We assessed whether cannabis use was associated with altered immune activation in antiretroviral-treated, HIV-infected individuals. Heavy cannabis use was associated with decreased frequencies of activated T cells and inflammatory monocytes, providing evidence of a potential immunological benefit of cannabinoids.
Keywords: cannabis, HIV, immune activation, innate immunity, adaptive immunity
Abstract
Background
Cannabis is a widely used drug in the United States, and the frequency of cannabis use in the human immunodeficiency virus (HIV)–infected population is disproportionately high. Previous human and macaque studies suggest that cannabis may have an impact on plasma viral load; however, the relationship between cannabis use and HIV-associated systemic inflammation and immune activation has not been well defined.
Methods
The impact of cannabis use on peripheral immune cell frequency, activation, and function was assessed in 198 HIV-infected, antiretroviral-treated individuals by flow cytometry. Individuals were categorized into heavy, medium, or occasional cannabis users or noncannabis users based on the amount of the cannabis metabolite 11-nor-carboxy-tetrahydrocannabinol (THC-COOH) detected in plasma by mass spectrometry.
Results
Heavy cannabis users had decreased frequencies of human leukocyte antigen (HLA)-DR+CD38+CD4+ and CD8+ T-cell frequencies, compared to frequencies of these cells in non-cannabis-using individuals. Heavy cannabis users had decreased frequencies of intermediate and nonclassical monocyte subsets, as well as decreased frequencies of interleukin 23– and tumor necrosis factor-α–producing antigen-presenting cells.
Conclusions
While the clinical implications are unclear, our findings suggest that cannabis use is associated with a potentially beneficial reduction in systemic inflammation and immune activation in the context of antiretroviral-treated HIV infection.
Despite long-term, consistent antiretroviral therapy (ART), human immunodeficiency virus (HIV)–infected individuals have a higher risk of developing non-AIDS comorbidities, including cardiovascular disease, cancer, kidney, liver, and neurologic disease [1]. Furthermore, ART-treated, HIV-infected individuals have shorter life expectancy compared to uninfected individuals [2]. This increased morbidity and mortality is linked to chronic immune activation and inflammation, which persists despite successful ART [3]. Indeed, ART-treated, HIV-infected individuals experience higher levels of T-cell activation compared to levels in uninfected individuals [4], and the frequency of CD4+ T cells often does not return to preinfection levels, particularly in mucosal tissues [5, 6]. Thus, there is a critical need for novel therapies that work in conjunction with ART to reduce inflammation and immune activation.
Cannabis (Cannabis sativa) is a widely used drug in the United States and the frequency of cannabis use is particularly high in the HIV-infected community, ranging from 20% to 38.5% [7–9]. Common medical reasons for cannabis use in the HIV-infected population include relief of anxiety and nausea, and appetite stimulation, among others [7]. Conflicting information exists regarding the positive or negative impacts of cannabis use in ART-treated persons living with HIV. Previous reports have suggested that cannabis use has no impact on enrollment in ART care, ART adherence, or successful viral load suppression [9–11], while others have demonstrated that cannabis use was associated with missed clinic appointments [12] and is a risk factor for cardiovascular disease in middle-aged HIV-infected men [13].
Another study demonstrated that cannabis use was associated with lower viremia following seroconversion [14], suggesting a possible beneficial impact of cannabinoids in viral control. In addition, other work demonstrated that Δ9-tetrahydrocannabinol (THC) administration to male simian immunodeficiency virus (SIV)–infected rhesus macaques resulted in decreased early mortality, lowered plasma viral load, and reduced tissue inflammation, although these findings were not reproducible in female SIV-infected rhesus macaques, suggesting a potential sex-specific effect [15–17]. It is possible that these beneficial outcomes in HIV/SIV infection are due to the immunomodulatory capabilities of cannabis, including decreasing cellular proliferation and reducing cytokine production [18]. Few studies, however, have directly assessed the associations between cannabis use, inflammation, and immune activation in the context of ART-treated HIV infection.
The goal of this study was to determine the impact of cannabis use on indicators of inflammation and immune activation in HIV-infected, ART-treated individuals. We hypothesized that regular cannabis use would be associated with decreased frequencies of inflammatory cells and lowered expression of markers of immune activation and dysfunction. We assessed the frequency, phenotype, and cytokine-producing capacity of innate and adaptive immune cells in peripheral blood mononuclear cells (PBMCs) from HIV-infected, ART-treated individuals who self-identified as cannabis users or cannabis abstainers. Furthermore, we assessed plasma markers of microbial translocation, gastrointestinal barrier dysfunction, and inflammation. We found that heavy cannabis use was associated with decreased frequencies of activated T cells and inflammatory antigen-presenting cell (APC) subsets, suggesting a potential immunologic benefit of cannabinoids through decreased immune activation in HIV-infected individuals.
MATERIALS AND METHODS
Study Participants
Cryopreserved PBMCs and plasma samples were obtained from 198 HIV-1–infected, ART-treated participants enrolled in the University of California, San Francisco (UCSF) Study on the Consequences of the Protease Inhibitor Era (SCOPE) cohort, an ongoing study of >1500 chronically HIV-infected individuals and HIV-uninfected controls. All participants gave written informed consent using protocols approved by the Committee on Human Research, UCSF. Sixty-five subjects self-identified as daily cannabis users, while 133 participants self-identified as never using cannabis. All participants reported no use of other drugs of abuse. Cannabis use or nonuse was verified in all individuals by liquid chromatography coupled to mass spectrometry (LC-MS), and subjects were excluded from analysis if their self-reported cannabis use or nonuse was not reflected by the presence or lack of cannabis metabolites in their plasma (Figure 1). Study subject characteristics for those individuals who were included in our analysis are detailed in Table 1. Study participant ART regimens are categorized in Supplementary Table 1.
