Abstract
Background
T lymphocytes are an essential component of allograft rejection and tolerance. The aim of the present study was to analyze and compare the characteristics of T cell subsets in patients who underwent deceased donor liver transplantation (DDLT) versus living donor liver transplantation (LDLT).
Material/Methods
Between April 2013 and June 2014, 64 patients underwent adult liver transplantation. The distribution of peripheral blood T lymphocyte subsets before transplantation and at 4, 8, 12, and 24 weeks post-transplantation were monitored serially.
Results
In the serial peripheral blood samples, the absolute CD3+ T cell counts in the LDLT group were higher than those in the DDLT group (p=0.037). The CD4+, CD8+, CD4/CD8, Vδ1, Vδ2, and γδ T cell counts did not change significantly over time in either group. The Vδ1/Vδ2 ratio was higher in patients with cytomegalovirus (CMV) infection than in patients without CMV infection (0.12 versus 0.26; p=0.033). The median absolute CD3+ and CD8+ T cell counts in patients with biopsy-proven acute rejection (BPAR) were 884 (range, 305–1,320) and 316 (range, 271–1,077), respectively, whereas they were 320 (range, 8–1,167) and 257 (range, 58–1,472) in patients without BPAR. The absolute CD3+ and CD8 T cell counts were higher in patients with BPAR than in patients without BPAR (p=0.007 and p=0.039, respectively).
Conclusions
With the exception of CD3+ T cells, T cell populations did not differ significantly between patients who received DDLT versus LDLT. In liver transplantation patients, CMV infection and BPAR were closely associated with T cell population changes.
MeSH Keywords: Cytomegalovirus Infections, Graft Rejection, Graft Survival, Liver Transplantation, T-Lymphocyte Subsets
Background
Living donor liver transplantation (LDLT) has emerged as an alternative to deceased donor liver transplantation (DDLT) due to the rarity of deceased donors. Many comparative cohort studies have reported that LDLT yields long-term survival rates for adult patients comparable to those associated with DDLT. LDLT offers many advantages such as shorter waiting times, optimal donor grafts, and the ability to optimize the recipient’s health [1–3].
Alpha-beta (αβ) T lymphocytes have been shown to play an important role in various experimental models of allograft rejection and tolerance by acting T cells [4]. Gamma-delta (γδ) T lymphocytes function at the boundary of innate and acquired immunity and act in immune surveillance by contributing to anti-tumor and anti-infectious immune responses [4]. Recently, γδ T cells were shown to be involved in liver allograft tolerance [5]. In addition, increased numbers of circulating γδT cells were found in the peripheral blood of recipients with stable graft function [6–8]. Human γδ T cells contain both Vδ1 and Vδ2 due to Vδ chains rearrangement. Several studies have reported that circulating Vδ1 γδ T cell populations are significantly increased after transplantation and are associated with operationally tolerant liver recipients [6,8,9].
Changes in liver grafts are related to changes in T cell populations. However, the characteristics of DDLT and LDLT T lymphocyte subsets have not been previously compared. In addition, the association of these subsets with cytomegalovirus (CMV) infection, biopsy-proven acute rejection (BPAR), and graft failure in liver transplantation has not been explored. Moreover, the immunologic functions of γδ T cells in liver transplantation are unclear.
In the present study, we aimed to compare the characteristics of T cell populations in adult DDLT versus adult LDLT and to explore the changes of T cell populations in CMV infection, BPAR, and graft failure in liver transplant recipients.
Material and Methods
Patients
Sixty-four patients underwent liver transplantation between 2012 and 2013. The Institutional Review Board of Samsung Medical Center (Seoul, Republic of Korea) approved this study (SMC-2012-06-031). All patients received tacrolimus, a calcineurin inhibitor. Study exclusion criteria were CMV-seronegative recipients, ABO-incompatible LDLT, or recipient age <18 years. Recipients who used cyclosporine, everolimus, or sirolimus were also excluded. All demographic and clinical data were prospectively collected from medical records. All patients were followed for the first 24 weeks after LDLT. Graft failure was defined as retransplantation or death because of liver dysfunction.
