Abstract
It is increasingly appreciated that perinatal events can set an organism on a life-long trajectory for either health or disease, resilience or risk. One early life variable that has proven critical for optimal development is the nutritional environment in which the organism develops. Extensive research has documented the effects of both undernutrition and overnutrition, with strong links evident for an increased risk for obesity and metabolic disorders, as well as adverse mental health outcomes. Recent work has highlighted a critical role of the immune system, in linking diet with long term health and behavioral outcomes. The present review will summarize the recent literature regarding the interactions of diet, immunity, and behavior.
Keywords: diet, nutrition, immune, inflammation, microglia, cytokine, IL-18, obesity, calorie restriction, lipopolysaccharide
1. Introduction
The incidence of obesity has now reached epidemic proportions within our society, with upwards of 50 percent of the population classified as overweight or obese in many developed countries (Colagiuri et al., 2010; Ogden et al., 2014). Dietary factors clearly play a significant role in contributing to this phenomenon. Obesity is also characterized by systemic and central inflammation that causes and contributes to excess fat deposition (De Souza et al., 2005; Gregor & Hotamisligil, 2011; Spencer, 2013a). This inflammatory profile leaves the individual highly susceptible to disease, particularly to those relevant to immune dysfunction, including diabetes, cancers, mood disorders, vascular dysfunction, and susceptibility to infection (Haslam & James, 2005; Noria & Grantcharov, 2013).
The developmental origins of health and disease (DOHAD) hypothesis suggests there are certain ‘critical windows’ throughout early life when aspects of physiology, including brain development, are particularly vulnerable to environmental influences. In utero development and early postnatal life are two of these. At these times, the nutritional environment can alter the ability of an organism to integrate metabolic and feeding information, thus predisposing the organism to excess weight gain long-term. Outside these critical windows, metabolic and feeding pathways are potentially less plastic, but can still be shaped by dietary influences. For instance, medium to long-term ‘dieting’ (calorie restriction) in adult life can improve health and lifespan, in part by altering the hypothalamic inflammatory milieu. This central immune profile is important in regulating hypothalamic pathways involved in feeding, and life-long alterations in cytokine balance can greatly influence how one processes nutritional information. Diet, and the associated changes in the immune profile are not only critical in programming metabolic and feeding-related information, but have significant influence extending beyond the hypothalamus and into brain regions governing executive function. As such, our diet can be incredibly important in establishing several aspects of our behavioral and immune profile across our lifespan (Figure 1). In this review we will address these aspects of the importance of diet throughout life.
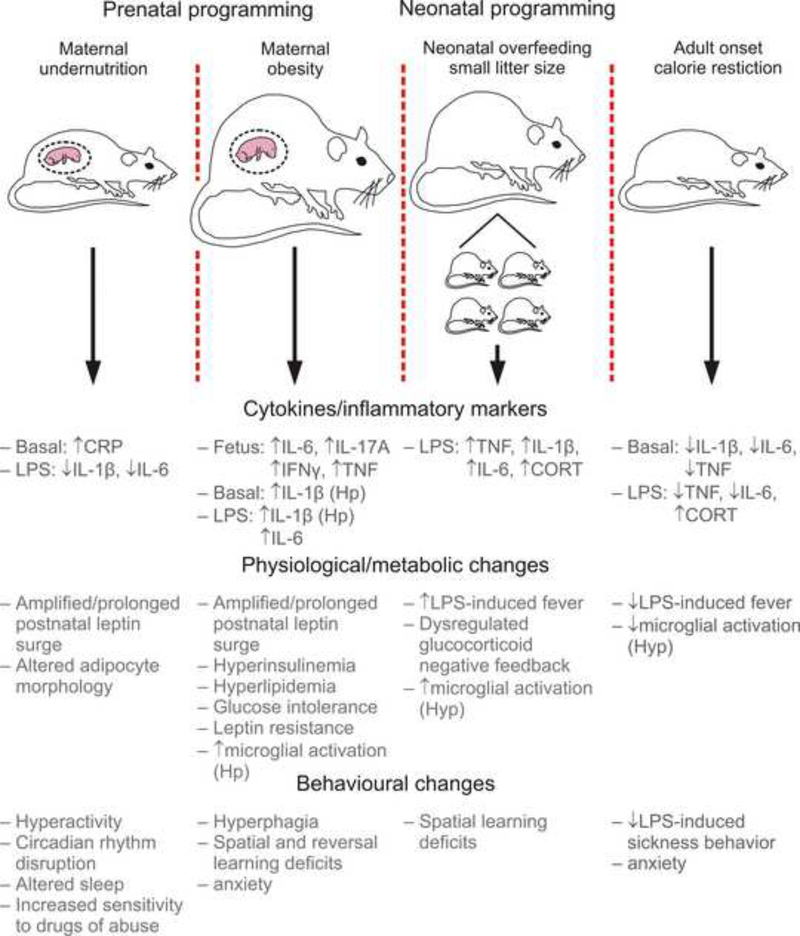
Figure 1.
Exposure to maternal under- or over-nutrition during gestation, or early life overfeeding can have life-long consequences for immune system functioning, metabolism and behavior. The schematic illustrates two rodent models of prenatal programming, maternal undernutrition and maternal obesity; and one rodent model of neonatal programming, suckling rats in small litters, along with some examples of the physiological, metabolic, and behavioral changes that occur as a result. The schematic also illustrates a model of adult onset calorie restriction, which can have a number of health promoting benefits.
Abbreviations: CORT, corticosterone; CRP, C-reactive-protein; Hp, hippocampus; Hyp, hypothalamus; LPS, lipopolysaccharide; TNF, tumor necrosis factor
2. Prenatal dietary programming of behavior and immunity across the lifespan
Nutrition during gestation can have lifelong impacts on an individual’s health and behavior. In this period of embryonic and fetal development the mother’s nutrition, particularly as it relates to her body weight and metabolism, appears to be absolutely critical to the later life health of her offspring.
This concept was first appreciated when epidemiological studies examined the long-term health outcomes of individuals who were in utero during the Dutch Hunger Winter (1944–1945). The offspring of these undernourished mothers had low birth weight and impaired glucose tolerance later in life compared to individuals born in flanking years (Ravelli et al., 1998). These observations inspired the concept that an individual’s risk of disease across their lifespan could be shaped by events that occurred much earlier in their lives; indeed during their time in the womb (Barker, 2004). Interestingly, the timing of exposure to famine within the gestation period is also critically important. Thus, people exposed to famine in early gestation in the Dutch Hunger Winter were at greater risk of developing coronary heart disease and obesity in later life, whereas those exposed to famine in mid and late gestation were not similarly affected (Ravelli et al., 1976; Ravelli et al., 1999; Roseboom et al., 2000b). Moreover, impaired glucose tolerance in offspring appears more likely to result from undernutrition in late than early gestation (Ravelli et al., 1998). Within the Barker study, and a subsequent study that examined the Chinese Famine in the late 1950s-60s, was the further observation that offspring who were most affected were those who, as their lives progressed, consumed a relatively high-fat Western diet and became obese (Ravelli et al., 1998; Li et al., 2010). Thus, maternal undernutrition elevates the risk of low birth weight babies, which in turn elevates the risk for obesity and diabetes later in life (Barker, 2006; Gluckman et al., 2008). In addition to elevating the risk for obesity, exposure to famine in utero has also been linked to an increased risk for adverse mental health outcomes, including schizophrenia (Susser & Lin, 1992; Clair et al., 2005), addiction (Franzek et al., 2008), and affective disorders (Brown et al., 1995).
With the recent increase in obesity across the Western world, much focus has now shifted to understanding the long-term health outcomes of individuals whose mothers were obese prior to or during pregnancy. Curiously, the epidemiological data paint a similar picture to undernutrition. Individuals whose mothers were obese during pregnancy show rapid postnatal growth, and a significantly higher risk for later life obesity, and “the metabolic syndrome” (Law et al., 1992; Gale et al., 2007; Armitage et al., 2008; Crozier et al., 2010; Tamashiro & Moran, 2010; Alfaradhi & Ozanne, 2011; Ornoy, 2011). Further, they also show increased risk for a constellation of behavioral and mental health problems, including autism, attention deficit/hyperactivity disorder, developmental delay, anxiety, and depression (Herva et al., 2008; Rodriguez, 2010; Van Lieshout & Boyle, 2011a; Van Lieshout & Boyle, 2011b; Colman et al., 2012; Halmoy et al., 2012; Krakowiak et al., 2012; Moore et al., 2012; Wojcik et al., 2013), risks which are also shared between those that experience undernutrition in utero (Grissom & Reyes, 2012). Finally, maternal dietary micronutrients and exposure to toxicants independent of body weight have both been reported to alter offspring metabolic and neurobehavioral disease risk. For example, in Indian populations it was found that low maternal vitamin B12 increased offspring risk for insulin resistance, and exposure in utero to the fungicide tibutyltin elevates offspring adiposity (Heindel, 2003; Yajnik et al., 2008; Heindel & Schug, 2013).
Although nutrition during gestation and nutrition after birth are key contributors to neurobehavioral disease risk across the lifespan, the mechanisms that link these factors, either alone or together, to health risks and outcomes remain poorly understood. Animal models, typically rodent, sheep, or non-human primate (NHP), have been invaluable in shedding light on the cellular and molecular details occurring behind the scenes. In these models, research has traditionally focused on defining the altered function of offspring peripheral organs involved in metabolism, such as the pancreas, liver, adipose depots, and skeletal muscle whose function is impaired in obesity and the metabolic syndrome. However, since body weight and metabolic function are coordinately regulated by the brain (Stanley & Leibowitz, 1984; Fan et al., 1997; Cowley et al., 1999; Horvath et al., 1999; Cone et al., 2001; Cowley et al., 2001; Elmquist et al., 2005), and since maternal nutrition during pregnancy programs an assortment of offspring neurological abnormalities, studies examining changes in the offspring brain have become increasingly prevalent.
2.1. Animal models of maternal undernutrition during pregnancy
Two common models of undernutrition have been used: global undernutrition ranging from 20–70% of normal calories; and protein restriction, typically ~8% instead of 20% in normal diet. Both produce intrauterine growth restriction (IUGR) and low birth weight in animal models. After birth, IUGR leads to rapid postnatal catch-up growth, an accompanying increased risk of metabolic pathologies later in life (Cottrell, 2007; Fernandez-Twinn, 2006), and a significantly reduced lifespan (Jennings et al., 1999; Ozanne & Hales, 2004). It is now becoming apparent that limiting this catch-up growth / very rapid postnatal weight gain can eliminate the risk of obesity developing. Thus, IUGR rodents do not develop obesity if their food is also restricted during the suckling period, but do become obese if allowed ad libitum food during this time (Desai et al., 2005; Berleze et al., 2009). In humans, data from the 1941–44 Siege of Leningrad Famine also support the notion of the importance of catch-up growth. Unlike in the Dutch Famine, the Leningrad Siege saw victims having poor nutrition for long periods before and after the famine, which likely meant there was no opportunity for significant catch-up growth (Leon et al., 1997; Roseboom et al., 2000a). Consequently, there was no association between perinatal malnutrition and adult obesity in this cohort (Stanner et al., 1997). The dilemma for physicians is that catch-up growth in IUGR babies can be essential for appropriate brain and lung development, so strategies to limit accelerated early postnatal weight gain need to be approached with caution (Brandt et al., 2003).
The very early postnatal responses of the offspring to maternal undernutrition are signaled in the neonatal period by a premature, and kinetically altered postnatal leptin surge (Yura et al., 2005). Hypothalamic development has been shown to be vulnerable to maternal malnutrition, with offspring demonstrating alterations in hypothalamic metabolism and plasticity (Alexandre-Gouabau et al., 2012), as well as extensive alterations in neuropeptide expression (Núñez et al., 2008; Remmers et al., 2008; Coupé et al., 2009). Thereafter, the later-life risks are underpinned by persistent changes in the function of peripheral metabolic tissues in adulthood. For example, liver function appears to be biased toward conservation of energy stores, including altered gluconeogenesis, greater glycogen content, and enhanced glucose storage (Burns et al., 1997; Gosby et al., 2003; Lie et al., 2014). Adipocyte morphology may be altered, response to hormones disrupted, and expression of inflammatory markers and macrophage infiltration increased (Guan et al., 2005; Ferland-McCollough et al., 2012; Reynolds et al., 2013b). Accompanying the inflammatory changes in adipose tissue, bone marrow macrophages appear hypersensitive to challenge, at least when presented in vitro with an acute inflammatory stimulus (lipopolysaccharide; LPS) (Reynolds et al., 2013a), while circulating immune cells appear to respond in an opposing manner, with attenuated peripheral T-cell responses (Badr & Mohany, 2011) and decreased responses to LPS (Desai et al., 2009). In the brain, for example, the development of hypothalamic neurons and their neural circuits in the postnatal period is disrupted (Delahaye et al., 2008; Coupe et al., 2010; Rocha et al., 2014), markers of oxidative stress may be increased, and myelination defects stemming from disrupted oligodendrocyte differentiation have been observed (Reid et al., 2012). Behaviorally, growth-restricted offspring have shown hyperactivity (Vucetic et al., 2010b), circadian disruptions (Sutton et al., 2010; Orozco-Solís et al., 2011), altered sleep homeostasis (Shimizu et al., 2013), and increased sensitivity to drugs of abuse (Shultz et al., 1999; Valdomero et al., 2007; Palmer et al., 2008; Vucetic et al., 2010b). For example, Shimizu et al showed that 50% dietary restriction from embryonic day (E)12 to birth leads to an enhancement of electroencephalogram (EEG) slow wave activity (SWA) during non-rapid eye movement (NREM) sleep without changing the diurnal pattern or amounts of wakefulness, NREM, or rapid eye movement (REM) sleep. In addition, restricted mice also displayed an enhancement of EEG-SWA rebound after 6 hours sleep deprivation and a higher threshold for waking in the face of external stimuli (Shimizu et al., 2013). Accompanying these behavioral changes, alterations in dopamine (Palmer et al., 2008; Vucetic et al., 2010b), glutamate (Palmer et al., 2008), and catecholamine (Petry et al., 2000) expression have been observed. Reductions in circulating levels of ACTH, adrenaline, and noradrenaline, but not leptin or testosterone have also been reported (Govic et al., 2008; Levay et al., 2008; Levay et al., 2010a).
