Abstract
Medicaid program criteria for accessing hepatitis C treatment are changing. Medicaid drug utilization data from 2014 to 2016 show that programs that have relaxed their criteria have seen significant increases in treatment utilization, as have states with Medicaid expansions.
Keywords: hepatitis C, Medicaid, direct-acting antivirals, policy, utilization
An estimated 5 million Americans are infected with chronic hepatitis C virus (HCV) [1], one of the leading causes of infection-related deaths in the United States [2]. This disease, once difficult to treat, has recently become almost universally curable because of new medications known as direct-acting antivirals (DAAs). DAA-containing regimens are highly effective and well tolerated, and the expansion of DAA treatment for Medicaid enrollees is a key component of HCV elimination efforts [3]. Despite their promise, DAAs have also drawn attention for their high cost, contributing to difficulty accessing treatment for a variety of patient groups.
The prevalence of HCV is disproportionately high in Medicaid enrollees compared with the commercially insured [4]. With the introduction of sofosbuvir in 2014, many state Medicaid programs began instituting treatment eligibility criteria to access DAAs. These criteria frequently required advanced liver fibrosis, abstinence from substance use, and consultation with a specialist medical provider [5]. Data from 2014 suggested that more restrictive criteria had a negative effect on DAA uptake [6]. These policies, however, have evolved rapidly: by 2016, many Medicaid programs had changed some of their criteria, largely relaxing restrictions [5]. Although criteria used by Medicaid-managed care plans vary, they are legally prohibited from being more restrictive than fee-for-service standards in the state where they operate [5]. The effect of recent changes in eligibility criteria on Medicaid DAA utilization has not, to our knowledge, previously been reported. In the current study, we aimed to characterize variation in DAA utilization among states and explore the association between changes in Medicaid treatment eligibility criteria and trends in utilization.
METHODS
We conducted an analysis of the Medicaid Drug Utilization File from the Centers for Medicare & Medicaid Services, containing counts of prescriptions dispensed to all Medicaid enrollees by National Drug Code for each state and quarter [7]. Codes with <12 prescriptions are suppressed in the raw data and treated as zero. We analyzed data from the second quarter of 2014, after the release of sofosbuvir but just before the release of the first all-oral DAA regimen, until the third quarter of 2016, the most recent available data. We excluded Connecticut from the analysis because DAAs prescribed in Connecticut were limited to 2-week quantities, rather than the 30 days used in other states [8].
Data from 2 states (North Dakota and Utah) for the third quarter of 2016 were excluded owing to incomplete data. The primary outcome was the number of DAA prescriptions per 1000 HCV-infected nonelderly adult Medicaid enrollees. The number of HCV-infected enrollees was estimated by applying state-specific age distributions among Medicaid enrollees [9] to the total number of Medicaid enrollees [10], and then applying age-specific HCV prevalence rates [11] to each state/age group. Enrollees aged >65 years are likely to be dually covered by Medicare and Medicaid and receive pharmacy benefits from Medicare Part D [12], and they were therefore excluded from the denominator.
States were categorized based on the change in Medicaid policy between 2014 and 2016 [5]. In either 2014 or 2016, a requirement of advanced fibrosis (with a METAVIR fibrosis score of F3 or F4) and requirement of proof of abstinence from substance use were considered high restriction; less restrictive policies were considered no-to-low restriction. Category definitions and state categorizations are shown in Supplementary Tables S1 and S2. We did not consider specialist consultation in our analysis because 25 of 26 states continued to be restrictive with this policy over the study years.
We plotted DAA prescriptions per 1000 HCV-infected nonelderly adult enrollees over time both overall and by each policy change category. We then estimated a linear regression of the outcome as a function of study quarter, the release of sofosbuvir-ledipasvir, change in fibrosis policy, change in abstinence policy, Medicaid expansion, and the interactions between policy change indicators and time.
RESULTS
Over the 2½ -year study period, across 49 states and the District of Columbia, a total of 273158 DAA prescriptions were dispensed to Medicaid enrollees. The number of prescriptions declined from 21061 in the second quarter of 2014 to 13555 in the fourth quarter of 2014 (probably reflecting delays in treatment in anticipation of the release of sofosbuvir-ledipasvir), and then rose to 40546 by the third quarter of 2016. Sofosbuvir-containing medications accounted for the majority, but a declining proportion of prescriptions in each quarter (86% in the second quarter of 2014 and 63% in the third quarter of 2016).
