Abstract
In-spite of significant advancement in hydrogel technology, low mechanical strength and lack of electrical conductivity has limited their next level biomedical applications for skeletal muscles, cardiac and neural cells. Host-guest chemistry based hybrid nanocomposites systems have gained attention as they completely overcome these pitfalls and generate bio-scaffolds with tunable electrical and mechanical characteristics. In recent years, carbon nanotubes (CNT)-based hybrid hydrogels have emerged as innovative candidates with diverse applications in regenerative medicines, tissue engineering, drug delivery devices, implantable devices, bio-sensing and bio-robotics. Present article is an attempt to recapitulate the advancement in synthesis and characterization of hybrid hydrogels and provide deep insights towards their functioning and success as biomedical devices. The improved comparative performance and biocompatibility of CNT-hydrogels hybrids systems developed for targeted biomedical applications are addressed here. Recent updates towards diverse applications and limitations of CNT hybrid hydrogels is the strength of the review. This will provide a holistic approach towards understanding of CNT based hydrogels and their applications in nanomedicine.
Keywords: Hydrogel hybrids, Biomaterials, Carbon nanotubes, Biosensors, Tissue engineering, Drug delivery, Imaging systems
1. Towards hybrids hydrogels
Hydrogels are the soft materials consisting of 3D polymeric networks and have the ability to imbibe large amount of water inside them. These crosslinked networks are synthesized using natural as well as synthetic polymers. [1, 2, 3] The two common forms of hydrogel consist of physical hydrogels and chemical hydrogels depending upon the crosslinks present inside them. Based on cross-linking patterns, hydrogels are categorized as a) physical hydrogel formed by electrostatic interactions or hydrogen bonding and b) chemical hydrogels involve the covalent bonding and utilize various crosslinking agents and numerous polymerization techniques. The salient features of hydrogels including tunable degree of porosity, biocompatibility, biodegradability, stability, mechanical strength and stimuli responsive behavior which is easily amenable to modification simply by altering their polymeric composition or adopting suitable polymerization technique.[4] Due to such characteristics, hydrogels have emerged as smart micr/nano-structures needed for next gerenation technology development. Other important applications of hybrid hydrogels include drug delivery, biosensing, tissue engineering, stem cell engineering, hyperthermia treatment, imaging and dental implants.
In context with drug delivery application, the drug loading and release potential of the hydrogels based pharmacological nanoformulation can be regulated simply by fine tuning of the response to various external stimuli (pH, light, temperature, pressure, electric field, radiations).[5] This makes them ideal carriers in therapeutics for drug delivery and their translation to clinics. Their application is not only limited to drug delivery but they have found remarkable biomedical applications (Figure 1), which includes tissue engineering,[6, 7] imaging,[8] diagnostics,[9] bone replacements,[10] dental implants,[11] water purification, biosensing,[12] contact lenses,[13] and drug delivery[14] to various major organs of human body[2].
Figure 1.
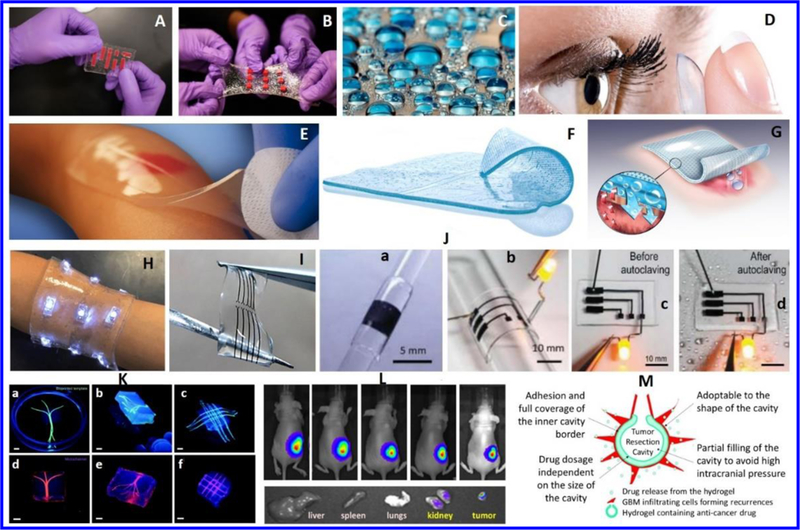
Biomedical applications of hydrogels. A) Stretchable hydrogel electronics, B) Water-based band-aid designed to senses temperature, lights up and delivery for specific medicine to the skin (Source: http://news.mit.edu/2016/tough-hydrogel-hybrid-artificial-skin-0627).C) Hydrogel based contact lenses made of silicone hydrogel (source: http://coopervision.com/about-contacts/silicone-hydrogel-contact-lenses. http://www.seetheclarity.com/contact-lens-basics/. D) Hydrogel pad prepared by Neoheal® Sterile company, designed for moist wound management (Source: http://kikgel.com.pl/produkty/neoheal/).E) Hydrogel sheet prepared by AquaDerm company for pain relief and wounds healing (http://dermarite.com/product/aquaderm/). F) A hydrogel sheet designed to cure (Source: http://www.asocorp.com/products/wound-care/). H) A hydrogel based bandage for temperature sensing and medicine delivery (Source: http://insights.globalspec.com/article/1815/hydrogel-bandage-senses-temperature-delivers-medicine). I) a hydrogel based stretchable electrodes deigned for health care [Ref. Nature Asia Materials http://www.nature.com/am/journal/v3/n1/full/am20119a.html. J) Stretchable hydrogel electronics (a-c) designed to develop implantable devices aiming for tissue engineering. (Source: http://onlinelibrary.wiley.com/doi/10.1002/adhm.201400209/epdf). K) Bio-printed hydrogel microchannel networks developed for real time monitoring with potential application in tissue engineering and organs on chip applications Reprinted with copyright permission from reference [15]. L) Injectable hydrogels fabricated using triblock copolymers of vitamin E-and functionalized polycarbonate and poly (ethylene glycol). This hydrogel was used to deliver a specific of antibodies to cure cancer.Reprinted with copyright permission from reference [16]M) Hydrogel designed for the delivery of anticancer therapeutic agent for the treatment of glioblastoma (GBM). Reprinted with copyright permission from reference [17]
Recent advancements in nano-enabled polymer technology led to the development of a new class of novel hydrogels known as “Hydrogel Hybrids” which are able to address the existing shortcomings of conventional hydrogels such as low mechanical strength, low biodegradability and increased toxicity levels.[18] Hydrogel hybrids have proven a potential platform of desired properties for various applications such as gene targeting,[19] scaffolds,[20] toxic metal ion adsorption,[21] biosensing and imaging,[22] microfluidics,[23] remote controlled materials[24] and targeted drug delivery.[25] After the initial assessment of the drug release kinetics and mechanism associated with hydrogels, the nano-enabled smart hydrogels systems, host-guest chemistry based organic-inorganic nanocomposites, are now being explored for targeting the cells and other acute diseases like cancer[26] and tuberculosis[27] etc. Recently, alginate-polyacrylamide hydrogels have been shown to impart a fracture energy of 9000 J m−2 which supports their application in replacements of cartilage[28]. Various nanoparticles in combination with the polymeric hydrogels are now being formulated to design the new generations chip based systems used for sensing, imaging [29] and diagnostics purposes[30]. Based on their physical states, many hybrid hydrogels can be used as sprays, films and particles. In addition to the aforementioned applications of hydrogels hybrids, the other interesting aspects of these biomaterials are their existence in diverse forms, which includes liquid form, spray, particles, films and most importantly nanocomposite hydrogels or hybrid hydrogels are discussed. [5, 31, 32]
The multi-responsive features of hybrid hydrogels have shown great promise to enhance the performance of hydrogels in targeted application.[33] Nano-fillers like graphene oxide,[34]clay nanoparticles,[35] metal and metal oxide nanoparticles, silica nanoparticles,[36] and polymeric nanoparticles based hybrid hydrogels have increased application of hydrogels resulting in improved outcomes such as pulsatile drug release, near IR responsive systems,[37] chondrogenic differentiation of stem cells.[38]
Among numerous hybrid hydrogel systems, carbon nanotubes (CNTs)-based hybrid hydrogels have gained significant attention due to their high mechanical strength, effective surface area, and high electrical conductivity. The well-demonstrated features of CNTs like high cellular uptake, high stability, and electromagnetic behavior advocate them as one of the most promising nano-filler for diverse applications. Beside this, CNTs exhibit high surface area, which allow a very high loading of the chemotherapeutic drugs [39] and can thus penetrate deep inside the tumor tissue resulting in high retention in the solid tumors.[40] The increasing significance of hybrid nanocomposite hydrogel can be assessed with the increasing number of research publications (Figure 2) related with hybrid hydrogel development.
Figure 2.
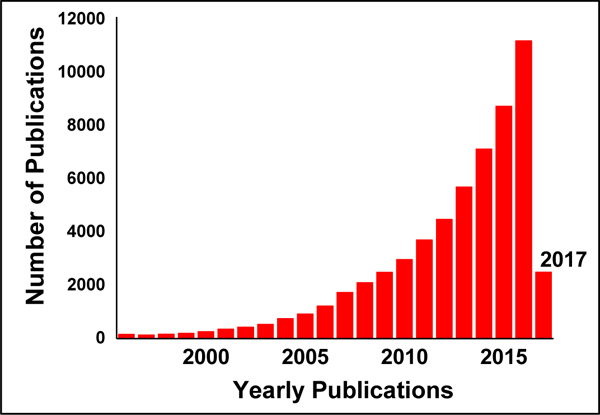
Estimate of the number of publication published in last 20 years in field of NC hydrogels.
Therefore, the present review is an attempt to explain carbon nanotubes, synthesis of hybrid hydrogels, CNTs based hybrid hydrogels, and potential biomedical application such as biosensing, imaging, drug delivery and tissue engineering of CNTs-hybrid hydrogels. [41] The clinical relevance of CNTs based hydrogel hybrids in nanomedicine is also discussed in detail.
2. Carbon Nanotubes: Types and Characteristics
The CNTs, allotropic form of carbon, are cylindrical nanostructure with hexagonal arrangement of sp2 hybridized carbon atom. Basically, it is rolled up graphene sheet having one single-walled (SW) i.e., SWCNTs or multiple walled (MW) i.e., MWCNTs. The wall of the CNTs discriminates it into two forms and both these forms have capped ends of tubes with a hemispherical arrangement of carbon networks known as fullerenes which is wrapped by graphene sheet. The unique feature of these two forms of CNTs is that the MWCNTs have interlayer separation of 0.34 nm in average and forms individual tube with an outer diameter of 2.5 −100 nm whereas SWCNT has comparatively very lesser outer diameter of 0.6 to 2.4 nm. The differences in the outer diameter brings versatile applications. SWCNTs have the perfect quality control as drug carrier whereas the MWCNTs are accompanied by defects in the nanostructure which are quite unstable structure and can be easily modified.[42] Conducting, semi-conducting or non-conducting nature of material/reinforcing agent under ambient temperature conditions have been greatly explored for various applications of the resultant hybrid material. Chirality in CNTs leads to two different conduction pathways, imparting distinct electrical behaviour. In general, CNTs with armchair tubes structures have relatively higher conductivity or ballistic conduction ability compared to other configurations of CNTs (i.e. zigzag and chiral). For example, SWCNTs can be metallic or semi-conducting depending on the relationship in indices of CNTs, which determines the electronic structure of the material. This electronic property has been helpful in fabrication of several programmable CNTs-hydrogel polymer nanocomposites. Xiaobo Zhang et al., have fabricated dual responsive actuators (thermal and optical) using poly(N-isopropylacrylamide) (pNIPAM) loaded SWCNTs with enhanced water molecule transport mechanism.[43] The composites are flexi-programmed to self-fold to give different structural geometries like cubes and flowers (Figure 3). The concept of lower critical solution temperature (LCST) was used for the development of actuators hinges and moreover, heating above this temperature can result in flipping of the hydrophilic nature of hydrogel to hydrophobic state forcing the expulsion of water from the 3D hydrogel matrix.[44]
Figure 3.
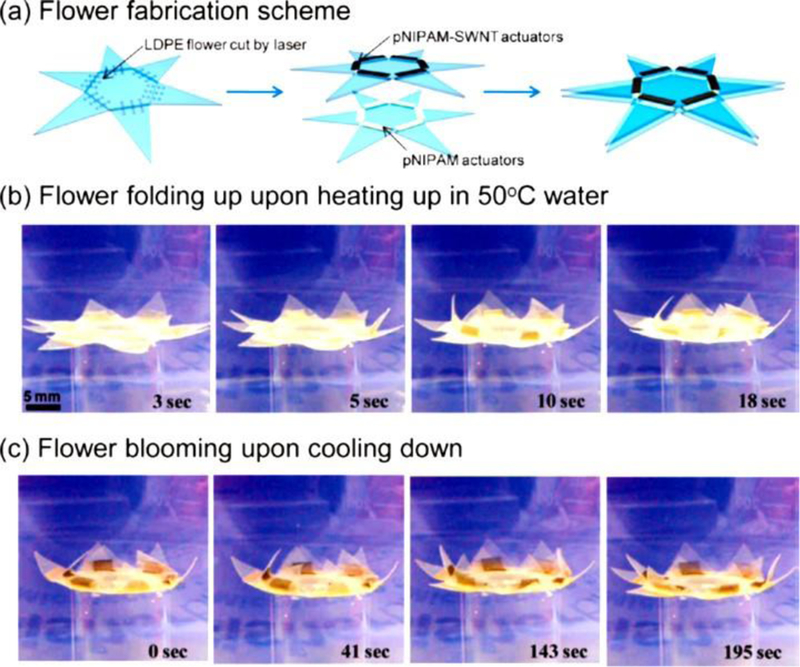
Programmable “flower’ made by heterogeneous integration of pNIPAM and SWCNTs-pNIPAM bilayer actuators. (a) Fabrication scheme for making a programmable flower, consisting of two layers of actuators. (b) Flower folding (i.e., closes) when heated in a water bath to 50 °C. (c) Flower blooming by cooling down the water bath. Reprinted with permission from ACS [43]
Such hybrid nanocomposite hydrogels have the ability to selectively change the surface charge.[45] However, purified semiconducting or metallic CNTs have been used in fixed proportions in medical devices or prosthetics for micro/nano electronic, electrical stimulation, and in-vitro electrical reading using CNTs based hydrogel materials. The mechanical strength, electrical, and electronic properties of the CNTs reinforced hydrogels have been improved due to high aspect ratio of CNTs and metallic nature of the CNTs. Structural integrity of the composite hydrogel can simply be improved due to higher tensile strength of CNTs. Further, till threshold concentrations of CNTs, the viability of the cells are high.[46] Thus, resulting hybrid conducting materials can easily mimic workability of certain tissues, such as cardiac muscle and nerve tissues propagating electrical potentials within the body.[46] Tissue engineering, scaffold for organs growth, 3D printing and bio sensing application are few of the various applications where these reinforced hydrogels can greatly be applied.