2.2. Animal models of maternal obesity during pregnancy
Animal models of maternal obesity typically use a high fat or high fat / high sugar (“cafeteria”) diet to induce obesity prior to mating, although variations including high fat feeding at the time of mating, or high fat feeding in a particular window of gestation, have also been reported. The offspring of obese pregnancies from a variety of animal models, including rodent, sheep and NHP, show changes in peripheral metabolic tissues that are remarkably similar to those observed in the offspring from undernourished mothers. For example, among rats, neonates from obese pregnancies show an amplified and prolonged leptin surge accompanied by elevated leptin mRNA expression in abdominal white fat (Kirk et al., 2009). In rat, mouse and sheep models, the offspring of obese mothers become hyperinsulinemic and hyperlipidemic, and show glucose intolerance, leptin resistance, increased adiposity (Ferezou-Viala et al., 2007; Samuelsson et al., 2008; Morris & Chen, 2009; Nivoit et al., 2009; Long et al., 2010), and disrupted insulin signaling in adipose tissue (Fernandez-Twinn et al., 2014). In mouse and NHP models, altered liver function, including evidence of oxidative damage, altered mitochondrial function, and lipid accumulation with a propensity to develop non-alcoholic fatty liver disease have also been reported (Bruce et al., 2009; McCurdy et al., 2009; Alfaradhi et al., 2014).
Maternal obesity can also have adverse consequences for offspring immune system functioning. Maternal obesity during pregnancy is a risk factor for offspring obesity and is associated with increased circulating high sensitivity C-reactive protein (hs-CRP) in offspring at 12 years of age (Leibowitz et al., 2012). Consistent with preclinical findings, in a rat model of maternal obesity, exposure to a high fat diet during pregnancy increases basal and LPS-stimulated interleukin (IL)-1β concentrations in the hippocampus and increased LPS-stimulated IL-6 serum concentrations in adult offspring compared with controls (Bilbo & Tsang, 2010). Furthermore, exposure to maternal high fat diet increases basal and LPS-induced microglial activation in the hippocampi of neonatal and adult offspring compared with controls (Bilbo & Tsang, 2010).
Behaviorally, among rats and mice, offspring born to obese dams can exhibit hyperphagia (Samuelsson et al., 2008; Nivoit et al., 2009). This is likely to be driven by a combination of changes taking place in the regions of the brain that regulate body weight and homeostatic food intake, including the arcuate nucleus of the hypothalamus (ARC) and the paraventricular nucleus of the hypothalamus (PVN). Additionally, brain areas associated with food seeking and reward are known to be altered by maternal obesity and/or consumption of a high fat diet. These changes include altered expression of appetite-regulating neuropeptides, altered proliferation of body weight-regulating neurons (Chang et al.; Chen et al., 2009), ARC neuron leptin resistance, as judged by reduced phosphorylated signal transducer and activator of transcription 3 (pSTAT3) in response to exogenous leptin administration, and developmental alterations in the neural circuitry that regulates body weight (Bouret et al., 2008; Kirk et al., 2009; Sanders et al., 2014a). In the reward pathways, altered gene expression in the opioid system (Grissom et al., 2014), and changes in the mesolimbic dopamine pathways, including alterations in gene expression of DA-related genes (Vucetic et al., 2010a), increased dopamine synthesis in the nucleus accumbens (NAc) and ventral tegmental area (VTA), and altered dopamine responsiveness in the NAc accompanied by reduced dopamine receptor D2 expression in the VTA (Naef et al., 2008; Naef et al., 2011). In the offspring hippocampus, abnormalities have been observed in neural circuit formation, neurogenesis and cell death both in the late gestation fetus and in the early postnatal period (Niculescu & Lupu, 2009; Tozuka et al., 2009; Tozuka et al., 2010).
Although relatively less studied compared to the effects of in utero growth restriction, the effects of maternal obesity or excessive gestational weight gain during pregnancy on the developing child have begun to be explored (Lieshout et al., 2011). Epidemiological studies have found support for a link between maternal obesity and adverse neurobehavioral development, including cognitive delay (Casas et al., 2013; Tanda et al., 2013), and inattention and negative emotionality (Rodriguez, 2010), although negative findings have also been reported (Brion et al., 2011). Maternal conditions, including diabetes, hypertension and/or obesity were also found to increase the risk for developmental delay and autism (Krakowiak et al., 2012)..
In animal models, offspring from obese and/or high fat fed dams also exhibit behavioral changes that are consistent with the associations between maternal obesity and some aspects of offspring mental illness observed in humans. For example, in rat and mouse models, the offspring of obese mothers show defects in spatial learning in the Barnes maze test (Tozuka et al., 2010), elevated anxiety (Bilbo & Tsang, 2010; Bland et al., 2010; Can et al., 2012; Sasaki et al., 2014) and altered reward seeking behaviors (Naef et al., 2008; Naef et al., 2011), with an increased locomotor response to a D2/D3 agonist, and increased operant responding for fat, but not sugar, in rats born to dams fed a high fat diet during gestation (Naef et al., 2011). Similarly, dams that were fed either a high fat diet or a highly palatable diet (high in carbohydrates) had offspring that demonstrated deficits in reversal learning, as well as decreased dopamine transporter (DAT) binding in the caudate, deficits that existed even in the absence of differences in body weight or serum glucose, insulin, or leptin (Wu et al., 2013). In the postnatal period, hippocampal changes appear to be underpinned by increased oxidative stress and a deficiency in brain derived neurotrophic factor (BDNF) (Tozuka et al., 2009; Tozuka et al., 2010).
Although not comprehensive, the above summary demonstrates that substantial progress has been, and is being, made in defining the spectrum of changes that develop after birth when an individual has undergone gestation in an obese or undernourished mother. What remains to be discovered is what happens during gestation: what molecular factors are important, and how do they act on the developing organism to affect the changes that increase disease risk much later in life?
2.3. Inflammation during pregnancy - A causative role?
Although many factors are involved in programming an individual’s disease risk in later life, the immune system stands out for its importance in contributing to obesity and its sequelae because it has a critical and highly regulated role at the fetal-maternal interface during normal pregnancy; for example, ensuring appropriate trophoblast invasion, and tolerance of the mother’s immune system to the developing, immunologically distinct, fetus (Lin et al., 1993; Arck & Hecher, 2013). Thus, the focus here will be on factors produced by the immune system that may have the ability to modulate the course of development and/or developmental programming. Cells of the immune system produce a vast number of molecules whose purpose is mainly to modulate immune system function, be it local inflammation following tissue injury or the more systemic, whole body, response to foreign substances. These regulatory molecules, called cytokines, fall into two broad classes: pro-inflammatory and anti-inflammatory. In the classic immune response the two antagonize one another in a carefully orchestrated manner in order to precisely regulate the vigor and duration of the immune response. These interactions are highly complex; so much so that some cytokines may have both pro- and anti-inflammatory actions in distinct situations. In addition, cytokines act via receptors that are present on many cell types that are classically considered outside the immune system, including neurons, hepatocytes, cardiac and skeletal muscle (Fahmi et al., 2013; Kammoun et al., 2013; Sanders et al., 2014b). Moreover, cytokine:receptor interactions are able to initiate signaling pathways, such as kinase cascades, that can regulate biochemical processes and changes in cellular physiology in a variety of cell types. Not surprisingly, they are beginning to surface as candidates that when aberrantly present, could disrupt developmental processes before birth.
2.3.1. Maternal inflammatory cytokines
Low grade, chronic activation of the immune system and the elevation of inflammation-modulating cytokines are hallmarks of both undernourished and obese adults (Hotamisligil et al., 1993; Fried et al., 1998; Das, 2001; Sartipy & Loskutoff, 2003; Weisberg et al., 2003; Khaodhiar et al., 2004; De Souza et al., 2005; Bastard et al., 2006; Wisse et al., 2007). This has led to the idea that in the pregnant state, maternal body weight-associated inflammatory factors from the mother’s circulation may impinge upon the embryo, fetus or both, and act upon responsive cells. A variety of cytokines, including, IL-1β, IL-6, monocyte chemoattractant protein 1 (MCP1), interferon gamma (IFNγ), IL-10, and tumor necrosis factor (TNF) have been observed to be elevated in association with obese pregnancy in both humans and rodents (Catalano et al., 2009; Madan et al., 2009; Roberts et al., 2011; Kepczynska, 2013). Studies that have in turn examined the cytokine profile in the fetal circulation in maternal obesity have identified elevated IL-6, TNF, chemokine (C-C motif) ligand 2, IL-17A, and IFNγ (Desai et al., 2013; Kim et al., 2014). Although the majority of these cytokines are pro-inflammatory, one of them (IL-10) is anti-inflammatory, and another (IFNγ) is considered regulatory (i.e., neither pro- nor anti-inflammatory). This raises the question of whether there might be opposing actions among the cytokines. This has yet to be addressed in detail, but it seems reasonable to suspect that a balance of biochemical activity would result from the combined actions of a panel of cytokines acting on particular tissues at particular developmental times.
In keeping with the notion that cytokines in the maternal circulation may affect the fetus, injection of isolated cytokines into pregnant dams appears sufficient to alter offspring development. For example, IL-6 or TNF injected into pregnant rat dams in mid-pregnancy (gestational days 8 (GD 8), 10 and 12) produced offspring with elevated body weight, adiposity, and decreased insulin sensitivity at birth (Dahlgren et al., 2001). Injection of IL-6 on these days or during late pregnancy (GD 16, 18, 20) additionally produced neuronal loss in the hippocampus, impaired learning manifested as longer escape latencies in the Morris Water Maze, and hypertension (Samuelsson et al., 2004; Samuelsson et al., 2006a; Samuelsson et al., 2006b). Further, administration of IL-6 into pregnant mice on GD12.5 caused deficits in pre-pulse inhibition in the offspring (Smith et al., 2007b). Also, injection of IL-1β in late pregnancy (GD17–21) resulted in offspring with altered stress response and impaired learning (Gotz et al., 1993).
The transfer of cytokines in the maternal circulation to the fetus in vivo has been demonstrated directly using radiolabeled IL-6 (Dahlgren et al., 2006). For IL-1α, IL-1β, and TNF, in vitro assays of placental permeability also suggest that transfer can occur in vivo (Kent et al., 1994). Importantly, only a fraction of the cytokine present in the maternal circulation appears to reach the fetus (Kent et al., 1994), and whether these levels are sufficient to produce cellular and molecular alterations in fetal tissues in vivo is not clear. This is in contrast to reports that the human term placenta does not appear to be permeable to IL-1β, TNF, or IL-6 (Aaltonen et al., 2005). This discrepancy may be explained by differences in preparations, species, or developmental timing, and a clear picture of maternal cytokine transfer to the embryo or fetus in vivo has not yet come into focus.
2.3.2. Placental inflammatory cytokines
In addition to maternal production of cytokines, the placenta also produces substantial cytokines in obese pregnancies across species, including humans (Hauguel-de Mouzon & Guerre-Millo, 2006; Radaelli et al., 2006; Challier et al., 2008; Zhu et al., 2010a; Heerwagen et al., 2013; Kim et al., 2014). Based on these and similar observations, it has also been suggested that the placenta may amplify, dampen or otherwise transform maternal inflammation, so as to gate the inflammatory environment that the fetus is exposed to (Zaretsky et al., 2004; Aaltonen et al., 2005; Dahlgren et al., 2006; Hauguel-de Mouzon & Guerre-Millo, 2006; Challier et al., 2008; Zhu et al., 2010b). In keeping with this, a recent study reported that inflammatory markers in the fetal circulation do not change significantly until late gestation in the mouse, despite elevated maternal inflammation much earlier (Kim et al., 2014). This is consistent with the idea that the placenta is able to protect the developing fetus from elevated maternal inflammation, at least up until late gestation (Lenz, 1997; Houser, 2012).
2.3.3. Fetal inflammatory cytokines
Finally, it is also likely that the fetus itself may be the source of its own inflammatory cytokines that may exert pathological effects on its own development. The literature to date has focused mainly on postnatal offspring inflammatory responses, however, several tissues have been described to show altered inflammatory cytokine profiles in fetuses developing in obese mothers. For example, in a sheep model, fetal large intestine expressed mRNA for a variety of cytokines and inflammation-mediating receptors (IL1α, IL-1β, TNF, IL-6, toll-like receptor (TLR)2, TLR4, TGF, IL-17) (Yan et al., 2011). In the mouse, fetal adipose tissue showed elevated CCR2 and TNF mRNA expression (Murabayashi et al., 2013). In the NHP, fetal liver cytokines have not been examined, however, triglyceride levels are increased, which may stimulate production of inflammatory mediators (McCurdy et al., 2009).
Upon reaching the embryo or fetus, irrespective of route, it is imagined that inflammatory factors may either change the course of tissue development or alter the regulation of tissue-specific gene expression that is necessary for normal adult function later in life; in either case ultimately impairing tissue function to work less efficiently later in life. Studies in this domain are just beginning to emerge. For example, we have shown recently, that IL-6, which is elevated in the maternal and fetal circulation, is able to alter the prenatal formation of body weight-regulating neural circuits in the ARC (Sanders et al., 2014a). However, the full impact of aberrant exposure to inflammatory mediators associated with maternal nutritional status on developing tissues, particularly the brain, remains a mostly open question.
In considering how cytokines or other factors in the peripheral fetal circulation might affect the brain, one additional consideration is whether cytokines actually reach the fetal brain to effect cellular and molecular changes that increase offspring risk for behavioral abnormalities. In the adult, factors such as IL-6 and leptin, when present in the peripheral circulation, can impinge on ARC neurons via specific transport across the blood brain barrier (BBB; Banks, 2006; Pohl et al., 2013; Koch et al., 2014). There is some controversy regarding the exact time of maturation of the BBB during embryonic development (Engelhardt & Liebner, 2014). Thus, the timing of BBB maturation and adult-like function will determine when during gestation specific factors in the fetal circulation could gain access to the fetal brain. At early times during gestation when the BBB is not yet fully functional, blood-borne factors may be able to gain access via free diffusion or some other process that is not present in the mature BBB. Tagging of peripheral factors, as has been done with leptin in the adult brain (Vauthier et al., 2013; Balland, 2014), may be one approach to resolving this important question (for example, see Banks et al., 2004).
Finally, there is the possibility that inflammatory mediators may be aberrantly produced by brain-resident cells in maternal under or overnutrition. In the NHP model of maternal obesity, the fetal hypothalamus showed increased expression of IL-1 and C-C chemokine signaling family genes, as well as an increase in activated microglia (Grayson et al., 2010). Although the presence of inflammatory cells within the brain may at first glance seem to obviate the need to get cytokines across the BBB, there is still the question of how these brain resident cells receive their information regarding maternal nutritional status. Microglia develop from the yolk sac and enter the brain beginning on GD10 in the mouse (Takahashi et al., 1989). During this time they could be exposed to cytokines of maternal, placental or fetal origin, making it likely that they enter the brain already primed or activated. Unfortunately, the early stages of microglia development in utero have remained largely elusive, and how early circulating factors may influence microglial function later in life are unknown.