Immunosuppression
The immunosuppression protocol in our center has been previously described [10]. Briefly, basiliximab (20 mg) was used as an induction agent in all recipients during LDLT and on day four after LDLT. All patients were infused with prostaglandin E1 (PGE1), gabexate mesilate, and methylprednisolone (MPD). Maintenance immunosuppressive therapy consisted of corticosteroids, tacrolimus, and mycophenolate mofetil (MMF). Corticosteroids were withdrawn three months after transplantation. Tacrolimus treatment was initiated on postoperative day three; optimal blood level was adjusted to maintain a trough plasma concentration of 10 ng/mL during the first month and was then reduced to 5–8 ng/mL. Beginning on postoperative day one, 750 mg of MMF was administered twice daily. A liver biopsy was performed if acute rejection was suspected.
Cytomegalovirus infection
Cytomegalovirus (CMV) infection was continuously monitored after LT. CMV infection was diagnosed as a CMV pp65 antigen-positive cell count greater than one positive cell per 200,000 white blood cells in patients with previously undetectable CMV antigen. CMV disease presented either as CMV syndrome or as tissue-invasive CMV disease [11,12].
Peripheral blood lymphocyte subpopulations
Lymphocytes were analyzed in fresh whole blood samples obtained preoperatively and at 4, 8, 12, and 24 weeks after liver transplantation. For lymphocyte subset analysis using multicolor flow cytometry, whole blood was incubated with various monoclonal antibodies specific for CD lineage markers according to the manufacturers’ instructions [13]. All monoclonal antibodies were purchased from eBioscience (San Diego, CA, USA), BD Biosciences (Franklin Lakes, NJ, USA), or Thermo Fisher Scientific, Rockford, IL, USA). The cocktail for γδ T cells consisted of CD3-PerCP Cy5.5 (SK7, eBioscience), CD4-APC-eFluor780 (RPA-T4, eBioscience), CD8-PE-Cy7 (SK1, eBioscience), TCR V delta 1-FITC (TS8.2, Thermo Fisher Scientific, Rockford, IL, USA), and Vδ2 TCR-PE (B6, BD Biosciences). The cocktails for regulatory T cells and natural killer (NK) cells were as follows: CD4-PerCP Cy5.5 (RPA-T4, eBioscience), CD25-APC (BC96, eBioscience) and FOXP3-PE (PCH101, eBioscience) for regulatory T cells, and CD3-PerCP Cy5.5 (SK7, eBioscience), CD16-APC-H7 (3G8, BD Biosciences) and CD56-FITC (MEM188, eBioscience) for NK cells. Briefly, 100 μl of whole blood containing different combinations of antibodies was incubated for 15 minutes at room temperature in the dark. Next, red blood cell (RBC) lysis was performed, and the remaining cells were washed in phosphate-buffered saline (PBS). Cells were then resuspended in PBS containing 0.5% albumin, after which cell surface staining was analyzed by fluorescence-activated cell sorting (FACS). For intracellular staining, surface stained cells were fixed and permeabilized with Fix/Perm reagent (eBioscience, San Diego, CA, USA) and then incubated with anti-Foxp3 antibody. Various lymphocyte subsets were defined as follows: CD3+CD4+ for helper T cells, CD3+CD8+ for cytotoxic T cells, CD3−CD56+CD16+ for NK cells, and TCR Vδ1 and Vδ2 for CD3+CD4−CD8− γδ T cells. Cells were analyzed on a FACSCanto II using FACSDIVA software (BD Biosciences).