One of important properties of CNTs includes their high stiffness and the capability to de-fold and fold reversibly. This results in the high stiffness value of 1 TPa and a high tensile strength of 150 GPa. This make CNTs as one of the stiffest material and perform under compression.[47] Moreover this feature make CNTs to exhibit different forms such as metallic semiconducting or semi metallic with the variation in the chirality and diameter. Therefore, chirality determines their conductivity and gives added applications to SWCNTs based nano-electronic devices. Other major application of CNTs is their use as interconnect chip, these chips have replaced the use of aluminum and copper. The high conductivity and the lesser dimensions of CNTs may provide an interconnect option to copper. One of research group highlighted the use of the SWCNTs for batteries as they have high storage capacity. The electrodes composed of CNTs are ten times thinner and light weight than the amorphous carbon electrodes.[48] The other interesting aspect of CNTs is their solubility in aqueous solvents and this is prerequisite for biocompatibility and should be met to be used in drug delivery. The uniform dispersion of CNTs is quite important as CNTs tends to assemble in bundles due to hydrophobic character of graphene sidewalls and the strong p-p interactions between the individual tubes. Thus various approaches such as surfactant assisted dispersion, solvent or biomolecular dispersion, and functionalization of CNTs are followed for obtaining a homogeneous dispersion. In context with the dispersion tendency of CNTs in the hydrogel matrix, they can physically dispersed or covalently conjugated to the polymeric hydrogel matrix.[49] The other common methods to design CNTs based hydrogel hybrids are discussed in detail in subsequent sections.
They constitute of only carbon, hence have superior biocompatibility, low toxicity, and immunogenicity, which make them ideal candidate to be used for biomedical applications.[50] The nm space inside the CNTs, protect the active compound from timely degradation and the surface functionalization (amide, amino, carboxyl, hydroxyl groups, etc.) promises the targeted delivery of the drugs and other bio-actives. Their hybrid materials are highly tunable, possessing the advantageous properties of CNTs in synergism with that of polymeric three-dimensional structures. The important features include high stiffness, increased adsorption, high stability, tunable improved electrical behavior, improved magnetism, flexibility, biocompatibility, and biodegradability. It has been reported that the improvement in the shape recovery stress, compressive performance, tensile strength, thermal, and electrical conductivity due to reinforcements done by CNTs makes them smart as well as adaptive. The mechanical properties are dependent on the amount of incorporated CNTs into the hydrogel system. The nano-fibrous networks with electrical conductivity of the hybrid hydrogel results in improved cell adhesion, their organization, cell–cell coupling and have shown potential for constructs used for neuron and other muscle cells. These features provide ability to recover or reorganize their structure under the influence of external stimuli like pH, temperature, magnetic field, electric field etc.[51] Carboxyl-functionalized CNTs dispersed poly(acrylamide-co-sodium methacrylate) [P(AM-co-SMA)] hydrogels exhibited higher swelling %, good reversibility, and pH responsiveness.[52] CNTs based systems have great potential as an extracellular matrix to bio mimic heart tissues for myocardial tissue engineering. These constructs are electrically conducting (DC conductivity heart muscles around 0.1 S/m) and these scaffolds have nano-fibrous architectures at sub-micro-meter scale (similar to naturally underlined electrically conductive purkinje fibers) and also have highly ordered CNTs structure.[53, 54]. The next section will elaborate various methods for synthesis and development of CNTs based hydrogel hybrids.
3. Synthesis and designing of CNTs based hybrid hydrogels
The unique properties exhibited by hybrid hydrogel are associated with the properties of hydrogel polymeric networks. In this section, we briefly discussed methodologies employed for the synthesis of hydrogel hybrids. Efforts have also been made to discuss, comparatively, the methodologies adopted to design nanocomposite hydrogels.[55]
3.1. Covalent cross-linking; In situ polymerization technique
Generally, the hydrogel hybrids are synthesized by using the covalent cross-linking method using the in-situ polymerization technique. The introduction of the nano-filler is done during the reaction and thus results in development of hydrogel hybrids with excellent mechanical strength. This technique allows synthesis of hybrid gels in variety of shapes and surface forms. This polymerization method involves mild conditions and the product is obtained in high yield. A very interesting study by Haraguchi et al [56] gained lot of attention using the in situ polymerization technique to develop NC gels composed of acrylamide derivative, poly(N-alkyl acrylamides), in the presence of inorganic clay. Hybrid gel with no phase separation and 100% yield was developed. This study highlighted that by varying the composition of initial reaction solution, the composition of final hybrid gels can be controlled. This study also proved that the use of organic cross-linker, bisacrylamide (BIS) mediated covalent interactions may make the gel weaker and brittle. The increased mechanical and modulus strength, improved deformability and fracture energy were some of the important parameters obtained in comparison to the conventional hydrogels. Other studies also utilized the covalent crosslinking interactions to achieve high mechanical strength and stability.[57] Beside this, non-covalent techniques based on coating on polymeric materials is also utilized to synthesize CNTs-hydrogel hybrids.[58]
3.2. Physical cross-linking technique
Other recent research showed the synthesis of nanoparticles in-situ during the formation of the cross-linked networks. These types of system are constituted by physical cross-linking. One of the interesting study shows that the physical inclusion of rod like cellulose nanocrystals can overcome weak mechanical strength and show 35-fold increase in storage modulus. These systems showed potential application in tissue engineering and promote the cell growth.[59] Physically crosslinked hydrogels are known to show high biocompatibility. On-going research utilizes molecular dynamics simulations to study their structure formation. However, some of contrasting studies revealed that the physical hydrogels may not impart high mechanical strength. They exhibit features to be responsive to the various external stimuli. Structure formation in physically cross-linked gels also suggests that the filler concentration is quite responsible for the caging effect in the gel. This also suggests that disk like nano-fillers can strengthen the hydrogels more as compared to spherical nano-fillers. This simple technique has been used by various research groups to synthesize the physical crosslinked hydrogel hybrids. Hybrid gelatin hydrogels with CNT has been synthesized using the physical mixing method.[60] The physical entrapment process approaches a very high affinity of nano-fillers for the polymeric matrix and thus limit the non-homogeneous distribution and agglomeration of the nano-fillers.
3.3. Ex-situ polymerization technique
Besides the in-situ polymerization method the incorporation of the nanoparticles in the polymer matrix is also done by using ex-situ polymerization technique.[61] This polymerization technique involves the dispersion of pre made nanoparticles into the polymeric matrix. This method is well known for large-scale production in comparison to in-situ methods. Sonication is also used to disperse the nano-filler in the matrix.
A group synthesized composite hydrogels having silver nanoparticle by ex-situ polymerization, which showed excellent antibacterial feature. Moreover, the newly developed amphoteric nanocomposite hydrogels have also gained lot of attention in recent years. These hydrogels exhibited high tensile strength and stretch ability with the incorporation of nano-fillers. For example, Du Juan et al [62] group demonstrated the in-situ copolymerization technique to develop highly robust gels having tensile strength of 316 kPa. The concentration of laponite affected the fracture strain of 2,317% with 3 wt. % laponite.[62]
3.4. Grafting technique
Another interesting and commonly used method employed to functionalize the surface of the CNTs includes grafting of the polymer chains into the surface of the nano-fillers. Many recent research showed that this type of grafting resulted into shielding the surface of the CNTs.[63] This method of shielding resulted in good dispersion of the nano-fillers and better interaction between the polymer and the nano-filler. This technique may be used for various protein-CNTs systems, which are applied for various biomedical applications.[64] The fabrication of the CNTs-polymer hybrids can be done by “grafting from” and “grafting to”. These two different approaches utilize the pre formed polymer chains with the surface of the functionalized CNTs. Moreover the “grafting to” method involved the reactive polymers having the reactive functional groups and there was a reaction with the surface of CNTs. More prominently the limitation of such methods were low grafting density and to overcome these limitation “grafting from” approach was used to synthesize the hydrogel hybrids. This process involved the monomer polymerization from the surface derived initiators on CNTs. No stearic hindrance was involved and high molecular weight polymers could be grafted and high grafting density was achieved.[55, 65]
3.5. Smart devices enable technique
One of the remarkable synthesis opted by Liu et al [66] for the development of responsive nanocomposite hydrogels was based on smart devices. This group utilized the cationic and anionic monomers, which demonstrate a very simple and versatile design of bilayer actuators by combining the oppositely charged hydrogels via electrostatic interactions. Thus the developed hydrogels were responsive to pH and ionic strength of the buffer and giving the features of polyelectrolyte hydrogels. These types of hydrogels are in high demand for the development of bilayer actuators.[66]
4. Polymers used for fabrication of CNTs-based hydrogel hybrids
4.1. Chitosan-CNTs hydrogel hybrid systems
Chitosan, a well-known biodegradable and biocompatible biopolymer have been extensively used in biomedical applications such as drug delivery, wound healing,[67] biosensing,[68] tissue engineering,[69] antibacterial activity,[70] blood coagulant,[71] cosmetics etc.[72] This linear polysaccharide consists of D-glucosamine and N-acetyl glucosamine unit, and derived from shrimps. Efforts have been made to improve the mechanical strength of chitosan based hydrogels, as the films and other forms of hydrogels comprising chitosan alone are quite brittle.[73] Thus the addition of the various nano-filler to the chitosan matrix have been done for improved characteristic properties. Herein, we discuss few interesting studies, which utilize CNTs as a nano-filler in chitosan based hydrogel matrix. For example, a recent study revealed the fabrication of the mechanically robust 3D nanocomposite hydrogels based on the chitosan and owe the feature of self-healing. The study came up with superior mechanical and electrical properties upon incorporation of CNTs as nano-filler. The crosslinking by the zinc phthalocyanine tetra-aldehyde (ZnPcTa) framework resulted in schiff-base linkage with chitosan to form the 3D structure and the rheological recovery is attributed to the covalent schiff-base linkage between the amino groups of chitosan and benzaldehyde groups of cross-linker.[18] Many studies revealed that the incorporation of MWCNTs in chitosan matrix resulted in enhanced features of the composite hydrogels. Many sensing applications have been demonstrated using chitosan and its hybrid nanocomposites including hydrogel forms. For instance, an amperometric cholesterol biosensor was developed using sol-gel chitosan/silica and MWCNTs. It was revealed that the hybrid film resulted in improved analytical performance of developed biosensor. These hybrids were utilized to measure the free cholesterol concentration in real human blood samples.[74] Composite scaffolds are also being exploited by the combination of chitosan and CNTs. One of recent study utilized two main features of CNTs viz., piezoelectricity and high mechanical strength in bone tissue engineering. This group demonstrated the development of composite scaffold consisting of MWCNTs, medium molecular weight chitosan and β-glycerophosphate. The electric cell compatibility studies revealed that these scaffolds can be used for cell electrical applications.[75] The metallic feature of CNTs has always been in scrutiny raising questions about its toxicity and preliminary studied declared it as a carcinogenic material. CNTs and chitosan are also being explored for electronic devices.[76] The features, come up with increased stretch ability and folding ability, which give these electronic devices compatibility concerning the tissues and various bodily motions when in contact with tissue. More recently, CNTs and natural polymer based conductive inks are also being invented for printing technology.[77]
4.2. Guar gum-CNTs based hydrogel hybrid systems
Guar gum is also a natural polymer, which occur in abundance naturally and owe many desirable features to be explored in biomedical applications.[78] The easy modification of guar gum by grafting or by derivatization and its ability to respond to various pH make it an ideal candidate to be used in drug delivery. This polymer had been used in transdermal patches and colon specific delivery systems.[79] In context to be used as nanocomposite hydrogels it has been exploited using various nano-fillers though intensive research has not been carried out for CNTs based systems. Yet few studies revealed that the carboxymethyl guar gum (CMG)-chemically modified MWCNTs hybrid hydrogels were developed for transdermal drug delivery.[80] Diclofenac sodium was used as model drug in the study. Overall the study implicated the concentration of CNTs influenced the drug encapsulation and release aspects. In another interesting study, the hydrophobic character of CNTs was improved by modifying it by covalently grafting the hydrophilic guar gum. Fe3O4 was used to add the magnetic feature to the developed system. The matrix of the guar gum and CNTs was found to act as template for the growth of iron oxide particles and also exhibited superparamagnetic characters, which were further applied in red and methylene blue dye adsorption. These research show great potential of guar gum and CNTs based systems for other biomedical application.[81]
4.3. Collagen-CNTs based hydrogel hybrid systems
Collagen is one of the most important structural protein used as a biomaterial and has been eternal part of the research undergoing in tissue engineering.[82] This protein was found in the extracellular space in the various connective tissues in animal bodies. The degree of mineralization make it more miscellaneous as it is rigid in case of bone and compliant in tendon and for cartilage, a gradient from rigid to compliant. The other forms of collagen included the elongated fibrils, which is the feature found in fibrous tissues like tendon, skin and ligaments. They are found in abundance in cornea, blood vessels, bones, intervertebral discs. Thus, ophthalmology, cancer therapy, tumor treatment are the common applications of collagen based biomaterials. It has been diversely applied for biotechnological application for the cellular growth and also been used in drug delivery. The present section highlights the use of CNTs when reinforced with collagen resulted in enhanced mechanical and electrical properties. One of the interesting study revealed its application as fiber-reinforced composite matrix. Living muscle cells were inserted into the composite materials of collagen and CNTs matrix. Cell viability was 85% on day 3 and day 7. These system show great potential to be used as scaffold and may be employed as a component for biosensors or medical devices.[83] In a similar kind of study these fiber- reinforced composites were exploited for human dermal fibroblast cells (HDF). The high aspect ratio of SWCNTs was utilized for enhancing the electrical properties. The conductivity was found to vary from 3 to 7 Mscm−1, with different concentrations of loading of SWCNTs. Thus this study also implicated the protein-SWCNT composite materials owe great potential in tissue engineering as substrate and can act as transducers and possess biosensing ability.[46] Other studies showed the use of SW/MW-CNTs to improve the young’s modulus of collagen hydrogels.[84] Many cross-linking methods have been proposed to cross-link collagen using poly (ethyleneimine) (PEI) and CNTs using carbodiimide chemistry.[85] Efforts have been made to utilize these features of cross-linking to enhance the modulus and use for novel biomedical applications.
