Summary
In a quasi-experimental, crossover trial involving 1508 patients receiving antibiotics whose providers received either preprescription authorization or postprescription review with feedback, we found that postprescription review with feedback was associated with 2 fewer antibiotic days of therapy per patient.
Keywords: antibiotics, ASP, days of therapy, antimicrobial stewardship.
Abstract
Background.
The optimal approach to conducting antibiotic stewardship interventions has not been defined. We compared days of antibiotic therapy (DOT) using preprescription authorization (PPA) vs postprescription review with feedback (PPRF) strategies.
Methods.
A quasi-experimental, crossover trial comparing PPA and PPRF for adult inpatients prescribed any antibiotic was conducted. For the first 4 months, 2 medicine teams were assigned to the PPA arm and the other 2 teams to the PPRF arm. The teams were then assigned to the alternate arm for an additional 4 months. Appropriateness of antibiotic use was adjudicated by at least 2 infectious diseases–trained clinicians and according to institutional guidelines.
Results. There were 2686 and 2693 patients admitted to the PPA and PPRF groups, with
29% and 27% of patients prescribed antibiotics, respectively. Initially, antibiotic DOTs remained relatively unchanged in the PPA arm. When changed to the PPRF arm, antibiotic use decreased (−2.45 DOT per 1000 patient-days [PD]). In the initial PPRF arm, antibiotic use decreased (slope of −5.73 DOT per 1000 PD) but remained constant when changed to the PPA arm. Median patient DOTs in the PPA and PPRF arms were 8 and 6 DOT per 1000 PD, respectively (P = .03). Antibiotic therapy was guideline-noncompliant in 34% and 41% of patients on days 1 and 3 in the PPA group (P < .01) and in 57% and 36% of patients on days 1 and 3 in the PPRF group (P = .03).
Conclusions.
PPRF may have more of an impact on decreasing antibiotic DOTs compared with PPA. This information may be useful for institutions without sufficient resources to incorporate both stewardship approaches.
Antibiotic stewardship programs (ASPs) have been shown to reduce antibiotic use, improve patient outcomes, and decrease adverse drug events such as Clostridium difficile infections (CDI) and antibiotic resistance [1–12]. However, the optimal approach to conducting antibiotic stewardship interventions has yet to be defined. The 2016 Infectious Diseases Society of America and Society for Healthcare Epidemiology of America guidelines for “Implementing an Antibiotic Stewardship Program” consider both preprescription authorization (PPA) and postprescription review with feedback (PPRF) approaches as “strong recommendations” for reducing antibiotic use in the healthcare setting [13]. However, they do not provide additional data on which approach is preferred for optimizing antibiotic use.
PPA requires that, for certain antibiotics, the prescriber seeks input from the stewardship program prior to the first administered dose. PPRF allows clinicians to prescribe any empiric antibiotic regimen but, in the ensuing 48–72 hours, the ASP advises the clinician with recommendations for stopping, discontinuing, or adjusting therapy if the ASP believes the available diagnostic tests or clinical course warrant changes. Both approaches have pros and cons [14]. The potential benefits of PPA include the following: (1) assuring that patients who need antibiotics receive the most appropriate agents when they are particularly vulnerable to negative sequelae from their infections; (2) increasing the likelihood of appropriate culture collection prior to antibiotic initiation; and (3) limiting patient exposure to antibiotics when no anti-infective therapy is warranted. However, PPA impacts only select agents, allowing prescribers to use unrestricted agents freely; it also has minimal impact on prescribers’ decisions about narrowing the antibiotic spectrum, stopping therapy, or on the duration of therapy after more clinical data are available. Additionally, PPA is resource intensive as it requires someone to be “on-call” to answer requests in real time.
In contrast, PPRF allows for greater flexibility regarding when the review of antibiotic use and feedback to the prescriber occur. Additionally, PPRF allows for more evidence-based discussions with prescribers, including microbiological and clinical data that have evolved since antibiotics were started. However, this approach can be time-consuming for ASPs as more clinical data need to be reviewed in the ensuing period since antibiotics were first initiated. Furthermore, uptake of PPRF is generally optional as once antibiotics have been initiated, stewardship teams generally do not have the authority to discontinue or alter orders. Additionally, PPRF does not address the large burden of empiric antibiotics started unnecessarily. We conducted a quasi-experimental, crossover trial of PPA and PPRF in 4 medical wards at The Johns Hopkins Hospital (JHH) to compare these 2 antibiotic stewardship strategies in the acute-care setting.