Statistical analysis
Patient data were collected prospectively from electronic medical records (EMRs). Categorical variables were expressed as percentages and compared using the χ2 test or Fisher’s exact test. Correlations were evaluated using Spearman’s rank order correlation. Continuous variables were expressed as medians and ranges and compared using the Mann-Whitney U test. Repeated measures of lymphocyte populations after liver transplantation were analyzed using a mixed model. Statistical significance was defined as a p-value <0.05. Data analysis was performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of LDLT and DDLT patients
Patient demographic characteristics are summarized in Table 1. All patients were CMV-seropositive and received tacrolimus. The median recipient and donor ages were 54 years (range, 42–77) and 33 years (range, 10–66), respectively, in the DDLT group and 54 years (range, 27–69) and 30 years (range, 18–62), respectively, in the LDLT group. No significant differences were observed between the two groups regarding gender, age, diagnosis, past history, body mass index, macrosteatosis, microsteatosis, warm ischemic time, length of intensive care unit stay after LT, or length of hospitalization. However, the Child-Pugh classes and Model for End-Stage Liver Disease (MELD) scores were worse in the DDLT group than in the LDLT group. The median graft-recipient weight ratio (GRWR) with LDLT was 1.02 (range, 0.67–1.40). The cold ischemic time was shorter in the LDLT group than in the DDLT group (91 minutes versus 341 minutes; p<0.001). The median donor operative times for DDLT and LDLT were 210 minutes (range, 135–330) and 369 minutes (range, 290–581), respectively. The donor operative time was shorter for the DDLT group than for the LDLT group (p<0.001). Most patients received tacrolimus, MMF, and steroids. There were no statistically significant differences in the use of tacrolimus, MMF, or steroids between the two groups. CMV infection, BPAR, and graft failure did not vary between the two groups in the first six months after liver transplantation.
Table 1.
Characteristics of LDLT and DDLT in adult liver transplant recipients.
| DDLT (n=11) | LDLT (n=53) | P-value | |
|---|---|---|---|
| Recipient | |||
| Gender (Male) | 7 (63.6%) | 47 (88.7%) | 0.060 |
| Age | 54 (42–77) | 54 (27–69) | 0.493 |
| Diagnosis | 0.193 | ||
| Alcoholic | 1 (9.1%) | 8 (15.1%) | |
| Alcoholic, HCC | 0 (0%) | 1 (1.9%) | |
| HBV | 0 (0%) | 9 (17.0%) | |
| HBV, HCC | 6 (54.5%) | 28 (52.8%) | |
| HCV, HCC | 0 (0%) | 2 (3.8%) | |
| NBNC | 1 (9.1%) | 2 (3.8%) | |
| NBNC, HCC | 0 (0%) | 1 (1.9%) | |
| Others | 3 (27.3%) | 2 (3.8%) | |
| Child-Pugh class | 0.045 | ||
| A | 1 (9.1%) | 24 (45.3%) | |
| B | 5 (45.5%) | 12 (22.6%) | |
| C | 5 (45.5%) | 17 (32.1%) | |
| Past history | |||
| Hypertension | 4 (36.4%) | 8 (15.1%) | 0.196 |
| Diabetes | 4 (36.4%) | 11 (20.8%) | 0.268 |
| Graft | <0.001 | ||
| Whole | 10 (90.9%) | 0 (0%) | |
| Right lobe | 0 (0%) | 52 (98.1%) | |
| Left lobe | 1 (9.1%) | 1 (1.9%) | |
| Body mass index | 22.8 (18.2–34.8) | 24.7 (17.6–35.6) | 0.096 |
| MELD | 21 (8–37) | 10 (6–52) | 0.020 |
| Macrosteatosis (%) | 5 (1–40) | 5 (1–15) | 0.978 |
| Microsteatosis (%) | 1 (1–90) | 5 (1–40) | 0.215 |
| Operative time (min) | 408 (306–625) | 595 (410–838) | <0.001 |
| Cold ischemic time (min) | 341 (287–450) | 91 (45–141) | <0.001 |
| Warm ischemic time (min) | 38 (22–135) | 35 (14–96) | 0.358 |
| Intensive care unit stay after LT (days) | 7 (5–9) | 6 (3–19) | 0.059 |
| Hospitalization (days) | 31 (17–55) | 24 (17–119) | 0.224 |
| Immunosuppression | |||
| Tacrolimus | 11 (100%) | 53 (82.8% | 0.853 |
| MMF | 10 (90.9%) | 39 (73.6%) | 0.434 |
| Steroids | 6 (75.0%) | 39 (76.5%) | 0.928 |
| Outcomes | |||
| CMV infection | 8 (72.7%) | 27 (50.9%) | 0.319 |
| Biopsy-proven acute rejection | 3 (27.3%) | 4 (7.5%) | 0.091 |
| Graft failure | 1 (9.1%) | 2 (3.8%) | 0.438 |
HCC – hepatocellular carcinoma; HBV – hepatitis B virus; HCV – hepatitis C virus; NBNC – non B non C; MELD – model for end-stage liver disease; MMF – mycophenolate mofetil; CMV – cytomegalovirus.