4.4. Polyethylene Glycol-CNTs hydrogel hybrid systems
Polyethylene glycol (PEG) is one of well-known synthetic polyether and has been used for developing medicine at industrial scale. It is a water soluble and non-toxic polymer that does not demonstrate any inflammatory response. The low molecular weight chains are referred to PEG whereas the high molecular weight above 20,000 is referred as poly (ethylene oxide) PEO. [86, 87] The most exciting feature of this polymer is that it gets rapidly cleared from the body and thus has been utilized for diverse biomedical applications. It has the capacity to bind covalently to other molecules, which make them very useful for functional modification of various bio-active and thus change the properties of toxic molecules to non-toxic. It is well known when they are coupled with biologically active molecules and they contribute to their activity. There is no denaturation of proteins when PEG is tethered.[87] The distinctive features of PEG make it a perfect candidate to be used in development of hydrogels for biomaterial applications. Recent research showed that PEG based hydrogel hybrids using CNTs find significant application in cancer therapy. Targeted bio-conjugates based on the anticancer agent and epidermal growth factor-SWCNTs were developed. The squamous cancer was targeted and it was shown that the SWCNTs-QDs-EGF conjugates were internalized into the cancer cells. These studies support the targeted killing of cancer cells and thus exhibit great potential to be clinically translated.[88] Another very known degenerative disease of articular joints is osteoarthritis (OA). Presently, the medical therapy is not that effective for anti-OA drugs. Thus various drug delivery systems are being explored for the cartilage penetration and an effective concentration of the medication may be delivered to the chondrocytes. Recently, a study showed the use of nano-system based on SWCNTs, which was modified using the PEG chains (PEG-SWCNTs). This study demonstrated that these nano-system were able to retain themselves in the joint cavity for a longer time and had an entry to the cartilage matrix. Moreover, they showed effective delivery of the gene inhibitors into the chondrocytes. Thus these kind of hybrid nano-sytem reveal great potential of nano-carriers for intraarticular delivery of various agents to the chondrocytes.[89] Other very important recent contribution of PEG based hydrogel hybrids in combination with CNTs is in neural engineering. The combined properties of PEG and CNTs based hydrogels, such as hydrophilicity, visco-elasticity, biocompatibility and on the other hand CNTs show high mechanical stability, can guide the neuronal cell behavior and may produce axon regeneration and thus being utilized for various drug delivery applications.[90]
4.5. Polyvinyl alcohol-CNTs hydrogel hybrid systems
Polyvinyl alcohol (PVA) is widely known synthetic polymer, which shows interesting and important properties like hydrophilicity, biodegradability and biocompatibility. These properties made PVA as a perfect candidate for biomedical applications.[91] The most exciting application of PVA based hydrogels is the tissue engineering applications and it has demonstrated potential results as scaffold material by substituting the current available for the artificial grafts.[92] Various studies showed the utilization of PVA and CNTs to achieve high mechanical strength for various applications.[93] The combination is also used in the development of PVA/MWCNTs/PANI hydrogels which showed high performance in the dye sensitized solar cells.[94] It is also seen that the combination of CNTs and PVA gel formed without any chemical crosslinking results in the high mechanical and electrical properties. These systems are being extensively exploited for biosensors, neural tissue engineering,[95] and cartilage tissue engineering.[96]
Owing to the toxicity associated with use of CNTs, the related hybrid hydrogels always received criticism and is always a matter of debate. Thus in the next section, we will describe the bio-compatibility of such systems and a function of precursors concentration.
5. Biocompatibility of CNTs-based hybrid hydrogels
Advancements in the synthesis of CNTs[97] and hydrogels [98] of desired functionality, shape, and size designed for targeted application have remarkable scientific significance. However, challenges such as poor dispersion and process-ability associated with CNTs and less chemical/physical stability of hydrogel limits their biomedical applications, especially in-vivo. To overcome these challenges, considerable efforts were made to develop nanocomposites of CNTs and hydrogel, and the success of early stage research of higher significance motivated to conduct more innovative research in this direction.[31, 99] Based on organic-inorganic hybrid nanocomposites and host-guest chemistry, CNTs-hydrogel hybrid nanostructures have demonstrated unique optical, electrical, and physical properties, which are absent in conventional hydrogels.[31, 99, 100] The CNTs based hydrogel performed as expected in the area of biocompatible electroactive electrode for sensor development, alignment of CNTs for optoelectronic devices, stretchable biopolymers for therapeutic patches, targeted drug delivery, and tissues engineering. Along with functional and structural characteristics, the selection of CNTs-hydrogel hybrids was also dependent on the biocompatibility of designed composite nanostructure.[31, 99] Recently, various cell models, according to targeted application, have been developed to explore the effects of selected CNTs-hydrogel nanocomposites and precursors on the cell viability. These models were useful for the optimization of a biocompatible dose of CNTs-hydrogel nanocomposites to achieve desired performance without any adverse effects. The assessment of biocompatibility of CNTs based hydrogel hybrids become essential to promote them for biomedical applications, especially for in-vivo application. This section of review explains various cell-lines models established to evaluate non-toxicity of CNTs-hydrogel hybrids for targeted application.
Since CNTs have been developed, this fantastic nanostructure was always a matter of debate due to its metallic nature and toxicity which limited their use in biomedical application.[101] Initial reports declared CNTs as carcinogenic carbon nanomaterial.[101] Aiming to use the unique electrical and optical properties of CNTs in biomedical application, efforts were continuously made to develop CNTs more feasible by improving their functionality, structural, and biocompatibility features.[102] For example, CNTs has been synthesized of desired functionality for the delivery of biomolecules such as DNA, genes, etc. and the matrix features can be tuned compatible to the cells such as neuron (Figure 4). [97] Moreover, the electrical conductivity of CNTs has been utilized for the cell growth. However, very few efforts were being made to improve the biocompatibility of CNTs. Considering the scientific application value of CNTs, the best methods to improve CNTs bio-compatibility was to develop its nanocomposite with hydrogel based on host-guest chemistry.[97] Two approaches were opted, i) cytotoxicity assay to estimate cell viability as dose dependent and ii) histopathology of organs after injecting hydrogel-CNTs hybrids, have been tested to evaluate biocompatibility of this smart nanocomposite systems. The common approach used for biocompatibility assessment was blood toxicity profile as a function of ingested dose and exposure time.
Figure 4.
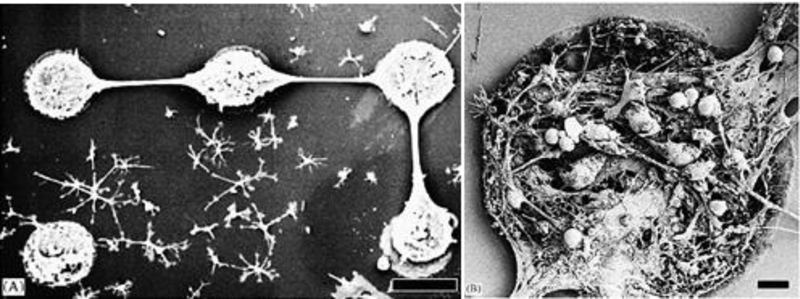
Neuronal network of cells grown on carbon nanotubes array, fabricated using chemical vapor deposition method. A) SEM image of cortical cells, dissociated from 1-dayold Charles River rats, grown on CNTs network. (B) Morphology of grown pattern at high magnification showed that cells are well confined on CNTs.[97]
The concept of hydrogel-CNTs hybrids addressed the issues related with low stability and conductivity of hydrogel along with less biocompatibility and dispensability of CNTs. A 3D biomimetic scaffold composite structure was developed using nanocrystalline hydroxyapatite, magnetically synthesized SWCNTs (synthesized using ac discharge method), and chitosan for bone regeneration application. The biocompatibility of this developed nano-crystalline composite system was assessed using osteoblasts density (cells/cm2) estimation. The findings confirmed that 20% nHA+ B-SWCNTs in chitosan scaffolds (till 7 days) was good for biomedical application (Figure 5 A).[103] The lyophilization procedure was used to explore the nature of optimized dose of scaffold to support the growth of human osteoblasts (bone-forming cells). Results confirmed that 20% nHA + B-SWCNTs chitosan scaffold showed a higher osteoblast density and can be explored for bone regeneration. Another 3D biocompatibility SWCNTs reinforced alginate composite scaffolds was prepared by Yildirim et al utilized freeform fabrication technique.[104] To claim thus developed biomaterials suitable for tissues engineering, an in-vitro study on rat heart endothelial cell in the presence of SWCNTs-alginate structure composite was conducted as function of time (till 7 days) using TM assay. The finding of TM assay, live/dead cell viability, confirmed that SWCNT-alginate scaffolds exhibited improve cell adhesion and proliferation (Figure. 5 B).[104]
Figure 5.
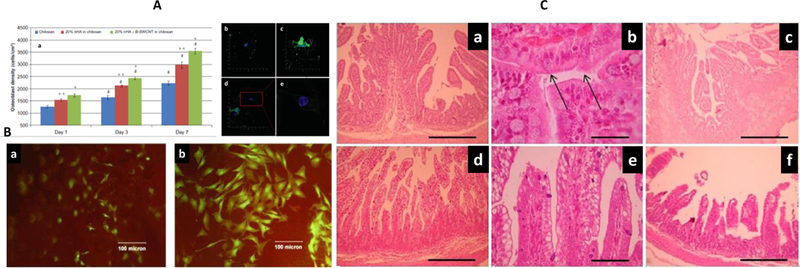
A) Demonstration of osteoblast proliferation on chitosan, 20% nHA in chitosan scaffold, and 20% nHA+B-SWCNTs in chitosan scaffolds. The morphological analysis of cells in the presence of chitosan controls (b), 20% nHA in chitosan scaffolds (c), 20% nHA + B-SWCNTs in chitosan scaffolds (d), and enlarged cell morphology (E) showed extended cell spreading confirming developed materials as a suitable biomimetic bone scaffold structure.[103] B) Microscopy of rat heart endothelial cell in the presence of alginate (a) and SWCNTs-alginate composite scaffold (b) on Day 7. [104]C) H&E staining microscopy of duodenum and Jejunum-ileum in the presence of composite hydrogel protected IgY. Duodenum jtransversal section and hydrogel protected IgY (a) as 100 X, and (b) at 400 X with reference to +ve control (c). Jejunum-ileum transversal section and IgY at 100X (d) 100 X and (e) at 400 X with reference +ve control (f).
A nanocomposite of CNTs-chitosan developed by Alustizaa et al was able to protect egg yolk immunoglobulin (IgY) against enterotoxigenic Escherichia coli (ETEC, 1011 CFU/mL) in experimentally challenged piglets (Figure 5 C).[105] The performance of these hydrogel composites was assessed clinically through monitoring dehydration, body weight, rectal temperature etc. including organs and blood specific toxicity assessment. The animal treated with protected IgY showed better performance than treated with unprotected IgY. The histological results analysis confirmed no significant structural and morphological changes. The results of this study confirmed that chitosan wrapped CNTs did not cause toxicity in-vivo and claimed this systems as a potential biomaterial for medication, especially in pigs.[105]
Shin et al engineered cardiac patches fabricated using a CNTs-gelatin methacrylate (GelMA) hydrogels based nanocomposite.[53] This cardiac structures exhibited better mechanical integrity, required electrophysiological functions, and resist damage to the cells during the secretion of cytotoxic agents. Based on these findings, author claimed this material as multifunctional scaffold with potential application for therapeutics and other in-vivo studies.[53] In this research, a non-toxic dose of this composite, via estimating cell viability as a function of time and varying concentration of CNTs (1 to 5 mg/mL), was used to grow neuronal rats for functionality assessment (Figure 6 A). The results of cell retention percentage and cellular DNA content studies showed better performance of CNTs-GelMA than GelMA. The cell % retention at day 1 was higher than GelMA confirmed significance of CNT-GelMA composite in terms of viability and retention. The cellular DNA on day 3 and day 6 did not exhibit CNTs dependent behavior. Author claimed that CNTs helped in cell spreading and elongation as well without altering cell proliferation. Though a non-significant cardiac fibroblasts cytotoxicity was obtained but no cytotoxicity was produced by CNTs over the period of 7 days.[53]
Figure 6.
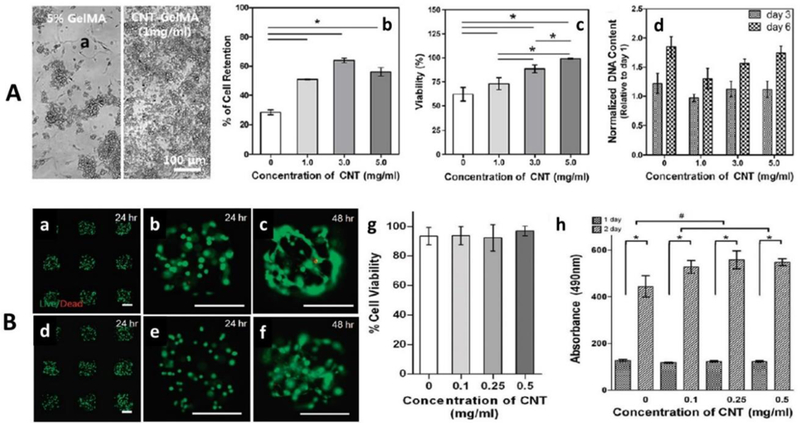
A) Biocompatibility assessment of CNTs-GelMA designed for cardiac construct a) morphology of GelMA and CNTs-GelMA in the presence of cell confirming good cell adhesion and cell retention, b) cell retention, c) cell viability, and normalized DNA quantities (at day 3 and 7) as a function of CNTs concentration (1 to 5 mg/mL).[53] B) Biocompatibility assessment of CNTs-GelMA system designed for ECM. a) Calcein-AM (green)/ethidium homodimer (red) Stained optical image of 3T3 fibroblasts cells embedded in GelMA (a to c) and micro-patterned CNTs-GelMA composite hydrogel (0.5 mg/mL) (d to f) for 24 and 48 h after encapsulation. The live/dead assay confirmed that developed system caused no toxicity, g) cell viability assay (after 24 h encapsulation) and h) MTS assay (absorption at 490 nm, after 24 and 48 h encapsulation) as a function of varying CNTs concentration.[106]
This research group also explored the CNTs-GelMA nanocomposites as scaffold microgels for cell encapsulation materials (ECM), through photo encapsulation mechanism.[106] Authors claimed hydrogel as a desired biological ECM exhibited the challenge of less mechanical strength. This can be overcome via incorporating CNTs in matrix of GelMA to develop a biocompatible nanocomposite of better cell-responsive platform due to its ability to create cell-laden 3D structures without affecting porosity of GelMA.[106] To grow NIH-3T3 cells and human mesenchymal stem cells (hMSCs) onto the hybrid structure, the composition of CNTs-GelMA hybrid was optimization in a way to achieve maximum cell viability. The varying concertation of CNTs (0 to 0.5 mg/mL) was used to fabricate 3D scaffold and tested for biocompatibility before claimed as a potential ECM (Figure 6 B).[106] This composite hydrogel exhibited 90% cell viability for 48 hours (Figure 6 B, a-c) in comparison of GelMA alone. The viability % found inversely proportional to CNTs concentration and exposure time. Authors also optimized the non-toxic wavelength (4 or 6 mW/cm2) of stimulation and time (3080 s minutes). The effect of CNTs concentration of cellular viability was also estimated and results confirmed that no significant cytotoxicity was produced by CNTs-GelMA composites containing CNTs 0.5 μg/mL (Figure 6 B, g). Moreover, the viable cell population in the suspension of 50 μg/mL bare CNTs compared to that of GelMA-coated CNTs suspension was found to be significantly lower, similar to a trend reported previously. In a previous study, the use of chemically modified CNTs, which were treated with acidic reagents were found to decrease cellular viability. The metabolic activity of cell inside the hydrogel was evaluated using MTS assay, as established method to know cellular proliferation. The results of this study, 24 −48 h follow up process and compared with gel, confirmed that CNTs-GelMA caused no cytotoxicity and the presence of CNTs found to support cell growth with a good rate of cellular proliferation rate. Based on tunable biocompatibility this well characterize and optimize CNTs-GelMA hydrogel composites systems can be used for tissues engineering.