METHODS
Study Setting and Participants
The JHH is a 1194-bed tertiary care facility in Baltimore, Maryland. Adult patients admitted to the general wards at JHH are cared for by 1 of 4 medical teams known as firms. Each firm admits patients to their own non–intensive care unit (ICU)–specific medicine floor. General medicine patients admitted to non–firm services were excluded from this study. The medical firms are managed by internal medicine housestaff (specific to each firm) who rotate on a 2- to 4-week basis. Each firm has an Assistant Chief of Service (ie, attending physician) who is present on daily patient rounds and oversees the medical care of all patients on the firm for the entire academic year. The internal medicine firm structure provided the unique advantage of having the same firm-specific physicians for the duration of the study, limiting variability in general antibiotic prescribing practices on each firm. Patient demographic characteristics, preexisting medical conditions, and severity of illness are similar between firms.
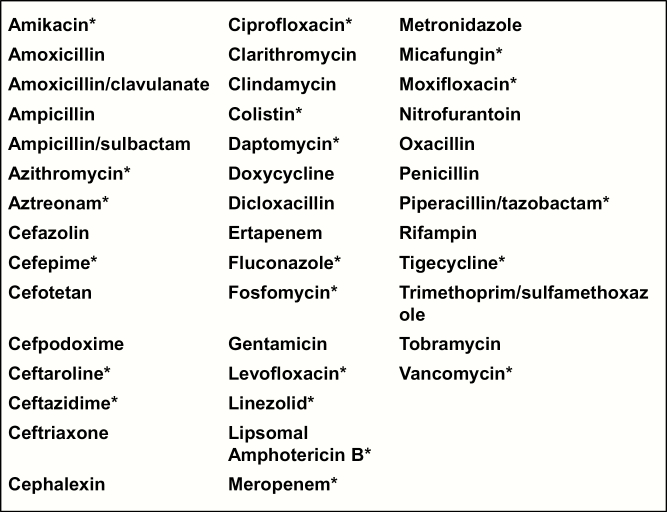
The present study took place from September 2013 to June 2014. Prior to study initiation, clinicians at JHH were required to obtain PPA for restricted antibiotics by a member of the ASP via a telephone conversation, and PPRF was not in place for any of the firms (Figure 1). Antibiotics were selected as “restricted” if they had a broad spectrum of activity, were associated with serious adverse events, or were costly.
Figure 1.
Anti-infectives reviewed by the antibiotic stewardship team as part of the current study. *Anti-infectives that were restricted as part of the preprescription authorization policy.
Eligibility Criteria
Patients were included in the study if they were admitted to 1 of the 4 medicine firms and initiated on any of 42 anti-infectives during the study period for at least 24 hours (Figure 1). Prophylactic antibiotics with no clear stop date were excluded, as were antibiotics used for reasons other than to treat infectious diseases (eg, rifaximin for hepatic encephalopathy). Patients prescribed antibiotics during their time on a nonstudy unit and transferred to a study unit were included in the study, but only antibiotics received during their time on the study unit were included in the analysis. However, patients admitted to a study unit who were transferred to a nonstudy unit or discharged within 24 hours of hospital admission were excluded from the study, as duration of therapy may not have been reflective of medicine firm decisions.
Interventions
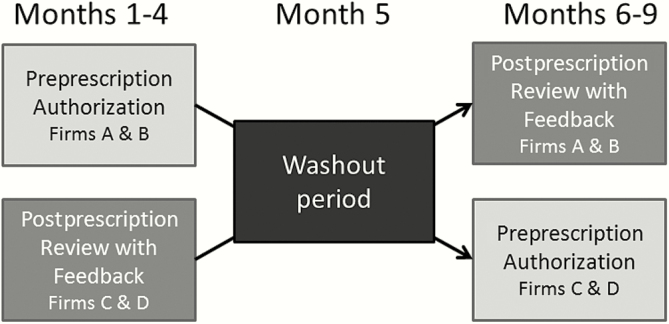
For the first 4 months of the study, firms A and B were assigned to PPA and firms C and D were assigned to PPRF; for months 6–9, the firms were assigned to the opposite arm. PPA was conducted in the same manner as before study initiation (8:00 am to 10:00 pm daily). There was a 1-month washout period between the 2 study periods (Figure 2). When a firm was assigned to the PPA arm, housestaff contacted a clinical pharmacist or infectious diseases fellow to request approval for restricted antibiotics listed in Figure 1. No PPRF was performed in the PPA after antibiotics were initiated.