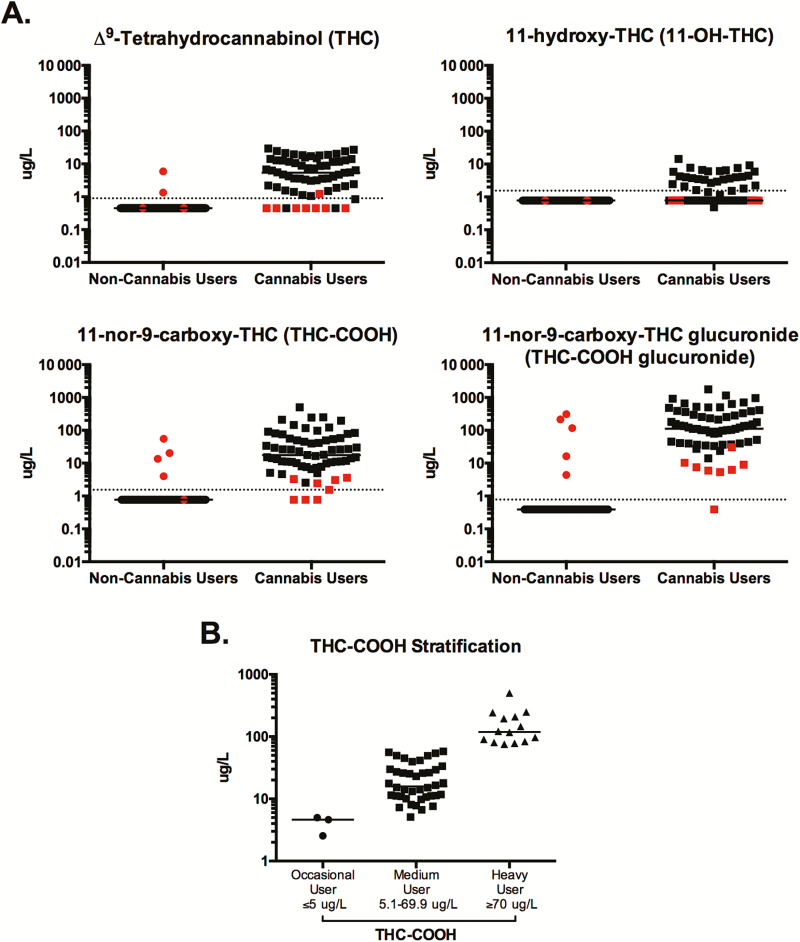
Figure 1.
Detection of cannabis metabolites and stratification of cannabis users. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) was used to quantify cannabis metabolites in the plasma of human immunodeficiency virus (HIV-1)–infected, antiretroviral-treated individuals who self-identified as noncannabis users or daily cannabis users. A, Plasma levels of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-carboxy-THC (THC-COOH), and 11-nor-carboxy-THC glucuronide (THC-COOH glucuronide) were evaluated in each individual. Each data point represents a single individual. Data points in red indicate individuals who were excluded from future analysis due to detection of ≥2 cannabis metabolites in noncannabis users and ≤2 metabolites in cannabis users. B, Among daily cannabis users, levels of THC-COOH were used to stratify individuals into occasional, moderate, and heavy cannabis use groups. Individuals with plasma THC-COOH levels ≥70 μg/L were classified as heavy cannabis users, ≤5 μg/L as occasional cannabis users, and 5.1–69.9 μg/L as medium cannabis users. In all plots, horizontal bars indicate the median and dotted horizontal lines indicate the limit of detection.
Table 1.
Study Subject Characteristics
| Characteristic | Noncannabis Users (n = 128) |
Cannabis Users | |
|---|---|---|---|
| Moderate Usersa (n = 40) |
Heavy Usersa (n = 14) |
||
| Male sexb | 111 (86) | 37 (93) | 10 (71) |
| Agec, y | 53 (46–59) | 51 (45–58) | 51 (45–54) |
| CD4+ countc, cells/µL | 533 (434–755) | 533 (476–675) | 550 (364–893) |
| CD8+ countc, cells/µL | 972 (684–1218) | 810 (650–1110) | 887 (706–2349) |
| CD4/CD8 ratioc | 0.64 (0.44–0.93) | 0.63 (0.38–0.98) | 0.51 (0.39–1.08) |
| Viral loadc, log10 copies/mL | <75 | <75 | <75 |
| Current tobacco use | 16 (13) | 11 (28) | 4 (29) |
| Alcohol use, >3 drinks/d | 17 (14) | 6 (15) | 6 (43) |
| Estimated time since HIV diagnosis, yc | 16.5 (11–21) | 17 (12–22.5) | 20.5 (12.75–27) |
| Duration of viral suppression, yc | 4.4 (2.4) | 4.4 (2.7–6.6) | 4.9 (3.1–7.8) |
| HCV-positive serostatusd | 21 (19) | 14 (36) | 6 (46) |
Data are presented as No. (%) or median (interquartile range).
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus.
aCannabis users were divided based on the level of 11-nor-carboxy-tetrahydrocannabinol detected in plasma from each individual.
bNo statistical difference between the number of males and females in each group was detected (χ2 test; P = .26).
cNo statistical difference was detected between the ages (P = .42), CD4+ count (P = .78), CD8+ count (P = .72), CD4/CD8 ratio (P = .88), viral load (P = .44), estimated time since HIV diagnosis (P = .28), and duration of viral suppression of individuals in each group (P = .7) (all Kruskal-Wallis test).
dModerate and heavy cannabis users had a higher frequency of HCV-infected individuals as compared to the non-cannabis-using individuals (χ2 test, P = .04).
Cannabis Metabolite Quantification in Plasma by LC-MS
THC, 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THC-COOH), and 11-nor-9-carboxy-THC glucuronide (THC-COOH glucuronide) were quantified in plasma using liquid chromatography–tandem mass spectrometry (LC-MS/MS) with an Agilent 1290 Ultra High Performance Liquid Chromatograph (Agilent, Santa Clara, California) coupled with an AB Sciex 5500 Quadrupole Linear Ion Trap Mass Spectrometer (QTRAP MS; AB Sciex, Foster City, California). The analyte and deuterated internal standards were purchased from Cerilliant (Round Rock, Texas). All solvents used were LC-MS grade from EMD Millipore (Billerica, Massachusetts) and Fisher Scientific (Hampton, New Hampshire).