Peripheral blood T cell subsets in LDLT and DDLT patients
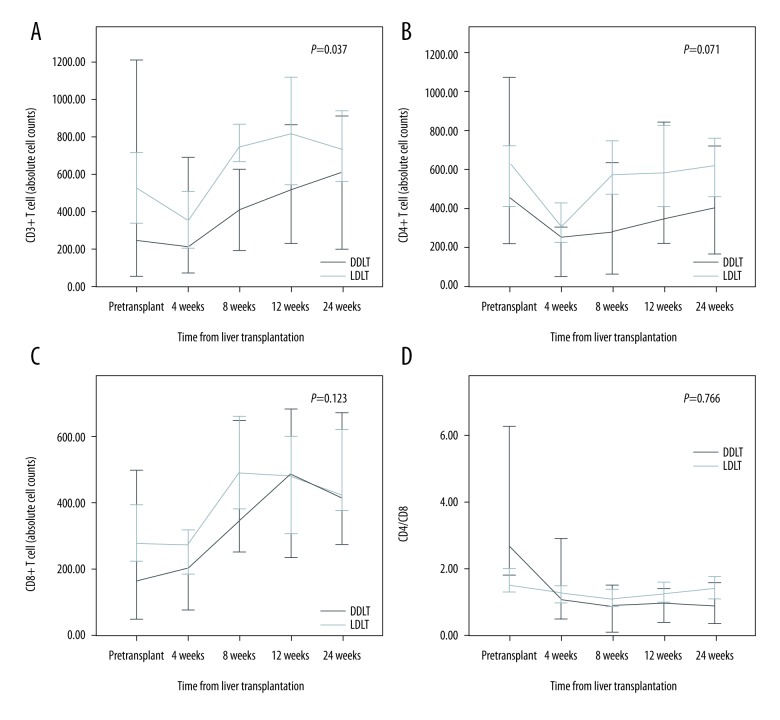
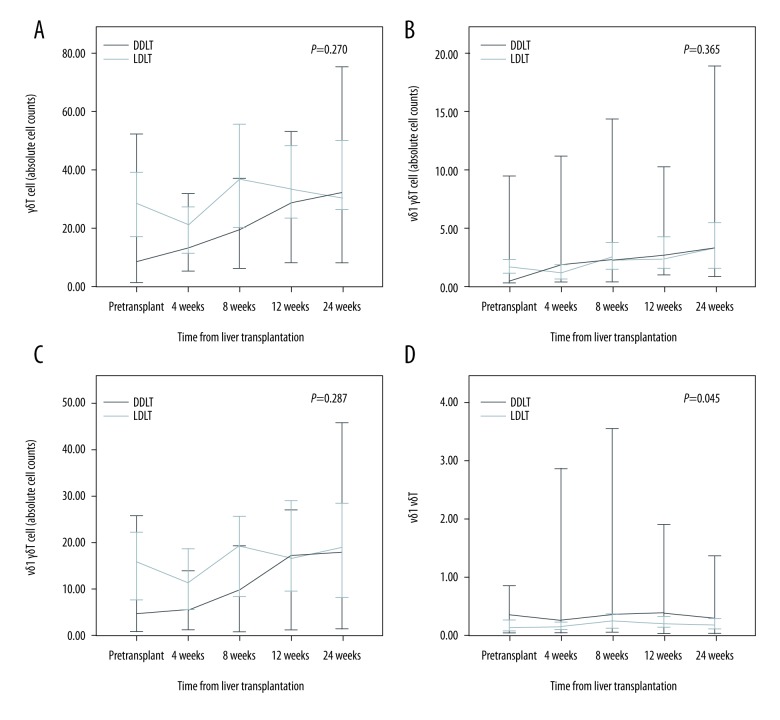
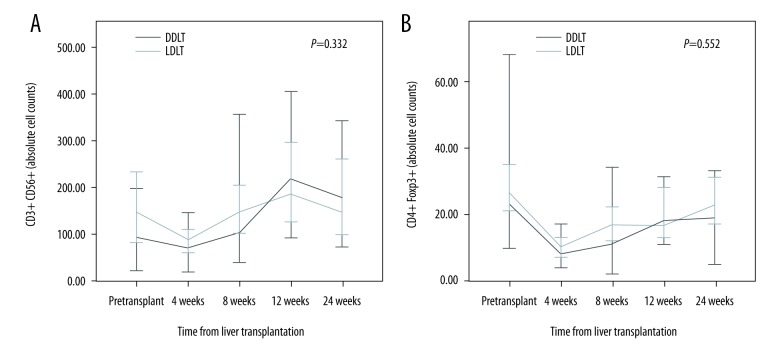
The absolute CD3+ T cell counts were higher in the LDLT group than in the DDLT group (p=0.037) (Figure 1). Moreover, the absolute CD4+ T cell counts were higher in the LDLT group than in the DDLT group after eight weeks. However, there was no statistically significant difference between the two groups. Comparison between the LDLT and DDLT groups revealed that the absolute cell counts of CD8+ T cells, CD4/CD8 ratio, Vδ1 cells, Vδ2 cells, and γδ T cells did not change significantly over time. However, the Vδ1/Vδ2 ratio was higher in the DDLT group than in the LDLT group (p=0.045) (Figure 2). In addition, the absolute cell counts of CD3-CD56+ T cells and CD4+Foxp3+ T cells in the DDLT group were not different from those in the LDLT group (Figure 3).
Figure 1.
Differences in αβT cells between LDLT and DDLT patients. (A) CD3+ T cell counts, (B) CD4+ T cell counts, (C) CD8+ T cell counts, and (D) CD4/CD8 ratios.
Figure 2.
Differences in γδ T cells between LDLT and DDLT patients. (A) γδ T cell counts, (B) Vδ1 γδ T cell counts, (C) Vδ2 γδ T cell counts, and (D) Vδ1/Vδ2 ratios.
Figure 3.
Differences in (A) CD3-CD56+ T cell counts and (B) CD4+FoxP3 T cell counts between LDLT and DDLT patients.
CMV infection