Figure 2.
Study design comparing antibiotic use among providers receiving preprescription authorization vs postprescription review with feedback antibiotic stewardship strategies.
When a firm was assigned to PPRF, there was no requirement for seeking approval from the ASP prior to the antibiotic being dispensed. At least 2 of the 3 ASP study team members made in-person visits at the same time every weekday to housestaff in firms in the PPRF arms to provide feedback on all patients who had been on study antibiotics for at least 48 hours. If results of diagnostic data were not yet available, recommendations were made once this information was known. If the patient was being seen by the infectious diseases consult service at the time of PPRF, the ASP discussed its recommendations with the consult service prior to making recommendations to the firm.
At least 2 members of the ASP (2 infectious diseases physicians [S. E. C. and P. D. T.] and an infectious diseases pharmacist [E. A.]) adjudicated the appropriateness of antibiotic use in every case to ensure consistency. In cases of disagreement, the third member of the ASP was involved. Appropriate antibiotic use was determined for all patients in the study on day 1 and on days 2–3 regardless of whether providers were assigned to the PPA or PPRF arms. Antibiotic use was considered appropriate if it was in accordance with the JHH Antibiotic Guidelines [15]. These guidelines are updated on an annual basis and provide detailed recommendations on diagnostic testing, antibiotic selection, and duration of antibiotic therapy for common inpatient infections. Appropriateness of antibiotic therapy was determined using the JHH “Four Moments in Antibiotic Decision-Making” approach: (1) Was antibiotic therapy indicated based on known clinical, microbiological, radiographic, and severity of illness findings of the patient? (2) Was the most appropriate empiric antibiotic regimen selected? (3) Was therapy appropriately adjusted or stopped after a reassessment by day 3 of antibiotics? (4) Was the duration of therapy appropriate for the infection being treated?
Known patient-specific adverse drug reactions were accounted for when determining antibiotic appropriateness.
Data Collection
Demographic data, preexisting medical conditions, severity-of-illness measures, detailed antibiotic data, microbiological data, and clinical outcomes data were collected on all eligible patients. Antibiotic usage during the same months in the year prior to the current study was collected to evaluate general prescribing trends over time. This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, with a waiver of informed consent.
Outcomes
The primary outcome was days of antibiotic therapy (DOT) per patient, hand-collected by the ASP study team through evaluation of electronic medical records. A single DOT was recorded for each individual antibiotic administered to a patient on a given day. Antibiotic use was normalized to patient days of therapy per 1000 patient-days (PD). Antibiotics prescribed upon discharge, according to patient discharge summaries, were included in the measurement of antibiotic use. Length of therapy (LOT)—each day a patient receives a systemic antibiotic, regardless of the number of agents or doses—was a secondary outcome [16]. Additional secondary outcomes included the following: (1) incident, symptomatic CDI within 60 days; (2) length of hospital stay from day 1 of antibiotics until hospital discharge; and (3) in-hospital mortality.
Statistical Approach
Demographic and clinical characteristics of patients, appropriateness of antibiotic use, and outcomes in the 2 study arms were summarized as percentages for categorical variables and medians and interquartile ranges for continuous variables. Comparisons between the treatment groups were made using the Student t test for continuous variables and the Pearson χ2 test for categorical variables. An interrupted time-series approach was used to assess changes in DOT per 1000 PD comparing PPA and PPRF across the 2 study periods. This methodology evaluates data collected at multiple time points before and after an intervention to detect whether the intervention had a greater effect than the expected secular trend. All included patients were assigned a 10-day period based on antibiotic start date. DOT and LOT for each patient were calculated and totaled for each 10-day interval, and then standardized to 1000 PD (DOT per 1000 PD and LOT per 1000 PD), using total PD for all admissions in the 10-month period. DOT or LOT were not artificially truncated at 10 days.