For analysis, 150 μL of cold ethanol and 2 μL of an internal standard mixture containing 1.98 μM THC-d3, 0.79 μM CBD-d3, 0.75 μM 11-OH-THC-d3, 3.63 μM THC-COOH-d9, and 2.4 μM THC-COOH-gluc-d3 was added to 48 μL of plasma, vortexed, kept on ice for 30 minutes, and centrifuged at 16000g for 5 minutes; the supernatant was collected for analysis, and 5 μL of sample was injected into the LC-MS. Cannabinoids were separated using a Supelco Ascentis Express RP amide column (2.1 × 150 mm, 2.7 μM) at 50°C. Mobile phase was water (A) and acetonitrile (B) with 0.1% formic acid. Gradient elution at 0.5 mL/minute flow was from 10% B held for 1 minute to 100% B at 8 minutes, held for 1 minute before returning to initial conditions. The cannabinoids were detected by multiple reaction monitoring (MRM) in positive ion polarity using electrospray ionization. For each analyte 2 MRM transitions were monitored to confirm identity of the detected analyte and one of the transitions was used for quantification (listed in Supplementary Table 2). The limits of quantification were 2.5 nM for CBD and THC, 4.7 nM for 11-OH-THC, 4.5 nM for THC-COOH, and 1.5 nM for THC-COOH-glucuronide. To confirm presence of the cannabinoids in plasma, both ion transitions monitored had to be detected in the samples. The data were processed using Multi-Quant (AB Sciex, Foster City, California).
Flow Cytometry
Multiparameter flow cytometric analysis was performed on PBMCs according to standard procedures using optimized antihuman monoclonal antibodies (mAbs). Predetermined optimal concentrations of the following mAbs were used: (1) from BD Biosciences, San Jose, California: anti-CD3-PE-CF594 (clone SP34-2), anti-CD8-APC-H7 (clone SK1), anti-CD14-BV786 (clone M5E2), anti-CD16-FITC (clone 3G8), anti-CD38-PE-Cy5 (clone HIT2), anti-human leukocyte antigen (HLA)-DR-BV711 (clone G46-6), anti-CD11c-BV650 (clone B-ly6), anti-CD123-PE-Cy7 (clone 7G3), anti-IgG-PE-Cy5 (clone G18-145); (2) from BioLegend, San Diego, California: anti-CD4-BV605 (clone OKT4), anti-CD20-BV570 (clone 2H7), anti-CD45-PerCP (clone HI30); and (3) from Miltenyi Biotech, Auburn, California: anti-IgA-PE-Vio770 (clone IS11-8E10). All cells were stained with the Live/Dead Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific, Grand Island, New York) to exclude dead cells. For intracellular cytokine staining, cells were stimulated with phorbol myristate acetate (PMA; 5 ng/mL; Sigma-Aldrich, St Louis, Missouri) and ionomycin (1 μM/mL; Thermo Fisher Scientific) for 10–14 hours, in the presence of brefeldin A (1 μg/mL; Sigma-Aldrich). All samples were permeabilized and fixed using the CytoFixPerm Kit (BD Pharmingen, San Jose, California) and intracellularly stained to detect anti–IL-6-FITC (clone MQ2-6A3, BD), anti–IL-10-eFluor450 (clone JES3-9D7), anti–IL-23-PerCP-eFluor710 (clone 23dcdp), and anti–TNF-α-AF700 (clone MAb11; all 3 from eBioscience). Flow cytometric acquisition was performed on a BD LSRII cytometer using FACS Diva Software (version 8; BD Pharmingen). Analysis of the acquired data was performed using FlowJo Software (version 10.0.8; Tree Star, Ashland, Oregon).
Detection of Plasma Markers of Microbial Translocation and Inflammation
Plasma levels of bacterial endotoxin (lipopolysaccharide [LPS]) were evaluated using the Limulus amebocyte lysate assay (Lonza, Switzerland). Commercial enzyme-linked immunosorbent assay kits were used to evaluate plasma levels of soluble CD14 (sCD14), lipopolysaccharide-binding protein (LBP), intestinal fatty acid binding protein (I-FABP) (all from R&D Systems, Minneapolis, Minnesota), Zonulin (MyBioSource, San Diego, California), and Luminex (21-plex Human High Sensitivity T-cell Panel, Millipore Sigma, Billerica, Massachusetts), according to the manufacturers’ recommended protocols.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism statistical software version 6 (GraphPad Software, San Diego, California) and the R statistical computing environment. Statistically significant differences between the number of males and females and hepatitis C virus (HCV) serostatus in each group were evaluated using the χ2 test; between age, CD4+ and CD8+ count, CD4/8 ratio, viral loads, duration of viral suppression, and duration of HIV diagnosis of individuals in each group using the Kruskal-Wallis test; and between experimental parameters between individuals in each group using the Mann-Whitney test. Nominal P values of <.05 were considered significant, but for each marker we further computed false discovery rate (FDR) Q values to accommodate multiple testing. In this study, the nominal .05 level corresponded to an FDR of 15%. As supplemental analysis, we considered adjustment for potential confounders. Specifically, each marker was regressed on an indicator of heavy cannabis use vs no cannabis use or moderate cannabis use vs no cannabis use with binary gender, smoking, and drinking and quantitative CD4 T-cell count and age as covariates. For adjusted analyses, due to the nonnormality, the standard inverse-normal transformation was applied to each marker to reduce impact of influential points and outliers. Results from the adjusted analysis remained significant at the nominal level unless otherwise noted. All median values with interquartile ranges, P values, and Q values for univariable and multivariable analyses for each biomarker assessed are listed in Supplementary Table 3.
RESULTS
Verification of Cannabis Use in Study Subjects and Grouping of Cannabis-Using Individuals
To verify and quantify the self-reported cannabis usage of all individuals, the level of 4 cannabis metabolites, including THC, 11-OH-THC, THC-COOH, and THC-COOH glucuronide, were quantified in plasma (Figure 1A). Individuals who self-reported as non-cannabis-using individuals, yet who had detectable levels of ≥2 plasma cannabis metabolites (n = 5), and individuals who reported as daily cannabis users, yet who had detectable levels of ≤2 cannabis metabolites (n = 8), were excluded from their respective groups and from all future analysis (shown in red, Figure 1A).
