Abstract
Kaposi’s sarcoma (KS) is an incurable, HIV-associated malignancy. We reviewed 320 immunotherapy-treated patient records. Seventeen had HIV-associated malignancies, including nine men with KS. Median viral load was 20 copies/mL (range: undetectable to 549,704) and median CD4 count: 256 cells/μL (range: 10 to 603). Eight patients received nivolumab and one received pembrolizumab. Six patients (67%) achieved partial (N=5) or complete remission (N = 1). No drug-related grade >2 toxicities occurred. In seven patients, CD4 counts increased (P = 0.09). Tissue and/or blood-derived circulating tumor DNA (ctDNA) was evaluated by next generation sequencing. Four evaluable patients each showed anomalies in distinct genes: TP53, KRAS, TLL2, PTPN6 (tissue and/or ctDNA), and NF1 (ctDNA). Tumor mutational burden was low, and PD-L1 immunohistochemistry was negative (three and four assessable patients, respectively). Responders included patients with low CD4 counts, high HIV load, and/or visceral disease. In summary, checkpoint blockade demonstrated significant antitumor activity and low toxicity in patients with HIV-associated KS.
Keywords: Kaposi’s sarcoma, HIV, checkpoint inhibitors, nivolumab, pembrolizumab
Introduction:
Acquired immunodeficiency syndrome (AIDS)-related Kaposi’s sarcoma (KS) is a vascular tumor associated with human immunodeficiency virus (HIV) and human herpesvirus-8 (HHV-8) co-infection (1). Advanced KS typically presents with extensive cutaneous and visceral (gastrointestinal and pulmonary) involvement in antiretroviral therapy (ART)-naïve AIDS patients with low CD4 counts (<200 cells/μL). HIV-infected individuals with a CD4 count less than 200 cells/μL have an 18.9-fold increased rate of developing KS compared to individuals with CD4 counts greater than 500 cells/μL (2). Widespread use of ART has led to a decline in the incidence of HIV-related KS. The standardized incidence ratio (SIR) for KS compared to the general population fell from 22,100 to 3,640 since the introduction and prevalent use of ART (3). In addition to low CD4 counts, corticosteroid therapy is also associated with the induction and/or exacerbation of KS (4). Initiation of ART therapy, with subsequent improvement in CD4 counts, can lead to partial KS tumor regression, thus, providing substantial evidence for the role of weakened cellular immunity in the pathogenesis of KS. However, despite a reduced incidence of KS in the post-ART era, about 15% of HIV-infected patients, with high CD4 count and low viral load, still go on to develop KS (5,6), which, therefore, presents an unmet medical need.
An association has been demonstrated between chronic viral infection, malignancy, and upregulation of programmed death receptor 1 (PD-1) on CD8+ cytotoxic T-lymphocytes (CTLs) (7). In patients with chronic HIV infection, CD8+ T cells are functionally impaired, with a reduced capacity to secrete cytokines and carry out cellular cytotoxicity, which may decrease immune surveillance of neoplasms (8). HIV-specific CD8+ T cells have increased PD-1 expression, which further promotes a cellular milieu conducive to oncogenesis (9). Hence, overexpression of programmed death ligand 1 (PD-L1), seen in several virally associated tumors including Epstein Barr virus (EBV)-positive Hodgkin lymphoma, presents a clear target for PD-1 inhibitors and has been associated with excellent response to checkpoint blockade (10). Viral disease may upregulate specific genes, such as APOBEC (apolipoprotein B mRNA editing enzyme), which might create immunogenic neoantigens that may confer sensitivity to additional immune-based therapies (11,12). Thus, PD-L1 blockade has been shown to increase survival, proliferation, and cytokine production by HIV-specific CD8+ T cells in vitro (9).
Systemic chemotherapy is generally used for patients with advanced KS in the setting of disease progression. Standard therapy includes liposomal doxorubicin, paclitaxel, bleomycin, vinblastine, vincristine, and etoposide (13). However, chemotherapy is mostly palliative and often associated with myelosuppression, which may not be compatible with the already immunosuppressed environment and low CD4 counts in the majority of newly diagnosed KS patients in need of urgent therapy. Immunomodulating agents, including lenalidomide and bortezomib, have been used with variable efficacy (14). Of interest, PD-1/PD-L1 checkpoint blockade has been shown to be an effective therapy in numerous malignancies, including virally mediated tumors (15).