Although this review has so far been restricted to inflammatory factors, in reality any diffusible substance whose expression or presence in fetal tissues is altered by maternal nutrition would be a candidate for mediating the changes associated with disease programming. Other factors which are gaining ground for their importance in disease risk programming by maternal nutritional status are fatty acids (de Vries et al., 2014; Loomans et al., 2014), which are able to signal through TLRs to initiate an inflammatory response (Hwang & Rhee, 1999; Brikos & O’Neill, 2008; Yin et al., 2014), and growth factors such as BDNF (Tozuka et al., 2009; Tozuka et al., 2010), epidermal growth factor (Serrero et al., 1993), and fibroblast growth factors (Hutley et al., 2004; Huang et al., 2007). Recently, the role of the gut microbiome in health and disease has received significant attention (Palma et al., 2014), and in regard to early life programming, parental consumption of a Western diet was shown to alter concurrently the offspring immunity and gut microbiome (Myles et al., 2013). In a NHP model, maternal high fat diet was also shown to alter the offspring gut microbiome, independent of offspring obesity (Ma et al., 2014).
3. Postnatal dietary programming of behavior and immunity across the lifespan
3.1. Neonatal nutrition programs physiology long-term
As discussed, prenatal life is clearly a time of significant vulnerability to long-term programming of health and disease. A second such critical window of vulnerability occurs in the days to weeks after birth (Spencer et al., 2006; Spencer & Tilbrook, 2011; Spencer, 2012). As such, the neonatal nutritional environment can have substantial long-term programming effects on an individual’s feeding behavior, satiety signaling, and metabolism, and can play an important role in susceptibility to obesity (Spencer, 2013a; Spencer, 2013b). This neonatal nutritional environment also influences the development of obesity-associated disorders, including those related to a dysfunctional immune system, such as diabetes, cancers, and susceptibility to infection (Spencer, 2013a; Spencer, 2013b).
3.2. Neonatal nutrition affects metabolism and susceptibility to obesity long-term
In humans, childhood overweight and obesity are considered substantial risk factors for the development of obesity in later life (Ong et al., 2000; Stettler et al., 2005). Incredibly, one study has found for every 100 g of weight a baby puts on in the seven days after birth, the likelihood of that baby becoming an obese adult increases by 28 percent (Stettler et al., 2005). Early work has also outlined that the likelihood of becoming an obese adult increases from 10 percent to approximately 50 percent if the subject was classified obese as a child, with this risk magnifying with degree of childhood obesity (Whitaker et al., 1997). While a portion of the risk associated with neonatal nutrition and neonatal obesity may be linked to genetic and later environmental factors, this does not seem to fully explain the effects. For instance, genetic factors may predispose a child to both metabolic disturbances and poor nutritional choices throughout life. However, body mass index (BMI) has to date been identified as having anything from a 40 to a 70 percent genetic component, with only around two percent of obesity-susceptible genetic loci identified, indicating other factors are likely to contribute (Loos, 2009). Likewise, poor nutritional choices made by parents on a child’s behalf early on may instill a culture or habit of poor eating practices that are continued throughout life (Colapinto et al., 2007). However, this potential variable is not included in studies using animal models of neonatal overfeeding, where early neonatal nutrition is still able to program metabolic phenotype long-term.
3.3. Neonatal nutritional programming of neuroimmune function
One such animal model of neonatal overfeeding, suckling rats in small litters, reveals the neonatal nutritional environment has substantial impact on long-term metabolic function and weight gain. Thus, pups suckled in small litters, where they have greater access to the dam’s milk and receive milk that is higher in fat (Fiorotto et al., 1991), have accelerated growth and development, weigh more early on than their control-litter counterparts, and maintain excessive weights and fat deposition into adulthood (Clarke et al., 2012). These rats also have differences in metabolic function (Stefanidis & Spencer, 2012) and in such diverse physiological functions as stress responses (Spencer & Tilbrook, 2009), puberty onset (Smith & Spencer, 2012), bone structure (de Albuquerque Maia et al., 2014), and wound healing (Sabol et al., 2014). As is seen with adult-onset obesity (Pohl et al., 2009; Clarke et al., 2012), neonatally overfed rats also have exacerbated febrile and pro-inflammatory cytokine responses to a neuroimmune challenge (Clarke et al., 2012).
In humans, obesity is linked to a dysregulated immune system, with systemic and central inflammation and an inability to appropriately respond to an infection. For instance, obese people are twice as likely to contract pneumonia as lean people are. They are up to six times as likely to develop post-surgical infections, and are more than twice as likely to die in intensive care from an infection-related complication (Falagas & Kompoti, 2006). Childhood obesity in particular is linked to disorders of the immune system, including autoimmune disorders such as asthma and atopy (Celedon & Kolls, 2014). Childhood obesity is also linked to increases in morbidity and mortality after hospitalization for acute illness or organ transplants, with increased incidence of infections playing a role (Bechard et al., 2013). Probably contributing to this increased morbidity and mortality, obese people can have exacerbated neuroimmune responses to an immune challenge. For instance, obese diabetic patients undergoing laproscopic Roux-en-Y gastric bypass surgery have a significantly greater increase in pro- and anti-inflammatory cytokines IL-6 and IL-10 than lean patients do after a similar surgery (Lin & Gletsu-Miller, 2013). Similarly, circulating cytokine levels following hip surgery have been correlated with BMI, implying the immune system is primed to over-react to immune stimuli in obese people (Motaghedi et al., 2013). Consistent with these findings in obese adult humans, neonatally overfed rats have exacerbated febrile and circulating pro-inflammatory cytokine responses to an immune challenge with bacterial endotoxin, LPS (Clarke et al., 2012). They also have increased inguinal fat TLR4 expression, and increased inguinal fat phosphorylated inhibitory factor κB (IκB) (Clarke et al., 2012).
Unlike adult-onset obesity in humans, or adult high fat diet in rodent models, however, neonatal overfeeding does not seem to affect sickness behavior responses to LPS to the same degree, including activity levels or anorexia (Clarke et al., 2012). The reasons for this specificity of response are currently unknown. However, it may be that the pathways regulating these aspects of sickness behavior remain intact in the neonatally overfed rats, while those affecting TLR4- and febrile-dependent responses are selectively affected. For instance, leptin plays a role in sickness-induced anorexia (Sachot et al., 2004), and leptin levels are not different between neonatally overfed and control rats after LPS (Clarke et al., 2012). However, the pro-inflammatory cytokine IL-1 is also known to play a critical role in mediating sickness-induced anorexia (Reyes & Sawchenko, 2002; Nadjar et al., 2010), and plasma levels of IL-1 do differ in response to neonatal overfeeding, highlighting the complexity of these physiological responses.
Neonatal overfeeding in this model also does not affect febrile responses to a viral mimetic, polyinosinic:polycytidylic acid (poly I:C). Responses to poly i:c are similar in neonatally overfed and control rats, despite increased inguinal fat TLR3 expression in the former group (Clarke et al., 2012). Immune responses to viruses in adult humans and rodents are affected by metabolic status, with obese adult people and mice being more likely to contract and die from influenza (Smith et al., 2007a; Fuhrman et al., 2011). Although data on the susceptibility to viral challenges in obese children are lacking (Esposito et al., 2012), it may be that neonatal overfeeding programs up-regulation of TLR3, but not the associated cell-transport mechanisms that would traffic the viral ligand into the cell (Clarke et al., 2012). The result is somewhat encouraging for those with early life obesity, at least in terms of their potential to combat a viral, if not a bacterial, infection.
The mechanism for the overactive immune response in those with obesity and the difference in how this is manifest in those with obesity programmed early in life remain to be determined. However, the exacerbated response to a bacterial immune challenge that occurs in neonatally overfed rats is likely to be due, at least in part, to changes in hypothalamic-pituitary-adrenal (HPA) axis function. The HPA axis is normally activated in response to an immune challenge and culminates in the release of glucocorticoids into circulation (Sapolsky et al., 2000; Papadimitriou & Priftis, 2009). Glucocorticoids then act on immune cells to suppress nuclear factor κB (NFκB)-mediated transcription of pro- and anti-inflammatory cytokines, thus dampening further activation of the immune response (Cartmell et al., 2003; Conti et al., 2004; Galic et al., 2009; Spencer, 2013a). Synthetic glucocorticoids are used therapeutically to suppress overactive immune function (Flammer & Rogatsky, 2011) and an underactive HPA axis, or glucocorticoid resistance, is linked to exacerbated immune responses (Silverman & Sternberg, 2012). This negative feedback effect of the HPA axis, when appropriately functional, allows the immune response to effectively combat the invading pathogen, but helps prevent it from over-activation and excessive sickness (Spencer, 2013a).
There is evidence HPA axis function is disturbed in neonatally overfed animals. Thus, females (but not males) have greater neuronal activation in the PVN, the apex of the HPA axis, in response to a psychological stress if they were raised in small litters (neonatally overfed) compared with those raised in control litters (Spencer & Tilbrook, 2009). Both male and female neonatally overfed rats also have significantly greater PVN neuronal activation after an immune challenge with LPS than control rats (Clarke et al., 2012). Neonatally overfed rats also have a glucocorticoid response to LPS that is significantly slower than that of control rats (Clarke et al., 2012). Thus, control rats have a peak plasma glucocorticoid response to LPS at around 30 to 60 minutes that is resolved back to baseline levels by 90 minutes. In neonatally overfed rats, however, glucocorticoid levels continue to remain elevated at 90 minutes after LPS exposure (Clarke et al., 2012). We hypothesize neonatally overfed rats have an enhanced febrile and cytokine profile in response to LPS at least partly because the HPA axis is slow to release immuno-suppressive levels of glucocorticoids. This response is also reflected in less negative feedback onto central steps of the HPA axis, hence enhanced PVN neuronal activation. These effects of neonatal nutrition on HPA axis function are supported by data from a recent study showing neonatally overfed rats have an accelerated HPA axis maturation, coupled with changes in HPA axis sensitivity to stress in adulthood and enhanced mesenteric adipose tissue glucocorticoid receptor (GR) mRNA expression (Boullu-Ciocca et al., 2005). As such, rats made overweight by being suckled in small litters have substantial increases in basal circulating adrenocorticotropic hormone (ACTH) and corticosterone at postnatal days 14 and 21, as well as reduced PVN corticotropin-releasing hormone (CRH) mRNA and enhanced PVN GR mRNA early on (Boullu-Ciocca et al., 2005).
3.4. Neonatal nutritional programming of central inflammation
It is likely that, additional to changes in the glucocorticoid response to LPS, neonatally overfed animals are also more susceptible to a bacterial immune challenge due to changes in their central inflammatory profile. It is increasingly apparent that obesity and high fat diet lead to central, as well as peripheral inflammation (Pickup & Crook, 1998; Weisberg et al., 2003; Wellen & Hotamisligil, 2003; De Souza et al., 2005; Zhang et al., 2008; Milanski et al., 2009; Posey et al., 2009; Thaler et al., 2012). Obesity in humans is associated with increases in indices of inflammation in the brain, including microgliosis (Thaler et al., 2012). Similarly, rodent models have revealed increased pro-inflammatory gene expression and cytokine production in the hypothalamus, including the ARC, after a short period of high fat diet feeding (De Souza et al., 2005; Zhang et al., 2008; Thaler et al., 2012). Recently, Thaler and colleagues demonstrated this high fat diet-induced central inflammation in rodents was linked to pronounced changes in the resident immune cells of the brain, microglia and astrocytes (Thaler et al., 2012). Human brains also show microgliosis after sustained obesity (Thaler et al., 2012). As little as three days after the onset of high fat feeding, mice and rats have increased numbers of cells immunopositive for the microglial-specific marker, ionized calcium-binding adapter molecule (Iba1) (Thaler et al., 2012). At this time they also have an elevated expression profile of pro- and anti-inflammatory cytokines in the hypothalamus (Thaler et al., 2012). This inflammatory profile is likely to reflect an initially protective effect of microglial activation and proliferation, since the inflammation normalizes by seven days only to reappear by three weeks if the high fat diet is continued (Thaler et al., 2012). With such prolonged high fat diet exposure, the central inflammation is associated with neuronal injury markers and an attrition of pro-opiomelanocortin (POMC) neurons in the ARC (Thaler et al., 2012). Other groups have shown this hypothalamic inflammation can lead to leptin and insulin resistance within the brain and thus directly contribute to impairments in satiety signaling and further obesity (De Souza et al., 2005; Posey et al., 2009). When a diet high in fat is continued long-term in rodent models (e.g., 14 to 20 weeks), central inflammation and damage to extra-hypothalamic regions can also ensue (Jeon et al., 2012; Puig et al., 2012). Thus, rats fed high fat diet for 14–20 weeks have hippocampal (Jeon et al., 2012) and frontal cortex (Pepping et al., 2013) microgliosis and increased levels of hippocampal pro-inflammatory cytokines (Jeon et al., 2012). This type of inflammatory profile is also associated with alterations in tasks of cognitive function. For instance, mice with indices of hippocampal inflammation perform less well in the Morris Water Maze and this can be rescued with the anti-inflammatory agent, resveratrol (Jeon et al., 2012). This potential link between central inflammation in obesity and cognitive dysfunction has recently been extensively reviewed (Miller & Spencer, 2014).
Commencing an inappropriate diet in adulthood can thus facilitate disruption of feeding- and metabolic pathways, but there is increasing evidence that the early life programming period is equally if not more important in this regard. As discussed in Section 2, in utero exposure to excess cytokines can alter the brain’s inflammatory milieu and disrupt axon growth and appropriate neuronal activity. The brain is also still vulnerable to this type of influence in the early postnatal period. For example, cytokines are present in breast milk (Garofalo, 2010), and have been shown to affect fat and lean mass accrual in humans (Fields & Demerath, 2012).
The early postnatal period, in the rodent, is one when the brain’s microglia complete their maturation from being few and amoeboid in morphology at E14 to 18 to being fully mature with an adult-like ramified morphology by Postnatal day (P)14 (Bilbo & Schwarz, 2009). Any stimulus that interferes with this maturation process is likely to alter the central inflammatory profile throughout life and thus susceptibility to future challenges. Bilbo and colleagues have shown early life exposure to bacterial infection has the effect of maintaining microglia in an immature ‘active’ state so they are effectively primed to over-respond to future immune stimuli (Bland et al., 2010). Additionally, the predominant polyunsaturated fatty acid (PUFA) in the typical Western diet is n-6, with a relative deficiency in n-3 PUFAs, and deficiencies in n-3 PUFAs have also been linked to adverse neurobehavioral outcomes. Recently it was reported that n-3 PUFA deficiency during pregnancy led to altered microglial morphology and increased expression of hippocampal proinflammatory cytokines (Madore et al., 2014). Importantly, we have recently reported neonatal overfeeding also leads to hypothalamic microgliosis early on, and this persists into adulthood despite resumption of a normal diet at weaning. These effects are associated with changes in hypothalamic pro-inflammatory cytokine gene expression and exacerbated microglial responses to LPS. These findings suggest neonatal overfeeding can also sensitize hypothalamic microglia, contributing to basal central inflammation and the hypersensitive response to an immune challenge throughout life (Ziko et al., 2014).