Previous pharmacokinetic studies investigating the level of various cannabinoids in the blood of individuals after cannabis use have demonstrated the stability of THC-COOH after ingestion, which has led to the use of this metabolite to differentiate heavy from occasional cannabis users [19–21]. Specifically, plasma level of THC-COOH at ≥70 µg/L was associated with heavy cannabis consumption, and plasma concentrations of ≤5 µg/L were correlated with occasional cannabis use [19]. Thus, these criteria were used to stratify cannabis-using individuals in our study into occasional (n = 3), moderate (n = 40), and heavy (n = 14) cannabis-using groups (Figure 1B). This grouping was used for all further analyses in this report. Furthermore, as this distribution identified only 3 occasional cannabis users, to ensure sufficient statistical power, our analysis focused on the moderate and heavy cannabis-using groups.
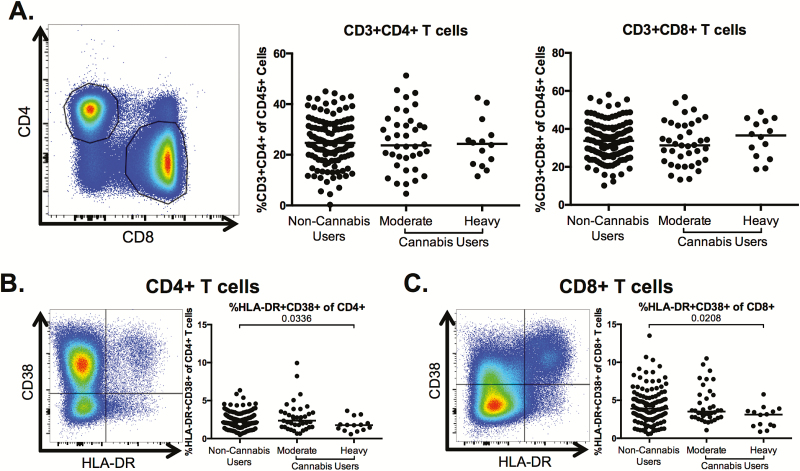
Lower Frequencies of Activated CD4+ and CD8+ T Cells in Heavy Cannabis Users Compared With Nonusers
We assessed whether cannabis use was associated with alterations in markers of CD4+ and CD8+ T-cell activation, a hallmark of HIV disease pathogenesis [3, 4]. The overall frequency of CD4+ and CD8+ T cells among PBMCs was not altered in moderate or heavy cannabis-using individuals, compared with non-cannabis-using individuals (Figure 2A). The frequency of activated (HLA-DR+CD38+) T cells was significantly lower in the heavy cannabis-using group compared to non-cannabis-using individuals for both CD4+ (P = .0336; Figure 2B) and CD8+ T cells (P = .0208; Figure 2C).
Figure 2.
CD4+ and CD8+ T-cell frequencies and phenotype in cannabis users and nonusers. Multiparameter flow cytometry was used to identify the frequency and phenotype of CD4+ and CD8+ T cells within total peripheral blood mononuclear cells of cannabis-using and nonusing individuals. A, Representative staining demonstrating CD4+ and CD8+ T-cell populations. Cells were identified by first excluding doublets using forward and side scatter properties, gating on total leukocytes as determined by CD45 expression, and removing dead cells with an Aqua Live/Dead viability dye. The frequency of T cells was then determined by gating on CD3+ cells and identifying CD4+- and CD8+-expressing T cells within this subset. Pooled data shows the percentage of CD3+CD4+ and CD3+CD8+ T cells in all individuals. B and C, Frequency of human leukocyte antigen (HLA)-DR+CD38+-expressing CD4+ (B) and CD8+ (C) T cells. Pooled data is accompanied by a representative flow plot showing gating for the indicated marker. In all plots, individuals are classified as noncannabis users or cannabis users stratified by moderate or heavy cannabis use as determined by plasma quantities of 11-nor-carboxy-tetrahydrocannabinol. Each individual is represented by a single point. Horizontal bars indicate the median value. The statistical significance of differences between each of the cannabis-using groups and the noncannabis users was determined using the Mann-Whitney test.
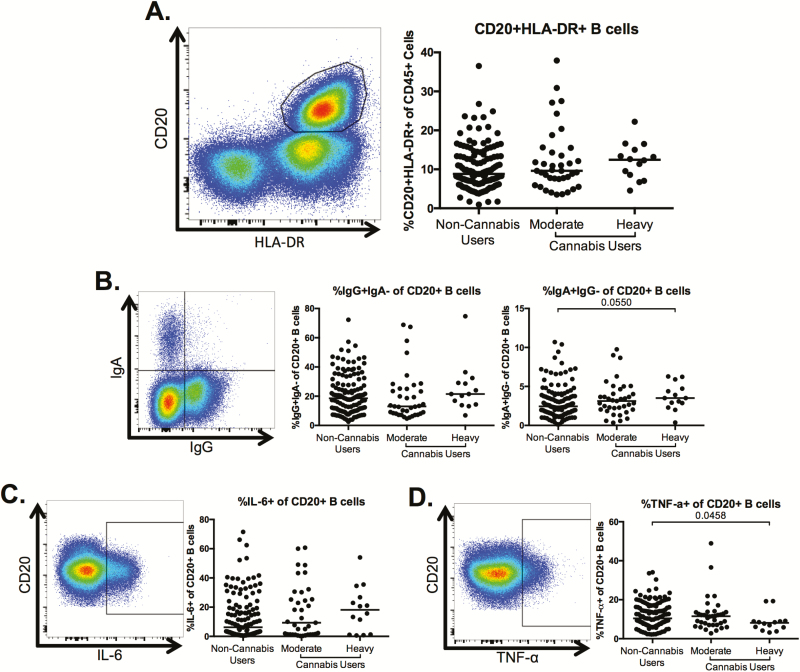
Altered B-Cell Phenotype in Heavy Cannabis Users Compared With Nonusers
We assessed B-cell frequency and phenotype and observed no significant alterations in the frequency of CD20+HLA-DR+ B cells among total PBMCs between the moderate or heavy cannabis-using groups compared to non-cannabis-using individuals (Figure 3A). We observed similar frequencies of immunoglobulin G (IgG)+ B cells between the cannabis-using groups and the non-cannabis-using individuals (Figure 3B); however, the frequency of immunoglobulin A (IgA)+ B cells trended toward being increased in the heavy cannabis-using individuals compared with non-cannabis-using subjects (P = .055; Figure 3B). Finally, although cannabis use was not associated with changes in the frequency of interleukin 6 (IL-6)+ B cells (Figure 3C), the frequency of tumor necrosis factor alpha (TNF-α)–producing B cells was significantly lower in heavy cannabis users compared to frequencies within noncannabis users (P = .0458; Figure 3D). However, after adjustment for clinical covariates (Table 1), the statistical significance of the difference of this population in the heavy cannabis-using group compared to noncannabis users increased to P = .148, potentially due to co-linearity with gender and CD4 count.