We analyzed the records of 320 patients treated at the Moores Cancer Center with immune checkpoint inhibitors. Of these patients, 17 patients with HIV-associated disease received immunotherapy, including nine individuals with KS. The latter are the subjects of this analysis, which includes reports on the next-generation sequencing (NGS) of tissue and blood-derived circulating tumor DNA (ctDNA) in KS patients, as well as the clinical outcomes and biologic correlates of PD-1 inhibitor administration in these patients. Overall, we demonstrated a high response rate for PD-1/PD-L1 checkpoint inhibitors in KS, even in the absence high tumor mutational burden and/or PD-L1 expression.
Methods:
Study patient population
We analyzed the medical records from patients treated from August 2013 through December 2017 and identified 320 individuals who had been given immunotherapy at the Moores Cancer Center at the University of California San Diego (UCSD). Of these patients, 17 had HIV-associated malignancies, of which nine had KS. Eight patients had received nivolumab (3 mg/kg; IV every two weeks) and one patient had received pembrolizumab (200 mg; IV every three weeks). The study was conducted in accordance with the Declaration of Helsinki and with UCSD Institutional Review Board-approved study guidelines. Written informed consent was obtained from each patient.
Pathology review of tumors and determining HHV-8 positivity
All patients had a pathologic confirmed diagnosis of KS. All tissue slides were re-reviewed by a dermatopathologist (PRC) to confirm diagnosis of KS. The tumor, present in the dermis or submucosa, consisted of a proliferation of vascular spaces containing erythrocytes and lined by spindle-shaped endothelial cells. The vascular tumor cells showed positive immunoperoxidase staining for either human herpesvirus-8 (HHV-8, three patients) and/or latency-associated nuclear antigen (LANA) for all nine patients.
Laboratory tests
CD4 and CD8 counts and HIV and HHV-8 viral load quantification:
Peripheral blood T-cell subsets were determined by flow cytometry. HIV-1 viral load was determined using HIV-1 RNA Ultra Quant detection test by reverse transcriptase polymerase chain reaction (RT-PCR; Roche HIV-1 v 2.0) with a detection range of 20–10,000,000 copies/mL. HHV-8 viral load was determined using PCR by the Associated Regional and University Pathologists (ARUP) laboratory with a detection range of 6,670–667,000,000 copies/mL. PD-1/PD-L1 status was determined by immunohistochemistry (IHC) performed by Foundation Medicine using antibodies against PD-1 (clone NAT105; CellMarque; Rocklin, CA) and PD-L1 (CD274, clone SP142; Spring Bioscience; Pleasanton, CA)
Next Generation Sequencing (NGS):
Formalin-fixed paraffin embedded (FFPE) tumor samples were analyzed by comprehensive genomic profiling (Foundation Medicine, a clinical laboratory improvement amendments (CLIA)-certified lab) using the FoundationOne hybrid-capture–based assay able to detect 405 genes (http://www.foundationone.com/). Average sequencing depth of coverage was greater than 250x, with >100x at >99% of exons (16).
For tumor mutational burden (TMB), the number of somatic mutations detected by NGS was quantified, and that value was extrapolated to the whole exome using a validated algorithm (16,17). Alterations with known and likely effects on functional status were not counted. TMB was measured in mutations per megabase (Mb). TMB levels were divided into three groups: low (1–5 mutations/mb), intermediate (6–19 mutations/mb), and high (≥ 20 mutations/mb).
For some patients, blood-derived ctDNA testing by the Guardant panel (73 genes detected by NGS) was obtained using Guardant360, Biopsy-Free™ Tumor Sequencing (https://guardanthealth.com).
Outcome Evaluation and Statistical Analysis
Outcome evaluation:
Patients’ tumors were staged in accordance with the AIDS Clinical Trials Group staging classification (ACTG) (Supplementary Table S2) (18). Patients were evaluated for response approximately every four weeks (KS response criteria as defined by the AIDS Malignancy Consortium (AMC)) (Supplementary Table S3)(18,19). According to these criteria, a partial response (PR) requires partial regression in either the cutaneous or noncutaneous sites of the disease, and no evidence of progression. A complete response (CR) requires disappearance of disease in both the cutaneous and noncutaneous (if applicable) sites of disease and no evidence of progression.
Progression-free survival (PFS) was calculated using the Kaplan and Meier method, with P values by log-rank (Mantel-Cox) test. PFS was considered from the start of checkpoint blockade. Patients were censored at date of last follow up for PFS if they had not progressed.
Statistical Analysis:
Comparison of values before and after treatment was done by the signed rank test (two-tailed p values). Statistical analyses were performed using Graph-Pad Prism version 7.0 (San Diego, CA, USA).