Thirty-five patients developed CMV infection. The absolute γδ T cell counts were lower in patients with CMV infection than in patients without CMV infection (17 versus 27; p=0.050). Subgroup analysis revealed that the absolute Vδ2 γδ T cell counts in patients with CMV infection were lower than the Vδ1 γδ T cell counts in patients without CMV infection (6 versus 15; p=0.025). Therefore, the Vδ1/Vδ2 ratio in patients with CMV infection was higher than that in patients without CMV infection (0.26 versus 0.12; p=0.033). The absolute cell counts of CD3+, CD4+, CD8+, CD3−CD56+, and CD4+Foxp3+ T cells and the CD4/CD8 ratio in patients with CMV infection were not different from those in patients without CMV infection (Table 2).
Table 2.
CMV infection in adult liver transplantation.
| No CMV infection (n=29) | CMV infection (n=35) | P-value | |
|---|---|---|---|
| White blood cells | 5,540 (2,670–15,160) | 5,220 (2,910–18,580) | 0.604 |
| Absolute lymphocyte counts | 851 (230–1830) | 640 (140–2220) | 0.147 |
| CD3+ T cells | 479 (8–1167) | 306 (47–1134) | 0.104 |
| CD4+ T cells | 388 (99–1083) | 279 (39–1303) | 0.171 |
| CD8+ T cells | 306 (73–1472) | 277 (78–860) | 0.691 |
| CD4/CD8 | 1.28 (0.20–3.72) | 1.04 (0.09–4.38) | 0.207 |
| γδT cells | 27 (1–468) | 17 (1–88) | 0.050 |
| Vδ1 γδT | 2 (1–54) | 1 (1–45) | 0.762 |
| Vδ2 γδT | 15 (1–458) | 6 (1–70) | 0.025 |
| Vδ1/Vδ2 | 0.12 (0.01–44.77) | 0.26 (0.01–11.02) | 0.033 |
| CD3−CD56+ T cells | 111 (28–473) | 77 (14–1137) | 0.171 |
| CD4+Foxp3+ T cells | 10 (4–103) | 9 (1–54) | 0.050 |
CMV – cytomegalovirus.