Figure 3.
B-cell frequency, isotype, and cytokine production in heavy users of cannabis compared to nonusers. Multiparameter flow cytometry was used to identify the frequency of CD20+human leukocyte antigen (HLA)-DR+ B cells within total peripheral blood mononuclear cells of cannabis-using and non-cannabis-using individuals. A, Representative staining demonstrating the CD20+HLA-DR+ B-cell population. Cells were identified by first excluding doublets using forward and side scatter properties, gating on total leukocytes as determined by CD45 expression and removing dead cells with an Aqua Live/Dead viability dye. The frequency of B cells was then determined by gating on CD3– cells and identifying CD20+HLA-DR+ cells within this subset. Pooled data shows the percentage of CD20+HLA-DR+ B cells in all individuals. B–D, Frequency of immunoglobulin G (IgG)+, immunoglobulin A (IgA)– and IgA+IgG– (B) cells and tumor necrosis factor alpha (TNF-α)– (C) and interleukin 6 (IL-6)–producing cells (D) within the B-cell population. Pooled data is accompanied by a representative flow plot showing gating for the indicated markers. In all plots, individuals are classified as noncannabis users or cannabis users stratified by moderate or heavy cannabis use as determined by plasma quantities of 11-nor-carboxy-tetrahydrocannabinol. Each individual is represented by a single point. Horizontal bars indicate the median value. The statistical significance of differences between each of the cannabis-using groups and the noncannabis users was determined using the Mann-Whitney test.
Altered Innate Immunity in Cannabis Users
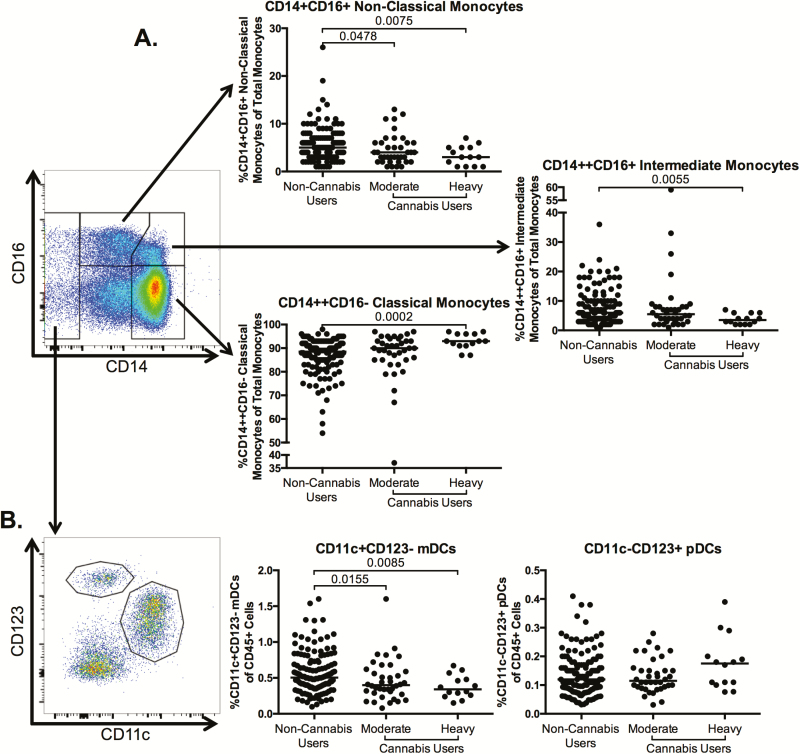
Given that previous studies have demonstrated that innate immune activation, including inflammatory CD16+ monocytes, contributes to end-organ complications in HIV-infected individuals [22–27], we characterized innate immune cell frequencies in cannabis users and nonusers. The frequency of classical monocytes was significantly increased in the heavy cannabis-using group compared to noncannabis users (P = .0002; Figure 4A). The frequency of intermediate monocytes (CD14++CD16+) was significantly lower in the heavy cannabis-using group compared to noncannabis users (P = .0055; Figure 4A). After adjustment for clinical covariates (Table 1), the statistical significance rose to P = .244, potentially due to co-linearity with mainly CD4 count, with additional contribution by alcohol, tobacco use, and gender. Finally, the frequency of nonclassical monocytes (CD14+CD16+) was significantly decreased in the moderate and heavy cannabis-using group compared to frequencies among noncannabis users (P = .0478 and P = .0075, respectively; Figure 4A). After adjustment for clinical covariates (Table 1), the statistical significance between the moderate cannabis-using group and the noncannabis users rose to P = .132, potentially due to co-linearity with all clinical covariates.
Figure 4.
Frequency of monocyte and dendritic cell subsets in cannabis-using individuals as compared to noncannabis users. Multiparameter flow cytometry was used to identify the frequency of monocyte subsets and dendritic cells (DCs) within total peripheral blood mononuclear cells (PBMCs) of cannabis-using and nonusing individuals. A, Classical (CD14+CD16–), intermediate (CD14++CD16+), and nonclassical (CD14+CD16+) monocytes were identified within total PBMCs by first excluding doublets using forward and side scatter properties, gating on total leukocytes as determined by CD45 expression, and removing dead cells with an Aqua Live/Dead viability dye. The frequency of each monocyte subset was then determined by gating on CD3–CD20–human leukocyte antigen (HLA)-DR+ cells and identifying monocytes within this population by CD14 and CD16 expression. B, DCs were identified within total PBMCs by gating on the CD14–CD16+/– population, in order to exclude any contaminating monocytes. Myeloid DCs (mDCs) were identified as CD11c+CD123– and plasmacytoid DCs (pDCs) were identified as CD11c–CD123+. The representative flow plots (A and B) demonstrating gating of monocyte and DC populations are accompanied by graphs showing pooled data for the indicated population. In all plots, individuals are classified as noncannabis users or cannabis users stratified by moderate or heavy cannabis use as determined by plasma quantities of 11-nor-carboxy-tetrahydrocannabinol. Each individual is represented by a single point. Horizontal bars indicate the median value. The statistical significance of differences between each of the cannabis-using groups and the noncannabis users was determined using the Mann-Whitney test.