Results
Patient characteristics
Nine HIV-associated, biopsy-confirmed KS patients receiving care at the UCSD Moores Cancer Center were analyzed (Supplementary Fig. S1). Baseline characteristics are shown in Table 1 and Supplementary Table S1. All patients were men, median age was 44 years (range: 33 to 63 years), and median disease duration was 4 years (range: 0 to 12 years). Four of the nine patients (45%) had cutaneous only (T0) disease, and five patients presented with visceral (gastrointestinal, pulmonary, or nodal) involvement (T1), according to the AIDS Clinical Trials Group (ACTG) criteria (Supplementary Table S2)(18). All patients were receiving antiretroviral therapy, with well-controlled HIV viral load in 7 of the 9 patients (median: 20 copies/mL, range: 0 to 549,704). Five patients had a good-risk immune status (I0), defined by a CD4 T-cell count of ≥200 cells/μL, and four patients were poor-risk (I1; CD4 < 200 cells/μL).
Table 1:
Patient profiles and treatment response
| Case | Age (years)/ gender1 | ARV/yrs since KS diagnosis & Stage | # of prior lines of therapy | HIV-1 viral load (copies/mL) | Best response | PFS months2 | DNA (characterized) alterations (no VUSs) Tumor mutation burden (TMB in mutations/megabase)* | Immunohistochemistry for PD-L1 and PD1 | Comments | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-therapy | Post-therapy | (Tissue NGS)* | ctDNA** | PD-L1 /PD1 TIL*** | PD-L1/PD1 tumor*** | |||||||
| 1 | 63 M | Yes/4 Cutaneous, LN (T0I0S1) |
2 (Bortezomib, Lenalidomide) | <20 | 29 | SD | 6.5+ | KRAS Q61H TP53 R273C TMB = 4 | TP53 R175H TP53 K164R TP53 S240R TP53 F134* TP53 G244S | Negative/5% | Negative/not reported | History of DLBCL in CR s/p ASCT (reached maximum lifetime dose of doxorubicin). Platelets increased from 30 to 90 K/ul); hemoglobin, from 8 to 11 g/dl |
| 2 | 47 M | Yes/12 Cutaneous (T0I0S1) |
1 (Lenalidomide) | ND | 92 | PR | 5+ | NA | No alterations | NA | NA | Regressed nodularity, hyperpigmentation and size |
| 3 | 55 M | Yes/9 Cutaneous w/ lymphedema (T1I0S1) |
4 (Liposomal doxorubicin, Lenalidomide, Paclitaxel, Bortezomib) | ND | ND | PR | 3.5+ | NA | NF1 Q1370fs | NA | NA | Regressed nodularity and hyperpigmentation. |
| 4 | 44 M | Yes/1 Cutaneous (T0I1S1) |
0 | 22 | <20 | SD | 6.5+ | TLL2 G465E TMB = 3 | No alterations | Negative/50% | Negative/not reported | Refused chemotherapy. |
| 5 | 49 M | Yes/0 Cutaneous (T0I1S1) |
0 | 116,706 | 118 | PR | 6.5+ | Inadequate tissue | N/A | Negative/20% | Negative/not reported | Concomitant active TB at the time of KS therapy. Lesions regressed in size, nodularity, pigmentation and are less painful. Some lesions have resolved. |
| 6 | 42 M | Yes/0 GI (T1IS1) |
0 | 24 | 64 | CR | 5.5+ | Inadequate tissue | No characterized alterations (ATM L2698F VUS found) | Negative/ negative | Negative/ negative | Refused chemotherapy. Bloody diarrhea resolved. Repeat colonoscopy was negative |
| 7 | 41 M | Yes/6 Cutaneous, LN, lung (T1I1S1) |
3 (Liposomal doxorubicin, Paclitaxel, Bortezomib) | ND | 84 | SD | 3.5+ | PTPN6M1 TMB = 1 | No alterations | NA | NA | Some lesions have flattened, regressed nodularity and hyperpigmentation |
| 8 | 38 M | Yes/2 Cutaneous, LN, bowel (T1I1S1) |
1 (Liposomal doxorubicin) | 549,704 | 1,210,000 | PR | 1.5+ | NA | NA | NA | NA | Improved right leg lymphedema, abdominal and leg pain. |
| 9 | 33 M | Yes/8 Cutaneous, GI (T1I1S1) |
1 ((Liposomal doxorubicin) | ND | <20 | PR | 1.5+ | NA | No alterations | NA | NA | All lesions have improved; some lesions have resolved completely. |
Tissue next generation sequencing (NGS) performed by Foundation (see Methods).
