase DJ-1 was originally identified in the setting of Parkinson’s disease, but more recently has been shown to have a more general and systemic antioxidant effect. In our study, although the levels were statistically different between the 3 groups, the difference between the lowest of the 3 and the highest of the 3 is not large in absolute terms. That DJ-1 is a significant player in the differences in mechanism between the 3 types of donation injury, therefore, seems unlikely. Albumin is, of course, the most common serum protein in humans, and, although it could be a non-specific finding, it could also reflect the amount of injury that has occurred to the preserved kidney. Collagen, on the other hand, is the most prevalent protein in the human body. Although this is most often considered to be a contaminant in proteomics studies, this could also reflect injury to tubular basement membranes, as we documented in our previous study [16]. Collagen and albumin may be useful in assessing the degree of injury, but further studies are needed to confirm this.
Few studies have been done looking at the proteins in the perfusate of kidneys preserved with machine cold perfusion. One group [29] compared the perfusates of kidneys from uncontrolled DCDD, controlled DCDD, and DNDD kidneys using 2-dimensional gel electrophoresis, and identified 19 proteins that differed between the groups. They also identified A1AT, albumin, and immunoglobulins as being significantly different between their 3 groups. Our study is unique in that perfusate from LDK was included and this should be a sharp contrast from the deceased donor kidneys due to the markedly shorter preservation time and the ideal pre-donation conditions (i.e., without hypotension or excessive cytokines as are seen in DCDD and DNDD, respectively). This likely increased the contrast between 3 groups and allowed the identification of functional or protective proteins such as peroxiredoxin and fatty acid binding protein (FABP). As for the other proteins such as albumin and collagen and immunoglobulins, it is difficult to make a strong case for these proteins in either study due to their ubiquitous nature.
One limitation of our study, and of any study of proteins in perfusates, is that the levels of proteins in the perfusate may reflect the levels at which the proteins are synthesized, actively released, or even passively released after an injury to the mitochondrial or cytoplasmic membranes, or from all of the above reasons. Whatever the mechanism whereby the protein enters the perfusate, these proteins are nonetheless still useful markers in documenting renal injury and still represent potential therapeutic targets.
Conclusions
The fact that these specific proteins differed between the 3 groups suggests they should be further explored to help in understand kidney transplant preservation injury. The inhibition or promotion of some of these proteins, such as A1AT, should be possible pharmacologically through the addition of drugs to the perfusion solution, as we have previously reported for doxycycline [16]. This would be a relatively simple clinical intervention that may yield significant benefits in terms of reduced delayed graft function or even increased graft survival, and tremendous cost savings for what is an inexpensive intervention. Experiments to see if these pharmacological agents will indeed protect the transplant kidney from injury are already underway.
Acknowledgements
We acknowledge the efforts of the team members of the Saskatchewan Transplant Program and the London Health Sciences Centre Multi-Organ Transplant Program for all their hard work and help in collecting samples
Abbreviations
- 2-DE
two-dimensional gel electrophoresis
- DCDD
donation after circulatory determination of death
- DGF
delayed graft function
- DNDD
donation after neurological determination of death
- ECD
extended criteria donor
- FABP
fatty acid binding protein
- KPS-1
kidney preservation solution-1
- LDH
lactate dehydrogenase
- LDK
living donor kidney
- MMP
matrix metalloproteinase
- NGAL
neutrophil gelatinase-associated lipocalin
- SGF
slow graft function
Footnotes
Source of support: Departmental sources
References
- 1.Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: Realities and costs. Am J Transplant. 2007;7:2769–74. doi: 10.1111/j.1600-6143.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 2.Lam VWT, Laurence JM, Richardson AJ, et al. Hypothermic machine perfusion in deceased donor kidney transplantation: A systematic review. J Surg Res. 2013;180:176–82. doi: 10.1016/j.jss.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 3.Cannon RM, Brock GN, Garrison RN, et al. To pump or not to pump: A comparison of machine perfusion vs. cold storage for deceased donor kidney transplantation. J Am Coll Surg. 2013;216:625–34. doi: 10.1016/j.jamcollsurg.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Moers C, Smits JM, Mathuis M-HJ, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 5.Yuan X, Theruvath AJ, GEX Machine perfusion or cold storage in organ transplantation: Indication, mechanisms, and future perspectives. Transpl Int. 2010;23:561–70. doi: 10.1111/j.1432-2277.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 6.Moers C, Varnav OC, van Heurn E, et al. The value of machine perfusion Perfusate Biomarkers for predicting kidney transplant outcome. Transplantation. 2010;90(9):966–73. doi: 10.1097/TP.0b013e3181f5c40c. [DOI] [PubMed] [Google Scholar]