The in utero and early postnatal periods are clearly of critical importance in dietary programming of behavior and immunity throughout life. This period appears to extend into the juvenile age as well. Recently, it was reported that consumption of a HFD in juveniles, but not adults, resulted in alterations in hippocampal inflammation, as well as deficits in spatial memory (Boitard et al., 2014). However, although feeding and immune pathways are somewhat less plastic outside these critical windows, they are certainly still potentially vulnerable to environmental and dietary influence.
4. Dietary influences on immune function in adulthood: Calorie restriction
As well as during the critical prenatal and perinatal periods, dietary influences during adulthood can have profound effects on immune functioning. For example, exposure to a calorie restricted diet during adulthood can alter a number of immune system processes. Calorie restriction (CR), which usually involves the reduction of daily food intake while maintaining dietary composition and avoiding malnutrition (Weindruch & Walford, 1988), is perhaps best known for extending mean and maximum lifespan in a number of species ranging from yeast to mammals (Weindruch et al., 1986). For example, in a population of rhesus monkeys at the Wisconsin National Primate Research Center (WNPRC) an adult-onset 30% CR regimen improved age-related and all-cause survival rate and delayed the onset of age-related diseases (Colman et al., 2009; Colman et al., 2014). In contrast, however, among a population of rhesus monkeys at National Institute on Aging (NIA), old-onset or young-onset 30% CR has shown no differences in survival compared with controls (Mattison et al., 2012). Interestingly, control NIA animals were provided regulated portions of food according to dietary standards rather than fed ad libitum, possibly indicating that they underwent a mild CR themselves. Indeed, control animals at NIA had a lower than average body weight when compared to a national database of healthy, captive non-human primates (Colman et al., 2014). Therefore, the lack of CR-induced differences in survival rate in NIA monkeys may result from the control monkeys experiencing survival benefits following a mild-CR. Given the control monkeys at WNPRC were provided unlimited access to nutrient dense food, an alternative explanation for the contrasting findings could be that these monkeys are over eating, resulting in a higher number of age-related diseases in this group. Of note, an earlier longitudinal study at the University of Maryland demonstrated that CR monkeys had a 2.6 fold reduced risk of death, an increased median survival age, and reduced age-related pathologies compared with ad libitum-fed control monkeys (Bodkin et al., 2003).
Aside from effects on longevity, CR has been shown to elicit many health promoting benefits, including delaying the onset of multiple age-related pathologies, attenuating the progression of neurodegeneration in animal models of Parkinson’s disease (Maswood et al., 2004), Alzheimer’s disease (Halagappa et al., 2007), and multiple sclerosis (Piccio et al., 2008), delaying the process of age-related immunosenescence (Koubova & Guarente, 2007), and reducing the growth of cancerous tumors (Berrigan et al., 2002). Furthermore, CR can improve learning and memory (Komatsu et al., 2008; Grayson et al., 2013), increase social behavior (Govic et al., 2009), reduce anxiety-like behavior (Levay et al., 2007) and can dose-dependently increase circulating corticosterone and decrease circulating testosterone concentrations (Levay et al., 2010b). In this section we will focus on the effects of a CR diet on immune system function.
4.1. Calorie restriction and immune function
As the effectiveness of the immune system often declines with age (Murasko & Goonewardene, 1990) and age-related diseases often have immune components (Jolly, 2004), considerable research has focused on elucidating the effects of CR on immunity. CR has been demonstrated to delay the age-related decline in immune efficiency (Weindruch & Walford, 1988) and can reduce the age-associated elevation in cytokines to levels comparable to that of young animals (Spaulding et al., 1997; Muthukumar et al., 2000). The majority of research has demonstrated that CR results in decreased (or unchanged) concentrations of pro-inflammatory mediators (e.g., Dong et al., 1998; Matsuzaki et al., 2001; MacDonald et al., 2011), coupled with increased concentrations of anti-inflammatory mediators (e.g., MacDonald et al., 2011; MacDonald et al., 2014) both at basal levels and following immune stimulation. However, due to significant variability between studies, the effect of CR on the immune response is not entirely clear.
Several lines of evidence have demonstrated unchanged basal pro-inflammatory cytokine concentrations following a CR regimen (Conn et al., 1995; Sun et al., 2001; Fenton et al., 2009; Crissey et al., 2014). For example, in rats, an eight week, 30% CR did not alter the serum concentrations of IL-6 or TNF; interestingly however, 30% CR did reduce expression of IL-6 and TNF mRNA in retroperitoneal and periaortic adipose tissue, but not in subcutaneous or interscapular adipose tissue (Crissey et al., 2014). In contrast, other studies, also in rats, have shown that CR reduces circulating basal levels of pro-inflammatory cytokines, including IL-1β, IL-6, and/or TNF (Dong et al., 1998; Ugochukwu & Figgers, 2007), and/or enhances the basal concentration of anti-inflammatory cytokines, such as IL-10 and IL-4 in rats and mice (Ugochukwu & Figgers, 2007; Fenton et al., 2009).
In contrast to the equivocal effects of CR on basal concentrations of cytokines, the majority of findings suggest that CR attenuates peripheral inflammation and enhances immune function following immune stimulation. For example, 25% CR improves phagocytic function of alveolar macrophages, evident by a CR-induced enhanced clearance of a bacterial infection (Streptococcus zooepidemicus) from the lungs (Dong et al., 1998). Further, macrophages of CR rats show decreased expression of TNF and IL-6 mRNA and decreased nitric oxide synthesis compared with ad libitum fed rats following LPS (Dong et al., 1998). In addition, 40% CR attenuates the LPS-induced increase in IL-1β, IL-6, and TNF, which is associated with reduced serum aspartate and alanine aminotransferase activities, indications of liver injury (Matsuzaki et al., 2001), suggesting improved immune function. Consistent with this finding, a 40% CR regimen causes a reduction in LPS-induced IL-6 and NFκB activity in splenocytes (Bhattacharya et al., 2006). Peritoneal macrophages isolated from 40% CR mice produce less IL-6 and IL-12, but not TNF, following LPS compared to ad libitum fed mice. Additionally, CR animals have lower LPS-induced expression of TNF, IL-6, and IL-12 mRNA than ad libitum fed mice (Sun et al., 2001). Exposure to a 40% CR diet also reduces IL-1β concentration in peritoneal macrophages following LPS; though there is no effect on levels of TNF, IL-6, or the anti-inflammatory cytokine IL-10 (Vega et al., 2004). These discrepant findings in two studies that utilized the same magnitude of CR (40%) and site of cytokine measurement (peritoneal macrophages) highlight the importance of considering methodological differences in the CR regimens. Differences in the duration of CR (five months in Sun et al., 2001; 15 months in Vega et al., 2004) and/or the length of exposure of the macrophages to LPS (24 hours in Sun et al., 2001; five hours in Vega et al., 2004) may explain the differences in CR-induced cytokine production. Of note, Vega and colleagues also demonstrated that an intermittent fasting (IF) regimen, where mice had access to food every other day, reduced IL-1β and TNF levels, increased IL-6 levels, but had no effect on IL-10 following LPS (Vega et al., 2004). Although IF regimens and CR are known to result in similar weight changes, approximately 70% of ad libitum controls for 3 weeks of an IF regimen (Chausse et al., 2014) or 3 weeks of 50% CR regimen (Levay et al., 2007), it is likely that these different dietary conditions result in subtle differences in the immune response.
Finally, recent work conducted by our research group found that 50% CR induced an anti-inflammatory bias both peripherally and centrally following immune stimulation (MacDonald et al., 2011; MacDonald et al., 2014). Specifically, CR reduced LPS-induced IL-6 production and increased corticosterone in serum (MacDonald et al., 2014). In addition, CR animals did not demonstrate the expected rise in hypothalamic pro-inflammatory signalling (COX-2, membrane prostaglandin E synthase 1 (mPGES-1)) at two hours post-LPS, but instead demonstrated delayed expression at four hours following LPS administration. At four hours post-LPS, there was also significantly enhanced expression of anti-inflammatory mediators suppressor of cytokine signaling 3 (SOCS3), IL-10, IκBα in the hypothalamus of CR animals (MacDonald et al., 2011).
4.2. Calorie restriction, fever, and sickness behavior
Limited research exists investigating the effects of CR on fever and sickness behavior following immune stimulation. The effects of food deprivation on fever and sickness however, have been investigated. Food deprivation is a very different dietary challenge to CR and IF regimens. Typical food deprivation paradigms involve the complete removal of food for a period of hours or days. Food deprivation of two days results in weight loss to approximately 82% of ad libitum fed controls (Inoue et al., 2008). Longer periods of food deprivation result in greater weight loss (78% and 71% of ad libitum fed controls for four and six days of food deprivation respectively (Shido et al., 1989). Previous research has shown, in various experimental animals, that food deprivation negatively affects their ability to physiologically produce a fever after exposure to a pyrogen (Kleitman & Satinoff, 1981; Shojoony, 1985; Shido et al., 1989; Inoue et al., 2008). In addition, food deprivation attenuates the anorexic response of rats in response to continuous infusion of IL-1 (Mrosovsky et al., 1989).
Recently, we demonstrated that a 50% CR regimen for four weeks completely attenuated LPS-induced sickness behavior, including fever, anorexia, and behavioral depression in both mice (MacDonald et al., 2011) and rats (MacDonald et al., 2014). Interestingly, this phenomenon is dose-dependent; CR for either shorter durations or smaller magnitude results in partial attenuation of the fever and other aspects of sickness behavior (MacDonald et al., 2011 and 2014).As outlined above, the suppression of sickness behavior was likely controlled by a reduction in pro-inflammatory signalling, coupled with an increase in anti-inflammatory signalling in CR mice (MacDonald et al., 2011; MacDonald et al., 2014).
4.3. Calorie restriction and central inflammation
To our knowledge, research investigating the effects of CR on microglial activation is limited. Mice exposed to an IF regimen for three months had reduced accumulation of microglia in the hippocampus one week following an intrahippocampal kainite injection designed to induce hippocampal damage as a consequence of excitotoxic seizure activity (Lee, Auyeung, & Mattson, 2003). Similarly, rats subjected to three months of 50% CR demonstrate a reduction in microglial activation, TNF expression, and neuronal cell death following cortical injury (Loncarevic-Vasiljkovic et al., 2012). Microglial cells surrounding the lesion site displayed predominantly reactive, amoeboid morphology in ad libitum fed rats compared with mainly ramified cells in CR rats, suggesting morphological differences between dietary groups (Loncarevic-Vasiljkovic et al., 2012). Furthermore, a chronic CR regimen for 19 months suppresses the age-related increases in microglial activation to levels similar to that of young mice in the corpus callosum, basal ganglia, and subregions of the dentate gyrus (Morgan et al., 1999). Finally, we have recently demonstrated that CR attenuates LPS-induced microglial activation in a subset of hypothalamic regions including the ARC and ventromedial hypothalamic nucleus (VMH) as well as the subfornical organ (Radler et al., 2014).
5. Cytokines and Energy Homeostasis
Prenatal and perinatal programming as well as dietary changes during adulthood can alter the inflammatory milieu, which can have consequences for the maintenance of energy and nutrient homeostasis throughout life. Indeed, the clinical observation that sickness is often associated with anorexia, loss of appetite, or even cachexia, was historically the main evidence that molecules of the immune system may be able to influence appetite and energy homeostasis. Three cytokines have been recognized as main modulators of these actions and remain to date the most investigated: IL-1β, IL-6 and TNF. These three cytokines mediate several of the symptoms associated with sickness including sleepiness, malaise, fever, and loss of appetite (Dantzer, 2001; Kelley et al., 2003). Indeed, it was generally believed that the appetite-suppressing effects of exogenous IL-1, IL-6, and TNF are inevitably linked to fever and malaise. However, recent data suggest a physiologic negative feedback role for these cytokines in energy homeostasis to curb fat accrual in the same manner as leptin. For example, IL-1β and IL-6 are hypersecreted from activated macrophages in white adipose tissue, and mice that lack the IL-1 receptor, IL-1/IL-6, or IL-1 become obese by 5–6 months of age (Wallenius et al., 2002; Chida et al., 2006; Garcia et al., 2006). Conversely, although leptin historically has been primarily characterized as an adipocyte hormone that regulates energy homeostasis, data clearly indicate sickness/inflammatory-like functions of leptin (Lord, 2006). For example, inflammatory stimuli, such as LPS, induce leptin expression, and exogenous leptin can induce fever via induction of IL-1 (Luheshi et al., 1999). Thus, the functional boundaries between “sickness” cytokines and “energy homeostasis” regulating cytokines have been blurred. Perhaps it is not surprising that components of the adaptive “sick syndrome” response to infection, often involving heat production and reduced food intake, may have been co-opted to also subserve energy balance.
Although some of the mediators have been identified, the mechanisms by which inflammation can affect nutrient homeostasis remain poorly understood. In this section, we review the evidence for the possible effects of maternal, placental or fetal inflammation in contributing to the programming of energy homeostasis. The sites of the anorexigenic action of pro-inflammatory cytokines also remain to be conclusively demonstrated. Cytokines are relatively easy to measure and are typically highly inducible, two features that makes them an attractive subject of investigation. However, they are also redundant, do not readily cross the BBB, and often regulate each other’s production making it difficult to determine the hierarchical order of action and whether these molecules can act centrally to modulate hypothalamic functions. Finally, the cytokine-mediated regulation of nutrient homeostasis appears to be finely regulated and the experimental evidence collected so far is not always easy to reconcile. For instance, while IL-1β, IL-6, TNF, as well as IL-18, are recognized to have anorexigenic effects, obesity is associated with a low grade, chronic inflammation characterized by the elevated level of pro-inflammatory cytokines. At present this is viewed to be similar to what is observed for the adipokine leptin as an ultimately failed attempt to maintain homeostasis and an acquired resistance state (Lago et al., 2007). This attempt was shown to be at least in part mediated by the ability of cytokines whose levels increase with obesity to oppose positive energy balance via endocrine or vagally-mediated negative feedback to the mediobasal hypothalamus and caudal hindbrain (Plata-Salamán, 2001; Wong & Pinkney, 2004; Lago et al., 2007). For example, leptin, a pro-inflammatory, adipocyte-derived type I cytokine, reduces food intake and increases energy expenditure. Conversely, deficiency of leptin or its signaling receptor (Ob-Rb) results in hyperphagia, energy thriftiness, and obesity (Halaas et al., 1995; Chen et al., 1996; Lago et al., 2007).