Denotes Guardant360 plasma-derived circulating tumor DNA sequencing (see Methods).
Antibody used: PD-1/PD-L1 status was determined with immunohistochemistry (IHC) performed by Foundation Medicine, INC (Programmed Death 1 (clone NAT105 by CellMarque) Programmed Death Ligand 1, CD274 (clone SP142 by Spring Bioscience)). 1Patient’s age at start of treatment with PD1 blockade. 2+ means response is ongoing at the time of data censoring. Abbreviations: ARVs = anti-retrovirals; ASCT = autologous stem cell transplant; CR = complete response; DLBCL = Diffuse large B-Cell lymphoma; Dx = diagnosis; GI = gastrointestinal; HHV8 = human herpes virus 8; HIV = human immunodeficiency virus; KS = Kaposi sarcoma; LN = lymph nodes: M = male; MB = megabase; N/A = not available; ND = not detected; NGS = next generation sequencing; PFS = progression free survival; PR = partial response; RNA = ribonucleic acid; R/R = relapsed /refractory; SD = stable disease; TIL = tumor infiltrating lymphocytes; TMB = tumor mutational burden; VL = viral load; VUS = variants of unknown significance
Patients received a median of one line of prior therapy (range: 0 to 4), with the most common drug classes being anthracyclines (liposomal doxorubicin (N=4)), taxanes (paclitaxel (N=2)), proteasome inhibitors (bortezomib (N=3), and immune modulators (lenalidomide (N=3)). Three patients had no prior therapy (two patients declined standard cytotoxic therapy and one patient had active tuberculosis). One patient had a prior lymphoproliferative disorder and was treated with the standard EPOCHR (etoposide, prednisone, oncovin (vincristine) cyclophosphamide, hydroxydaunorubicin (doxorubicin), rituximab) (20), followed by radiation and autologous stem cell transplantation (patient #1, Table 1). Concomitant co-infections with human papilloma virus (N=9), hepatitis B virus (N=4), cytomegalovirus (N=3), and Mycobacterium tuberculosis (N=1) were identified.
Therapy and clinical outcome after checkpoint blockade
Eight patients received nivolumab and one patient pembrolizumab. The response rate (RR) was 66.6% (six of nine patients), with one complete remission (CR) (a patient with gastrointestinal disease) and five partial remissions (PR). The remaining three patients experienced ongoing stable disease (SD) that has lasted more than 3.5, 6.5, and 6.5 months, respectively (Table 1, Fig. 1, and Fig. 2), according to the response evaluation of the ACTG criteria (18) (Supplementary Table S3). No patient has exhibited disease progression and all remain on treatment. The median progression-free survival (PFS) has not been reached in the nine patients at a median follow up of 5 months. One patient with chronic idiopathic thrombocytopenia and anemia and stable skin lesions had significant improvement in platelet count (from ~30,000/μL to ~90,000/μL) and anemia (increased from 8 to 11 gm/dL) following initiation of nivolumab (patient #1, Table 1).
Figure 1. Baseline and post-therapy lesion photographs.
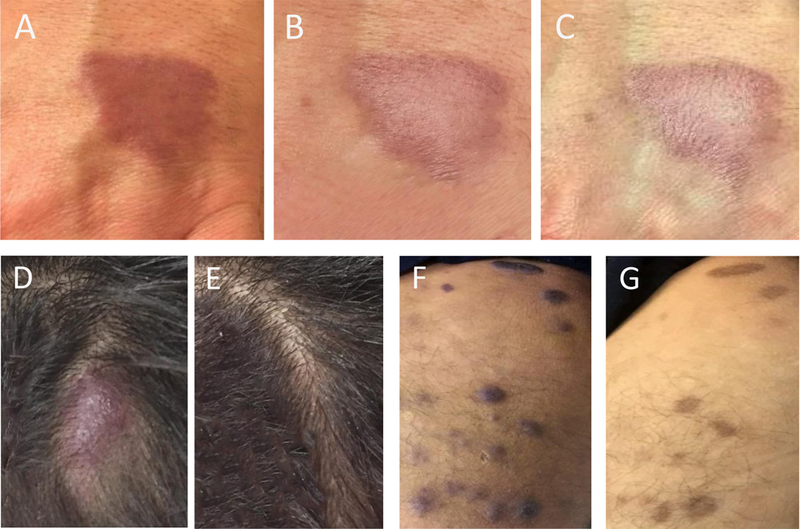
(A-C) Case example (patient 2). Left hand lesion (A) pre-therapy, (B) post 4 weeks and (C) post 8 weeks of therapy. (D-G) Case example (patient 9). Scalp lesion (D) pre-therapy and (E) post 6 weeks of therapy. Right medial thigh lesion (F) pre-therapy and (G) post 6 weeks of therapy.