To adjust for autocorrelation between the error terms, the generalized least-squares method was applied to estimate the parameters in a linear regression model in which errors are assumed to follow a first-order autoregressive pattern. The models generated included a constant, a baseline slope term to control for secular trends, and terms estimating changes in level and slope of outcome rates. To ensure the validity of the model, a sensitivity analysis was performed using regression-based time-series methods to evaluate the adequacy of the model and test the error distribution [17]. An interrupted time-series model was also created using antibiotic usage data from the year prior to the study (during the same months) to evaluate general antibiotic usage trends in the absence of a targeted PPRF intervention. All analyses were performed using Stata software version 13 (StataCorp, College Station, Texas).
RESULTS
Baseline Characteristics of Patients
During the study period, 2686 and 2693 patients were admitted to the PPA and PPRF groups, respectively. Of these, 778 (29%) and 730 (27%) patients were started on anti-infective therapy for at least 24 hours in the PPA and PPRF groups, respectively (P = .90). The PPA and PPRF groups were generally similar with regard to demographic characteristics, preexisting medical conditions, and severity of illness (Table 1). Approximately 13% of patients in both arms required ICU care prior to being transferred to a study firm. About 1% of patients had surgery in the days preceding study entry. The median McCabe score was the same across both study periods [18]. Sixty-seven (9%) and 79 (10%) patients in the PPA and PPRF groups, respectively, received infectious diseases consultations during their current hospital admission.
Table 1.
Baseline Characteristics of Patients in the Preprescription Authorization and the Postprescription Review With Feedback Groups
| Characteristic | Preprescription (n = 778) |
Postprescription (n = 730) | P Value |
|---|---|---|---|
| Age, y, median (IQR) | 59 (48–70) | 58 (45–69) | .10 |
| Female sex | 409 (52.6) | 362 (49.6) | .26 |
| Race | |||
| African American | 440 (56.6) | 422 (57.8) | .62 |
| White | 267 (34.3) | 239 (32.7) | .55 |
| Other | 71 (9.0) | 69 (9.3) | .86 |
| Preexisting medical conditions | |||
| Diabetes | 269 (34.6) | 224 (30.7) | .11 |
| Congestive heart failure with ejection fraction <40% | 101 (13.0) | 81 (11.1) | .26 |
| Structural lung disease | 196 (25.2) | 143 (19.6) | .01 |
| HIV | 17 (2.2) | 19 (2.6) | .62 |
| End-stage liver disease | 53 (6.8) | 39 (5.3) | .23 |
| End-stage renal disease requiring dialysis | 56 (7.2) | 51 (7.0) | .92 |
| Chronic corticosteroid use and/or immunomodulator therapy | 77 (9.9) | 54 (7.4) | .10 |
| Solid organ transplant | 31 (4.0) | 32 (4.4) | .70 |
| Chemotherapy within 6 mo | 24 (3.1) | 23 (3.2) | 1.00 |
| Length of stay from hospital admission until study enrollment, median (IQR)a | 0 (0–1) | 0 (0–1) | .71 |
| ICU admission during current hospitalization prior to study enrollment | 102 (13.1) | 97 (13.3) | .94 |
| Surgery during current hospitalization prior to study enrollment | 11 (1.4) | 16 (2.2) | .33 |
| McCabe classification, median (IQR) | 3 (2–3) | 3 (2–3) | .46 |
| Number of SIRS criteria on day of study enrollment, median (IQR) | 2 (1–2) | 2 (1–2) | .32 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; SIRS, systemic inflammatory response syndrome.
aStudy enrollment is defined as day 1 of antibiotic prescription in the study unit.
Indications for Antibiotic Therapy
The day 1 indication for antibiotic therapy was determined by the prescribing clinician. The most common indications for initiating therapy on day 1, according to the prescribing team, were urinary tract infections (24%), community-acquired pneumonia (16%), and skin and soft tissue infections (13%), with no differences observed between the 2 study arms. Antibiotic therapy was adjudicated as not JHH guideline compliant in 34% of patients in the PPA group and 41% of patients in the PPRF group on day 1 (P < .01), generally because anti-infective therapy was not indicated (Table 2). There were 417 (54%) PPA patients and 462 (63%) PPRF patients who remained on antibiotic therapy on the third day after therapy was initiated (P < .01). Indications for therapy on day 3 were determined by the ASP. Approximately 36% of patients in the PPA group and 24% of patients in the PPRF group had no indication for continued antibiotic therapy on day 3 (P = .03; Table 3). For those patients for whom antibiotics were indicated on day 3, common indications for antibiotic use included the following: urinary tract infections (24%), skin and soft tissue infections (15%), and community-acquired pneumonia (13%), with no differences noted between the PPA and PPRF groups (Table 4).