In addition to these “classical cytokines”, there is now strong evidence that the IL-18 also helps regulate energy homeostasis. Circulating IL-18 levels in humans positively correlate with BMI, adiposity, type 2 diabetes or insulin resistance, hypertriglyceridemia, and metabolic syndrome (Esposito et al., 2002b; Esposito et al., 2003; Olusi et al., 2003; Hung et al., 2005). Such a relationship is consistent with the chronic mild inflammatory state that accompanies obesity and associated metabolic syndrome disorders, due to increased adipocytokine release from accumulating white adipose tissue (Fain, 2006; Lago et al., 2007). Indeed, “obesity-recruited” fat-resident monocyte/macrophage lineage cells are major sources of IL-18, and adipocytes from obese humans secrete 3-fold more IL-18 than those from lean donors (Skurk et al., 2005; Fain, 2006). Subcutaneous adipose tissue IL-18 mRNA also is elevated in human obesity, correlating with insulin resistance (Leick et al., 2007). The results suggest an adipocytokine-like action of IL-18 in obesity. Yet, and once more similar to leptin, deletion of IL-18 induces hyperphagia and late-onset body weight gain.
Here we review work carried out in mice to understand the significance of IL-18 as modulator of feeding and energy efficiency and its mechanism of action. We believe the results obtained so far serve as a valuable example of how cytokines may influence energy homeostasis.
5.1. Interleukin 18 and energy homeostasis
IL-18 is a cytokine originally identified as interferon gamma inducing factor (IGIF) in mice (Dao et al., 1996) and subsequently cloned in human (Ushio et al., 1996) and rat (Conti et al., 1997). IL-18 has pleiotropic biological activity and is capable of modulating both the humoral or the cellular immune response depending on the cytokine milieu, influencing an exceptional variety of processes and diseases (for review, see Dinarello et al., 1998; Nakanishi et al., 2001). Mouse IL-18 is a 157 amino acid (aa) polypeptide produced as a 193 aa precursor matured into the secretable biologically active protein by IL1β converting enzyme (ICE). Its tertiary structure is similar to that of IL-1β (Bazan et al., 1996) and its receptor belongs to the TLR IL-1 superfamily activating the MyD88 dependent IRAK-TRAF6 pathway. Despite these similarities with IL-1β, IL-18 differs functionally from the “sickness” cytokines in key respects. For example, IL-18 is constitutively expressed, rather than being primarily inducible in response to inflammatory stimuli. Also, systemic IL-18 is not pyrogenic and can even reduce the amplitude of IL-1induced fever (Gatti et al., 2002) and does not promote taste aversion (Zorrilla et al., 2007). Unlike IL-1, IL-6 and TNF, IL-18 reduces food intake without producing fever or malaise. In summary, the lack of these pathologic properties and its constitutive expression place IL-18 in a position of possibly regulating food intake under non-pathological conditions and/or to be a therapeutically useful pathway for curbing food intake.
Work on the possible role of IL-18 in modulating energy homeostasis began following the observation that mice deficient for IL-18 (Il18−/−) developed increased body weight gain (Ushio et al., 1996; Plata-Salamán, 2001; Wallenius et al., 2002; Olusi et al., 2003; Wong & Pinkney, 2004; Skurk et al., 2005; Thorand et al., 2005; Netea et al., 2006; Zorrilla et al., 2007; Zorrilla & Conti, 2014). Such an increase was recorded starting at mid adulthood but not earlier and was larger in female mice. Chow-reared female Il18−/− mice weighed 27% more than WT mice at 20, but not at 15 weeks of age; male Il18−/− mice however were 14% heavier than WT mice at 27, but not at 15 weeks of age. Il18−/− mice were not only heavier, but also fatter, than age and gender matched WT mice. While brown fat mass was not disproportionately greater in Il18−/− mice, white fat pad mass of Il18−/− mice was ~ 2–3-fold greater than that of WT mice, with subcutaneous (subcutaneous, inguinal) versus intra-abdominal pads (gonadal, mesenteric, retroperitoneal) equally larger (Zorrilla et al., 2007; Zorrilla & Conti, 2014).
IL-18 system deficient mice not only become fatter, but also develop steatosis associated with increased insulin levels and insulin resistance (Netea et al., 2006; Zorrilla et al., 2007). These data are suggestive of a late onset metabolic syndrome, which is of particular interest because it resembles what often occurs in adult humans. Circulating levels of IL-18 have consistently been reported to be elevated in patients with type 2 diabetes, and have also been suggested to contribute to nephropathy in type 2 diabetes (Aso et al., 2003; Esposito et al., 2003; Fischer et al., 2005). Elevated levels of IL-18 have been shown to predict the development of type 2 diabetes (Esposito et al., 2002a; Thorand et al., 2005; Hivert et al., 2009). Elevated circulating levels of IL-18 are also reported to be associated with obesity in mice.
Measurements of food intake reveal that Il18−/− mice overeat (~ 60–120% more) before they start to gain weight, indicating that increased food intake may be a cause of overweight. Hyperphagia in Il18−/− mice is larger in female mice and although no overall alteration of the circadian feeding profile is observed, both genders eat more during the late dark cycle and first three hours of the light cycle. However, this is observed only on regular (10% fat diet) but not on high (60%) fat diet where normalization per body weight eliminates any significant difference in calorie intake. These observations suggest that IL-18 may regulate lipid intake. In addition, if loss of IL-18 increases feeding, IL-18 itself may act as anorexigenic signal.
This was soon demonstrated by showing that intracerebroventricular injection of recombinant IL-18 blunted recovery of baseline body weight and re-feeding of food deprived mice in a dose dependent way. The possibility that the anorexigenic effects of recombinant IL-18 could be at least in part due to the presence of endotoxin in the preparation was tested by measuring taste aversion and fever response. The results not only excluded such possible confounding variables, but also confirmed that IL-18 could reduce food intake without inducing fever (Gatti et al., 2002; Zorrilla et al., 2007). Altogether these observations are indicative of the central anorexigenic action of IL-18.
Studies on cells of the immune system showed that IL-18 acts through a heterodimer receptor comprised of an alpha subunit (IL-18Rα) required for ligand interaction, and a beta subunit (IL-18Rβ) necessary for activation of signal transduction. Binding of this heterodimer complex by IL-18 induces the activation of the IL-1R-associated kinase (IRAK) transduction pathway and eventually the activation of NF-κB. The action of IL-18 is also highly regulated by negative modulators. The soluble IL-18 binding protein (IL18BP) can bind circulating IL-18 and prevent it from activating IL-18R. In addition, at least two alternative isoforms of both IL-18Rα and β have been described. One of these isoforms, arbitrarily named IL-18Rα type II to distinguish it from the canonical Type I form, lacks the intracellular toll/interleukin receptor (TIR) domain required for activation of the IRAK pathway and was thus proposed to be a decoy receptor. A short splice variant of the IL-18Rβ, previously described in rat, where it was named short IL-18Rβ (sIL-18Rβ), also exists (Alboni et al., 2011). sIL-18Rβ is comprised of only one of the extracellular IgG domains of the receptor chain and it is thus believed to be a soluble form that can interfere with the dimerization of the canonical receptor complex. The full length as well as the short length and presumed negative regulators isoforms of IL-18R were all demonstrated to be expressed in neurons throughout the brain including several hypothalamic regions involved in nutrient homeostasis (Alboni et al., 2009; Alboni et al., 2010). In fact, high level of expression was observed in the hypothalamus, where IL-18Rα and β were found in several nuclei including the ARC, lateral hypothalamus (LH), VMH, PVN and dorsomedial (DMN) nuclei that represent important elements of nutrient sensing and major regulators of feeding and energy homeostasis. The relative distribution and amount of total, type I or type II IL-18Rα in the hypothalamus differ in specific areas and nuclei. Although it was not possible to demonstrate the localization of all isoforms, both IL-18Rβ and sIL-18Rβ are strongly induced in the brain following LPS injection indicating that the IL-18 system may modulate its central action during infection.
Hypothalamic expression of IL-18Rβ has a distribution pattern that is similar to that of IL-18Rα with especially high levels in the PVN lateral magnocellular part - PaLM, in the PVN dorsal cap - PaDC and in the ventromedial hypothalamic nucleus dorsomedial part - VMHDM. Interestingly, the levels of IL-18Rβ mRNA in the CNS are elevated following LPS.
Several questions remain to be answered to understand the exact mechanisms of action of the IL-18 system on feeding and energy efficiency. For instance, IL-18 does not readily cross the BBB and although it can be produced by microglial cells during inflammation the source of IL-18 and its ability to regulate feeding in a naïve animal remains to be determined. In addition, despite the evidence that IL-18 may act centrally via IL-18R, IL-18 also exerted anorexigenic effects when injected intraperitoneally, indicating that peripheral mechanisms may also be at play. These may include fat-resident monocyte/macrophage lineage cells which are major source of IL-18 and also respond to it. Adipocytes from obese humans secrete 3-fold more IL-18 than those from lean donors (Skurk et al., 2005; Fain, 2006) correlating with insulin resistance (Leick et al., 2007) and suggesting an adipocytokine-like action of IL-18 in obesity. Interestingly, work in mice demonstrated that IL-18 is capable of affecting not only food intake but also energy efficiency. Yet how this is achieved remains to be investigated (Zorrilla et al., 2007).
As mentioned above, the anorexigenic action of IL-18 is in apparent contradiction with the fact that obese individuals have increased circulating level of IL-18. In fact, circulating IL-18 levels in humans positively correlate with BMI, adiposity, type 2 diabetes or insulin resistance, hypertriglyceridemia, and metabolic syndrome (Esposito et al., 2002b; Esposito et al., 2003; Olusi et al., 2003; Hung et al., 2005). The development of IL-18 resistance was proposed as a likely explanation. One possibility is that this may occur via the negative regulators of IL-18 functions including IL-18BP, IL-18Rα type II and sIL-18Rβ, although this hypothesis remains untested. The same is true for the possible role of another member of the IL-18 family, the IL-18 binding protein (IL-18BP). IL-18BP binds IL-18 and prevents IL-18 from binding to its receptor complex thus serving as yet another negative regulator of IL-18 activity that could have a role in the development or maintenance of IL-18 resistance in obesity. Finally, the possibility that IL-18 may act during development to affect the tuning of energy homeostasis should also be considered and addressed.
Although IL-18 can contribute to the regulation of feeding also indirectly by stimulating the production of other anorexigenic cytokines such as IL-1β and TNF (Dinarello et al., 1998) the information so far indicates that one of the mechanisms by which IL-18 is capable of regulating energy homeostasis is by direct action on specific brain nuclei that regulate feeding. In this respect it is a true immune-modulator of neuronal functions that may serve as communicator between the immune and the central nervous systems.
6. Conclusions and perspectives
The literature presented herein reviews the powerful impact that diet and nutrition (and the subsequent physiological responses to diet) can have on physiology and behavior; in particular, highlighting a role for the immune system as a critical link between diet and health. There is a clear and profound impact of early life nutrition, both in utero and in the early postnatal period, on physiology, and as reviewed here, specifically on brain development. Hypothalamic systems that underlie metabolic functions can be modulated by early life under- and overnutrition, establishing a risk for later life obesity. Additionally, other brain regions (reward circuitry, hippocampus) are affected by early life nutritional challenges, and can lead to deficits in reward related behaviors, executive function, memory and cognition. Beyond the early life time period, nutrition continues to affect health via effects on the immune system, and the literature regarding the impact of caloric restriction on immune and behavioral endpoints was summarized. Cytokines in particular have strong effects on long term health and physiology, and one such exemplar cytokine, IL-18, was reviewed and discussed.
A number of unanswered questions remain. With regard to diet composition, there is a great deal of variance across the particular animal models. For example, some diet manipulations alter macronutrient levels (high fat or low protein), while others hold the diet composition constant, and alter the amounts (e.g., 50% of ad libitum levels, or altering litter size in the early neonatal studies). It is also important to parse out the relative contributions of the diet per se, as opposed to the response to the diet (e.g., an effect of a high fat diet versus the resultant obesity with increases in adipokines and cytokines). However, the strength of these multiple approaches emerges as convergent findings are documented across divergent models. Another area that requires additional focus is the functioning of specific barriers (e.g., the BBB and the maternal-fetal divide within the placenta) in communicating information. Whether cytokines pass through these barriers and/or act directly on these barriers at specific points of development requires additional clarification. And the same questions apply to the trafficking of immune cells, which can themselves secrete cytokines.
Because diet and nutrition are modifiable variables, a better understanding of their effects on the immune system and how these effects underlie related physiological changes (obesity, behavior) is critical. Modification of diet represents not only a therapeutic strategy for the treatment of a wide range of poor health outcomes, but should remain a focus of preventative health strategies and the establishment of optimal health and resilience.
Diet, behavior and immunity across the lifespan.
Undernutrition and overnutrition, perinatally and throughout life, cause increased risk for obesity and metabolic disorders
Nutrition also influences adverse mental health outcomes.
The immune system is critical in this programming, linking diet with long term health and behavioral outcomes.