Figure 2.
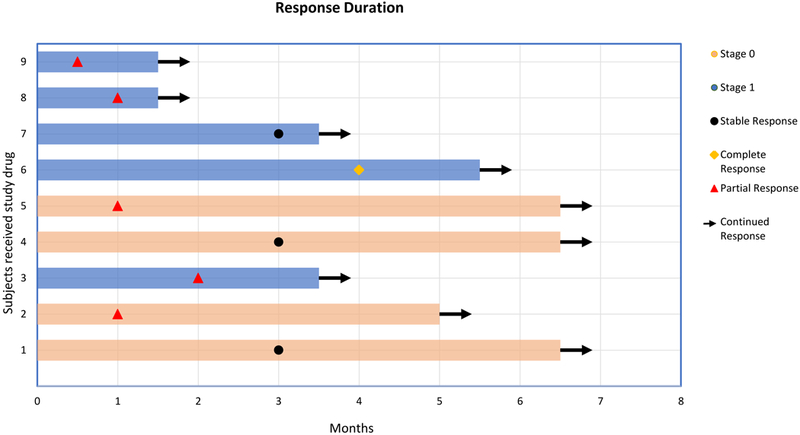
Swimmer response assessment plot. Each bar represents one patient in the study and their response duration. Right arrow cap: continued response. Stage 0 (T0): cutaneous only; stage 1 (T1): visceral/nodal disease. N=9.
Biological data
Seven of the nine patients (78%) on checkpoint inhibitor treatment experienced an improvement in CD4+ cell count, with an overall change in median increase of +104 cells/μL (p = 0.09) (Fig. 3). A similar increase was observed in CD8+ cell count in seven of the nine patients, although also non-significant, by a median of +166 cells/μL (p = 0.26). HHV-8 viral load status determined post-therapy was undetectable in all patients (pre-therapy status was not evaluated). However, tissue examination of 4 patients revealed positive immunoperoxidase staining for either HHV-8 (three patients) and/or latency-associated nuclear antigen (LANA, all nine patients). PD-L1 expression on both tumors and tumor-infiltrating lymphocytes (TILs) was negative in the four patients evaluated (Table 1).
Figure 3.
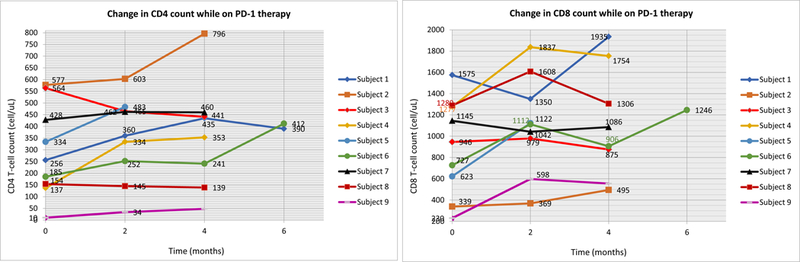
Change in CD4 and CD8 count while on checkpoint blockade therapy. Each line represents one patient in the study and their fluctuations in cell counts while on therapy. Left: CD4. Right: CD8. N=9.
Molecular Data
Eight patients were tested for genomic alterations in tissue and/or in blood-derived circulating tumor DNA (ctDNA) (Table 1). Tissue tumor mutational burden (TMB) was assessed in three patients, and NGS was performed on the tissue of five patients. In two cases, the sample was inadequate. However, one patient showed a KRAS and TP53 mutation, one showed a TLL2 (Tolloid-Like 2) mutation, and one had a PTPN6 (protein tyrosine phosphatase, non-receptor type 6) mutation (all characterized alterations, not variants of unknown significance (VUSs)) (Table 1).All three assessable patients had a low TMB (1, 3, and 4 mutations/Mb).
NGS was performed on blood-derived ctDNA for seven patients. In four of these individuals, no alterations in ctDNA were seen. However, one patient, who’s tissue showed a TP53 mutation, also had several mutations in ctDNA TP53, one patient had an NF1 alteration, and one patient had no characterized ctDNA alterations but did show an ATM VUS (in the latter two patients, tissue was either not done or inadequate) (Table 1).