Dendritic cells (DCs) also promote inflammation and have been demonstrated to contribute to HIV pathogenesis [28]. We found that the frequency of myeloid DCs (CD11c+CD123–) was significantly lower in the moderate and heavy cannabis-using groups as compared to noncannabis users (P = .0155 and .0085, respectively; Figure 4B), although plasmacytoid DC frequencies were similar between the 2 groups (Figure 4B).
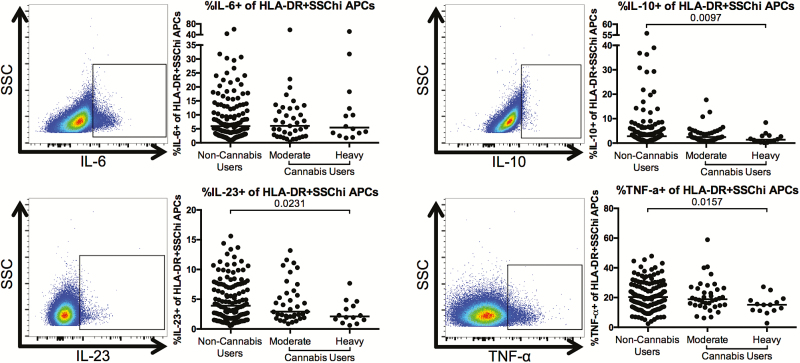
Finally, we determined whether cannabis use was also associated with alterations in the frequency of cytokine-producing APCs after overnight stimulation with PMA/ionomycin. We found that the frequencies of interleukin 10 (IL-10)+, interleukin 23 (IL-23)+, and TNF-α+ APCs were significantly lower in heavy cannabis-using individuals as compared to noncannabis-using individuals (IL-10: P = .0097; IL-23: P = .0231; TNF-α+: P = .0157; Figure 5).
Figure 5.
Lower frequency of cytokine-producing antigen-presenting cells (APCs) in cannabis-using individuals as compared to noncannabis users. Multiparameter flow cytometry was used to identify the frequency of APCs within total peripheral blood mononuclear cells of cannabis-using and nonusing individuals that expressed interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 23 (IL-23), and tumor necrosis factor alpha (TNF-α) after overnight culture in the presence of phorbol myistate acetate, ionomycin, and brefeldin A. Cells were identified by first excluding doublets using forward and side scatter properties, gating on total leukocytes as determined by CD45 expression and removing dead cells with an Aqua Live/Dead viability dye. Total APCs were then identified by gating on CD3–CD20–HLA-DR+ cells. Expression of the indicated cytokine was then determined within this population. Pooled data is accompanied by a representative flow plot showing gating for the indicated cytokine. In all plots, individuals are classified as noncannabis users or cannabis users stratified by moderate or heavy cannabis use as determined by plasma quantities of 11-nor-carboxy-tetrahydrocannabinol. Each individual is represented by a single point. Horizontal bars indicate the median value. The statistical significance of differences between each of the cannabis-using groups and the noncannabis users was determined using the Mann-Whitney test. Abbreviations: SSC, side scatter; SSChi, side scatter high.
Similar Plasma Levels of Markers of Microbial Translocation, Barrier Integrity, and Inflammation in Cannabis Users Compared With Nonusers
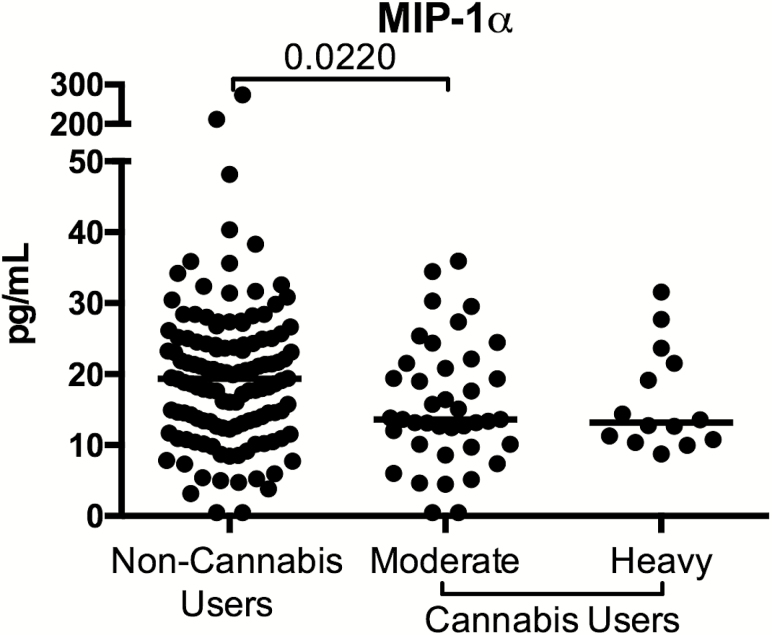
Finally, we assessed whether cannabis use was associated with soluble indicators of microbial translocation (LPS, LBP, sCD14), intestinal barrier integrity (I-FABP, Zonulin), and inflammation (21 cytokines and chemokines) in HIV-infected, ART-treated individuals. We observed significantly lower macrophage inflammatory protein-1α in moderate cannabis users compared to levels in noncannabis users (P = .022; Figure 6), but no evidence of a dose-response effect. All other analytes assessed were similar among the cannabis-using groups and the noncannabis users.
Figure 6.
Altered plasma levels of macrophage inflammatory protein (MIP) 1α in plasma of heavy cannabis users compared with noncannabis users. Plasma levels of MIP-1α was assessed as part of a multiplex Luminex assay. Data are shown as pg/mL. Individuals are classified as noncannabis users or cannabis users stratified by moderate or heavy cannabis use as determined by plasma quantities of 11-nor-carboxy-tetrahydrocannabinol. Each individual is represented by a single point. Horizontal bars indicate the median value.