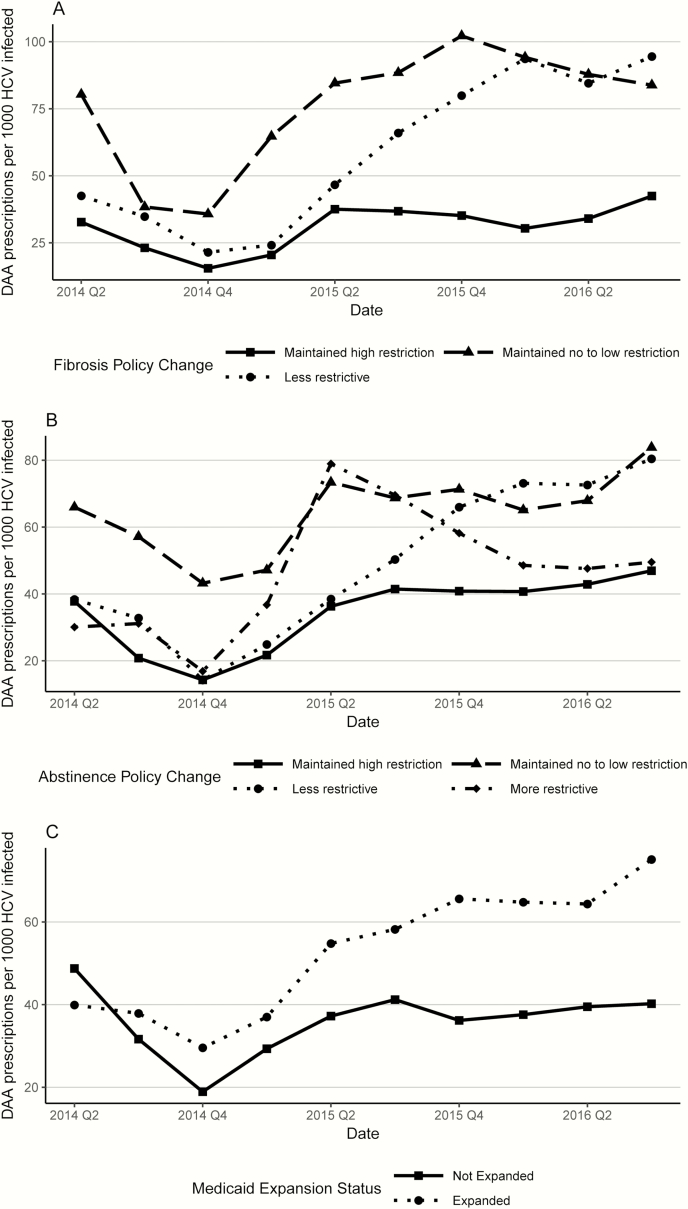
Among the 28 states with known fibrosis requirements in both 2014 and 2016, 10 became less restrictive, 16 maintained high restriction, and 2 maintained no-to-low restriction. Of the 32 states with known abstinence requirements in both years, 5 became less restrictive, 4 became more restrictive, 4 maintained no-to-low restriction, and 19 maintained high restriction. Only 2 states became less restrictive in both categories, and 10 maintained high restriction in both categories. For both the fibrosis and abstinence requirements, although all policy change categories saw an increase in DAA utilization and the states that stayed highly restrictive maintained a high level of use throughout the study period, states that became less restrictive had the most rapid increase, ending the third quarter of 2016 at a rate comparable to that in states that maintained no-to-low restriction. (Figure 1A and 1B).
Figure 1.
Mean number of direct-acting antiviral (DAA) prescriptions per 1000 hepatitis C virus (HCV)–infected nonelderly adult Medicaid enrollees, categorized by fibrosis policy change (A), abstinence policy change (B), and Medicare expansion status (C). A, High restriction is defined as a METAVIR score of F3 or F4; no-to-low restriction, no restriction or a score of F1 or F2; less restrictive, a change from high restriction to no-to-low restriction; and more restrictive, a change in the opposite direction. B, High restriction is defined as any documentation of abstinence from substance use; no-to-low restriction, no documentation needed; less restrictive, a change from high restriction to no-to-low restriction; and more restrictive, a change in the opposite direction. Abbreviations: Q2, quarter 2; Q4, quarter 4.