The effects of SWCNTs on cell viability was explored by Pok et al aiming to develop and SWCNTs-gelatin chitosan hydrogel composite scaffold that could match with the electrical conductivity of the heart (Figure 7A).[107]Though hydrogel based myocardial patches have been developed to repair the heart defects but the insulating nature of scaffold polymeric walls was the major challenge. This barrier resulted in weak electrical signals between cardiomyocytes and caused arrhythmias. The results of this study showed that SWCNTs-gelatin chitosan system can perfectly act as electrical nano-bridges among cardiomyocytes to produce desired electrical coupling, synchronous beating, and cardiomyocyte function needed to develop a cardiovascular patch. The morphological and electrical properties of this system were found to be SWCNTs concentration dependent. Thus optimization of SWCNTs concentration to develop a suitable biocompatible plateful to grow neonatal rat ventricular myocytes (NRVM) was very crucial. The NRVM cell grown in this composite system (SWCNTs, 69 ppm) exhibited cell viability of > 80% viability, as compared with hydrogel and as correlated with immune-staining analysis (day 7) (Figure 7 B).[107]
Figure 7.
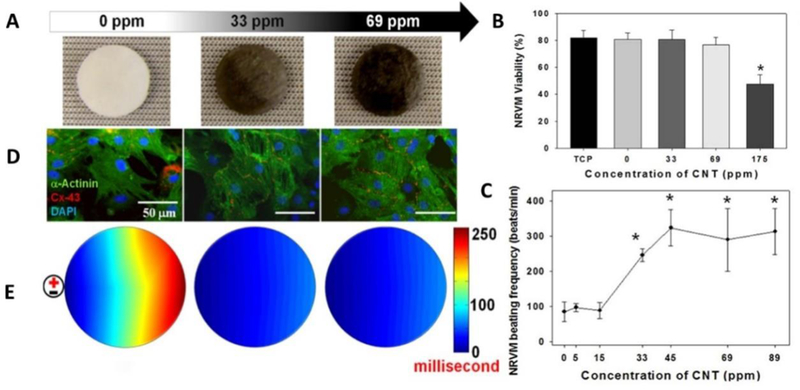
A) Macro- and microscopic structural properties of SWNT-incorporated hydrogels. The Effects of SWCNTs concentration on ventricular myocyte viability (B) and spontaneous beating frequency(C). The developed composite hydrogel, containing 175 ppm CNTs, showed significantly toxicity after 4 days in culture (viability % 47) and cells grown on structure containing 69 ppm or higher concentrations of SWNTs exhibited heart beat ∼310 beats/min (close to rat). D) Ventricular myocytes cultured on hydrogels stained and E) Optical mapping of activation time. The cells were stained suing Di-8-ANEPPS after 7 days in culture.[107]
A significant cytotoxicity (viability ~ 45 %) was observed in case of SWCNTs concentration at 175 ppm (Figure 7B). The response of SWCNTs doses was studied for the assessment of beating frequency (beat/min) of the NRVM cells. The SWCNTs (33 ppm) showed beating frequency similar to control (100 beats/min, below the rat heart beat 330– 480 beats/min). On increasing SWCNTs concentration (more than 33 ppm), the heart of NRVM increased to ∼240 beats/min and gradually approaching to rat hearts (310 beats/minutes) (Figure 7 C). The action potential properties of NRVM in composites system at day 7 was assessed using a voltage-sensitive dye (Di-8-ANEPPS) (Figure 7 E) and results confirmed that SWCNTs in composite strongly affected the propagation of conduction velocity. Thus engineered biocompatible electroactive hydrogel composite system perfectly matches with heart electrical functioning with no risk of inducing cardiac arrhythmias. [107]
Most of the hydrogel-CNTs hybrid systems have dominancy of hydrogels, but interestingly a biocompatible and biodegradable scaffold was prepared using MWCNTs as major (89%) and chitosan as minor constituent. This platform showed a very good viability, support cell adhesion, and proliferation with respect to C2C12 cell line, a myoblastic mouse cell type (Figure 8A).[108] The MTT-assay was performed to assess the viability of C2C12 cells as a function of time and results confirmed no cytotoxicity generated by this hydrogel nanocomposites. Further, cylindrical shaped MWCNTs-chitosan (10 mm diameter and 2 mm thickness) bind with recombinant human bone morphogenetic protein-2 (rhBMP-2) and implanted in muscle tissues of mice for 3 weeks to evaluate the ectopic formation of bone tissue. The results immunostaining confirmed that MWCNTs-CHI scaffold was biocompatible and did not produce chronic inflammation during implantation (Figure 8 B).[108] Over the period of 3 week, the bone tissue regeneration and degradation in scaffold was observed significantly. Author claimed that prepared structure is biocompatible, even on using MWCNTs in high amount, is due to adopted purification process for MWCNTs synthesis which eliminate metal traces. [108, 109]
Figure 8.
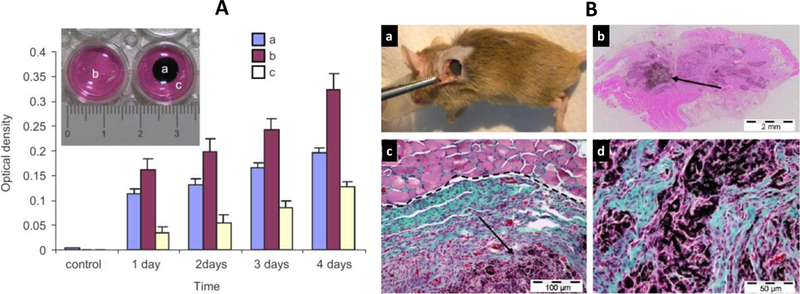
A) Cytotoxicity assays (assayed sampled shown in insert) of C2C12 cell seeded onto MWCNTs-chitosan scaffolds surface (a) in comparison of control (b), and cells culture adjacent to scaffold (c). B) The optimized biocompatible MWCNTs-chitosan was bind with rhBMP-2 and implanted for surgery, mouse subcutaneous muscular pocket (a), and scaffold showed bone tissues regeneration (b). Microscopic image of regenerated bone tissue for detailed analysis on the bases of collagen expressing cells (blue–green colored). Further detailed analysis of remained MWCNTs-chitosan scaffold as indicated by purple color (d).
An endovascular stent device was developed by Paul et al using a nano-bio-hybrid hydrogel system capable to prevent post angioplasty in-stent restenosis (ISR) and demonstrated significant vascular endothelial recovery (Figure 9).[110] For this research, hybrid nanogel was prepared by layer-by-layer (LbL) on a stent surface using endosomolytic Tat peptide/DNA nanoparticles hybridized to polyacrylic acid (PAA) wrapped single-walled carbon nanotubes (NP-CNTs) for the target specific delivery of proangiogenic, vascular endothelial growth factor (Vegf) and Angiopoietin-1(Ang1). Authors developed nanobiohybrid fibrin hydrogels formulations of concentrations of agents. The plasmid DNA (500 mg gfp) was binded with hydrogel formulations, varying CNTs concentration. The results of in-vitro, cultured for 15 days, demonstrated that hydrogel (2.5 mg/ml, containing 25 mg/mL of CNTs) produced no significant cytotoxicity, therefore was used for gfp transgene expression in human aortic smooth muscle cells (HASMCs) (Figure 9). The results also suggested that CNTs made hydrogel more bioactive and a suitable reservoirs for plasmid need to retain its longer activity. An extended iv-vivo study demonstrated that nano-bio-hybrid carrying NPvegf+Ang1, significantly enhanced re-endothelialization of injured artery compared to control. Thus developed methodology can be used to fabricate other biomedical devices wherein a controlled delivery of multi-therapeutics agent is required. [110]
Figure 9.
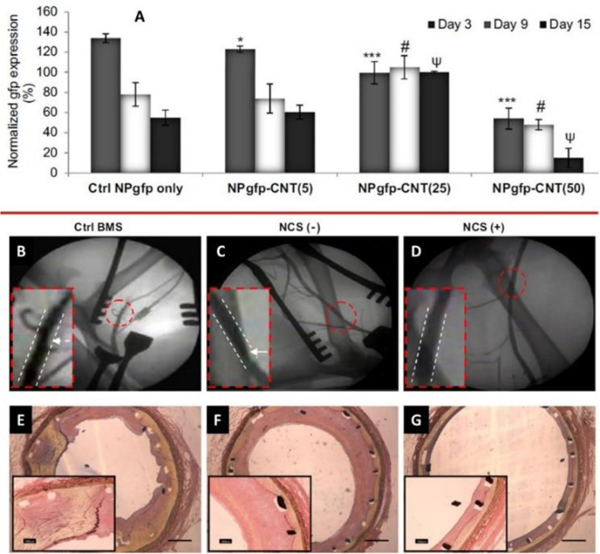
A) Persistent gfp expression in HASMCs grown on control NPgfp and NPgfp-CNTs (5 – 50) as a function of time (day 3 – 9). Evaluation of stent efficacy to prevent ISR, angiographic images of canine femoral arteries with BMS, NCS (−) and NCS (+) at week 6 post stent deployment (B-D) and cross-sectional images of elastic Van Gieson stained stented femoral arteries of the three groups on week 6 (E-G). [110]
6. Biomedical Application of CNTs based hydrogel hybrids
The CNTs, specially SWCNTs have emerges as one of desired nanostructure for biomedical application. Table 1 elaborates the recent development in CNTs based hydrogel hybrids for biomedical applications. This section highlights the various biomedical applications of CNTs ranging from tissue engineering to gene/drug delivery, biosensor development, and imaging purpose.
Table 1.
Recent development in CNTs based hydrogel hybrids in biomedical fields.
| Application | Polymers used | Nano-fillers | Disease targeted/Drugs used | Ref. |
|---|---|---|---|---|
| Drug delivery | Molecularly imprinted polymers methacrylic acid as the functional monomer, ethylene glycol dimethacrylate as crosslinking agent | CNTs | Diclofenac Sodium | [111] |
| Methacrylic acid around carbon nanotubes in the presence of Quercetin as biologically active | CNTs | flavonoid | [112] | |
| Electro-responsive MWCNTs nanotube/poly(methylacrylic acid) (MWCNT/PMAA) | CNTs | Pulsatile drug delivery of various drugs. | [113] | |
| Electro-responsive hybrid hydrogel films developed using Acrylamide and N,N′-ethylene bisacrylamide | CNTs | The external electric voltage resulted in a faster release of anionic drugs, whereas a slower release was observed for cationic drugs. | [114] | |
| Hydrogel nanocomposite developed using grafting of acrylic acid (AA) on to kappa-carrageenan (κC) | MWCNTs | Adsorption of crystal violet and holds potential for similar kind of drugs. | [115] | |
| Tissue engineering/ scaffolds | Functional cardiac patches by seeding neonatal rat cardiomyocytes onto CNT-incorporated photo-cross-linkable gelatin methacrylate (GelMA) hydrogels. | CNTs | Multifunctional cardiac scaffolds | [53] |
| Reinforced CNT–gelatin methacrylate (GelMA) hybrid system as cell-responsive hydrogel platform for the creation of cell-laden three dimensional (3D) constructs | CNTs | Fabricating complex 3D biomimetic tissue-like structures. | [106] | |
| CNTs embedded aligned poly(glycerol sebacate):gelatin (PG) electrospun nano-fibers | CNTs | Hybrid scaffolds for engineering cardiac constructs | [116] | |
| Align CNTs within gelatin methacrylate (GelMA) hydrogels | CNTs | To Fabricate Contractile Muscle Myofibers | [117] | |
| Hybrid hyaluronic acid (HA) hydrogels with SWNTs were then formed by cross-linking with divinyl sulfone | SWCNT | HA plays a dual role of matrix and linker for the rigid reinforcing nano-fibers. | [118] | |
| Aligned CNT forest microelectrode arrays and incorporating them into scaffolds for cell stimulation | CNTs | To engineering biohybrid tissue actuators | [119] | |
| hybrid hydrogel tissue scaffolds of calcium alginate/single- SWCNTs composite | SWCNT | Tissue engineering applications | [120] | |
| GelMA | CNTs | Tissue Engineering Cardiac Constructs and Bioactuators | [53, 121, 122] | |
| Biosensors | Pt nanoparticle (PtNP)-modified conducting polymer hydrogels CPH electrode. | CNTs | Biosensors for Human Metabolite Detection | [53] |
| Composite films developed by incubating an enzyme in a SWNTs solution and crosslinking done by poly[(vinylpyridine)Os(bipyridyl)2Cl2+/3+] polymer film | SWCNTs | Amperometric Biosensors | [123] | |
| Aligned MWCNT arrays instead of bulk random CNTs to fabricate composite films based on a poly(N-isopropyl acrylic amide) (PNIPAm) hydrogel. | MWCNTs | Potential applications in temperature or humidity sensors | [124] | |
| Fabrication as well as integration of active microstructures based on composites of 3DCNT frameworks and hydrogels. | CNTs | Useful in directing the actuation of gels and measuring stimuli responsiveness | [125] | |
| PAAm and MWCNT hydrogel composite | MWCNTs | Electromechanical actuation | [126] | |
| Orthopedics | ||||
| Hydroxyapatite and magnetically synthesized chitosan Nanocomposite | SWCNT | Bone Regeneration | [103] | |
| Nanocrystalline Hydroxyapatite, poly(2-hydroxyethyl methacrylate), | Helical rosette nanotubes | Bone Substitutes | [127] | |
| Chitosan and hydroxyapatite | MWCNTs | bone tissue engineering | [128] | |
| Polyethylene Glycol functionalization of SWCNT 3D nanocomposite scafold | SWCNT | Chondrocyte Growth and Cartilage Tissue Engineering | [129] | |
| Hydroxyapatite, chitosan and glycerol phosphate based thermosensitive hydrogels | CNTs | Injectable Scaffolds for Bone Regeneration | [130] | |
| poly(N-isopropylacrylamide) | MWCNTs | thermosensitive cell sheet engineering | [131] | |
| Chitosan, lactic acid, chitosan modified hydroxyapatite | CNTs | calcium deposits for bone regeneration | [132] | |
| Alginate | MWCNTs | Robust biological bone, breast, cardiac or tumor tissues engineering. | [133] | |
| Polylactic acid | CNTs | tendon and ligament applications | [134] | |
| Theranostic | ||||
| Chitosan, Graphite, designed peptide | CNTs | cancer diagnosis and therapeutics | [135] | |
6.2. CNTs-based hydrogel hybrids in tissue engineering
Serious attempts have been made to repair the defective tissues or impaired organs in the regenerative medicine. It was the general tradition to transplant the somatic cells in the defected area though this process involved the limitation that the cells used to die at very early stage of its transplantation due to less supply of the blood to the tissues and the inflammation. Further progress was made by combining the cells and the bio-actives together in the tissue engineering scaffolds. This technique restricted the initial cell death and increased the probability of cell survival. Thus the cell encapsulation enhanced the cell transplantation and prevented the anoikis signaling and thus easing the diffusion of the gas, essential nutrients and tuning up the fine size of the pores. Advancements urged the innovation of hydrogels and hydrogel hybrids having the 3D microenvironment to have better cellular activities.[136]
The hydrogels should meet certain criteria to be effectively function for the new tissue formation. The basic features include both the physical and biological considerations like they should be degraded and mechanically strong as well should possess the biological cell adhesion. The other most important parameter is that they should be completely biocompatible and sustain unharmed itself and to the surrounding cellular environment. Both natural and synthetic polymers have been exploited for the tissue engineering and the preferred polymers are always natural. Though the recent research have demonstrated that the inclusion of the synthetic polymers and nano-fillers to a certain concentration give extraordinary enhancement in the mechanical strength of the constructs and improve the various properties. The most important and critical feature of the hydrogel is to maintain mechanical strength for the tissue engineering purpose and maintain the space for tissue development. Certain gels having the very less temperature rise during the polymerization process are quite useful for the bone tissue development. Many properties like adhesion and gene expression to the cells are important and require specific mechanical strength.