Table 2.
Overview of Antibiotic Therapy on Day 1 in the Preprescription Authorization and the Postprescription Review With Feedback Groups
| Therapy | Preprescription (n = 778) | Postprescription (n = 730) | P Value |
|---|---|---|---|
| Antibiotic regimen inappropriatea | 262 (33.7) | 300 (41.1) | <.01 |
| Antibiotic therapy not indicated | 138 (17.7) | 161 (22.1) | .04 |
| No bacterial infection | 122 (15.7) | 141 (19.3) | .07 |
| Treatment course completed | 9 (1.2) | 11 (1.5) | .65 |
| Prophylaxis not indicated | 7 (0.9) | 9 (1.2) | .62 |
| Antibiotic therapy too broad | 113 (14.5) | 131 (17.9) | .08 |
| Unnecessary redundant coverage | 13 (1.7) | 16 (2.2) | .57 |
| Unnecessary MRSA coverage | 39 (5.0) | 36 (4.9) | 1.00 |
| Unnecessary broad-spectrum, gram-negative coverage |
33 (4.2) | 49 (6.7) | .04 |
| Unnecessary gram-negative coverage | 14 (1.8) | 11 (1.5) | .69 |
| Unnecessary gram-positive coverage | 5 (0.6) | 3 (0.4) | .73 |
| Unnecessary anaerobic coverage | 9 (1.2) | 16 (2.2) | .16 |
| Antimicrobial therapy too narrow | 9 (1.2) | 5 (0.7) | .43 |
| Equally effective but more cost-effective options existed | 2 (0.3) | 3 (0.4) | .68 |
Data are presented as No. (%).
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
aFor antibiotic therapy considered inappropriate, a single reason was selected whenever possible.
Table 3.
Overview of Antibiotic Therapy on Day 3 in the Preprescription Authorization and the Postprescription Review With Feedback Groups
| Therapy | Preprescription (n = 417) | Postprescription (n = 462) | P Value |
|---|---|---|---|
| Antibiotic regimen inappropriatea | 239 (57.3) | 168 (36.4) | <.01 |
| Antibiotic therapy not indicated | 148 (35.5) | 109 (23.6) | <.01 |
| No bacterial infection | 128 (30.7) | 74 (16.0) | <.01 |
| Treatment course completed | 12 (2.9) | 27 (5.8) | .03 |
| Prophylaxis not indicated | 8 (1.9) | 8 (1.7) | 1.0 |
| Antibiotic therapy too broad | 87 (20.9) | 57 (12.3) | <.01 |
| Unnecessary double coverage | 9 (2.2) | 5 (1.1) | .28 |
| Unnecessary MRSA coverage | 27 (6.5) | 17 (3.7) | .06 |
| Unnecessary broad-spectrum, gram-negative coverage | 26 (6.2) | 19 (4.1) | .17 |
| Unnecessary gram-negative coverage | 16 (3.8) | 8 (1.7) | .06 |
| Unnecessary gram-positive coverage | 4 (1.0) | 0 | … |
| Unnecessary anaerobic coverage | 5 (1.2) | 8 (1.7) | .59 |
| Antibiotic therapy too narrow | 3 (0.7) | 0 | … |
| Equally effective but more cost-effective options existed | 1 (0.2) | 2 (0.4) | 1.00 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
aFor antibiotic therapy considered inappropriate, a single reason was selected whenever possible.
bInappropriate regimens are inclusive of any reason in table.
Table 4.