Safety
No drug-related grade >2 toxicities were observed. (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf) Most common side effects included fatigue (56%) up to four days post-infusion, pruritus (44%), muscle/joint ache (22%), abdominal discomfort (11%), and onycholysis (11%). Following an insect bite while on therapy, one patient developed cellulitis of the affected extremity, with subsequent Staphylococcus aureus bacteremia, which improved after treatment with intravenous vancomycin. The patient resumed therapy without additional problems. One patient developed a delayed hypersensitivity reaction following initiation of an antibiotic (trimethoprim sulfamethoxazole) prophylaxis while on nivolumab treatment. The latter two events were determined to be unrelated to checkpoint inhibitor treatment.
Discussion
Herein, we reported that six of the nine patients (67%) with HIV-associated Kaposi’s sarcoma achieved an objective response (PR: N=5; CR: N=1) after treatment with immune checkpoint blockade with nivolumab or pembrolizumab. An additional two patients have had ongoing stable disease for over 6 months, and one patient remains stable for more than 3.5 months. Similar findings were reported by Delyon et al., demonstrating major clinical and metabolic responses in two patients with endemic, non-HIV–associated KS following the administration of nivolumab (21).
Patients with advanced HIV-associated KS tend to have a poor prognosis and limited duration of response to conventional chemotherapy. The current standard of care for advanced HIV-associated KS is liposomal doxorobucin, with response rates of approximately 58% (22). However, responses are usually not durable, with a median PFS of less than 150 days (22,23). A significant risk of neutropenia is also associated with liposomal doxorubicin, which can further exacerbate immunosuppression in the already immunocompromised KS patients in need of urgent therapy. Consequently, 10% of patients are likely to terminate chemotherapy early due to toxicity and infections (22).
Most of our patients received one to four prior lines of therapy but still responded to checkpoint blockade. No drug-related toxicity more than grade 2 have been reported, with low-grade fatigue, pruritus, and muscle aches being the most common side effects. Neutropenia was not observed. One patient, had significant improvement in both platelet count and hemoglobin while on nivolumab. Taken together, the side effect profile of PD-1 antibodies, unlike that of cytotoxic chemotherapy, is not associated with further myelosuppression, making the use of these agents an attractive option for patients with HIV. Similar to other monoclonal antibody cancer therapies, PD-1 blocking antibodies may have limited drug interactions, making them appealing for patients receiving ART.
Evidence indicates that PD-1/PD-L1 blockade might be effective in controlling HIV infection, thus, allowing faster reconstitution of the immune system (24). Our data support this notion, with CD4 and CD8 counts both increasing in most patients, although not to levels statistically significant and perhaps due to the limited sample size. Seven patients had an HIV viral load less than 50 copies/mL, but two had higher viremia. Both patients with high viral loads achieved a PR on treatment with immune checkpoint blockade (and HIV viral load decreased in one patient, but increased in the other). HHV-8 viral load was not tested pre-therapy, but it was undetectable in all patients after therapy.
In contrast to other virally related malignancies, including EBV-associated classical Hodgkin lymphoma and extra-nodal NK/T-cell lymphoma, HHV-8–associated KS does not typically have high expression of PD-L1 (25). In our data set, PD-L1 expression on both tumors and TILs was not detected in the four patients tested. However, despite these findings, we demonstrated that HHV-8–associated KS could respond to PD-1 blockade. Two of the four patients achieved a CR and PR, respectively, and the other two patients have had ongoing stable disease for over six months. Although PD-L1 expression by IHC is a common biomarker for response to PD-1/PD-L1 blockade, its use has limitations. Across cancers, response rates are approximately 0% to 17% in PD-L1–negative tumors versus 36% to 100% in PD-L1–positive tumors (26). Technical and other factors may limit the predictive ability of PD-L1 IHC. The higher-than-anticipated objective response rates in tumors with low PD-L1 expression have also been reported in Polyoma virus (MCPyV)-associated Merkel-cell carcinoma and other virally driven cancers, suggesting that the presentation of viral antigens on tumors may confer an increased response rate to anti–PD-1 therapy (27). The presence of oncogenic viruses in virus-mediated cancers, wherein, viral antigens serve as tumor-specific antigens, has been postulated as a potential marker that can predict response to anti–PD-1 therapy (28).