Adhesive gels are quite important for the specific cell type and is quite dependent on the interactions of cell receptors and thus could alter with the adsorption ability of the different hydrogels.[137] Many NC hydrogels have shown potential application in tissue engineering. [121
The most vital aspect of tissue engineering is the development of suitable biological scaffold. In this context, CNTs hybrid hydrogel have shown to exert exceptional performance. Although conventional hydrogels are biocompatible and are suitable for culturing and/or fabricating different cell types and tissues, they possess little electrical conductance and mechanical strength. [6, 49, 138] Inclusion of mechanical and electrical properties in the scaffold inculcates electrical stimulation ability into cell and tissue construct, which is essential to regulate the electro-active behaviors of cells during the tissue regeneration process.[139, 140] CNTs have high electrical conductivity and therefore when reinforced with hydrogel composites, it serves as an excellent platform to engineer different electrically conductive tissues. Insolubility of raw CNTs remains a debatable issue among scientific community.[141] Nonetheless, several studies have shown remarkable CNTs solubility due to surface modifications using amines, hydroxyls, and carboxyl functional groups.[140] These modifications allow physical dispersion of CNTs in hydrogels owing to their covalent conjugation with hydrogel polymer chains.[142] This promotes exceptional mechanical strength in the cross linked networks of CNTs hybrid hydrogels. The mechanical strength of matrix increases by an order of magnitude even by introducing only < 1% of chemical bonds between matrix and CNTs.[143] Tissue specific tailoring of mechanical properties in CNTs hybrid hydrogel based scaffold is very essential for successful tissue engineering (replacement/repairing). In fact, mimicking of scaffold similar to the biological milieu of extracellular matrix can guide cellular differentiation to acquire near real-time stiffness. Shin et al (2013) synthesized a novel matrix by incorporating CNTs in photocross-linkable gelatin methacrylate (GelMA) hydrogel.[106] Thus developed matrix was used to engineer cardiac patches by seeding neonatal rat cardiomyocytes, which resulted in the development of a unique cardiac constructs with greater mechanical integrity and electroactive physiological functions. Myocardial tissues developed on CNTs-GelMA matrix reflected 3 times higher beating rate in comparison to those cultured on hydrogel framework only. The intrinsic electric conductive and nano-fibrous network of CNTs in the CNTs-GelMA matrix allowed better adhesion and organization to cardiac cells and it significantly strengthened intercellular junctions as well. Notably, the developed cardiac tissue demonstrated higher resistibility against cardiac inhibitor and cytotoxic compounds. Thus, a multifunctional cardiac scaffold was developed, which possess applicability for both therapeutic purposes and in vitro studies and thus can be used as a model for developing similar construct for neurons and other muscle cells. In a similar study, Ahadian et al (2014, 2016) evaluated CNTs-GelMA matrixes, which were developed by aligning CNTs into GelMA matrix.[144, 145] Differentiation of embryoid bodies on the scaffold of this GelMA- CNTs matrix showed superior performance upon application of electrical stimulation in comparison to control gel only. Same research group showed the applicability of the developed system for the muscle myofiber fabrication. Thus a manually controllable platform for electrically induced differentiation and stimulation of stem cells was developed. [145]
Recently, biomimetic scaffolds was used to design the cardiac constructs. In this study, the type 1 collagen with combination with CNTs (Figure 10) was utilized.[146] Cardiomyocytes were seeded within the hybrid hydrogels of CNTs-collagen. This system demonstrated the great potential of the system with improved cardiac cell function as compared to the convnetional pure collagen hydrogels. The outcomes of this study demonstrated that the hybrid hydrogels show potential improvements in the important properties such as the strength, appropriate conductivity required for a cardiac constructs.
Figure 10.
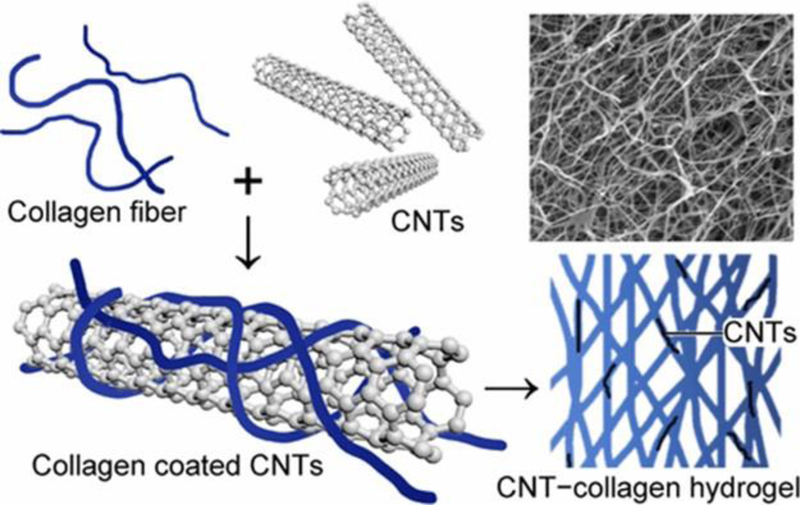
Mechanically and Electrically Enhanced CNTs−Collagen Hydrogels As Potential Scaffolds for Engineered Cardiac Constructs. Reprinted with Copyright permission from ACS reference [146]
6.3. CNTs-based hybrid hydrogels for drug delivery
Significant efforts have been made towards the real-time development of controlled drug delivery systems from conventional approaches.[147] Several nanotechnology projects in the last two decades have lead the foundation of smart drug delivery systems. Hydrogel based drug delivery carriers came up with extraordinary features with combination of controlled and sustained release giving ideal features to the drug delivery system. It is essential to highlight the important features of hydrogels, which make them ideal candidate to be used for drug delivery applications. Hydrogels posses the porous structure, which can be modulated by keeping a control on the cross-linking and thus have complete control o their swelling and diffusion features by which the drugs are being released from them. The stability of the drug and therelase behaviour can be modulated by having control on their structure during the synthesis. The pharmokinectic control for the drugs from hydrogel matrix is very important to maintain the local drug concentartion in th`e targeted region of body and thus can be effcetively imployed for systemic drug delivery. The high water content in hydrogels make them quite biocompatible and biodegradable and their dissolution can be controlled by varying the functional group modification and thus control the enzymatic and hydrolytic degradtion. Moreover their ability to respond to the external stimuli like electric field, pressure, pH, and temperature also give them add on advantages to be used in drug delievery application. Hydrogels can be deformed to various shapes and forms which make them versatile. The mucoadhesive nature of the hydrogels ease their application on different shape sites.[3]
Further, the invention of hydrogel hybrids with the inclusion of the nano-fillers gave an additional advantages to the overall system eliminating many limitations. Considering the CNTs based hydrogel hybrids, these system introduced a new approach for the spatial and temporal control for the release of the cargos.[148] The increased bio-availability and efficacy of the therapeutics due to the active permeation and high loading efficiency turn up with marvelous structural stability and effective dual-stage release features.[149] It was observed that the inclusion of CNTs in the hydrogel matrix hold ample control on the structural transformation of the hydrogel owing to the tough photo-thermal effect of NIR light giving the advantage of on demand drug release by having the external control on the light source.[149, 150]
In this framework, CNTs-hybrid hydrogel have been experimented at pre-clinical levels for on-demand drug delivery systems.[151] Many efforts have been invested to control the release of drugs bound to CNTs-hybrid hydrogel in response to external triggers such as electric field, near infra-red ray, etc.[41] For example a study by Feng et al showed a thermosensitive hydrogel comprising of PCL-PEG-PCL in combination with CNTs for NIR triggered drug delivery. This study demonstrated a crucial role of CNTs in sol-gel transition of the gel as well as enhanced the sustained effect by dual stage release by controlling the source from outside.
Many studies have utilized the electric stimulation of the CNTs based hybrid hydrogel for obtaining effective release of the therapeutic interventions. Likewise, an interesting study by Spizzirri et al (2013) engineered a spherical CNTs-hybrid hydrogels composed of gelatin and multi-walled CNTs.[152] The electric sensitivity of this composite was modulated using different concentration of CNTs and it was found that electric stimulation exerts additive effect on the release profile of loaded diclofenac sodium salt. Other research group developed an electro-responsive transdermal drug delivery system composed of multi-walled CNTs and poly (vinyl alcohol)/poly (acrylic acid). Modulation of CNTs content together with oxyfluorination of the carrier resulted in adequate electro-sensitivity to release the drugs.[153] Servant et al (2013) engineered a pulsatile drug delivery carrier using poly(methylacrylic acid) and MWCNTs.[154] Similarly, several electro-responsive CNTs-hybrid hydrogel have been experimented.[41]
Application of CNTs-hybrid hydrogel for NIR mediated drug release has also been studied extensively.[151] In these cases SWCNTs are the NIR-responsive constituent of the matrix. NIR absorbance ability of single-walled CNTs is due to their electronic transitions between the first and second van hove singularities.[155] NIR mediated drug delivery of CNTs-hybrid hydrogels have largely been explored to cancer therapy. Liu et al (2014)[156] synthesized a doxorubicin nanoformulation with poly (ethylene glycol) and SWCNTs. Exposure of 800 nm NIR with 1mW/cm2 power on nanoformulations resulted in burst drug release.[157] Similarly, NIR (808 nm laser; 0.7 W/ cm2) triggered doxorubicin release from a nanocarriers constituted from PEG modified mesoporous silica coat on SWCNTs. NIR responsive drug release from MWCNTs have also been shown and been explained elsewhere.[158]
The rapid development in the field of CNTs based hydrogel hybrids for drug delivery application offer numerous challenges and aspects to be addressed. The high versatility of the CNTs as a nano-filler make them appealing to be utilized for developing therapies. These system offer unique platform for managing the chronic illness that require multiple dosages. The diverse and extraordinary properties of these hybrids (stimuli responsive) make them ideal candidate to be explored further for drug delivery.[113]
5.3. CNTs-based hybrid hydrogels for imaging applications
Hydrogels have shown high efficacy in delivering targeted bio-actives. Beside this, these systems also possess easy detection in-vivo by imaging tools due to the fluorescent properties exhibited by hydrogels or nano-fillers. The advancements in the technology have come up with hydrogels, which are functionalized with contrast agents and have emerged as non- invasive tools for imaging the path and fate of the therapeutics delivered by using hydrogel carriers. Additionally, the CNTs-hydrogel hybrids offer enormous surface area, ease of surface modulations, active towards catalyst, and electronic transportability results in enhancing the sensing of targeted analytes and bio-actives. The ability of strong absorption in the NIR window (750–1000 nm) make them ideal to be used in photoacoustic imaging. Due to high uptake of tubular structures of CNTs/hybrid system, the fluorescent contrast feature has been employed for deep tissue imaging, for example photo thermal ablation therapy.[159]
Inclusion of multi-functionality is always a demanding feature exhibited by materials selected for biomedical application, especially imaging. CNTs, one of such nanostructure, have exhibited properties of a potential imaging contrast agent along with other biomedical applications. Singh et al. (2014) developed (CNTs@MNPs@mSiO2) system [160] to achieve improved drug loading and delivery. Superparamagnetic characteristic of the MNPs nanocrystals was expected to accelerate the transverse relaxation of water protons which made them suitable as a T2 contrast agent in MRI. Accordingly, CNT@MNP@mSiO2 hybrid showed characteristics of T2 contrast agent as measured by a clinical 4.7T MR system. The in-vivo contrast-enhancing effect of CNTs@MNPs@mSiO2 hybrid was evaluated in mice liver, spleen, and kidney (Figure 11). Some previous studies explored the potential of CNTs@MNPs for imaging [161] and diagnostics.[162] Thus recent trends in research and development showed that CNTs based hydrogel hybrids holds great potential in imaging.
Figure 11.