Indication for Antibiotic Therapy in the Preprescription Authorization and the Postprescription Review With Feedback Groups According to the Antibiotic Stewardship Team on Day 3 of Therapy, if Antibiotic Therapy Was Considered Indicateda
| Indication | Preprescription (n = 269) |
Postprescription (n = 353) | P Value |
|---|---|---|---|
| Meningitis | 2 (0.7) | 2 (0.6) | 1.00 |
| Endocarditis | 6 (2.2) | 9 (2.5) | 1.00 |
| Community-acquired pneumonia | 32 (11.9) | 51 (14.4) | .41 |
| Healthcare-associated pneumonia | 17 (6.3) | 18 (5.1) | .60 |
| Aspiration pneumonia | 7 (2.6) | 15 (4.2) | .39 |
| Chronic obstructive pulmonary disease exacerbation | 20 (7.4) | 28 (7.9) | .88 |
| Biliary tract infection | 1 (0.4) | 1 (0.3) | 1.00 |
| Intra-abdominal infection | 18 (6.7) | 21 (5.9) | .74 |
| Infectious diarrhea | 1 (0.4) | 3 (0.8) | .64 |
| Clostridium difficile infection | 21 (7.8) | 30 (8.5) | .77 |
| Osteoarticular infection | 27 (10.0) | 32 (9.1) | .68 |
| Urinary tract infection | 67 (24.9) | 83 (23.5) | .71 |
| Cystitis | 32 (11.9) | 20 (5.7) | <0.01 |
| Pyelonephritis | 15 (5.6) | 20 (5.7) | 1.00 |
| Urosepsis | 12 (4.5) | 19 (5.4) | .71 |
| Catheter-associated UTI | 5 (1.9) | 19 (5.4) | .03 |
| Nephrostomy-tube associated infection | 3 (1.1) | 5 (1.4) | 1.00 |
| Skin and soft tissue infection | 45 (16.7) | 47 (13.3) | .26 |
| Sepsis not otherwise specified | 2 (0.7) | 5 (1.4) | .71 |
| Prophylaxis | 3 (1.1) | 8 (2.3) | .37 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: UTI, urinary tract infection.
aOnly including patients who were still receiving inpatient antibiotics on day 3.
Antibiotic Days of Therapy and Length of Therapy
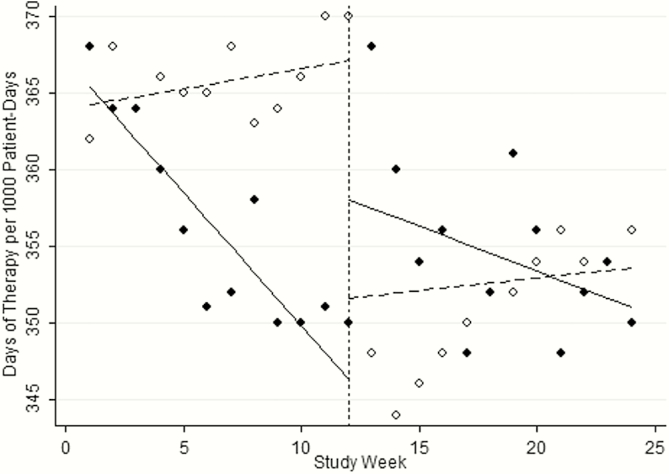
Figure 3 displays the results of the time-series analysis. During the first 4 months of the study, antibiotic DOT remained steady in the PPA arm (dotted line to the left of the vertical line; slope of 1.42 DOT per 1000 PD, P = .16; Figure 3). When these firms received PPRF after the washout period, antibiotic use decreased (dotted line to the right of vertical line; slope of −2.45 DOT per 1000 PD, P = .02; Figure 3). In contrast, in the first 4 months of the study, DOT steadily decreased in the PPRF arm (solid line to the left of the vertical line; slope of −5.73 DOT per 1000 PD, P < .01; Figure 3). For the second 4 months of the study when these firms were receiving PPA, DOT were stable (dotted line to the right of the vertical line; slope of 1.35, P = .18; Figure 3). The median patient DOT in the PPA and PPRF arms were 8 and 6 DOT per 1000 PD, respectively (P = .03). The median patient LOT in the PPA and PPRF arms was 7 and 5 per 1000 PD, respectively (P < .01). Approximately 48% of antibiotic use in the PPA arm was prescribed in the outpatient setting, compared with 34% in the PPRF arm (P < .01). Antibiotic usage for the same wards during the 8 months in the year prior generally decreased over time, but was not significant (slope of −1.21 DOT per 1000 PD, P = .23).
Figure 3.
Time-series analyses comparing days of antibiotic therapy per 1000 patient-days during the study period. Dotted lines indicate preprescription authorization and solid lines indicate postprescription review with feedback. Dotted vertical line represents the four week washout period, during which antibiotics were not adjudicated.
Clinical Outcomes
There were a total of 30 (4%) and 22 (3%) episodes of incident, clinically significant CDI in the PPA and PPRF groups, respectively (P = .40). The median duration of hospital stay from the time of study enrollment until hospital discharge or death was 3 days (interquartile range, 2–7) in both groups (P = .99). There was no difference in in-hospital mortality between the 2 study arms (11% and 14% in PPA and PPRF arms, respectively; P = .44).