Acknowledgements:
SJS: This work was supported by a Discovery Project Grant from the Australian Research Council (ARC; DP130100508), and a Project Grant from the National Health and Medical Research Council (NHMRC; APP1011274). SJS is an ARC Future Fellow (FT110100084) and an RMIT University VC Senior Research Fellow. TMR was supported by the NIH (MH087978). BC: This work was supported by National Institute of Health grant DK094026.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ARC
arcuate nucleus
- BDNF
brain derived neurotropic factor
- BMI
body mass index
- CR
calorie restriction
- CRH
corticotropin-releasing hormone
- GR
glucocorticoid receptor
- HPA axis
hypothalamic-pituitary-adrenal axis
- IF
intermittent fasting
- IκB
inhibitory factor κB
- IL
interleukin
- Iba1
ionized calcium-binding adapter molecule
- LPS
lipopolysaccharide
- NHP
nonhuman primate
- NFκB
nuclear factor κB
- PVN
paraventricular nucleus of the hypothalamus, poly I:C, polyinosinic:polycytidylic acid
- POMC
pro-opiomelanocortin
- TLR
toll-like receptor
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A (2005). Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol 106(4): 802–807. [DOI] [PubMed] [Google Scholar]
- Alboni S, Cervia D, Ross B, Montanari C, Gonzalez AS, Sanchez-Alavez M, et al. (2009). Mapping of the full length and the truncated interleukin-18 receptor alpha in the mouse brain. J Neuroimmunol 214(1–2): 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, Cervia D, Sugama S, Conti B (2010). Interleukin 18 in the CNS. J Neuroinflammation 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, Montanari C, Benatti C, Blom JM, Simone ML, Brunello N, et al. (2011). Constitutive and LPSregulated expression of interleukin-18 receptor beta variants in the mouse brain. Brain Behav Immun 25(3): 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre-Gouabau MC, Bailly E, Moyon TL, Grit IC, Coupé B, Drean GL, et al. (2012). Postnatal growth velocity modulates alterations of proteins involved in metabolism and neuronal plasticity in neonatal hypothalamus in rats born with intrauterine growth restriction. J Nutr Biochem 23(2): 140–152. [DOI] [PubMed] [Google Scholar]
- Alfaradhi MZ, Fernandez-Twinn DS, Martin-Gronert MS, Musial B, Fowden AL, Ozanne SE (2014). Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am J Physiol Regul Integr Comp Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaradhi MZ, Ozanne SE (2011). Developmental programming in response to maternal overnutrition. Front Genet 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck PC, Hecher K (2013). Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med 19(5): 548–556. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Poston L, Taylor PD (2008). Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res 36: 73–84. [DOI] [PubMed] [Google Scholar]
- Aso Y, Okumura K, Takebayashi K, Wakabayashi S, Inukai T (2003). Relationships of plasma interleukin18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care 26(9): 2622–2627. [DOI] [PubMed] [Google Scholar]
- Badr G, Mohany M (2011). Maternal perinatal undernutrition attenuates T-cell function in adult male rat offspring. Cell Physiol Biochem 27(3–4): 381–390. [DOI] [PubMed] [Google Scholar]
- Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falleul-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prevot V (2014). Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA (2006). The blood-brain barrier in psychoneuroimmunology. Neurol Clin 24(3): 413–419. [DOI] [PubMed] [Google Scholar]
- Banks WA, Niehoff ML, Zalcman SS (2004). Permeability of the mouse blood-brain barrier to murine interleukin-2: predominance of a saturable efflux system. Brain Behav Immun 18(5): 434–442. [DOI] [PubMed] [Google Scholar]
- Barker DJ (2004). The developmental origins of chronic adult disease. Acta Paediatr Suppl 93(446): 26–33. [DOI] [PubMed] [Google Scholar]
- Barker DJ (2006). Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49(2): 270–283. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. (2006). Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17(1): 4–12. [PubMed] [Google Scholar]
- Bazan JF, Timans JC, Kastelein RA (1996). A newly defined interleukin-1? [letter; comment]. Nature 379(6566): 591. [DOI] [PubMed] [Google Scholar]
- Bechard LJ, Rothpletz-Puglia P, Touger-Decker R, Duggan C, Mehta NM (2013). Influence of obesity on clinical outcomes in hospitalized children: a systematic review. JAMA Pediatr 167(5): 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleze KJ, Muller AP, Schweigert ID, Longoni A, Sordi F, de Assis AM, et al. (2009). Gestational and postnatal low protein diet alters insulin sensitivity in female rats. Exp Biol Med (Maywood) 234(12): 1437–1444. [DOI] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD (2002). Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 23: 817–822. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Chandrasekar B, Rahman MM, Banu J, Kang JX, Fernandes G (2006). Inhibition of inflammatory response in transgenic fat-1 mice on a calorie-restricted diet. Biochemical and Biophysical Research Communications 349(3): 925–930. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM (2009). Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V (2010). Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24(6): 2104–2115. [DOI] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, et al. (2010). Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun 24(3): 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC (2003). Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci 58(3): 212–219. [DOI] [PubMed] [Google Scholar]
- Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, et al. (2014). Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Boullu-Ciocca S, Dutour A, Guillaume V, Achard V, Oliver C, Grino M (2005). Postnatal diet-induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood: its relationship with the metabolic syndrome. Diabetes 54(1): 197–203. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB (2008). Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7(2): 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt I, Sticker EJ, Lentze MJ (2003). Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. J Pediatr 142(5): 463468. [DOI] [PubMed] [Google Scholar]
- Brikos C, O’Neill LA (2008). Signalling of toll-like receptors. Handb Exp Pharmacol(183): 21–50. [DOI] [PubMed] [Google Scholar]
- Brion MJ, Zeegers M, Jaddoe V, Verhulst F, Tiemeier H, Lawlor DA, et al. (2011). Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 127(1): e202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM (1995). Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–1945. Br J Psychiatry 166: 601–606. [DOI] [PubMed] [Google Scholar]
- Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, et al. (2009). Maternal highfat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50(6): 1796–1808. [DOI] [PubMed] [Google Scholar]
- Burns SP, Desai M, Cohen RD, Hales CN, Iles RA, Germain JP, et al. (1997). Gluconeogenesis, glucose handling, and structural changes in livers of the adult offspring of rats partially deprived of protein during pregnancy and lactation. J Clin Invest 100(7): 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can ÖD, Ulupinar E, Özkay ÜD, Yegin B, Öztürk Y (2012). The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav Pharmacol. 23(5–6): 582–592. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Ball C, Bristow AF, Mitchell D, Poole S (2003). Endogenous interleukin-10 is required for the defervescence of fever evoked by local lipopolysaccharide-induced and Staphylococcus aureus-induced inflammation in rats. J Physiol 549(Pt 2): 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Chatzi L, Carsin AE, Amiano P, Guxens M, Kogevinas M, et al. (2013). Maternal pre-pregnancy overweight and obesity, and child neuropsychological development: two Southern European birth cohort studies. Int J Epidemiol. 42(2): 506–517. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S (2009). Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32(6): 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celedon JC, Kolls JK (2014). An innate link between obesity and asthma. Nat Med 20(1): 19–20. [DOI] [PubMed] [Google Scholar]
- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. (2008). Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29(3): 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF (2008). Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28(46): 12107–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausse B, Solon C, Caldeira da Silva CC, Masselli Dos Reis IG, Manchado-Gobatto FB, Gobatto CA, et al. (2014). Intermittent fasting induces hypothalamic modifications resulting in low feeding efficiency, low body mass and overeating. Endocrinology 155(7): 2456–2466. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. (1996). Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84(3): 491–495. [DOI] [PubMed] [Google Scholar]
- Chen H, Simar D, Morris MJ (2009). Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS ONE 4(7): e6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida D, Osaka T, Hashimoto O, Iwakura Y (2006). Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes 55(4): 971–977. [DOI] [PubMed] [Google Scholar]
- Clair DS, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. (2005). Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA 294(5): 557–562. [DOI] [PubMed] [Google Scholar]
- Clarke MA, Stefanidis A, Spencer SJ (2012). Postnatal overfeeding leads to obesity and exacerbated febrile responses to lipopolysaccharide throughout life. J Neuroendocrinol 24(3): 511–524. [DOI] [PubMed] [Google Scholar]
- Colagiuri S, Lee CM, Colagiuri R, Magliano D, Shaw JE, Zimmet PZ, et al. (2010). The cost of overweight and obesity in Australia. Med J Aust 192(5): 260–264. [DOI] [PubMed] [Google Scholar]
- Colapinto CK, Fitzgerald A, Taper LJ, Veugelers PJ (2007). Children’s preference for large portions: prevalence, determinants, and consequences. J Am Diet Assoc 107(7): 1183–1190. [DOI] [PubMed] [Google Scholar]
- Colman I, Ataullahjan A, Naicker K, Van Lieshout RJ (2012). Birth weight, stress, and symptoms of depression in adolescence: evidence of fetal programming in a national Canadian cohort. Can J Psychiatry 57(7): 422–428. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325(5937): 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM (2014). Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5: 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ (2001). The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25 Suppl 5: S63–67. [DOI] [PubMed] [Google Scholar]
- Conn CA, Kozak WE, Tooten PC, Niewold TA, Borer KT, Kluger MJ (1995). Effect of exercise and food restriction on selected markers of the acute phase response in hamsters. Journal of Applied Physiology 78(2): 458–465. [DOI] [PubMed] [Google Scholar]
- Conti B, Jahng JW, Tinti C, Son JH, Joh TH (1997). Induction of interferon-gamma inducing factor in the adrenal cortex. Journal of Biological Chemistry 272(4): 2035–2037. [DOI] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, Bartfai T (2004). Cytokines and fever. Front Biosci. 9: 1433–1449. [DOI] [PubMed] [Google Scholar]
- Coupe B, Amarger V, Grit I, Benani A, Parnet P (2010). Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 151(2): 702–713. [DOI] [PubMed] [Google Scholar]
- Coupé B, Grit I, Darmaun D, Parnet P (2009). The timing of “catch-up growth” affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 297(3): R813–824. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD (1999). Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24(1): 155–163. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. (2001). Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411(6836): 480–484. [DOI] [PubMed] [Google Scholar]
- Crissey JM, Jenkins NT, Lansford KA, Thorne PK, Bayless DS, Vieira-Potter VJ, et al. (2014). Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. Americam Journal of Physiology. Regulatory, Integrative, and Comparative Physiology. 306(8): R596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. (2010). Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr 91(6): 1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A, et al. (2001). Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab 281(2): E326–334. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson AM, Jansson T, Holmang A (2006). Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res 60(2): 147–151. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2001). Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci 933: 222–234. [DOI] [PubMed] [Google Scholar]
- Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H (1996). Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T helper 1 cells. Cellular Immunology 173(2): 230–235. [DOI] [PubMed] [Google Scholar]
- Das UN (2001). Is obesity an inflammatory condition? Nutrition 17(11–12): 953–966. [DOI] [PubMed] [Google Scholar]
- de Albuquerque Maia L, Lisboa PC, de Oliveira E, da Conceicao EP, Lima IC, Lopes RT, et al. (2014). Bone Structure and Strength are Enhanced in Rats Programmed by Early Overfeeding. Horm Metab Res. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. (2005). Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146(10): 4192–4199. [DOI] [PubMed] [Google Scholar]
- de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G, et al. (2014). Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins Leukot Essent Fatty Acids. [DOI] [PubMed] [Google Scholar]
- Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, et al. (2008). Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 149(2): 470–475. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Babu J, Ross MG (2005). Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol 288(1): R91–96. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle DA, Casillas E, Boles J, Ross MG (2009). Early undernutrition attenuates the inflammatory response in adult rat offspring. J Matern Fetal Neonatal Med 22(7): 571–575. [DOI] [PubMed] [Google Scholar]
- Desai N, Roman A, Rochelson B, Gupta M, Xue X, Chatterjee PK, et al. (2013). Maternal metformin treatment decreases fetal inflammation in a rat model of obesity and metabolic syndrome. Am J Obstet Gynecol 209(2): 136 e131–139. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, et al. (1998). Overview of interleukin18: more than an interferon-gamma inducing factor. J Leukoc Biol 63(6): 658–664. [PubMed] [Google Scholar]
- Dong W, Selgrade MK, Gilmour IM, Lange RW, Park P, Luster MI, et al. (1998). Altered alveolar macrophage function in calorie-restricted rats. American Journal of Respiratory Cell and Molecular Biology 19(3): 462–469. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB (2005). Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493(1): 63–71. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Liebner S (2014). Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Giugliano F, Di Palo C, Ciotola M, Barbieri M, et al. (2003). Cytokine milieu tends toward inflammation in type 2 diabetes. Diabetes Care 26(5): 1647. [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. (2002a). Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106(16): 2067–2072. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G, et al. (2002b). Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab 87(8): 3864–3866. [DOI] [PubMed] [Google Scholar]
- Esposito S, Preti V, Consolo S, Nazzari E, Principi N (2012). Adenovirus 36 infection and obesity. J Clin Virol 55(2): 95–100. [DOI] [PubMed] [Google Scholar]
- Fahmi A, Smart N, Punn A, Jabr R, Marber M, Heads R (2013). p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal 25(4): 898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain JN (2006). Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 74: 443–477. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Kompoti M (2006). Obesity and infection. Lancet Infect Dis 6(7): 438–446. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD (1997). Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385(6612): 165–168. [DOI] [PubMed] [Google Scholar]
- Fenton JI, Nunez NP, Yakar S, Perkins SN, Hord NG, Hursting SD (2009). Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes, Obesity and Metabolism 11(4): 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, et al. (2007). Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 293(3): R1056–1062. [DOI] [PubMed] [Google Scholar]
- Ferland-McCollough D, Fernandez-Twinn DS, Cannell IG, David H, Warner M, Vaag AA, et al. (2012). Programming of adipose tissue miR-483–3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ 19(6): 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Alfaradhi MZ, Martin-Gronert MS, Duque-Guimaraes DE, Piekarz A, Ferland-McCollough D, et al. (2014). Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol Metab 3(3): 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DA, Demerath EW (2012). Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatr Obes 7(4): 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorotto ML, Burrin DG, Perez M, Reeds PJ (1991). Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am J Physiol 260(6 Pt 2): R1104–1113. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Perstrup LB, Berntsen A, Eskildsen P, Pedersen BK (2005). Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol 117(2): 152160. [DOI] [PubMed] [Google Scholar]
- Flammer JR, Rogatsky I (2011). Minireview: Glucocorticoids in autoimmunity: unexpected targets and mechanisms. Mol Endocrinol 25(7): 1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzek EJ, Sprangers N, Janssens AC, Duijn CMV, Wetering BJVD (2008). Prenatal exposure to the 1944–45 Dutch ‘hunger winter’ and addiction later in life. Addiction 103(3): 433–438. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS (1998). Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83(3): 847–850. [DOI] [PubMed] [Google Scholar]
- Fuhrman C, Bonmarin I, Bitar D, Cardoso T, Duport N, Herida M, et al. (2011). Adult intensive-care patients with 2009 pandemic influenza A(H1N1) infection. Epidemiol Infect 139(8): 1202–1209. [DOI] [PubMed] [Google Scholar]
- Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C (2007). Maternal size in pregnancy and body composition in children. J Clin Endocrinol Metab 92(10): 3904–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic MA, Spencer SJ, Mouihate A, Pittman QJ (2009). Postnatal programming of the innate immune response. Integrative and comparative biology 49(3): 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, et al. (2006). Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes 55(5): 1205–1213. [DOI] [PubMed] [Google Scholar]
- Garofalo R (2010). Cytokines in human milk. J Pediatr 156: S36–40. [DOI] [PubMed] [Google Scholar]
- Gatti S, Beck J, Fantuzzi G, Bartfai T, Dinarello CA (2002). Effect of interleukin-18 on mouse core body temperature. Am J Physiol Regul Integr Comp Physiol 282(3): R702–709. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008). Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1): 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosby AK, Maloney CA, Phuyal JL, Denyer GS, Bryson JM, Caterson ID (2003). Maternal protein restriction increases hepatic glycogen storage in young rats. Pediatr Res 54(3): 413–418. [DOI] [PubMed] [Google Scholar]
- Gotz F, Dorner G, Malz U, Rohde W, Stahl F, Poppe I, et al. (1993). Short- and long-term effects of perinatal interleukin-1 beta-application in rats. Neuroendocrinology 58(3): 344–351. [DOI] [PubMed] [Google Scholar]
- Govic A, Kent S, Levay EA, Hazi A, Penman J, Paolini AG (2008). Testosterone, social and sexual behavior of perinatally and lifelong calorie restricted offspring. Physiol Behav 94(3): 516–522. [DOI] [PubMed] [Google Scholar]
- Govic A, Levay EA, Kent S, Paolini AG (2009). The social behavior of male rats administered an adultonset calorie restriction regimen. Physiol Behav 96(4–5): 581–585. [DOI] [PubMed] [Google Scholar]
- Grayson BE, Fitzgerald MF, Hakala-Finch AP, Ferris VM, Begg DP, Tong J, et al. (2013). Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. International Journal of Obesity 38(3): 349–356. [DOI] [PubMed] [Google Scholar]
- Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL (2010). Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151(4): 1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS (2011). Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445. [DOI] [PubMed] [Google Scholar]
- Grissom NM, Lyde R, Christ L, Sasson IE, Carlin J, Vitins AP, et al. (2014). Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology 39(4): 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom NM, Reyes TM (2012). Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int J Dev Neurosci doi:pii: S0736–5748(12)00597–7 10.1016/j.ijdevneu.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Arany E, van Beek JP, Chamson-Reig A, Thyssen S, Hill DJ, et al. (2005). Adipose tissue gene expression profiling reveals distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab 288(4): E663–673. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. (1995). Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269(5223): 543–546. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, et al. (2007). Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiology of Disease 26(1): 212–220. [DOI] [PubMed] [Google Scholar]
- Halmoy A, Klungsoyr K, Skjaerven R, Haavik J (2012). Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 71(5): 474–481. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP (2005). Obesity. Lancet 366(9492): 1197–1209. [DOI] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, Guerre-Millo M (2006). The placenta cytokine network and inflammatory signals. Placenta 27(8): 794–798. [DOI] [PubMed] [Google Scholar]
- Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE (2013). Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS ONE 8(6): e67791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ (2003). Endocrine disruptors and the obesity epidemic. Toxicol Sci 76(2): 247–249. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Schug TT (2013). The perfect storm for obesity. Obesity (Silver Spring) 21(6): 1079–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herva A, Pouta A, Hakko H, Laksy K, Joukamaa M, Veijola J (2008). Birth measures and depression at age 31 years: the Northern Finland 1966 Birth Cohort Study. Psychiatry Res 160(3): 263–270. [DOI] [PubMed] [Google Scholar]
- Hivert MF, Sun Q, Shrader P, Mantzoros CS, Meigs JB, Hu FB (2009). Circulating IL-18 and the risk of type 2 diabetes in women. Diabetologia 52(10): 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN (1999). Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci 19(3): 1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM (1993). Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259(5091): 87–91. [DOI] [PubMed] [Google Scholar]
- Houser BL (2012). Decidual macrophages and their roles at the maternal-fetal interface. Yale J Biol Med 85(1): 105–118. [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL (2007). FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes 56(10): 2501–2510. [DOI] [PubMed] [Google Scholar]
- Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP (2005). Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol 25(6): 1268–1273. [DOI] [PubMed] [Google Scholar]
- Hutley L, Shurety W, Newell F, McGeary R, Pelton N, Grant J, et al. (2004). Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes 53(12): 3097–3106. [DOI] [PubMed] [Google Scholar]
- Hwang D, Rhee SH (1999). Receptor-mediated signaling pathways: potential targets of modulation by dietary fatty acids. Am J Clin Nutr 70(4): 545–556. [DOI] [PubMed] [Google Scholar]
- Inoue W, Somay G, Poole S, Luheshi GN (2008). Immune-to-brain signaling and central prostaglandin E2 synthesis in fasted rats with altered lipopolysaccharide-induced fever. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology 295(1): R133–R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BJ, Ozanne SE, Dorling MW, Hales CN (1999). Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett 448(1): 4–8. [DOI] [PubMed] [Google Scholar]
- Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, et al. (2012). Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 61(6): 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammoun HL, Kraakman MJ, Febbraio MA (2013). Adipose tissue inflammation in glucose metabolism. Rev Endocr Metab Disord. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. (2003). Cytokine-induced sickness behavior. Brain Behav Immun 17 Suppl 1: S112–118. [DOI] [PubMed] [Google Scholar]
- Kent AS, Sullivan MH, Elder MG (1994). Transfer of cytokines through human fetal membranes. J Reprod Fertil 100(1): 81–84. [DOI] [PubMed] [Google Scholar]
- Kepczynska MA, Wargent ET, Cawthorne MA, Arch JRS, O’Dowd JF, Stocker CJ (2013). Circulating levels of the cytokines IL10, IFNγ and resistin in an obese mouse model of developmental programming. Journal of Developmental Origins of Health and Disease 4(6): 491–498. [DOI] [PubMed] [Google Scholar]
- Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR (2004). Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr 28(6): 410–415. [DOI] [PubMed] [Google Scholar]
- Kim DW, Young SL, Grattan DR, Jasoni CL (2014). Obesity During Pregnancy Disrupts Placental Morphology, Cell Proliferation, and Inflammation in a Sex-Specific Manner Across Gestation in the Mouse. Biol Reprod. [DOI] [PubMed] [Google Scholar]
- Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, et al. (2009). Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE 4(6): e5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N, Satinoff E (1981). Behavioral responses to pyrogen in cold-stressed and starved newborn rabbits. American Journal of Physiology 241(3): R167–R172. [DOI] [PubMed] [Google Scholar]
- Koch CE, Lowe C, Pretz D, Steger J, Williams LM, Tups A (2014). High-fat diet induces leptin resistance in leptin-deficient mice. J Neuroendocrinol 26(2): 58–67. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Chiba T, Yamaza H, Yamashita K, Shimada A, Hoshiyama Y, et al. (2008). Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescenceaccelerated mouse strain P8. Experimental Gerontology 43(4): 339–346. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L (2007). How does calorie restriction work? Genes and Development 17(3): 313–321. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129(5): e1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O (2007). The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 18(3–4): 313–325. [DOI] [PubMed] [Google Scholar]
- Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ (1992). Early growth and abdominal fatness in adult life. J Epidemiol Community Health 46(3): 184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Auyeung WW, Mattson MP (2003). Interactive effects of excitotoxic injury and dietary restriction on microgliosis and neurogenesis in the hippocampus of adult mice. Neuromolecular Medicine 4(3): 179–196. [DOI] [PubMed] [Google Scholar]
- Leibowitz KL, Moore RH, Ahima RS, Stunkard AJ, Stallings VA, Berkowitz RI, et al. (2012). Maternal obesity associated with inflammation in their children. World J Pediatr 8(1): 76–79. [DOI] [PubMed] [Google Scholar]
- Leick L, Lindegaard B, Stensvold D, Plomgaard P, Saltin B, Pilegaard H (2007). Adipose tissue interleukin-18 mRNA and plasma interleukin-18: effect of obesity and exercise. Obesity (Silver Spring) 15(2): 356–363. [DOI] [PubMed] [Google Scholar]
- Lenz T (1997). Release of prorenin and placental hormones from superfused minced chorion laeve. Acta Obstet Gynecol Scand 76(10): 903–906. [DOI] [PubMed] [Google Scholar]
- Leon DA, Chenet L, Shkolnikov VM, Zakharov S, Shapiro J, Rakhmanova G, et al. (1997). Huge variation in Russian mortality rates 1984–94: artefact, alcohol, or what? Lancet 350(9075): 383–388. [DOI] [PubMed] [Google Scholar]
- Levay EA, Govic A, Penman J, Paolini AG, Kent S (2007). Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiology and Behavior 92(5): 889–896. [DOI] [PubMed] [Google Scholar]
- Levay EA, Paolini AG, Govic A, Hazi A, Penman J, Kent S (2008). Anxiety-like behaviour in adult rats perinatally exposed to maternal calorie restriction. Behav Brain Res 191(2): 164–172. [DOI] [PubMed] [Google Scholar]
- Levay EA, Paolini AG, Govic A, Hazi A, Penman J, Kent S (2010a). HPA and sympathoadrenal activity of adult rats perinatally exposed to maternal mild calorie restriction. Behav Brain Res 208(1): 202–208. [DOI] [PubMed] [Google Scholar]
- Levay EA, Tammer AH, Penman J, Kent S, Paolini AG (2010b). Calorie restriction at increasing levels leads to augmented concentrations of corticosterone and decreasing concentrations of testosterone in rats. Nutr Res 30(5): 366–373. [DOI] [PubMed] [Google Scholar]
- Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. (2010). Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes 59(10): 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie S, Morrison JL, Williams-Wyss O, Suter CM, Humphreys DT, Ozanne SE, et al. (2014). Impact of embryo number and maternal undernutrition around the time of conception on insulin signaling and gluconeogenic factors and microRNAs in the liver of fetal sheep. Am J Physiol Endocrinol Metab 306(9): E1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieshout RJV, Taylor VH, Boyle MH (2011). Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes Rev 12(5): e548–559. [DOI] [PubMed] [Google Scholar]
- Lin E, Gletsu-Miller N (2013). Surgical stress induces an amplified inflammatory response in patients with type 2 diabetes. ISRN Obes 2013: 910586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG (1993). Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol 151(9): 4562–4573. [PubMed] [Google Scholar]
- Loncarevic-Vasiljkovic N, Pesic V, Todorovic S, Popic J, Smiljanic K, Milanovic D, et al. (2012). Caloric restriction suppresses microglial activation and prevents neuroapoptosis following cortical injury in rats. PLoS ONE 7(5): e37215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, et al. (2010). Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci 88(11): 3546–3553. [DOI] [PubMed] [Google Scholar]
- Loomans EM, Van den Bergh BR, Schelling M, Vrijkotte TG, van Eijsden M (2014). Maternal long-chain polyunsaturated fatty acid status during early pregnancy and children’s risk of problem behavior at age 56 years. J Pediatr 164(4): 762–768. [DOI] [PubMed] [Google Scholar]
- Loos RJ (2009). Recent progress in the genetics of common obesity. Br J Clin Pharmacol 68(6): 811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM (2006). Leptin as a proinflammatory cytokine. Contrib Nephrol 151: 151–164. [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ (1999). Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci U S A 96(12): 7047–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, et al. (2014). High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 5: 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald L, Hazi A, Paolini AG, Kent S (2014). Calorie restriction dose-dependently abates lipopolysaccharide-induced fever, sickness behavior, and circulating interleukin-6 while increasing corticosterone. Brain Behav Immun Advance online publication. [DOI] [PubMed] [Google Scholar]
- MacDonald L, Radler M, Paolini AG, Kent S (2011). Calorie restriction attenuates LPS-induced sickness behavior and shifts hypothalamic signaling pathways to an anti-inflammatory bias. American Journal of Physiology. Regulatory, Integrative, and Comparative Physiology 301(1): R172–R184. [DOI] [PubMed] [Google Scholar]
- Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, et al. (2009). Maternal obesity and markers of inflammation in pregnancy. Cytokine 47(1): 61–64. [DOI] [PubMed] [Google Scholar]
- Madore C, Nadjar A, Delpech JC, Sere A, Aubert A, Portal C, et al. (2014). Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav Immun Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, et al. (2004). Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci U S A 101(52): 18171–18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki J, Kuwamura M, Yamaji R, Inui H, Nakano Y (2001). Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. Nutritional Immunology 131(8): 2139–2144. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489(7415): 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, et al. (2009). Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119(2): 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. (2009). Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29(2): 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ (2014). Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G (2012). Autism risk in small- and large-forgestational-age infants. Am J Obstet Gynecol 206(4): 314 e311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, et al. (1999). The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 89(3): 687–699. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Chen H (2009). Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes (Lond) 33(1): 115–122. [DOI] [PubMed] [Google Scholar]
- Motaghedi R, Bae JJ, Memtsoudis SG, Kim DH, Beathe JC, Paroli L, et al. (2013). Association of Obesity With Inflammation and Pain After Total Hip Arthroplasty. Clin Orthop Relat Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N, Molony LA, Conn CA, Kluger MJ (1989). Anorexic effects of interleukin 1 in the rat. American Journal of Physiology 257: R1315–R1321. [DOI] [PubMed] [Google Scholar]
- Murabayashi N, Sugiyama T, Zhang L, Kamimoto Y, Umekawa T, Ma N, et al. (2013). Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur J Obstet Gynecol Reprod Biol 169(1): 39–44. [DOI] [PubMed] [Google Scholar]
- Muthukumar AR, Jolly CA, Zaman K, Fernandes G (2000). Calorie restriction decreases proinflammatory cytokines and polymeric Ig receptor expression in the submandibular glands of autoimmune prone (NZB x NZW)F1 mice. Journal of Clinical Immunology 20(5): 354–361. [DOI] [PubMed] [Google Scholar]
- Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK (2013). Parental dietary fat intake alters offspring microbiome and immunity. J Immunol 191(6): 3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjar A, Sauvant J, Combe C, Parnet P, Konsman JP (2010). Brain cyclooxygenase-2 mediates interleukin-1-induced cellular activation in preoptic and arcuate hypothalamus, but not sickness symptoms. Neurobiol Dis 39(3): 393–401. [DOI] [PubMed] [Google Scholar]
- Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD (2011). Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 176: 225–236. [DOI] [PubMed] [Google Scholar]
- Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD (2008). Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology (Berl) 197(1): 83–94. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H (2001). Interleukin-18 regulates both Th1 and Th2 responses. Annual Reviews in Immunology 19: 423–474. [DOI] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, et al. (2006). Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 12(6): 650–656. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Lupu DS (2009). High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci 27(7): 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivoit P, Morens C, Van Assche FA, Jansen E, Poston L, Remacle C, et al. (2009). Established dietinduced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 52(6): 1133–1142. [DOI] [PubMed] [Google Scholar]
- Noria SF, Grantcharov T (2013). Biological effects of bariatric surgery on obesity-related comorbidities. Can J Surg 56(1): 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez H, Ruiz S, Soto-Moyano R, Navarrete M, Valladares L, White A, et al. (2008). Fetal undernutrition induces overexpression of CRH mRNA and CRH protein in hypothalamus and increases CRH and corticosterone in plasma during postnatal life in the rat. Neurosci Lett 448(1): 115–119. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311(8): 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusi SO, Al-Awadhi A, Abraham M (2003). Relations of serum interleukin 18 levels to serum lipid and glucose concentrations in an apparently healthy adult population. Horm Res 60(1): 29–33. [DOI] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB (2000). Association between postnatal catchup growth and obesity in childhood: prospective cohort study. BMJ 320(7240): 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A (2011). Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32(2): 205–212. [DOI] [PubMed] [Google Scholar]