In other cancers, various features such as high TMB might be associated with immunotherapy response (28,29). Our patients had low TMB. Previously, it was shown that only about 5% of patients harboring cancers with low TMB respond to checkpoint blockade. Therefore, the underlying biology leading to response of KS to anti–PD-1 agents remains unclear. However, it is well known that virus-associated cancers frequently have low or modest mutational burdens, owing to tumorigenesis driven by the dominant effects of viral oncogenes. Viral antigens are foreign and, thus, potentially strong immune stimulants that can lead to a robust response to checkpoint blockade (28). Kaposi sarcoma herpes virus (KSH) extensively modulates the immune system, activating both innate and adaptive immune responses including KSHV-specific T cells (30). Another mechanistic factor that may be of interest in this regard is upregulation of APOBEC (apolipoprotein B mRNA editing enzyme), resulting from viral infections. Increased APOBEC activity may cause clusters of localized hyper-mutations (designated kataegis) in human cancers and has, therefore, been termed ‘mutagenic fuel’ for cancer evolution and heterogeneity (11,12). The role of APOBEC and kataegis in KS merits further investigation. Finally, it is also plausible that HHV-8 viral-derived antigens are sufficient to elicit immune responses. Kaposi tumors occur mostly in severely immunocompromised patients, which suggests that HHV-8 might be immunogenic.
We also provide results of both tissue and blood-derived ctDNA molecular profiling in KS. The three evaluable cases each had distinct molecular profiles that included anomalies in TP53, KRAS, TLL2, and PTPN6 genes. In seven patients, interrogation of ctDNA was attempted, with four cases showing no alterations. The other three had genomic alterations in TP53, NF1, ATM genes in their ctDNA. These genes are involved in several distinct cellular pathways that play a role in tumorigenesis and immunity. Taken together, these results suggested that, as with many other malignancies, diverse genomic alterations could be associated with KS. Further interrogation of KS lesions with advanced molecular techniques are warranted.
In conclusion, our observations suggest that patients with HIV-associated KS have high response rates to PD-1 checkpoint blockade, without significant toxicity, even in the presence of low TBM and/or lack of PD-L1 expression. Suppression of blood counts was not observed, and one patient who suffered from chronic idiopathic thrombocytopenia and anemia had improvement in both platelet count and hemoglobin levels. CD4+ and CD8+ cell counts were also not adversely affected by this therapy, and most of our patients experienced a rise in counts. Genomic analysis of tissue and blood-derived ctDNA showed distinct molecular profiles in each patient with available data and tissue mutational burden was low. A more in-depth study with a larger number of patients will be required to ascertain if an association between the KS mutanome and immune responsiveness exists. One of the major limitations of our report, in addition to the small number of patients, was the paucity of archival tissue material available to conduct multiple analyses of interest. PD-L1 expression was negative in the four patients tested, yet all four individuals attained an objective response (PR or CR) or stable disease lasting more than six months. Responders included patients with low baseline CD4+ cell counts and those with visceral disease and/or high HIV load. Based on our observations, longer follow-up and larger prospective trials with immune checkpoint inhibitors are warranted. Importantly in this regard, the NCI/AIDS Malignancy Consortium is conducting a prospective study of combined nivolumab and ipilumumab in patients with HIV-related cancers (ClinicalTrials.gov Identifier: NCT02408861), as well as single agent pembrolizumab for patients with HIV and relapsed, refractory, or disseminated malignant neoplasms (NCT02595866).
Supplementary Material
Acknowledgments
Funding: Funded in part by National Cancer Institute grant P30 CA023100 and the Joan and Irwin Jacobs Fund philanthropic fund.
Footnotes
Conflict of Interests Disclosure statement: Dr. Kurzrock receives research funding from Incyte, Genentech, Merck, Serono, Pfizer, Sequenom, Foundation Medicine, and Guardant, as well as consultant fees from X Biotech, Loxo, and Actuate Therapeutics, speaker fees from Roche, and has an ownership interest in Curematch Inc. Garrett M. Frampton is an employee of Foundation Medicine. Drs. Galanina, Goodman and Cohen have no disclosures.