DISCUSSION
Our study compares outcomes related to 2 commonly used strategies for antibiotic stewardship: PPA and PPRF. Our results suggest that PPRF may have more of an impact on decreasing both antibiotic DOT and LOT. We found no difference in the clinical outcomes of patients in both groups including incident CDI, length of hospital stay, and in-hospital mortality. However, antibiotic usage, with all of its downstream effects, in itself is arguably a clinically relevant outcome.
Mehta and colleagues also compared PPA and PPRF in a quasi-experimental study at a tertiary care hospital [19]. These investigators found that after introduction of PPRF, antibiotic DOT per 1000 PD increased, in contrast to our findings. There are some important differences between the 2 studies. Mehta et al performed PPRF on 3 antibiotics even though their primary outcome included all inpatient antibiotic use, compared with our study in which PPRF was conducted on all antibiotics consumed during the study period for patients in the PPRF arm. Additionally, as their study was a quasi-experimental study, the 2 stewardship interventions occurred at different periods in time, and it is unclear if there were other ecologic changes impacting antibiotic prescribing practices (eg, increased rates of drug-resistant bacteria over time, changes in case-mix data, drug shortages, changes in local or national antibiotic treatment guidelines). Furthermore, the investigators only included inpatient antibiotic use and did not account for antibiotics prescribed upon hospital discharge. As many of their patients had relatively short hospital LOS, durations of therapy were likely artificially shortened for a large number of patients. We found that approximately 40% of all antibiotic use is prescribed for continuation in the outpatient setting. In fact, there were fewer DOT of antibiotics prescribed for outpatient completion in the PPRF arm compared with the PPA arm. Without including antibiotics prescribed at the time of hospital discharge, total antibiotic use is underestimated.
In the first 4 months of our study, DOT in the PPA group remained steady and decreased in the PPRF group. In the later 4 months of the study, DOT decreased in the PPRF group and remained unchanged in the PPA group. Overall, antibiotic usage decreased as the academic year continued. We cannot say with certainty that the stewardship interventions led to any of the changes in antibiotic use. It is possible that over time, the housestaff became more knowledgeable about antibiotic use and their antibiotic use would have improved in the second half of the study without any ASP interventions. Additionally, it is likely that some of the education provided to the PPRF group lingered when they were assigned to the PPA group in the second half of the study, as they already had received in-person feedback from the study investigators during the first half of the study during the time they were assigned to the PPRF arm. A third possibility is that the study findings we observed were a result of a Hawthorne effect [20]. To be more specific, it is possible that the medicine housestaff modified their antibiotic prescribing practices because they knew they were part of a study. However, housestaff in both the PPA and PPRF arms were aware that a study was ongoing and that their antibiotic usage was actively being evaluated, and prescribing differences between these stewardship approaches persisted. To explore the first hypothesis, that housestaff became more knowledgeable about appropriate antibiotic prescribing during the academic year and antibiotic use would have naturally decreased over time regardless of the current intervention, we evaluated antibiotic usage data from identical time periods in the year before the study when only PPA was occurring and there was no active PPRF. We found that in the year prior to the study when only PPA was in place, DOT per 1000 PD remained relatively constant during the course of the year, leading us to believe that a direct or indirect impact from the PPRF intervention was the most likely explanation.
There are a number of limitations to our study. First, as we have had a PPA system in place for several years with detailed antibiotic treatment guidelines, it is uncertain if our findings are representative of what would be observed at institutions where ASPs have not yet been established [21]. Second, although the practice of carrying the antibiotic prior-approval pager (pharmacist during the daytime and infectious diseases fellow on evenings and weekends) did not change throughout the study for units assigned to the prior-approval arm, we understand that some variability between antibiotic approval practices will differ between stewardship practitioners (eg, some are more restrictive than others), which may have impacted antibiotic usage patterns. Third, patients who were discouraged from initiating antibiotic therapy on day 1 or patients who refused to receive antibiotic therapy on day 1 were not included in the study. This likely overestimates the proportion of patients who would have received appropriate antibiotic therapy on day 1. Additionally, we used 1000 PD as the denominator for overall antibiotic use because a comprehensive denominator that captures both inpatient and outpatient antibiotic use has not been established. This denominator may be flawed as the numerator of days of outpatient antibiotic use is not captured in the denominator. Of note, we repeated the time-series analysis of DOT per 100 patient-admissions and found similar trends (data not shown).