- Orozco-Solís R, Matos RJ, Souza SLd, Grit I, Kaeffer B, Castro RMd, et al. (2011). Perinatal nutrient restriction induces long-lasting alterations in the circadian expression pattern of genes regulating food intake and energy metabolism. Int J Obes (Lond) 35(7): 990–1000. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN (2004). Lifespan: catch-up growth and obesity in male mice. Nature 427(6973): 411–412. [DOI] [PubMed] [Google Scholar]
- Palma GD, Collins SM, Bercik P, Verdu EF (2014). The Microbiota-Gut-Brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J Physiol. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Brown AS, Keegan D, Siska LD, Susser E, Rotrosen J, et al. (2008). Prenatal protein deprivation alters dopamine-mediated behaviors and dopaminergic and glutamatergic receptor binding. Brain Res 1237: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou A, Priftis KN (2009). Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 16(5): 265–271. [DOI] [PubMed] [Google Scholar]
- Pepping JK, Freeman LR, Gupta S, Keller JN, Bruce-Keller AJ (2013). NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am J Physiol Endocrinol Metab 304(4): E392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry CJ, Dorling MW, Wang CL, Pawlak DB, Ozanne SE (2000). Catecholamine levels and receptor expression in low protein rat offspring. Diabet Med 17(12): 848–853. [DOI] [PubMed] [Google Scholar]
- Piccio L, Stark JL, Cross AH (2008). Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol 84(4): 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup JC, Crook MA (1998). Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41(10): 1241–1248. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán CR (2001). Cytokines and feeding. Int J Obes Relat Metab Disord 25 Suppl 5: S48–52. [DOI] [PubMed] [Google Scholar]
- Pohl J, Luheshi GN, Woodside B (2013). Effect of obesity on the acute inflammatory response in pregnant and cycling female rats. J Neuroendocrinol 25(5): 433–445. [DOI] [PubMed] [Google Scholar]
- Pohl J, Woodside B, Luheshi GN (2009). Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology 150(11): 4901–4910. [DOI] [PubMed] [Google Scholar]
- Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, et al. (2009). Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296(5): E1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK (2012). Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat dietinduced obesity. PLoS ONE 7(1): e30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaelli T, Uvena-Celebrezze J, Minium J, Huston-Presley L, Catalano P, Hauguel-de Mouzon S (2006). Maternal interleukin-6: marker of fetal growth and adiposity. J Soc Gynecol Investig 13(1): 53–57. [DOI] [PubMed] [Google Scholar]
- Radler ME, Hale MW, Kent S (2014). Calorie restriction attenuates lipopolysaccharide (LPS)-induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav Immun 38: 13–24. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, Der Meulen JH, Osmond C, Barker DJ, Bleker OP (1999). Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 70(5): 811–816. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. (1998). Glucose tolerance in adults after prenatal exposure to famine. Lancet 351(9097): 173–177. [DOI] [PubMed] [Google Scholar]
- Ravelli GP, Stein ZA, Susser MW (1976). Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295(7): 349–353. [DOI] [PubMed] [Google Scholar]
- Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA, Grinspan JB (2012). Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol 71(7): 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers F, Verhagen LA, Adan RA, Waal HAD-vd (2008). Hypothalamic neuropeptide expression of juvenile and middle-aged rats after early postnatal food restriction. Endocrinology 149(7): 3617–3625. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Sawchenko PE (2002). Involvement of the Arcuate Nucleus of the Hypothalamus in Interleukin-1-Induced Anorexia. J. Neurosci. 22(12): 5091–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CM, Li M, Gray C, Vickers MH (2013a). Pre-weaning growth hormone treatment ameliorates bone marrow macrophage inflammation in adult male rat offspring following maternal undernutrition. PLoS ONE 8(7): e68262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CM, Li M, Gray C, Vickers MH (2013b). Preweaning growth hormone treatment ameliorates adipose tissue insulin resistance and inflammation in adult male offspring following maternal undernutrition. Endocrinology 154(8): 2676–2686. [DOI] [PubMed] [Google Scholar]
- Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, et al. (2011). Placental structure and inflammation in pregnancies associated with obesity. Placenta 32(3): 247–254. [DOI] [PubMed] [Google Scholar]
- Rocha ML, Fernandes PP, Lotufo BM, Manhães AC, Barradas PC, Tenorio F (2014). Undernutrition during early life alters neuropeptide Y distribution along the arcuate/paraventricular pathway. Neuroscience 256: 379–391. [DOI] [PubMed] [Google Scholar]
- Rodriguez A (2010). Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry 51(2): 134–143. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP (2000a). Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr 72(5): 1101–1106. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, et al. (2000b). Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 84(6): 595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol F, Bobrov N, Novotny M, Vasilenko T, Mozes S, Sefcikova Z, et al. (2014). Skin wound healing in obese and lean male adolescent rats submitted to pre-weaning litter size manipulation. Folia Biol (Praha) 60(1): 21–27. [PubMed] [Google Scholar]
- Sachot C, Poole S, Luheshi GN (2004). Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. J Physiol 561(Pt 1): 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Alexanderson C, Molne J, Haraldsson B, Hansell P, Holmang A (2006a). Prenatal exposure to interleukin-6 results in hypertension and alterations in the renin-angiotensin system of the rat. J Physiol 575(Pt 3): 855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A (2006b). Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol 290(5): R1345–1356. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, et al. (2008). Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51(2): 383–392. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Ohrn I, Dahlgren J, Eriksson E, Angelin B, Folkow B, et al. (2004). Prenatal exposure to interleukin-6 results in hypertension and increased hypothalamic-pituitary-adrenal axis activity in adult rats. Endocrinology 145(11): 4897–4911. [DOI] [PubMed] [Google Scholar]
- Sanders TR, Kim DW, Glendining KA, Jasoni CL (2014a). Maternal obesity and IL6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology: en 20131968. [DOI] [PubMed] [Google Scholar]
- Sanders TR, Kim DW, Glendining KA, Jasoni CL (2014b). Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology 155(7): 2566–2577. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21(1): 55–89. [DOI] [PubMed] [Google Scholar]
- Sartipy P, Loskutoff DJ (2003). Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 100(12): 7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, de Vega W, Sivanathan S, St-Cyr S, McGowan P (2014). Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 272C: 92–101. [DOI] [PubMed] [Google Scholar]
- Serrero G, Lepak NM, Hayashi J, Goodrich SP (1993). Impaired epidermal growth factor production in genetically obese ob/ob mice. Am J Physiol 264(5 Pt 1): E800–803. [DOI] [PubMed] [Google Scholar]
- Shido O, Nagasaka T, Watanabe T (1989). Blunted febrile response to intravenous endotoxin in starved rats. Journal of Applied Physiology 67(3): 963–969. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Chikahisa S, Nishi Y, Harada S, Iwaki Y, Fujihara H, et al. (2013). Maternal dietary restriction alters offspring’s sleep homeostasis. PLoS ONE 8(5): e64263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojoony MJ (1985). Effects of fasting on heat balance and nonshivering thermogenesis in febrile adult guinea pigs. Journal of Thermal Biology 10: 239–243. [Google Scholar]
- Shultz PL, Galler JR, Tonkiss J (1999). Prenatal protein restriction increases sensitization to cocaineinduced stereotypy. Behav Pharmacol 10(4). [DOI] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM (2012). Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 1261: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk T, Kolb H, Muller-Scholze S, Rohrig K, Hauner H, Herder C (2005). The proatherogenic cytokine interleukin-18 is secreted by human adipocytes. Eur J Endocrinol 152(6): 863–868. [DOI] [PubMed] [Google Scholar]
- Smith AG, Sheridan PA, Harp JB, Beck MA (2007a). Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr 137(5): 1236–1243. [DOI] [PubMed] [Google Scholar]
- Smith JT, Spencer SJ (2012). Preweaning over- and underfeeding alters onset of puberty in the rat without affecting kisspeptin. Biol Reprod 86(5): 145, 141–148. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007b). Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27(40): 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding CC, Walford RL, Effros RB (1997). Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mechanisms of Ageing and Development 93(1–3): 87–94. [DOI] [PubMed] [Google Scholar]
- Spencer SJ (2012). Early life programming of obesity: the impact of the perinatal environment on the development of obesity and metabolic dysfunction in the offspring. Curr Diabetes Rev 8(1): 55–68. [DOI] [PubMed] [Google Scholar]
- Spencer SJ (2013a). Perinatal nutrition programs neuroimmune function long-term: mechanisms and implications. Front Neurosci 7: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ (2013b). Perinatal programming of neuroendocrine mechanisms connecting feeding behavior and stress. Front Neurosci 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Martin S, Mouihate A, Pittman QJ (2006). Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 31(9): 1910–1918. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Tilbrook A (2009). Neonatal overfeeding alters adult anxiety and stress responsiveness. Psychoneuroendocrinology 34(8): 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Tilbrook A (2011). The glucocorticoid contribution to obesity. Stress. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF (1984). Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci 35(26): 2635–2642. [DOI] [PubMed] [Google Scholar]
- Stanner SA, Bulmer K, Andres C, Lantseva OE, Borodina V, Poteen VV, et al. (1997). Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. BMJ 315(7119): 1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis A, Spencer SJ (2012). Effects of neonatal overfeeding on juvenile and adult feeding and energy expenditure in the rat. PLoS ONE 7(12): e52130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, et al. (2005). Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation 111(15): 1897–1903. [DOI] [PubMed] [Google Scholar]
- Sun D, Muthukumar AR, Lawrence RA, Fernandes G (2001). Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clinical and Diagnostic Laboratory Immunology 8(5): 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser ES, Lin SP (1992). Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psychiatry 49(12): 983–988. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Centanni AV, Butler AA (2010). Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology 151(4): 1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamamura F, Naito M (1989). Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol 45(2): 87–96. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Moran TH (2010). Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav 100(5): 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda R, Salsberry PJ, Reagan PB, Fang MZ (2013). The impact of prepregnancy obesity on children’s cognitive test scores. Matern Child Health J 17(2): 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. (2012). Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122(1): 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorand B, Kolb H, Baumert J, Koenig W, Chambless L, Meisinger C, et al. (2005). Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes 54(10): 2932–2938. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Kumon M, Wada E, Onodera M, Mochizuki H, Wada K (2010). Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem Int 57(3): 235–247. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Wada E, Wada K (2009). Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J 23(6): 1920–1934. [DOI] [PubMed] [Google Scholar]
- Ugochukwu NH, Figgers CL (2007). Caloric restriction inhibits up-regulation of inflammatory cytokines and TNF-alpha, and activates IL-10 and haptoglobin in the plasma of streptozotocin-induced diabetic rats. The Journal of Nutritional Biochemistry 18(2): 120–126. [DOI] [PubMed] [Google Scholar]
- Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, et al. (1996). Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. Journal of Immunology 156(11): 4274–4279. [PubMed] [Google Scholar]
- Valdomero A, Velazquez EE, Olmos Sd, Olmos JSd, Orsingher OA, Cuadra GR (2007). Increased rewarding properties of morphine in perinatally protein-malnourished rats. Neuroscience 150(2): 449–458. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Boyle MH (2011a). Canadian youth born large or small for gestational age and externalizing and internalizing problems. Can J Psychiatry 56(4): 227–234. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Boyle MH (2011b). Is bigger better? Macrosomia and psychopathology later in life. Obes Rev 12(5): e405–411. [DOI] [PubMed] [Google Scholar]
- Vauthier V, Derviaux C, Douayry N, Roux T, Trinquet E, Jockers R, et al. (2013). Design and validation of a homogeneous time-resolved fluorescence-based leptin receptor binding assay. Anal Biochem 436(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Vega VL, De Cabo R, De Maio A (2004). Age and caloric restriction diets are confounding factors that modify the response to lipopolysaccharide by peritoneal macrophages in C57BL/6 mice. Shock 22(3): 248–253. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM (2010a). Maternal High-Fat Diet Alters Methylation and Gene Expression of Dopamine and Opioid-Related Genes. Endocrinology 151(10): 4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, et al. (2010b). Early life protein restriction alters dopamine circuitry. Neuroscience 168(2): 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, et al. (2002). Interleukin-6deficient mice develop mature-onset obesity. Nat Med 8(1): 75–79. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL (1988). The retardation of aging and disease by dietary restriction. edn. Charles C Thomas: Springfield, IL. [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D (1986). The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. Journal of Nutrition 116(4): 641–654. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr., (2003). Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12): 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS (2003). Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112(12): 1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH (1997). Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337(13): 869–873. [DOI] [PubMed] [Google Scholar]
- Wisse BE, Ogimoto K, Morton GJ, Williams DL, Schwartz MW (2007). Central interleukin-1 (IL1) signaling is required for pharmacological, but not physiological, effects of leptin on energy balance. Brain Res 1144: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik W, Lee W, Colman I, Hardy R, Hotopf M (2013). Foetal origins of depression? A systematic review and meta-analysis of low birth weight and later depression. Psychol Med 43(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S, Pinkney J (2004). Role of cytokines in regulating feeding behaviour. Curr Drug Targets 5(3): 251–263. [DOI] [PubMed] [Google Scholar]
- Wu T, Deng S, Li WG, Yu Y, Li F, Mao M (2013). Maternal obesity caused by overnutrition exposure leads to reversal learning deficits and striatal disturbance in rats. PLoS ONE 8(11): e78876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. (2008). Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 51(1): 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, et al. (2011). Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm Bowel Dis 17(7): 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Peng Y, Wu J, Wang Y, Yao L (2014). Toll-like receptor 2/4 links to free fatty acid-induced inflammation and beta-cell dysfunction. J Leukoc Biol 95(1): 47–52. [DOI] [PubMed] [Google Scholar]
- Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, et al. (2005). Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 1(6): 371–378. [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, Bawdon RE (2004). Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 103(3): 546–550. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D (2008). Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135(1): 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MJ, Du M, Nathanielsz PW, Ford SP (2010a). Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 31(5): 387391. [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Ma Y, Long NM, Du M, Ford SP (2010b). Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol 299(5): R1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziko I, De Luca S, Dinan T, Barwood JM, Sominsky L, Cai G, et al. (2014). Neonatal overfeeding alters hypothalamic microglial profiles and central responses to immune challenge long-term. Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Conti B (2014). Interleukin-18 null mutation increases weight and food intake and reduces energy expenditure and lipid substrate utilization in high-fat diet fed mice. Brain Behav Immun 37: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, et al. (2007). Interleukin18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci U S A 104(26): 11097–11102. [DOI] [PMC free article] [PubMed] [Google Scholar]