DISCUSSION
In this study, we found that heavy cannabis use, as defined by plasma quantity of THC-COOH, in HIV-infected, ART-treated individuals was associated with lower frequencies of activated (HLA-DR+CD38+) CD4+ and CD8+ T cells compared to frequencies of these cells in non-cannabis-using individuals. This novel finding is important given that elevated levels of T-cell activation have been associated with lower CD4+ T-cell gains following ART [4] and with mortality in this population [29]. Thus, our work suggests that cannabinoids may have an immunological benefit in the context of HIV infection, as lowering the frequency of activated T cells could limit the risk of development of non-AIDS-associated comorbidities [3, 30].
We observed that heavy cannabis use was associated with significantly lower frequencies of TNF-α+ B cells as compared with no cannabis use. It was previously reported that B cells from HIV-infected individuals spontaneously produce IL-6 and TNF-α in vitro, which increased viral production by infected cells [31]. Although we saw no differences in the frequency of IL-6+ B cells in heavy cannabis users compared to nonusers, the decreased frequency of TNF-α+ B cells in heavy cannabis users could indicate a generalized lowering of systemic inflammation. We also noted a trend toward higher frequencies of IgA+ B cells in heavy cannabis users, although this difference did not reach statistical significance. A previous murine study demonstrated that cannabinoid treatment could directly induce B-cell class switching from immunoglobulin M to immunoglobulin E [32]; thus, it is possible that cannabis use could directly influence B-cell functionality.
Another notable finding in our study was that heavy cannabis use, compared to noncannabis use, was associated with increased frequencies of classical monocytes and decreased frequencies of intermediate and nonclassical monocytes. Previous work has demonstrated that CD16+ monocytes have higher frequency and turnover in HIV- and SIV-infected subjects as compared to uninfected individuals [22–25, 33], and that these cells are more proinflammatory than their classical monocyte counterparts [34–36]. Thus, our work suggests that one mechanism by which cannabis may exert an anti-inflammatory impact in HIV-infected individuals is by increasing the frequency of CD16– monocyte subsets and decreasing the frequency of CD16+ monocyte subsets.
An additional way that altered APCs could contribute to lower T-cell activation could be through changes in cytokine production. Indeed, we observed in heavy cannabis users lower frequencies of IL-23+ and TNF-α+ APCs. These results are consistent with previous studies demonstrating that cannabinoids can reduce immune cell cytokine production and cellular proliferation [18, 37, 38]. Although the frequency of IL-23+ or TNF-α+ APCs did not correlate with the frequency of activated or exhausted CD4+ or CD8+ T cells, it is possible that decreased production of cytokines from these cells creates a less inflammatory milieu, which could influence the level of activation and exhaustion of T-cell subsets.
Coinfection with HCV is relatively common in the HIV-infected population (15%–30%), likely due to overlapping transmission routes [39]. Previous studies have indicated that HIV/HCV coinfection is associated with increased immune activation, as compared to monoinfected individuals [40]. In our study, the moderate and heavy cannabis-using individuals had a significantly higher frequency of HCV-coinfected individuals as compared to the non-cannabis-using individuals (Table 1). It is notable, therefore, that despite this higher frequency of HCV coinfection, these individuals still have significantly lower indicators of inflammation and immune activation as compared to the non-cannabis-using groups. In addition, we did not have cytomegalovirus (CMV) or Epstein-Barr virus (EBV) serostatus on most patients in this trial, but CMV has been associated with increased disease progression in HIV infection, and could be an important covariate [41–43]. Further investigation is necessary to clarify the exact influence that HCV and other common coinfections, such as CMV and EBV, may have in cannabis-using, HIV-infected individuals.
A major strength of our study is that we were able to quantify the level of 4 cannabis metabolites in the plasma of our study participants, which allowed us to confirm the self-reported cannabis usage of individuals included in the study, and stratify the cannabis-using group into heavy, moderate, and occasional use groups. Previous studies that have investigated the effect of cannabis use in HIV infection have solely relied on self-reported cannabis usage to stratify study participants, which may not always be accurate, as evidenced by the fact that we identified cannabis metabolites in the plasma of some self-reported non-cannabis users and a lack of cannabis metabolites in some self-reported regular cannabis users (Figure 1). It is possible that inaccuracies such as this could contribute to the conflicting results observed in previous studies conducted to assess the impact of cannabis use on clinical care in HIV-infected individuals [9–13]. To our knowledge, our work is the first to directly quantify and associate high plasma levels of a cannabis metabolite, namely THC-COOH, with decreased markers of immune activation in HIV-infected, ART-treated individuals.
A caveat of our study is that although our direct measurement of cannabis metabolites in the plasma of the study participants is a more accurate measure of cannabis use than self-reported status, we were not able to assess host factors that could influence the rate of cannabis metabolism in each individual, such as hepatic enzymes and genetics. Furthermore, while all participants reported no other use of drugs of abuse, inaccurate self-reporting could confound the results. In addition, while measurement of the cannabis metabolites was a strength of our study, it still does not provide information about route of cannabis intake (ie, smoking, vaping, eating), nor what type or strain of cannabis was used, which could contribute to differences in inflammation and health. Moreover, the microbiome could be impacted by cannabis use and the different routes by which it is taken, which may also contribute to alterations in levels of inflammation. Additionally, the cross-sectional design of our study limits us to drawing associations and not causation between heavy cannabis use and lower levels of immune activation in the study subjects. It will be important in the future to account for host factors, use of other drugs of abuse, cannabis types/routes, cigarette smoking, microbiome, and HCV and other coinfection status, and to perform longitudinal observations, in order to accurately evaluate relationships between drug level and immunological readouts. Finally, the impact of cannabis and the resulting decreased inflammation on the viral reservoir will be an important avenue of future research.
In summary, our work demonstrates that heavy cannabis use is associated with lower markers of inflammation and immune activation in HIV-infected, ART-treated individuals. Further work is necessary to determine the mechanism by which cannabis causes reduced immune activation. These findings have clinical implications, as cannabinoids may have an immunological benefit and nonpsychoactive cannabis derivatives could be investigated as novel therapeutics to be used in conjunction with ART to aid in reduction of persistent inflammation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. A. M. performed experiments, analyzed the data, and wrote the manuscript. J. S. K. performed experiments and analyzed data. T. M. G., E. C., T. H. M., and C. M. assisted with experiments. M. C. W. performed and aided with statistical analysis. N. T. F assisted with monocyte analysis. N. I. led cannabinoid quantification. J. N. M. and P. W. H. aided with sample collection and selection. A. C. C. supported clinical studies. N. K. R. conceived of and led the study.