These limitations notwithstanding, although both PPA and PPRF have an impact on overall antibiotic use, a favorable impact on antibiotic DOT and LOT may be more pronounced with PPRF. Both of these approaches are useful and impactful antibiotic stewardship techniques, but they both also require significant personnel time. We favor incorporating a combination of PPA and PPRF into stewardship activities, but in settings where resources are limited, precluding this possibility, our findings suggest it may be of more value to prioritize PPRF.
Financial support. This study was made by possible by an investigator-initiated grant from Pfizer Independent Grants for Learning and Change and The Joint Commission.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control 2013; 41:145–8. [DOI] [PubMed] [Google Scholar]
- 2. Solomon DH, Van Houten L, Glynn RJ, et al. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med 2001; 161:1897–902. [DOI] [PubMed] [Google Scholar]
- 3. Carling P, Fung T, Killion A, Terrin N, Barza M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol 2003; 24:699–706. [DOI] [PubMed] [Google Scholar]
- 4. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 2012; 33:354–61. [DOI] [PubMed] [Google Scholar]
- 5. Laible BR, Nazir J, Assimacopoulos AP, Schut J. Implementation of a pharmacist-led antimicrobial management team in a community teaching hospital: use of pharmacy residents and pharmacy students in a prospective audit and feedback approach. J Pharm Pract 2010; 23:531–5. [DOI] [PubMed] [Google Scholar]
- 6. White AC, Jr, Atmar RL, Wilson J, Cate TR, Stager CE, Greenberg SB. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis 1997; 25:230–9. [DOI] [PubMed] [Google Scholar]
- 7. Sick AC, Lehmann CU, Tamma PD, Lee CK, Agwu AL. Sustained savings from a longitudinal cost analysis of an internet-based preapproval antimicrobial stewardship program. Infect Control Hosp Epidemiol 2013; 34:573–80. [DOI] [PubMed] [Google Scholar]
- 8. Wagner B, Filice GA, Drekonja D, et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 2014; 35:1209–28. [DOI] [PubMed] [Google Scholar]
- 9. Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:1748–54. [DOI] [PubMed] [Google Scholar]
- 10. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. Am J Respir Crit Care Med 2000; 162:505–11. [DOI] [PubMed] [Google Scholar]
- 11. DiazGranados CA. Prospective audit for antimicrobial stewardship in intensive care: impact on resistance and clinical outcomes. Am J Infect Control 2012; 40:526–9. [DOI] [PubMed] [Google Scholar]
- 12. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Sys Rev 2013; CD003543. [DOI] [PubMed] [Google Scholar]
- 13. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:1197–202. [DOI] [PubMed] [Google Scholar]
- 14. Tamma PD, Cosgrove SE. Antimicrobial stewardship. Infect Dis Clin North Am 2011; 25:245–60. [DOI] [PubMed] [Google Scholar]
- 15. Johns Hopkins Medicine. Antibiotic guidelines 2015–2016 Available at: www.hopkinsmedicine.org/amp Accessed 19 December 2016.
- 16. Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53:1100–10. [DOI] [PubMed] [Google Scholar]
- 17. Jandoc R, Burden AM, Mamdani M, Lévesque LE, Cadarette SM. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol 2015; 68:950–6. [DOI] [PubMed] [Google Scholar]
- 18. McCabe WR, Jackson GG. Gram-negative bacteraeremia. Arch Int Med 1962; 110:847–91. [Google Scholar]
- 19. Mehta JM, Haynes K, Wileyto EP, et al. ; Centers for Disease Control and Prevention Epicenter Program Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol 2014; 35:1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yanes AF, McElroy LM, Abecassis ZA, et al. Observation for assessment of clinician performance: a narrative review. BMJ Qual Saf 2015; 25:46–55. doi:10.1136/bmjqs-2015–004171. [DOI] [PubMed] [Google Scholar]
- 21. Cosgrove SE, Seo SK, Bolon MK, et al. ; CDC Prevention Epicenter Program Evaluation of postprescription review and feedback as a method of promoting rational antimicrobial use: a multicenter intervention. Infect Control Hosp Epidemiol 2012; 33:374–80. [DOI] [PubMed] [Google Scholar]