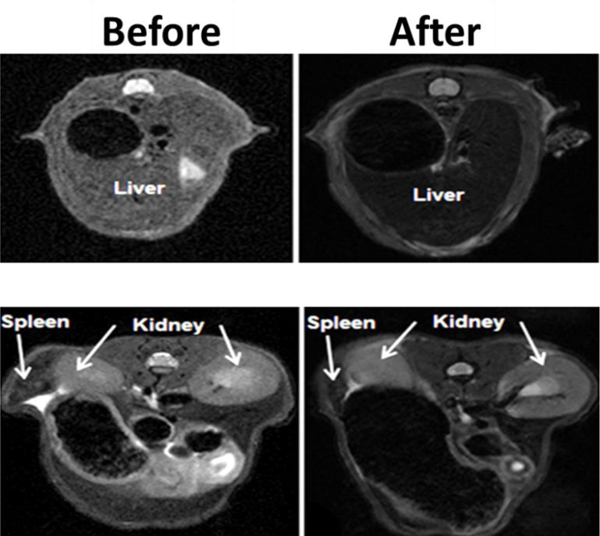
T2-weighted MRI images of liver (left) and kidney and spleen (right) before and after intravenous administration of CNTs@MNPs@mSiO2 nanocarriers. It shows their applicability of CNTs@MNPs@mSiO2 contrast agent for imaging purpose. Reprinted with permission from reference. [160]
5.5. CNTs-based hydrogel hybrid for bio-sensing applications
Hydrogels, an electro-active bio-compatible bio-sensing platform, have been used for the immobilization of the desired biomolecules. For biosensor development, a hydrogel provides a protecting layers to bio-actives, which have comprehensive control over the diffusion of the biomolecules and also possess enhanced biocompatibility. These features make hydrogels one of best suitable candidate to be used for bio-sensing applications. Moreover, the amplified stability of hydrogel based biosensors for enzyme entrapment on electrodes is being explored. Hydrogels also showed response to various external stimuli, which enable smart sensing and high actuating performance. The present section discusses the CNTs based hydrogel hybrids developed for various biosensing applications.[163]
The broader interest in development of CNTs-based hybrid hydrogel biosensors arose for improving their applications and understanding the mechanism behind the various diagnostic approaches. Depending on the biological sensing element and mode of sensing i.e. electrical, chemical, electrochemical, thermal, optical, such hybrid hydrogels can be effectively used for sensing pathogenic diseases (cancer, HIV), specific biomarkers, enzymes, hormones, environment monitoring, etc.[164] One of the prerequisite condition is the incorporation of complementary structures (antibody-antigen and receptor-hormones), which have strong affinity due to specific molecular recognition, followed by quantization of proportional signals (Figure 8). The availability of electron and fast conduction within CNTs helps in enhancing sensitivity of transducer for various diseases and hence, can be utilized for rapid detection of pathogens as well as amplification of signals.[165] Figure 12 demonstrated CNTs incorporated hydrogel hybrid with possible interaction with biological moieties or biologically active molecules. The CNTs can be incorporated with the bio-molecules, or polymeric matrix, or screen printed electrodes, with an idea in mind to avoid leakage. Hydrogels and CNTs both individually can be used as a suitable platform or substrate material, to implant the specific molecular structure (antibody-antigen and receptor-hormones) for sensing activity.
Figure 12.
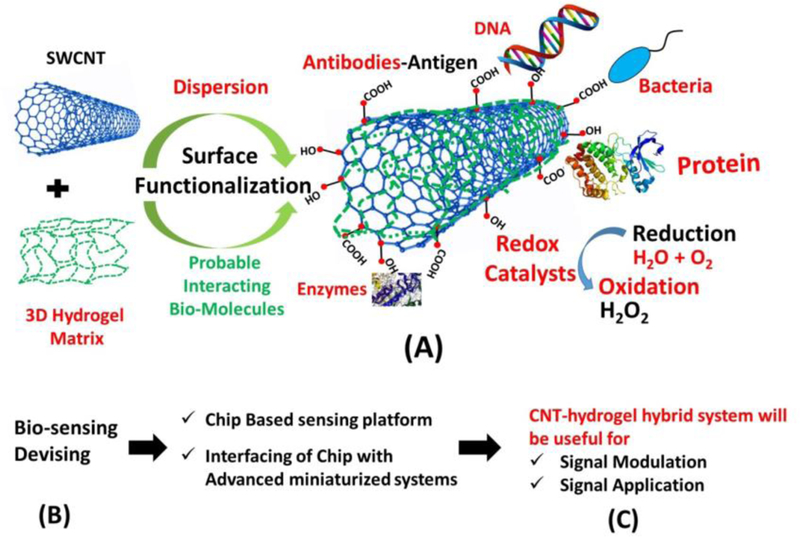
(A-C) showed a typical setup of hydrogel with inbuilt redox molecule/tag, which can be utilized for biosensing activity involving redox conversion, CNTs incorporated hydrogel matrix, functionalized with hydroxyl or carboxylic groups, used for interacting/ capturing/creating possible affinity sites for sensing activity.
CNTs due to intrinsic high electrical conductivity, good mechanical properties, its ability to get functionalized and 3-D construct, produces tuning ability to the electrochemical bio-sensing characteristic of the resultant hybrid material. CNTs based sensors can be utilized for detection of analytes like glucose, neurotransmitters, amino acids, insulin, DNA, cancer biomarkers, cholesterol, etc. In many cases SWCNT addition to a polymeric matrix lead to 2 to 10-fold increase in the peak currents during oxidation and reduction in cyclic voltammetry along with increase in electro-oxidation current during glucose sensing application. In another example, enhanced mass transport of water molecules in poly (N-isopropylacrylamide) (pNIPAM) loaded with SWCNTs hydrogel polymer composites, the thermal response time increased (5 times). Further, strong absorption properties of nanotubes aided in obtaining ultrafast NIR optical response, photoluminescence (PL) of the composite hydrogels for biosensing or actuator formation.[43, 166] Basically, the swelling and de-swelling of the hydrogel changes the cross-linking density, hydration state and dielectric around the CNTs, which causes lattice deformation to the dispersed CNTs, changing the PL emission maxima of the composite hydrogel.[166] Further, the higher reactivity rate on CNTs substrate, resistance to surface fouling, and efficient electron transfer to the redox sites of enzymes, also paved its path for electrochemical sensor in many ways like amperometric biosensor, etc. As CNTs possess higher electro-catalytic activity for oxygen reduction reaction i.e. redox reaction as in case for sensing of hydrogen peroxide (H2O2) or sensing the reaction (Figure 13), which results in production of H2O2 as during the enzymatic reaction between glucose oxidase/glucose or choline oxidase/choline.[167] Incorporation such SWCNT or MWCNTs in a proper matrix or substrate such as nano-gel of hyaluronic acid, conjugated with boronic acid (a reversible glucose-sensing material) have led to the formation of reversible torsional actuation that can be used for on demand drug delivery as well as glucose sensing.[168] A short response time and high sensitivity was achieved by this nano-gel MWCNTs yarn artificial muscle without any electrical power source as well as glucose oxidase (mostly used in any typical glucose sensor). Moreover, CNTs based hydrogels has been utilized to monitor the proper growth of human embryo by measuring the secretion of L-lactate within embryonic cell culture.[169] So, with proper surface functionalization and uniform dispersion CNTs high aspect ratio, tunable conductivity, non-cytotoxicity, specificity, selectivity, functionalization and compatibility with biological system renders their uses in fabrication of sensing and other biological devices.
Figure 13.
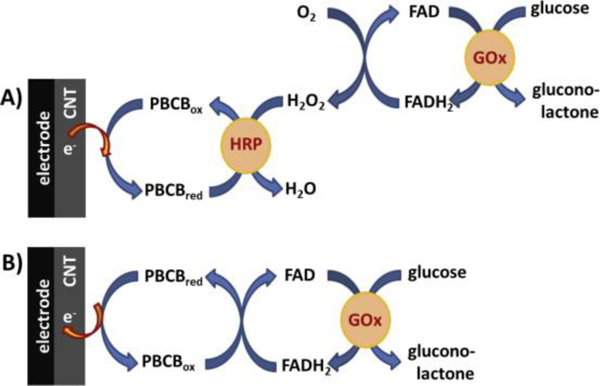
GOx biosensor mechanism (A) in a bi-enzymatic configuration, when detection is based on the detection of peroxide formed in the first GOx enzymatic reaction and (B) based on the direct regeneration of FAD at polyphenazine mediator. Reprinted with copyright permission from reference. [170]
7. Clinical aspects of CNTs based hydrogel hybrids
As of now, the fundamentals and advancements in hydrogel hybrids of desired properties for specific biomedical application has been demonstrated significantly (Figure 14 A and B )[171, 172]. However, the efforts are being continuously made to improve synthesis and processing strategies to develop novel hydrogels of much better improved biocompatibility and therapeutic efficacy. Due to tunable functionality, desired morphology, and electrical properties, the nano/micro gels has been scaled-up for clinical applications. Aiming to improve health care, various hydrogels have promoted as efficient drug delivery agent, wound healing agent, preservative of drug/vaccine/gene to make them long-acting therapeutic agent, organ transplantation, and tissue engineering material.
Figure 14.
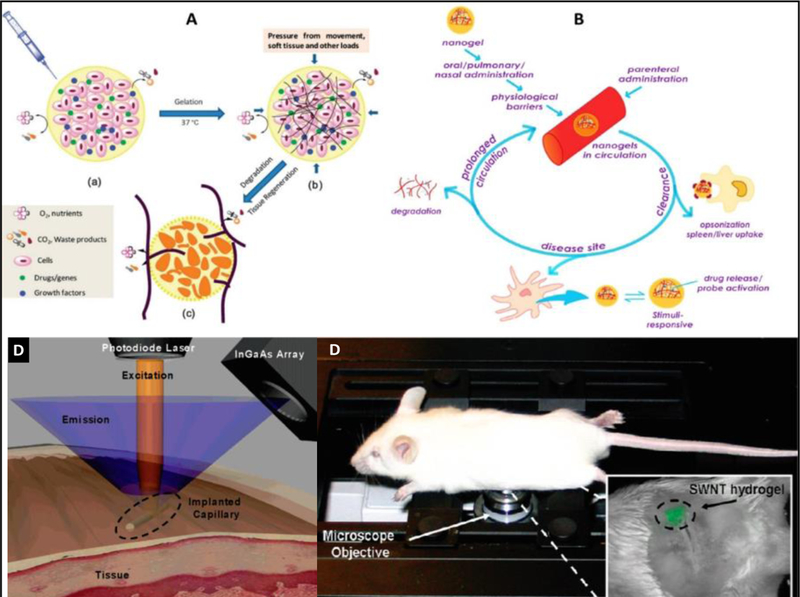
A) Injectable hydrogels developed for various biomedical applications with related mechanism,[171] B) demonstration of in-vivo behavior of a nano-gel developed for targeted application.[172] C) A hydrogel-fluorescent SWCNTs modified capillary based optical sensor implanted beneath the skin to detect glucose in blood. Through a biochemical reaction, The VEGF, released from hydrogel, induced neovascularization to cause SWCNTs fluorescence attenuation that can be detected using an InGaAs array. Very recently, a SWCNTs-hydrogel based sensor was developed in implanted in mice to detect blood glucose. [173] (D) The swelling of hydrogel affect photoluminescence of SWCNTs which dependent of glucose amount in blood. The fluorescence image of a PV-SWCNTs hydrogel in mouse and imaged using a CRi-Maestro in vivo imager. The excitation from 576 to 605 nm and emission from 840 to 950 nm with a 5 s exposure was selected as operational parameter.[166]
The major challenges of instability and low-conductivity in hydrogels have been overcome through designing hybrid hydrogels with CNTs, for further improved performance in biomedical applications (Figure 14 C).[173] At the same time using advanced technology, the toxicity of CNTs, the most challenging obstacle in its promotion for in-vivo use has been resolved. Since decades, many efforts have been made to demonstrate in-vivo application of CNTs through its coating with a suitable hydrogel. For example hydrogel coated CNTs capillary was implanted in mice to detect blood glucose through optical sensing as a function of photoluminescence (Figure 14 D).[173] In another approach, Brone et al developed a hydrogel-SWCNTs system to detect glucose in-vivo optically, based on the hydrogel swelling mechanism on NIR exposure (Figure 14D) using apo-glucose as a cross-linker in hydrogel.[166] The NIR stimulation, the swelled hydrogel induces solvatochromic shifting reversibly. This developed validated sensor reported glucose concentration, 10 nM within 60 s, at in-vivo as a function of swelled state of hydrogel.[166]
In spite of significant performance of hydrogels and CNTs in biomedical applications, the composites class of both materials is not well-promoted towards clinical level. We believe that a significantly increased attention should be paid globally to promote CNTs based hydrogel hybrids for extended research toward FDA approval and further for clinical applications.
8. Concluding comments and Future prospects
Next generation nano-composite hydrogel hybrids have turned up as an excellent platform for drug delivery systems for human body. The need of future research is to explore the strong and effective methods to develop hydrogel hybrids with reduced toxicity and enhanced efficacy. The unique and novel technologies using CNTs based interpenetrating networks and stem cell inclusion in the side chains is quite challenging and visionary applied. The other crucial areas of research which needs to be addressed in near future are (1) the study of the diverse forms of hydrogel hybrids for easy and suitable route of administration including (injections (in situ gels), patches in the form (hydrogel film), ocular, nasal and oral route, intrathecal or implanting the hydrogel hybrid inside the human body, (2) the complete understanding toxicity and compatibility to surrounding tissue with the side products after the degradation of the polymeric matrix used for the design of the hydrogel hybrids, (3) the interactions of these hydrogel hybrids with individual organs and their detection after consumption or implantation to see the real effect on the cell survival and the functional activity of all the cells and widely on individual organs, (4) The key effect on the inflammation and recovery of the damaged part, (5) to know the exact mechanism for the development for effective utilization of hydrogel hybrids and finally their excretion from human body. The assessment of the efficacy of these nanocomposite hydrogels in suitable animal model will give real picture for them to be completely accepted by the human health care system. The future research is emerging in the direction where hybrid hydrogels are involved in developing base materials for microchemotaxis devices, biosensing devices and for motifs used for DNA identification. The future goal is to pay attention to the development of safe hydrogel hybrids for tissue engineering, organ replacement, cardiac constructs and as innovative nanoreactors having controlled functions.
Acknowledgements:
Authors acknowledge NIH grants namely RO1DA034547, R01DA037838, R01DA040537, and RO1DA042706.