Acknowledgments. We thank all of the study participants for their generous donation of time and samples. We thank Dr Alexander S. Zevin for helpful discussions. Special thanks go to Dr Jag Khalsa of the National Institute on Drug Abuse (NIDA), National Institutes of Health, for support of these studies.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by NIDA (grant number 1DP1DA037979-01 to N. R. K.).
Potential conflicts of interest. N. T. F. has served as a consultant for Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS 2008; 22:2409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 2013; 254:326–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunt PW, Martin JN, Sinclair E et al. . T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 5. Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol 2008; 1:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robbins GK, Spritzler JG, Chan ES et al. ; AIDS Clinical Trials Group 384 Team Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis 2009; 48:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris GE, Dupuis L, Mugford GJ et al. . Patterns and correlates of cannabis use among individuals with HIV/AIDS in Maritime Canada. Can J Infect Dis Med Microbiol 2014; 25:e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mimiaga MJ, Reisner SL, Grasso C et al. . Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health 2013; 103:1457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okafor CN, Zhou Z, Burrell LE 2nd et al. . Marijuana use and viral suppression in persons receiving medical care for HIV-infection. Am J Drug Alcohol Abuse 2017; 43:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lake S, Kerr T, Capler R, Shoveller J, Montaner J, Milloy MJ. High-intensity cannabis use and HIV clinical outcomes among HIV-positive people who use illicit drugs in Vancouver, Canada. Int J Drug Policy 2017; 42:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slawson G, Milloy MJ, Balneaves L et al. . High-intensity cannabis use and adherence to antiretroviral therapy among people who use illicit drugs in a Canadian setting. AIDS Behav 2015; 19:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kipp AM, Rebeiro PF, Shepherd BE et al. . Daily marijuana use is associated with missed clinic appointments among HIV-infected persons engaged in HIV care. AIDS Behav 2017; 21:1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorenz DR, Dutta A, Mukerji SS, Holman A, Uno H, Gabuzda D. Marijuana use impacts midlife cardiovascular events in HIV-infected men. Clin Infect Dis 2017; 65:626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milloy MJ, Marshall B, Kerr T et al. . High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug Alcohol Rev 2015; 34:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molina PE, Amedee AM, Winsauer P, Nelson S, Bagby G, Simon L. Behavioral, metabolic, and immune consequences of chronic alcohol or cannabinoids on HIV/AIDs: studies in the non-human primate SIV model. J Neuroimmune Pharmacol 2015; 10:217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amedee AM, Nichols WA, LeCapitaine NJ et al. . Chronic Δ⁹-tetrahydrocannabinol administration may not attenuate simian immunodeficiency virus disease progression in female rhesus macaques. AIDS Res Hum Retroviruses 2014; 30:1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molina PE, Amedee AM, LeCapitaine NJ et al. . Modulation of gut-specific mechanisms by chronic δ(9)-tetrahydrocannabinol administration in male rhesus macaques infected with simian immunodeficiency virus: a systems biology analysis. AIDS Res Hum Retroviruses 2014; 30:567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med 1997; 156:1606–13. [DOI] [PubMed] [Google Scholar]
- 19. Fabritius M, Favrat B, Chtioui H et al. . THCCOOH concentrations in whole blood: are they useful in discriminating occasional from heavy smokers?Drug Test Anal 2014; 6:155–63. [DOI] [PubMed] [Google Scholar]
- 20. Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J Anal Toxicol 2008; 32:470–7. [DOI] [PubMed] [Google Scholar]
- 21. Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 1992; 16:276–82. [DOI] [PubMed] [Google Scholar]
- 22. Amirayan-Chevillard N, Tissot-Dupont H, Capo C et al. . Impact of highly active anti-retroviral therapy (HAART) on cytokine production and monocyte subsets in HIV-infected patients. Clin Exp Immunol 2000; 120:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol 1995; 25:3418–24. [DOI] [PubMed] [Google Scholar]
- 24. Funderburg NT, Zidar DA, Shive C et al. . Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120:4599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manuzak JA, Dillon SM, Lee EJ, Dong ZM, Hecht DK, Wilson CC. Increased Escherichia coli-induced interleukin-23 production by CD16+ monocytes correlates with systemic immune activation in untreated HIV-1-infected individuals. J Virol 2013; 87:13252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baker JV, Hullsiek KH, Singh A et al. ; CDC SUN Study Investigators Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014; 28:831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunt PW, Sinclair E, Rodriguez B et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dillon SM, Lee EJ, Kotter CV et al. . Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol 2016; 9:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hunt PW, Cao HL, Muzoora C et al. . Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011; 25:2123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rieckmann P, Poli G, Kehrl JH, Fauci AS. Activated B lymphocytes from human immunodeficiency virus-infected individuals induce virus expression in infected T cells and a promonocytic cell line, U1. J Exp Med 1991; 173:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agudelo M, Newton C, Widen R et al. . Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol 2008; 3:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burdo TH, Soulas C, Orzechowski K et al. . Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 2010; 6:e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belge KU, Dayyani F, Horelt A et al. . The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 2002; 168:3536–42. [DOI] [PubMed] [Google Scholar]
- 35. Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood 1996; 87:373–7. [PubMed] [Google Scholar]
- 36. Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007; 81:584–92. [DOI] [PubMed] [Google Scholar]
- 37. Srivastava MD, Srivastava BI, Brouhard B. Delta9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 1998; 40:179–85. [DOI] [PubMed] [Google Scholar]
- 38. Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol 2002; 133:124–31. [DOI] [PubMed] [Google Scholar]
- 39. Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–7. [DOI] [PubMed] [Google Scholar]
- 40. Feuth T, Arends JE, Fransen JH et al. . Complementary role of HCV and HIV in T-cell activation and exhaustion in HIV/HCV coinfection. PLoS One 2013; 8:e59302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith DM, Nakazawa M, Freeman ML et al. . Asymptomatic CMV replication during early human immunodeficiency virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis 2016; 63:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gianella S, Letendre S. Cytomegalovirus and HIV: a dangerous pas de deux. J Infect Dis 2016; 214(Suppl 2):S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gianella S, Anderson CM, Var SR et al. . Replication of human herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol 2016; 90:3944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.