States that implemented Medicaid expansion under the Affordable Care Act saw a high rate of increase in utilization compared with states that did not (Figure 1C). By the end of the study, the 31 expansion states had a mean of 70 prescriptions per 1000 HCV infected enrollees, compared to only 41 among the 19 nonexpansion states.
Results of the multivariate linear model using the 25 states with complete policy data yielded very similar findings as the descriptive analysis. Model results and plots of predicted outcomes for each policy change category are provided in Supplementary Table S3 and Supplementary Figure S1.
DISCUSSION
Increases in the use of DAAs by Medicaid enrollees over 2014–2016 were significantly associated with changes in state Medicaid programs’ treatment eligibility criteria over the same period. Our findings are consistent with analyses by Liao and Fischer [6], who reported lower Medicaid spending on sofosbuvir in states with strict behavioral criteria around drug and alcohol use in 2014. Our findings also suggest the potential impact of the Medicaid expansion on DAA utilization.
A limitation of the data used in this study is the suppression of values with a small number of prescriptions, although we conducted sensitivity analyses around this assumption (Supplementary Figure S2), and the results did not change overall findings. Policy changes were classified based on cross-sectional surveys in 2014 and 2016; as a result, the exact timing of a given policy change was not known. We could not determine the exact number of HCV-infected Medicaid enrollees in each state; instead, we based our estimates on state-specific age distributions of Medicaid enrollees and national data on HCV prevalence by age.
In conclusion, relaxation of Medicaid DAA treatment eligibility requirements in terms of disease severity and substance use abstinence may have contributed to increased treatment access for HCV-infected Medicaid enrollees. Despite the obvious implications for Medicaid program cost, this expansion is necessary to significantly reduce the burden of HCV-related mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies or the US government.
Financial support. This work was supported by the National Center for Advancing Translational Sciences (grant UL1 TR000457), the National Institute on Drug Abuse (grant P30 DA040500), and the National Institute of Mental Health (grant T32 MH073553).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015; 62:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003–2013. Clin Infect Dis 2016; 62:1287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Committee on a National Strategy for the Elimination of Hepatitis B and C. A national strategy for the elimination of hepatitis B and C: phase two report. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 4. Johnson RL, Blumen HE, Ferro C. The burden of hepatitis C virus disease in commercial and managed Medicaid populations Available at: http://us.milliman.com/uploadedFiles/insight/2015/milliman-hcv-burden.pdf. Accessed 7 February 2017.
- 5. National Viral Hepatitis Roundtable and the Center for Health Law and Policy Innovation. Hepatitis C: the state of Medicaid access. Washington, DC, 2016. [Google Scholar]
- 6. Liao JM, Fischer MA. Restrictions of hepatitis C treatment for substance-using Medicaid patients: cost versus ethics. Am J Public Health 2017; 107:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Medicare and Medicaid Services. State drug utilization data Available at: https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilization-data/index.html. Accessed 31 July 2017.
- 8. State of Connecticut Department of Social Services. CT Medical Assistance Program hepatitis C prior authorization (PA) request form Available at: https://www.ctdssmap.com/CTPortal/Pharmacy%20Information/tabId/65/~Information/Get%20Download%20File/tabid/44/Default.aspx?Filename=Hep%20C%20PA%20Request%20Form.pdf&URI=Forms/Hep%20C%20PA%20Request%20Form.pdf. Accessed 15 June 2017.
- 9. US Census Bureau. Table HI05: health insurance coverage status and type of coverage by state and age for all people: 2014 and 2015 Available at: https://www.census.gov/data/tables/time-series/demo/income-poverty/cps-hi.html. Accessed 8 February 2017.
- 10. Centers for Medicare and Medicaid Services. Medicaid and CHIP enrollment data Available at: https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/index.html. Accessed 27 March 2017.
- 11. Denniston MM, Jiles RB, Drobeniuc J et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014; 160:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaiser Family Foundation. Aged and disabled dual eligibles as a percent of total Medicaid beneficiaries Available at: http://www.kff.org/medicaid/state-indicator/ageddisabled-medicaid-beneficiaries/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed 15 June 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.