Biopsy-proven acute rejection
BPAR occurred in seven patients. All cases were diagnosed as acute cellular rejection. Three patients with mild grade BPAR were treated with increased immunosuppression by increasing their tacrolimus concentration. Four patients with moderate grade BPAR were treated with steroid pulse therapy. All patients recovered normal liver function after treatment. While the absolute lymphocyte counts in patients with BPAR were higher than those in patients without BPAR (1,130 versus 658), there was no statistically significant difference between the two groups. The absolute CD3+ T cell counts in patients with BPAR were higher than those in patients without BPAR (884 versus 320; p=0.007). Subgroup analysis revealed that the absolute CD8+ T cell counts in patients with BPAR were higher than those in patients without BPAR (316 versus 257; p=0.039). The absolute CD4+ T cell counts and CD4/CD8 ratio in patients with BPAR were not different from those of patients without BPAR. There were no statistically significant differences in γδ T cell count, Vδ1/Vδ2 ratio, CD3-CD56+ T cell count, or CD4+Foxp3+ T cell count between the two groups (Table 3).
Table 3.
Biopsy-proven acute rejection in adult liver transplantation.
| No BPAR (n=57) | BPAR (n=7) | P-value | |
|---|---|---|---|
| White blood cells | 5,540 (2,580–18,580) | 5,060 (3,410–6,950) | 0.289 |
| Absolute lymphocyte counts | 658 (100–2,220) | 1,130 (510–1,590) | 0.057 |
| CD3+ T cells | 320 (8–1167) | 884 (305–1320) | 0.007 |
| CD4+ T cells | 296 (37–1303) | 324 (207–766) | 0.486 |
| CD8+ T cells | 257 (58–1472) | 316 (271–1077) | 0.039 |
| CD4/CD8 | 1.22 (0.13–4.38) | 0.81 (0.22–1.74) | 0.226 |
| γδT cells | 19 (1–88) | 20 (9–468) | 0.289 |
| Vδ1 γδT | 9 (1–48) | 14 (1–85) | 0.260 |
| Vδ2 γδT | 61 (11–98) | 24 (1–98) | 0.270 |
| Vδ1/Vδ2 | 0.16 (0.01–2.00) | 0.80 (0.01–58.16) | 0.226 |
| CD3−CD56+ T cells | 96 (14–563) | 137 (65–250) | 0.341 |
| CD4+Foxp3+ T cells | 10 (1–57) | 11 (5–34) | 0.374 |
BPAR – biopsy-prove acute rejection.
Graft failure
Three patients developed graft failure. The absolute counts of lymphocytes, CD4+ T cells, γδ T cells, and Vδ2 γδ T cells were significantly lower in patients with graft failure compared to patients without graft failure. However, the absolute cell counts of CD3+, CD8+, CD3-CD56+, and CD4+Foxp3+ T cells, as well as the CD4/CD8 ratio and Vδ1/Vδ2 ratio, were not different in patients with versus without graft failure (Table 4).
Table 4.