- 7.Stroo I, Stokman G, Teske GJ, et al. Chemokine expression in renal ischemia/reperfusion is most profound during the reparative phase. Int Immunol. 2010;22(6):433–42. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humar A, Johnson EM, Payne WD, et al. Effect of initial slow graft function on renal allograft rejection and survival. Clin Transplant. 1997;11(6):623–27. [PubMed] [Google Scholar]
- 9.Cheung PY, Sawicki G, Wozniak M, et al. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–39. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 10.Fert-Bober J, Leon H, Sawicka J, et al. Inhibiting matrix metalloproteinase-2 reduces protein release into coronary effluent from isolated rat hearts during ischemia-reperfusion. Basic Res Cardiol. 2008;103:431–43. doi: 10.1007/s00395-008-0727-y. [DOI] [PubMed] [Google Scholar]
- 11.Sawicki G, Leon H, Sawicka J, et al. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: A new intracellular target for matrix metalloproteinase-2. Circulation. 2005;112:544–52. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- 12.Fert-Bober J, Basran RS, Sawicka J, Sawicki G. Effect of duration of ischemia on myocardial proteome in ischemia/reperfusion injury. Proteomics. 2008;8:2543–55. doi: 10.1002/pmic.200800022. [DOI] [PubMed] [Google Scholar]
- 13.Sawicki G, Dakour J, Morrish DW. Functional proteomics of neurokinin B in the placenta indicates a novel role in regulating cytotrophoblast antioxidant defenses. Proteomics. 2003;3:2044–51. doi: 10.1002/pmic.200300537. [DOI] [PubMed] [Google Scholar]
- 14.Sawicki G, Jugdutt BI. Detection of regional changes in protein levels in the in vivo canine model of acute heart failure following ischemia-reperfusion injury: Functional proteomics studies. Proteomics. 2004;4:2195–202. doi: 10.1002/pmic.200300746. [DOI] [PubMed] [Google Scholar]
- 15.Moser M, Arcand S, Lin H-B, et al. Protection of the transplant kidney from preservation injury by inhibition of matrix metalloproteinases. PLoS One. 2016;11(6):1–20. doi: 10.1371/journal.pone.0157508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marek C, Thomson B, Shoker A, et al. The prognostic value of time needed on dialysis in patients with delayed graft function. Nephrol Dial Transplant. 2013;29(1):203–8. doi: 10.1093/ndt/gft412. [DOI] [PubMed] [Google Scholar]
- 17.Singh RP, Farney AC, Rogers J, et al. Kidney transplantation from donation after cardiac death donors: Lack of impact of delayed graft function on post-transplant outcomes. Clin Transplant. 2011;25(2):255–64. doi: 10.1111/j.1399-0012.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- 18.Moser MAJ, Ginther N, Luo Y, et al. Early experience with hypothermic machine perfusion of living donor kidneys – a retrospective study. Transpl Int. 2017;30(7):706–12. doi: 10.1111/tri.12964. [DOI] [PubMed] [Google Scholar]
- 19.Sawicki G, Jugdutt BI. Detection of regional changes in protein levels in the in vivo canine model of acute heart failure following ischemia-reperfusion injury – functional proteomics studies. Proteomics. 2004;4:2195–202. doi: 10.1002/pmic.200300746. [DOI] [PubMed] [Google Scholar]
- 20.Hossain MA, De Souza AI, Bagul A, et al. HSP70, peroxiredoxin-3 and -6 are upregulated during renal warm ischaemia in a donation after circulatory death model. J Proteomics. 2014;108:133–45. doi: 10.1016/j.jprot.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Lee EG, Park JY, Woo HA. Protective role of peroxiredoxin V against ischemia/reperfusion induced acute kidney injury in mice. Int J Bioeng and Life Sci. 2016;3(5):1040. [Google Scholar]
- 22.Parikh CR, Hall IE, Bhangoo RS, et al. Associations of perfusate biomarkers and pump parameters with delayed graft function and deceased donor kidney Allograft function. Am J Transplant. 2016;16(5):1526–39. doi: 10.1111/ajt.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noiri E, Doi K, Negishi K, et al. Urinary fatty acid-binding protein 1: An early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296(4):F669–79. doi: 10.1152/ajprenal.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto T, Noiri E, Ono Y, et al. Renal l-type fatty acid binding protein in acute Ischemic injury. J Am Soc Nephrol. 2007;18(11):2894–902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 25.Toldo S, Seropian IM, Mezzaroma E, et al. Alpha-1-antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia–reperfusion injury. J Mol Cell Cardiol. 2011;51(2):244–51. doi: 10.1016/j.yjmcc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Daemen MARC, Heemskerk VH, van ’t Veer C, et al. Functional protection by acute phase proteins 1-Acid Glycoprotein and 1-Antitrypsin against Ischemia/Reperfusion injury by preventing Apoptosis and inflammation. Circulation. 2000;102(12):1420–26. doi: 10.1161/01.cir.102.12.1420. [DOI] [PubMed] [Google Scholar]
- 27.Blaurock N, Schmerler D, Hünniger K, et al. C-terminal alpha-1 antitrypsin peptide: A new sepsis biomarker with immunomodulatory function. Mediators Inflamm. 2016;2016:6129437. doi: 10.1155/2016/6129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zager RA, Johnson ACM, Hanson SY. Proximal tubular cytochrome c efflux: Determinant, and potential marker, of mitochondrial injury. Kidney Int. 2004;65(6):2123–34. doi: 10.1111/j.1523-1755.2004.00638.x. [DOI] [PubMed] [Google Scholar]
- 29.Snoeijs M, Pulinx B, van Dieijen-Visser M, et al. Characterization of the perfusate proteome of human donor kidneys. Ann Clin Biochem. 2013;50:140–46. doi: 10.1258/acb.2012.011144. [DOI] [PubMed] [Google Scholar]