Footnotes
Conflict of Interest: Authors declare no conflict of interest
References
- [1].Gupta P, Vermani K, Garg S, Drug Discovery Today 2002, 7, 569. [DOI] [PubMed] [Google Scholar]
- [2].Vashist A, Vashist A, Gupta Y, Ahmad S, J. Mater. Chem. B 2014, 2, 147. [DOI] [PubMed] [Google Scholar]
- [3].Hoare TR, Kohane DS, Polymer 2008, 49, 1993. [Google Scholar]
- [4].Ahmed EM, J. Adv. Res. 2015, 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Haraguchi K, Colloid Polym. Sci. 2011, 289, 455. [Google Scholar]
- [6].Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA, Adv. Mater. 2009, 21, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vashist A, Ahmad S, Curr. Pharm. Biotechnol. 2015, 16, 606. [DOI] [PubMed] [Google Scholar]
- [8].Cai H, Yao P, Nanoscale 2013, 5, 2892. [DOI] [PubMed] [Google Scholar]
- [9].Vashist A, Kaushik A, Vashist A, Jayant RD, Tomitaka A, Ahmad S, Gupta YK, Nair M, Biomater. Sci. 2016, 4, 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nicolais L, Ambrosio L, Netti PA, Callegaro L, Google Patents, 1997. [Google Scholar]
- [11].Pan H, Han JJ, Park Y-D, Cho TH, Hwang SJ, J. Cranio-Maxillofacial Surg. 2016, 44, 116. [DOI] [PubMed] [Google Scholar]
- [12].Urban G, Weiss T, in Hydrogel Sensors and Actuators, Springer, 2009, 197. [Google Scholar]
- [13].Diec J, Tilia D, Thomas V, Eye Contact Lens 2017. [DOI] [PubMed] [Google Scholar]
- [14].Vashist A, Ahmad S, Oriental Journal of Chemistry 2013, 29, 861; [Google Scholar]; Li J, Mooney DJ, Nat. Rev. Mater. 2016, 1, 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Lab Chip 2014, 14, 2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee AL, Ng VW, Gao S, Hedrick JL, Yang YY, Adv. Funct. Mater. 2014, 24, 1538. [Google Scholar]
- [17].Bastiancich C, Danhier P, Préat V, Danhier F, J. Controlled. Release 2016, 243, 29. [DOI] [PubMed] [Google Scholar]
- [18].Karimi AR, Khodadadi A, ACS Appl. Mater. Interfaces 2016, 8, 27254. [DOI] [PubMed] [Google Scholar]
- [19].Wan L, Chen Q, Liu J, Yang X, Huang J, Li L, Guo X, Zhang J, Wang K, Biomacromolecules 2016, 17, 1543. [DOI] [PubMed] [Google Scholar]
- [20].Mehrban N, Teoh GZ, Birchall MA, Int. J.Bioprint. 2016, 2. [Google Scholar]
- [21].Muya FN, Sunday CE, Baker P, Iwuoha E, Water Sci. Technol. 2016, 73, 983. [DOI] [PubMed] [Google Scholar]
- [22].Song HS, Kwon OS, Kim J-H, Conde J, Artzi N, Biosens. Bioelectron. 2016. [DOI] [PubMed] [Google Scholar]
- [23].Zhu Z, Yang CJ, Accounts Chem. Res. 2016, 50, 22. [DOI] [PubMed] [Google Scholar]
- [24].Zhang J, Du P, Xu D, Li Y, Peng W, Zhang G, Zhang F, Fan X, Ind. Eng. Chem. Res. 2016, 55, 4526. [Google Scholar]
- [25].Satarkar NS, Biswal D, Hilt JZ, Soft Matter 2010, 6, 2364. [Google Scholar]
- [26].Basu K, Baral A, Basak S, Dehsorkhi A, Nanda J, Bhunia D, Ghosh S, Castelletto V, Hamley IW, Banerjee A, Chem. Comm. 2016, 52, 5045. [DOI] [PubMed] [Google Scholar]
- [27].Zimenkov DV, Kulagina EV, Antonova OV, Zhuravlev VY, Gryadunov DA, J. Antimicrob. Chemother. 2016, 71, 1520. [DOI] [PubMed] [Google Scholar]
- [28].Sun JY, Zhao X, Illeperuma WR, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z, Nature 2012, 489, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang X, Ding S, Cao S, Zhu A, Shi G, Biosens. Bioelectron. 2016, 80, 315. [DOI] [PubMed] [Google Scholar]
- [30].Paul A, Nanomedicine 2015, 10, 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Haraguchi K, Curr. Opin. Solid State Mater. Sci. 2007, 11, 47. [Google Scholar]
- [32].Haraguchi K, Li HJ, Angewandte Chemie Int. Ed. 2005, 44, 6500. [DOI] [PubMed] [Google Scholar]
- [33].Liu F, Urban MW, Prog. Polym. Sci. 2010, 35, 3. [Google Scholar]
- [34].Shi K, Liu Z, Wei Y-Y, Wang W, Ju X-J, Xie R, Chu L-Y, ACS Appl. Mater. Interfaces 2015, 7, 27289; [DOI] [PubMed] [Google Scholar]; Patel M, Moon HJ, Ko DY, Jeong B, ACS Appl. Mater. Interfaces 2016, 8, 5160. [DOI] [PubMed] [Google Scholar]
- [35].Wang Q, Mynar JL, Yoshida M, Lee E, Lee M, Okuro K, Kinbara K, Aida T, Nature 2010, 463, 339; [DOI] [PubMed] [Google Scholar]; Kim YS, Liu M, Ishida Y, Ebina Y, Osada M, Sasaki T, Hikima T, Takata M, Aida T, Nat. Mater. 2015, 14, 1002. [DOI] [PubMed] [Google Scholar]
- [36].Kehr NS, Prasetyanto EA, Benson K, Ergün B, Galstyan A, Galla HJ, Angewandte Chemie Int. Ed. 2013, 52, 1156; [DOI] [PubMed] [Google Scholar]; Gaharwar AK, Schexnailder PJ, Jin Q, Wu C-J, Schmidt G, ACS Appl. Mater. Interfaces 2010, 2, 3119. [DOI] [PubMed] [Google Scholar]
- [37].Shi K, Liu Z, Wei Y-Y, Wang W, Ju X-J, Xie R, Chu L-Y, ACS Appl. Mater. Interfaces 2015, 7, 27289. [DOI] [PubMed] [Google Scholar]
- [38].Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VT, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, ACS Nano 2014, 8, 8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maeda H, Bharate G, Daruwalla J, Eur. J. Pham. Biopharmaceutics 2009, 71, 409. [DOI] [PubMed] [Google Scholar]
- [40].Liu Z, Sun X, Nakayama-Ratchford N, Dai H, ACS Nano 2007, 1, 50. [DOI] [PubMed] [Google Scholar]
- [41].Cirillo G, Hampel S, Spizzirri UG, Parisi OI, Picci N, Iemma F, BioMed Res. Int. 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Foldvari M, Bagonluri M, Nanomedicine: NBM 2008, 4, 173. [DOI] [PubMed] [Google Scholar]
- [43].Zhang X, Pint CL, Lee MH, Schubert BE, Jamshidi A, Takei K, Ko H, Gillies A, Bardhan R, Urban JJ, Nano Lett. 2011, 11, 3239. [DOI] [PubMed] [Google Scholar]
- [44].Schild HG, Prog. Polym. Sci. 1992, 17, 163. [Google Scholar]
- [45].Wang J, Nguyen TD, Cao Q, Wang Y, Tan MY, Chan-Park MB, ACS Nano 2016, 10, 3222. [DOI] [PubMed] [Google Scholar]
- [46].MacDonald RA, Voge CM, Kariolis M, Stegemann JP, Acta Biomater. 2008, 4, 1583. [DOI] [PubMed] [Google Scholar]
- [47].Trotter H, Phillips R, Ni B, Hu Y, Sinnott SB, Mikulski PT, Harrison JA, J. Nanosci. Nanotechnol. 2005, 5, 536. [DOI] [PubMed] [Google Scholar]
- [48].Rueckes T, Kim K, Joselevich E, Tseng GY, Cheung CL, Lieber CM, Science 2000, 289, 94; [DOI] [PubMed] [Google Scholar]; Jaber-Ansari L, Iddir H, Curtiss LA, Hersam MC, ACS Nano, 2014, 8, 2399; [DOI] [PubMed] [Google Scholar]; Lee SW, Yabuuchi N, Gallant BM, Chen S, Kim B-S, Hammond PT, Shao-Horn Y, Nat. Nanotechnol. 2010, 5, 531; [DOI] [PubMed] [Google Scholar]; Luo H, Shi Z, Li N, Gu Z, Zhuang Q, Anal. Chem. 2001, 73 915. [DOI] [PubMed] [Google Scholar]
- [49].Biondi M, Borzacchiello A, Mayol L, Ambrosio L, Gels 2015, 1, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sharma P, Kumar Mehra N, Jain K, Jain N, Curr. Drug Deliv. 2016, 13, 796; [DOI] [PubMed] [Google Scholar]; Shao H, Zhao P, Su L, Tian L, Zhang Y, Sun Y, Yue S, Xue W, Ramakrishna S, He L, Nanomedicine 2016, 12, 3087; [DOI] [PubMed] [Google Scholar]; Li J, Mo L, Lu C-H, Fu T, Yang H-H, Tan W, Chem. Soc. Rev. 2016, 45, 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chan BQY, Low ZWK, Heng SJW, Chan SY, Owh C, Loh XJ, ACS Appl. Mater. Interfaces 2016, 8, 10070. [DOI] [PubMed] [Google Scholar]
- [52].Liu Z, Yang Z, Luo Y, Polym. Composites 2012, 33, 665. [Google Scholar]
- [53].Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim S. b., Nikkhah M, Khabiry M, Azize M, Kong J, Wan K.-t., Palacios T, Dokmeci MR, Bae H, Tang X, Khademhosseini A, ACS Nano 2013, 7, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].You J-O, Rafat M, Ye GJ, Auguste DT, Nano Lett. 2011, 11, 3643. [DOI] [PubMed] [Google Scholar]
- [55].Spizzirri UG, Curcio M, Cirillo G, Spataro T, Vittorio O, Picci N, Hampel S, Iemma F, Nicoletta FP, Pharmaceutics 2015, 7, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Haraguchi K, “Nanocomposite gels: new advanced functional soft materials”, presented at Macromolecular Symposia, 2007, Vol. 256, No. 1, pp. 120–130. WILEY‐VCH Verlag. [Google Scholar]
- [57].Wu Y, Xia M, Fan Q, Zhu M, Chem. Comm. 2010, 46, 7790. [DOI] [PubMed] [Google Scholar]
- [58].Yang BX, Pramoda KP, Xu GQ, Goh SH, Adv. Funct. Mater. 2007, 17, 2062. [Google Scholar]
- [59].De France KJ, Chan KJ, Cranston ED, Hoare T, Biomacromolecules 2016, 17, 649. [DOI] [PubMed] [Google Scholar]
- [60].Li H, Wang DQ, Chen HL, Liu BL, Gao LZ, Macromolecular Biosci. 2003, 3, 720. [Google Scholar]
- [61].Lee WF, Tsao KT, J. Appl. Polym. Sci. 2006, 100, 3653. [Google Scholar]
- [62].Du J, Wu R, Liu H, Nie X, Li H, Xu S, Wang J, Polym. Composites 2015, 36, 538. [Google Scholar]
- [63].Roy S, Das T, Ming Y, Chen X, Yue CY, Hu X, J. Mater. Chem. A 2014, 2, 3961. [Google Scholar]
- [64].Mittal V, Properties of Polyurethane/Carbon Nanotube Nanocomposites, in Polymer Nanotube Nanocomposites: Synthesis, Properties, and Applications, John Wiley & Sons, Inc., 2010, 141. [Google Scholar]
- [65].Cirillo G, Leonhardt A, Haase D, Iemma F, Puoci F, Ritschel M, Picci N, Hampel S, Carbon Nanotubes-Imprinted Polymers: Hybrid Materials for Analytical Applications, in Materials Science and Technology. InTech; 2012. [Google Scholar]
- [66].Liu S, Gao G, Xiao Y, Fu J, J. Mater. Chem. B 2016, 4, 3239. [DOI] [PubMed] [Google Scholar]
- [67].Chen X, Zhang M, Chen S, Wang X, Tian Z, Chen Y, Xu P, Zhang L, Zhang L, Zhang L, Cell Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Krishnan SK, Prokhorov E, Bahena D, Esparza R, Meyyappan M, ACS Omega 2017, 2, 1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Arakawa C, Ng R, Tan S, Kim S, Wu B, Lee M, Tissue Eng J. Regenerative Med. 2017, 11, 164. [DOI] [PubMed] [Google Scholar]
- [70].Wu T, Huang J, Jiang Y, Hu Y, Ye X, Liu D, Chen J, Food Chem. 2017. [DOI] [PubMed] [Google Scholar]
- [71].Chen Z, Yao X, Liu L, Guan J, Liu M, Li Z, Yang J, Huang S, Wu J, Tian F, Carbohydr. Polym. 2017. [DOI] [PubMed] [Google Scholar]
- [72].Anitha A, Sowmya S, Kumar PS, Deepthi S, Chennazhi K, Ehrlich H, Tsurkan M, Jayakumar R, Prog. Polym. Sci. 2014, 39, 1644. [Google Scholar]
- [73].Vashist A, Gupta Y, Ahmad S, Carbohydr. Polym. 2012, 87, 1433. [Google Scholar]
- [74].Tan X, Li M, Cai P, Luo L, Zou X, Analytical Biochem. 2005, 337, 111. [DOI] [PubMed] [Google Scholar]
- [75].Correa-Duarte MA,Wagner N, Rojas-Chapana J, Morsczeck C, Thie M, Giersig M. Nano Lett. 2004, 4, 2233 [Google Scholar]
- [76].Adewunmi AA, Ismail S, Sultan AS, J. Inorg. Organometall. Polym. Mater. 2016, 26, 717. [Google Scholar]
- [77].Shin SR, Farzad R, Tamayol A, Manoharan V, Mostafalu P, Zhang YS, Akbari M, Jung SM, Kim D, Comotto M, Adv. Mater. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mody N, Agrawal U, Sharma R, Vyas S, Handbook of Polymers for Pharmaceutical Technologies: Biodegradable Polymers. John Willey & Sons; Vol. 3, 2015, 3, 243. [Google Scholar]
- [79].Seeli DS, Prabaharan M, Carbohydr. Polym. 2017, 158, 51; [DOI] [PubMed] [Google Scholar]; Sonawane PR, Katti SA, Int. J. Res. Pharm. Chem. 2016, 6, 534. [Google Scholar]
- [80].Giri A, Bhowmick M, Pal S, Bandyopadhyay A, Int. J. Biol. Macromol. 2011, 49, 885. [DOI] [PubMed] [Google Scholar]
- [81].Yan L, Chang PR, Zheng P, Ma X, Carbohydr. Polym. 2012, 87, 1919. [Google Scholar]
- [82].Glowacki J, Mizuno S, Biopolymers 2008, 89, 338. [DOI] [PubMed] [Google Scholar]
- [83].MacDonald RA, Laurenzi BF, Viswanathan G, Ajayan PM, Stegemann JP, J. Biomed. Mater. Res. Part A 2005, 74, 489. [DOI] [PubMed] [Google Scholar]
- [84].Camponeschi EL, Dispersion and alignment of carbon nanotube polymer based composites, Diss. Georgia Institute Technol, 2007. [Google Scholar]
- [85].Homenick CM, Sheardown H, Adronov A, J. Mater. Chem. 2010, 20, 2887. [Google Scholar]
- [86].Peppas NA, Keys KB, Torres-Lugo M, Lowman AM, J. Controlled Release 1999, 62, 81. [DOI] [PubMed] [Google Scholar]
- [87].J. Harris, Plenum Press, New York, 1992.