Graft failure in adult liver transplantation.
| No graft failure (n=61) | Graft failure (n=3) | P-value | |
|---|---|---|---|
| White blood cells | 5,240 (2,580–18,580) | 7,440 (5,080–10,900) | 0.641 |
| Absolute lymphocyte counts | 740 (100–2,220) | 260 (140–380) | 0.031 |
| CD3+ T cells | 334 (8–1167) | 106 (23–291) | 0.059 |
| CD4+ T cells | 296 (37–1303) | 57 (47–82) | 0.005 |
| CD8+ T cells | 276 (58–1472) | 196 (55–311) | 0.374 |
| CD4/CD8 | 1.20 (0.13–4.38) | 0.29 (0.15–1.51) | 0.152 |
| γδT cells | 22 (1–468) | 2 (1–8) | 0.013 |
| Vδ1 γδT | 2 (1–54) | 1 (1–2) | 0.161 |
| Vδ2 γδT | 12 (1–458) | 1 (1–5) | 0.019 |
| Vδ1/Vδ2 | 0.16 (0.01–44.77) | 0.47 (0.26–1.00) | 0.102 |
| CD3−CD56+ T cells | 16 (4–46) | 9 (4–30) | 0.485 |
| CD4+Foxp3+ T cells | 10 (1–103) | 4 (4–8) | 0.193 |
Discussion
The present study compared peripheral blood T lymphocyte populations in patients who underwent LDLT versus DDLT. The CD3+ T cell counts in the LDLT group were higher than those in the DDLT group. Subgroup analysis revealed that CD4+ T cell counts were higher in the LDLT group compared to the DDLT group after eight weeks. However, CD8+ T cell count were not significantly different between the LDLT and DDLT groups. Regarding γδ T cells, the Vδ1/Vδ2 ratio in the DDLT group was higher than that in the LDLT group. The present study showed that an increased absolute cell count of Vδ1 γδ T cells is common in liver allograft recipients, regardless of DDLT or LDLT. In addition, low counts of Vδ2 γδ T cells were closely associated with CMV infection and graft failure in liver transplant recipients.
Although LDLT allows optimal selection and provides timely grafts to save lives [14], this surgical procedure may lead to more unfavorable outcomes and increased surgical complications compared to DDLT [3]. Many cohort studies have reported that LDLT recipients exhibit decreased overall long-term survival compared to DDLT recipients [1,2]. Since LDLT and DDLT have different rates of chronic rejection [15], the similar proportions of patients identified in subgroups of LDLT and DDLT raise concerns regarding the quality of predicting these parameters during immunosuppression. The present study revealed that the counts of CD3+ T cells in patients who received LDLT were different than those in patients who received DDLT.
The present study found that γδ T cell counts were decreased in patients with graft failure compared to those in patients without graft failure. Another study reported similar results, specifically, that the presence of γδT cells in the blood of transplant patients was associated with stable liver or kidney allograft function [6,7]. However, γδ T cell counts were not associated with biopsy-proven acute rejection. Additionally, CD3+ and CD8+ T cell counts were elevated in patients with BPAR compared to patients without BPAR.
Recent studies have reported that operationally tolerant liver recipients exhibit significant alterations in their peripheral blood γδ T cell subsets. These changes include increased Vδ1 T cells and a marked increase in the Vδ1/Vδ2 ratio [6–9]. These results suggest that Vδ1 γδ T cells might be associated with spontaneous operational tolerance. However, the present study found that elevated Vδ1 γδ T and Vδ1/Vδ2 ratios were not related to acute rejection or graft failure. On the contrary, decreased Vδ2 γδ T cell counts were closely associated with graft tolerance.
Vδ1 T cells recognize heterogeneous and self-antigens induced by cell stress, such as bacterial or viral infections [4]. Populations of peripheral blood γδ T cells and Vδ1 cells expand substantially in CMV infection [16]. This expansion is associated with infection resolution, suggesting that these cells exert anti-rail effects in vivo [17]. However, the present study found that γδ T and Vδ2 T cell counts decreased with CMV infection, while the Vδ1/Vδ2 ratio increased. Our study did not show an increase in Vδ1 cell counts.
The present study did have several limitations. First, fewer cases of DDLT were studied than of LDLT. In addition, the small number of acute cellular rejection and CMV infection cases limited our ability to draw concrete conclusions. Second, all patients received immunosuppressive therapy. While this therapy undoubtedly alters γδ T cell populations, we did not determine the precise effects of immunosuppression on these populations. Third, T cell changes in peripheral blood do not necessarily reflect the T cell populations in liver tissue. Third, memory and effector T cells were not analyzed.