References:
- 1.Moore PS. Hot papers - Virology - Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and those without HIV infection by Moore PS, Chang Y - Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas by Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM - Comments. Scientist 1997;11(10):12-. [Google Scholar]
- 2.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K. Kaposi Sarcoma Incidence and Survival Among HIV-Infected Homosexual Men After HIV Seroconversion. Journal of the National Cancer Institute 2010;102(11):784–92 doi 10.1093/jnci/djq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids 2006;20(12):1645–54 doi DOI 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 4.Trattner A, Hodak E, David M, Sandbank M. The appearance of Kaposi sarcoma during corticosteroid therapy. Cancer 1993;72(5):1779–83. [DOI] [PubMed] [Google Scholar]
- 5.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. The New England journal of medicine 2007;357(13):1352–3 doi 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 6.Krown SE, Lee JY, Dittmer DP, Consortium AM. More on HIV-associated Kaposi’s sarcoma. The New England journal of medicine 2008;358(5):535–6; author reply 6 doi 10.1056/NEJMc072994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol 2017;14(4):203–20 doi 10.1038/nrclinonc.2016.168. [DOI] [PubMed] [Google Scholar]
- 8.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 2000;96(9):3094–101. [PubMed] [Google Scholar]
- 9.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006;12(10):1198–202 doi 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. The New England journal of medicine 2015;372(4):311–9 doi 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boichard A, Tsigelny IF, Kurzrock R. High expression of PD-1 ligands is associated with kataegis mutational signature and APOBEC3 alterations. Oncoimmunology 2017;6(3) doi ARTN e1284719 10.1080/2162402X.2017.1284719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanton C, McGranahan N, Starrett GJ, Harris RS. APOBEC Enzymes: Mutagenic Fuel for Cancer Evolution and Heterogeneity. Cancer discovery 2015;5(7):704–12 doi 10.1158/2159-8290.CD-15-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee FC, Mitsuyasu RT. Chemotherapy of AIDS--related Kaposi’s sarcoma. Hematol Oncol Clin North Am 1996;10(5):1051–68. [DOI] [PubMed] [Google Scholar]
- 14.Pourcher V, Desnoyer A, Assoumou L, Lebbe C, Curjol A, Marcelin AG, et al. Phase II Trial of Lenalidomide in HIV-Infected Patients with Previously Treated Kaposi’s Sarcoma: Results of the ANRS 154 Lenakap Trial. AIDS Res Hum Retroviruses 2017;33(1):1–10 doi 10.1089/AID.2016.0069. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909–20 doi 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature biotechnology 2013;31(11):1023–31 doi 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer immunology research 2016;4(11):959–67 doi 10.1158/2326-6066.CIR-16-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1989;7(9):1201–7 doi 10.1200/JCO.1989.7.9.1201. [DOI] [PubMed] [Google Scholar]
- 19.Krown SE, Testa MA, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1997;15(9):3085–92 doi 10.1200/JCO.1997.15.9.3085. [DOI] [PubMed] [Google Scholar]
- 20.Cabanillas F, Shah B. Advances in Diagnosis and Management of Diffuse Large B-cell Lymphoma. Clin Lymphoma Myeloma Leuk 2017;17(12):783–96 doi 10.1016/j.clml.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Delyon J, Bizot A, Battistella M, Madelaine I, Vercellino L, Lebbe C. PD-1 blockade with nivolumab in endemic Kaposi sarcoma. Ann Oncol 2018;29(4):1067–9 doi 10.1093/annonc/mdy006. [DOI] [PubMed] [Google Scholar]
- 22.Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol 1998;16(2):683–91 doi 10.1200/JCO.1998.16.2.683. [DOI] [PubMed] [Google Scholar]
- 23.Cooley T, Henry D, Tonda M, Sun S, O’Connell M, Rackoff W. A randomized, double-blind study of pegylated liposomal doxorubicin for the treatment of AIDS-related Kaposi’s sarcoma. Oncologist 2007;12(1):114–23 doi 10.1634/theoncologist.12-1-114. [DOI] [PubMed] [Google Scholar]
- 24.Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology 2015;12:14 doi 10.1186/s12977-015-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen BJ, Chapuy B, Jing OY, Sun HH, Roemer MGM, Xu ML, et al. PD-L1 Expression Is Characteristic of a Subset of Aggressive B-cell Lymphomas and Virus-Associated Malignancies. Clinical Cancer Research 2013;19(13):3462–73 doi 10.1158/1078-0432.Ccr-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015;14(4):847–56 doi 10.1158/1535-7163.Mct-14-0983. [DOI] [PubMed] [Google Scholar]
- 27.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. The New England journal of medicine 2016;374(26):2542–52 doi 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. The New England journal of medicine 2017;377(25):2500–1 doi 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther 2017;16(11):2598–608 doi 10.1158/1535-7163.Mct-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Host KM, Jacobs SR, West JA, Zhang Z, Costantini LM, Stopford CM, et al. Kaposi’s Sarcoma-Associated Herpesvirus Increases PD-L1 and Proinflammatory Cytokine Expression in Human Monocytes. MBio 2017;8(5) doi 10.1128/mBio.00917-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