- [88].Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS, Rusling JF, ACS Nano 2009, 3, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sacchetti C, Liu-Bryan R, Magrini A, Rosato N, Bottini N, Bottini M, ACS Nano 2014, 8, 12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shah K, Vasileva D, Karadaghy A, Zustiak S, J. Mater. Chem. B 2015, 3, 7950. [DOI] [PubMed] [Google Scholar]
- [91].Paradossi G, Cavalieri F, Chiessi E, Spagnoli C, Cowman MK, J. Mater. Sci.: Mater. Med. 2003, 14, 687. [DOI] [PubMed] [Google Scholar]
- [92].Stammen JA, Williams S, Ku DN, Guldberg RE, Biomaterials 2001, 22, 799. [DOI] [PubMed] [Google Scholar]
- [93].Huang Y, Zheng Y, Song W, Ma Y, Wu J, Fan L, Composites Part A: Appl. Sci. Manufact. 2011, 42, 1398; [Google Scholar]; Tong X, Zheng J, Lu Y, Zhang Z, Cheng H, Mater. Lett. 2007, 61, 1704. [Google Scholar]
- [94].Nath BC, Gogoi B, Boruah M, Khannam M, Ahmed GA, Dolui SK, Electrochim. Acta 2014, 146, 106. [Google Scholar]
- [95].Shokrgozar MA, Mottaghitalab F, Mottaghitalab V, Farokhi M, J. Biomed. Nanotechnol. 2011, 7, 276. [DOI] [PubMed] [Google Scholar]
- [96].Kai D, Prabhakaran MP, Stahl B, Eblenkamp M, Wintermantel E, Ramakrishna S, Nanotechnology 2012, 23, 095705. [DOI] [PubMed] [Google Scholar]
- [97].Harrison BS, Atala A, Biomaterials 2007, 28, 344. [DOI] [PubMed] [Google Scholar]
- [98].Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci‐Unal G, Dokmeci MR, Peppas NA, Khademhosseini A, Adv. Mater. 2014, 26, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gaharwar AK, Peppas NA, Khademhosseini A, Biotechnol. Bioeng. 2014, 111, 441; [DOI] [PMC free article] [PubMed] [Google Scholar]; Haraguchi K, Takehisa T, Adv. Mater. 2002, 14, 1120. [Google Scholar]
- [100].Kaushik A, Kumar R, Arya SK, Nair M, Malhotra B, Bhansali S, Chem. Rev. 2015, 115, 4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A, Adv. Drug Del. Rev. 2013, 65, 2078. [DOI] [PubMed] [Google Scholar]
- [102].Bianco A, Kostarelos K, Prato M, Chem. Comm. 2011, 47, 10182; [DOI] [PubMed] [Google Scholar]; Ali‐Boucetta H, Kostarelos K, Advanced Drug Delivery Reviews 2013, 65, 1897; [DOI] [PubMed] [Google Scholar]; Saito N, Haniu H, Usui Y, Aoki K, Hara K, Takanashi S, Shimizu M, Narita N, Okamoto M, Kobayashi S, Chem. Rev. 2014, 114, 6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Im O, Li J, Wang M, Zhang LG, Keidar M, Int. J. Nanomed. 2012, 7, 2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yildirim ED, Yin X, Nair K, Sun W, J. Biomed. Mater. Res. Part B: Appl. Biomater. 2008, 87, 406. [DOI] [PubMed] [Google Scholar]
- [105].Alustiza F, Bellingeri R, Picco N, Motta C, Grosso MC, Barbero CA, Acevedo DF, Vivas A, Vaccine 2016, 34, 3291. [DOI] [PubMed] [Google Scholar]
- [106].Shin SR, Bae H, Cha JM, Mun JY, Chen Y-C, Tekin H, Shin H, Farshchi S, Dokmeci MR, Tang S, ACS Nano 2011, 6, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Pok S, Vitale F, Eichmann SL, Benavides OM, Pasquali M, Jacot JG, ACS Nano 2014, 8, 9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Abarrategi A, Gutiérrez MC, Moreno-Vicente C, Hortigüela MJ, Ramos V, López-Lacomba JL, Ferrer ML, del Monte F, Biomaterials 2008, 29, 94. [DOI] [PubMed] [Google Scholar]
- [109].Serrano MC, Gutiérrez MC, del Monte F, Prog. Polym. Sci. 2014, 39, 1448. [Google Scholar]
- [110].Paul A, Shao W, Shum-Tim D, Prakash S, Biomaterials 2012, 33, 7655. [DOI] [PubMed] [Google Scholar]
- [111].Puoci F, Hampel S, Hassan A, Cirillo G, Picci N, J. Appl. Polym. Sci. 2013, 130, 829. [Google Scholar]
- [112].Cirillo G, Vittorio O, Hampel S, Iemma F, Parchi P, Cecchini M, Puoci F, Picci N, Eur. J. Pharm. Sci. 2013, 49, 359. [DOI] [PubMed] [Google Scholar]
- [113].Servant A, Methven L, Williams RP, Kostarelos K, Adv. Healthcare Mater. 2013, 2, 806. [DOI] [PubMed] [Google Scholar]
- [114].Curcio M, Spizzirri UG, Cirillo G, Vittorio O, Picci N, Nicoletta FP, Iemma F, Hampel S, RSC Adv. 2015, 5, 44902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hosseinzadeh H, Polish J Chem. Technol. 2015, 17, 70. [Google Scholar]
- [116].Kharaziha M, Shin SR, Nikkhah M, Topkaya SN, Masoumi N, Annabi N, Dokmeci MR, Khademhosseini A, Biomaterials 2014, 35, 7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ramón-Azcón J, Ahadian S, Estili M, Liang X, Ostrovidov S, Kaji H, Shiku H, Ramalingam M, Nakajima K, Sakka Y, Khademhosseini A, Matsue T, Adv. Mater. 2013, 25, 4028. [DOI] [PubMed] [Google Scholar]
- [118].Bhattacharyya S, Guillot S, Dabboue H, Tranchant J-F, Salvetat J-P, Biomacromolecules 2008, 9, 505. [DOI] [PubMed] [Google Scholar]
- [119].Shin SR, Shin C, Memic A, Shadmehr S, Miscuglio M, Jung HY, Jung SM, Bae H, Khademhosseini A, Tang X, Dokmeci MR, Adv. Funct. Mater. 2015, 25, 4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Yildirim ED, Yin X, Ko FK, Guceri S, Sun W, “Evaluation of 3D Hybrid Alginate/Single Wall Carbon Nanotube Tissue Scaffolds in Terms of Process and Cytocompatibility”, presented at Proceedings of the IEEE 32nd Annual Northeast Bioengineering Conference, 01–02 April 2006, 2006. [Google Scholar]
- [121].Motealleh A, Kehr NS, Adv. Healthcare Mater. 2016. [Google Scholar]
- [122].Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, ACS Nano 2013, 7, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Joshi PP, Merchant SA, Wang Y, Schmidtke DW, Anal. Chem. 2005, 77, 3183. [DOI] [PubMed] [Google Scholar]
- [124].Yang Z, Cao Z, Sun H, Li Y, Adv. Mater. 2008, 20, 2201. [Google Scholar]
- [125].De Volder M, Tawfick SH, Copic D, Hart AJ, Soft Matter 2011, 7, 9844. [Google Scholar]
- [126].Kalisadan V, Sreekumar S, Parthasarathy M, Asian J Sci. Res. 2014, 7, 262. [Google Scholar]
- [127].Lijie Z, Jose R, Jose R, Andrew JM, Hicham F, Thomas JW, Nanotechnology 2009, 20, 175101.19420581 [Google Scholar]
- [128].Chen L, Hu J, Shen X, Tong H, J. Mater. Sci.: Mater. Med. 2013, 24, 1843. [DOI] [PubMed] [Google Scholar]
- [129].Chahine NO, Collette NM, Thomas CB, Genetos DC, Loots GG, Tissue Eng., Part A 2014, 20, 2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yasmeen S, Lo MK, Bajracharya S, Roldo M, Langmuir 2014, 30, 12977. [DOI] [PubMed] [Google Scholar]
- [131].Chen Y-S, Tsou P-C, Lo J-M, Tsai H-C, Wang Y-Z, Hsiue G-H, Biomaterials 2013, 34, 7328. [DOI] [PubMed] [Google Scholar]
- [132].Cancian G, Tozzi G, Hussain AAB, De Mori A, Roldo M, J. Mater. Sci.: Mater. Med. 2016, 27, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Joddar B, Garcia E, Casas A, Stewart CM, Sci. Reports 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Correia Pinto V, Costa‐Almeida R, Rodrigues I, Guardão L, Soares R, Miranda Guedes R, J. Biomed. Mater. Res., Part A 2017. [DOI] [PubMed] [Google Scholar]
- [135].Subramanian A, Jaganathan S, Supriyanto E, RSC Adv. 2015, 5, 72638. [Google Scholar]
- [136].Lee HJ, J. Dermatolog. Treat. 2015. 26,77. [DOI] [PubMed] [Google Scholar]
- [137].Lee KY, Mooney DJ, Chem. Rev. 2001, 101, 1869. [DOI] [PubMed] [Google Scholar]
- [138].Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS, Adv. Mater. 2010, 22, 3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Mottaghitalab F, Farokhi M, Zaminy A, Kokabi M, Soleimani M, Mirahmadi F, Shokrgozar MA, Sadeghizadeh M, PLoS One 2013, 8, e74417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Huang YC, Hsu SH, Kuo WC, Chang‐Chien CL, Cheng H, Huang YY, J. Biomed. Mater. Res., Part A 2011, 99, 86. [DOI] [PubMed] [Google Scholar]
- [141].Marchesan S, Bosi S, Alshatwi A, Prato M, Nano Today 2016, 11, 398. [Google Scholar]
- [142].Ma P-C, Siddiqui NA, Marom G, Kim J-K, Composites Part A: Appl. Sci. Manufact. 2010, 41, 1345. [Google Scholar]
- [143].Frankland S, Caglar A, Brenner D, Griebel M, J. Phys. Chem. B 2002, 106, 3046. [Google Scholar]
- [144].Ahadian S, Ramón-Azcón J, Estili M, Liang X, Ostrovidov S, Shiku H, Ramalingam M, Nakajima K, Sakka Y, Bae H, Sci. Reports 2014, 4, 4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ahadian S, Yamada S, Ramón-Azcón J, Estili M, Liang X, Nakajima K, Shiku H, Khademhosseini A, Matsue T, Acta Biomater. 2016, 31, 134. [DOI] [PubMed] [Google Scholar]
- [146].Yu H, Zhao H, Huang C, Du Y, ACS Biomater. Sci. Eng. 2017. [DOI] [PubMed] [Google Scholar]
- [147].Barba M, Czosnek H, Hadidi A, Viruses 2014, 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Meng L, Zhang X, Lu Q, Fei Z, Dyson PJ, Biomaterials 2012, 33, 1689. [DOI] [PubMed] [Google Scholar]
- [149].Hou L, Yang X, Ren J, Wang Y, Zhang H, Feng Q, Shi Y, Shan X, Yuan Y, Zhang Z, Int. J. Nanomed. 2016, 11, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Huang H, Yuan Q, Shah JS, Misra RDK, Adv. Drug Deliv. Rev. 2011, 63, 1332. [DOI] [PubMed] [Google Scholar]
- [151].Sagar V, Atluri V, Tomitaka A, Shah P, Nagasetti A, Pilakka-Kanthikeel S, El-Hage N, McGoron A, Takemura Y, Nair M, Sci. Reports 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Spizzirri UG, Hampel S, Cirillo G, Nicoletta FP, Hassan A, Vittorio O, Picci N, Iemma F, Int. J. Pharm. 2013, 448, 115. [DOI] [PubMed] [Google Scholar]
- [153].Yun J, Im JS, Lee Y-S, Kim H-I, Eur. Polym. J. 2011, 47, 1893. [Google Scholar]
- [154].Servant A, Bussy C, Al-Jamal K, Kostarelos K, J. Mater. Chem. B 2013, 1, 4593. [DOI] [PubMed] [Google Scholar]
- [155].Kam NWS, O’Connell M, Wisdom JA, Dai H, Proc. Nat. Acad. Sci. USA 2005, 102, 11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Liu J, Wang C, Wang X, Wang X, Cheng L, Li Y, Liu Z, Adv. Funct. Mater. 2015, 25, 384. [Google Scholar]
- [157].Liu S, Ko AC-T, Li W, Zhong W, Xing M, J. Mater. Chem. B 2014, 2, 1125. [DOI] [PubMed] [Google Scholar]
- [158].Li R, Wu R. a., Zhao L, Hu Z, Guo S, Pan X, Zou H, Carbon 2011, 49, 1797. [Google Scholar]
- [159].Wang S, Lin J, Wang T, Chen X, Huang P, Theranostics 2016, 6, 2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Singh RK, Patel KD, Kim J-J, Kim T-H, Kim J-H, Shin US, Lee E-J, Knowles JC, Kim H-W, ACS Applied Mater. Interfaces 2014, 6, 2201. [DOI] [PubMed] [Google Scholar]
- [161].Yang D, Yang F, Hu J, Long J, Wang C, Fu D, Ni Q, Chem. Comm. 2009, 4447. [DOI] [PubMed] [Google Scholar]
- [162].Liu Z, Yang K, Lee S-T, J. Mater. Chem. 2011, 21, 586; [Google Scholar]; Yang F, Jin C, Yang D, Jiang Y, Li J, Di Y, Hu J, Wang C, Ni Q, Fu D, Eur. J. Cancer 2011, 47, 1873. [DOI] [PubMed] [Google Scholar]
- [163].Gerlach G, Arndt K-F, Hydrogel sensors and actuators: engineering and technology, Vol. 6, Springer Science & Business Media, 2009. [Google Scholar]
- [164].Kaushik A, Tiwari S, Jayant RD, Vashist A, Nikkhah-Moshaie R, El-Hage N, Nair M, Trends Biotechnol. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kaushik A, Shah P, Vabbina PK, Jayant RD, Tiwari S, Vashist A, Yndart A, Nair M, Anal. Methods 2016, 8, 6115. [Google Scholar]
- [165].Kumar S, Rani R, Dilbaghi N, Tankeshwar K, Kim K-H, Chem. Soc. Rev. 2017, 46, 158. [DOI] [PubMed] [Google Scholar]
- [166].Barone PW, Yoon H, Ortiz-García R, Zhang J, Ahn J-H, Kim J-H, Strano MS, Acs Nano 2009, 3, 3869. [DOI] [PubMed] [Google Scholar]
- [167].Xu X, Jiang S, Hu Z, Liu S, Acs Nano 2010, 4, 4292; [DOI] [PubMed] [Google Scholar]; Wang J, Electroanalysis 2005, 17, 7. [Google Scholar]
- [168].Lee J, Ko S, Kwon CH, Lima MD, Baughman RH, Kim SJ, Small 2016. [DOI] [PubMed] [Google Scholar]
- [169].Hernández-Ibáñez N, García-Cruz L, Montiel V, Foster CW, Banks CE, Iniesta J, Biosens. Bioelectron. 2016, 77, 1168. [DOI] [PubMed] [Google Scholar]
- [170].Barsan MM, Ghica ME, Brett CM, Anal. Chim. Acta 2015, 881, 1. [DOI] [PubMed] [Google Scholar]
- [171].Li Y, Rodrigues J, Tomás H, Chem. Soc. Rev. 2012, 41, 2193. [DOI] [PubMed] [Google Scholar]
- [172].Sekine Y, Endo H, Iwase H, Takeda S, Mukai S.-a., Fukazawa H, Littrell KC, Sasaki Y, Akiyoshi K, J. Phys. Chem. B 2016, 120, 11996. [DOI] [PubMed] [Google Scholar]
- [173].Barone PW, Parker RS, Strano MS, Anal. Chem. 2005, 77, 7556. [DOI] [PubMed] [Google Scholar]