Conclusions
T cell populations in patients who received LDLT were not significantly different from those in patients who received DDLT, with the exception of CD3+ T cells. Patients with BPAR showed elevated CD3+ and CD8+ T cell counts. In addition, Vδ2 T cells are closely associated with CMV infection and graft failure. The Vδ1/Vδ2 ratio is a potentially prognostic parameter for CMV infection after liver transplantation. Monitoring of CD3+ and CD8+ T cells after liver transplantation is a promising strategy. The contribution of T cells to liver transplant recipient immunology warrants further investigation.
Abbreviations
- LDLT
living donor liver transplantation
- DDLT
deceased donor liver transplantation
- CMV
cytomegalovirus
- BPAR
biopsy-proven acute rejection
- MMF
mycophenolate mofetil
Footnotes
Source of support: Departmental sources
Conflicts of interest
The authors have no competing interests to declare.
References
- 1.Lee SG. A complete treatment of adult living donor liver transplantation: A review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17–38. doi: 10.1111/ajt.12907. [DOI] [PubMed] [Google Scholar]
- 2.Reichman TW, Katchman H, Tanaka T, et al. Living donor versus deceased donor liver transplantation: A surgeon-matched comparison of recipient morbidity and outcomes. Transpl Int. 2013;26:780–87. doi: 10.1111/tri.12127. [DOI] [PubMed] [Google Scholar]
- 3.Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: A systematic review and meta-analysis. Liver Transpl. 2014;20:425–36. doi: 10.1002/lt.23836. [DOI] [PubMed] [Google Scholar]
- 4.Kalyan S, Kabelitz D. Defining the nature of human gammadelta T cells: A biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10:21–29. doi: 10.1038/cmi.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malone F, Carper K, Reyes J, Li W. gammadeltaT cells are involved in liver transplant tolerance. Transplant Proc. 2009;41:233–35. doi: 10.1016/j.transproceed.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Puig-Pey I, Bohne F, Benitez C, et al. Characterization of gammadelta T cell subsets in organ transplantation. Transpl Int. 2010;23:1045–55. doi: 10.1111/j.1432-2277.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- 7.Koshiba T, Li Y, Takemura M, et al. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007;17:94–97. doi: 10.1016/j.trim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Llordella M, Puig-Pey I, Orlando G, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309–19. doi: 10.1111/j.1600-6143.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Koshiba T, Yoshizawa A, et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant. 2004;4:2118–25. doi: 10.1111/j.1600-6143.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Kwon CH, Joh JW, et al. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol. 2013;59:1215–22. doi: 10.1016/j.jhep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Kotton CN. CMV: Prevention, diagnosis and therapy. Am J Transplant. 2013;13(Suppl 3):24–40. doi: 10.1111/ajt.12006. quiz. [DOI] [PubMed] [Google Scholar]
- 12.Kim JM, Kim SJ, Joh JW, et al. Is cytomegalovirus infection dangerous in cytomegalovirus-seropositive recipients after liver transplantation? Liver Transpl. 2011;17:446–55. doi: 10.1002/lt.22249. [DOI] [PubMed] [Google Scholar]
- 13.Human and Mouse CD Marker Handbook. 2010. [accessed Oct 1, 2016]. http://www.bdbiosciences.com/documents/cd_marker_handbook.pdf.
- 14.Kim TS, Joh JW, Moon H, et al. The different etiology of fulminant hepatic failure (FHF) in Korea and prognostic factors in patients undergoing liver transplantation for FHF. Clin Transplant. 2013;27:297–302. doi: 10.1111/ctr.12055. [DOI] [PubMed] [Google Scholar]
- 15.Gupta P, Hart J, Cronin D, et al. Risk factors for chronic rejection after pediatric liver transplantation. Transplantation. 2001;72:1098–102. doi: 10.1097/00007890-200109270-00020. [DOI] [PubMed] [Google Scholar]
- 16.Pitard V, Roumanes D, Lafarge X, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–24. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafarge X, Merville P, Cazin MC, et al. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis. 2001;184:533–41. doi: 10.1086/322843. [DOI] [PubMed] [Google Scholar]