Abstract
Endogenous hydrogen sulfide (H2S), mainly synthesized by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH), has been implicated in regulating placental angiogenesis; however, the underlying mechanisms are unknown. This study was to test a hypothesis that trophoblasts synthesize H2S to promote placental angiogenesis. Human choriocarcinoma-derived BeWo cells expressed both CBS and CTH proteins, while the first trimester villous trophoblast-originated HTR-8/SVneo cells expressed CTH protein only. The H2S producing ability of BeWo cells was significantly inhibited by either inhibitors of CBS (carboxymethyl hydroxylamine hemihydrochloride, CHH) or CTH (β-cyano-L-alanine, BCA) and that in HTR-8/SVneo cells was inhibited by CHH only. H2S donors stimulated cell proliferation, migration, and tube formation in ovine placental artery endothelial cells (oFPAECs) as effectively as vascular endothelial growth factor. Co-culture with BeWo and HTR-8/SVneo cells stimulated oFPAEC migration, which was inhibited by CHH or BCA in BeWo but CHH only in HTR-8/SVneo cells. Primary human villous trophoblasts (HVT) were more potent than trophoblast cell lines in stimulating oFPAEC migration that was inhibited by CHH and CHH/BCA combination in accordance with its H2S synthesizing activity linked to CBS and CTH expression patterns. H2S donors activated endothelial nitric oxide synthase (NOS3), v-AKT murine thymoma viral oncogene homolog 1 (AKT1), and extracellular signal-activated kinase 1/2 (mitogen-activated protein kinase 3/1, MAPK3/1) in oFPAECs. H2S donor-induced NOS3 activation was blocked by AKT1 but not MAPK3/1 inhibition. In keeping with our previous studies showing a crucial role of AKT1, MAPK3/1, and NOS3/NO in placental angiogenesis, these data show that trophoblast-derived endogenous H2S stimulates placental angiogenesis, involving activation of AKT1, NOS3/NO, and MAPK3/1.
Keywords: hydrogen sulfide, trophoblasts, endothelial cells, angiogenesis, placenta, human
Summary Sentence
Human trophoblast-derived endogenous H2S mediates trophoblast–endothelial cell interaction in stimulating placental artery endothelial cell angiogenesis in vitro.
Introduction
Angiogenesis is a process of new vessel formation that requires proliferation, migration, and differentiation of endothelial cells (ECs) from the preexisting blood vessels as they send out capillary sprouts to initiate the formation of new tube-like structures, and secondary vasodilatation to enhance circulation and nutrient uptake. This multistep process begins with a rise in local and/or systemic angiogenic factors, followed by breakdown of endothelial basement membrane to facilitate EC migration and proliferation. EC differentiation leads to newly formed tube-like structures that stabilize as mature vessels with the recruitment of pericytes or smooth muscle cells [1]. Deranged angiogenesis has a major impact on human health and contributes to the pathogenesis of numerous vascular diseases that are caused by either excessive angiogenesis in tumors, retinopathy, and cavernous hemangioma or insufficient angiogenesis in hypertension, diabetes, and restenosis [2]. Angiogenesis is a key mechanism for regulating maternal–fetal interface blood flows that are rate-limiting to fetal/placental growth and the mother's well-being during pregnancy [3]. Angiogenesis in the placenta takes similar steps as it occurs in any other organs; it also requires proliferation, migration, and differentiation of ECs from the preexisting placental microvessels [4,5]. Deranged placental vasculature has been recognized as the most common placental pathology in numerous pregnancy complications including preeclampsia and intrauterine growth restriction [6–9], implicating that placental angiogenesis must be tightly regulated during pregnancy.
Hydrogen sulfide (H2S) is a gaseous signaling molecule of the gasotransmitter family after nitric oxide (NO) and carbon monoxide (CO) [10]; it functions as a potent vasodilator [11] and angiogenic factor [12,13]. The biological function of H2S is elicited through several mechanisms. Activation of adenosine triphosphate (ATP)-sensitive K+-channel (KATP) in smooth muscle cells is important for the vasorelaxatory effect of H2S [14]. Activation of phosphoinositol-3-kinase (PI3K)-protein kinase B/v-AKT murine thymoma viral oncogene homolog 1 (AKT1) and extracellular signal-activated protein kinase (mitogen-activated protein kinase kinase 3/1, MAPK3/1) mediates the proangogenic effect of H2S in ECs [12]. H2S can alter numerous cellular processes via sulfhydration of the proteome post-translationally [15]. Moreover, H2S regulates cellular physiology via interaction with NO and CO synergistically [16,17].
Endogenous H2S is primarily synthesized from L-cysteine by two enzymes: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH) [18–20]; their expression can be tissue/cell specific as both are needed to generate H2S in some tissues while one enzyme is sufficient in others [21,22]. Our recent work first shows that H2S is a new uterine artery vasodilator since H2S production and CBS, but not CTH, mRNA/protein expressions are significantly stimulated by exogenous estrogens in ovine uterine arteries [23] and are augmented and positively linked to endogenous estrogens in human uterine artery, contributing to pregnancy-associated uterine vasodilation [24]. Dysregulation of H2S metabolism disrupts oviduct transport of embryos [25] and CBS insufficiency causes infertility [26]. The system is also downregulated in uterine myometrium during the transition of pregnant uterus from quiescence to contractile state with labor onset in mice and women [27], via suppressing myometrial contraction-associated proteins and inflammation [28]. In human placenta, CBS, but not CTH, protein is localized in trophoblasts and Hofbauer cells and CTH, but not CBS, protein is found in placental microvessel ECs [29–31]. Human placental CTH/H2S signaling is downregulated in preeclamptic vs. normotensive pregnancies [30,32]. H2S inhibition results in decreased placental angiogenesis with increased anti-angiogenic factors, shallow trophoblast invasion, and impaired uterine spiral remodeling during mouse and human pregnancy [33]. These observations suggest a critical role of H2S in placental angiogenesis. However, it is unknown how H2S regulates placental angiogenesis.
We hypothesize that human placental trophoblasts synthesize H2S to stimulate placental angiogenesis. We report herein that (1) exogenous H2S donors stimulate proliferation, migration, and formation of tube-like structures of placental artery ECs in vitro (i.e. angiogenesis); (2) endogenous H2S derived from CBS/CTH in trophoblasts stimulates placental artery EC migration in vitro; and (3) H2S activates AKT1-endothelial nitric oxide synthase (NOS3) and MAPK3/1, which we have previously shown to be important for placental artery EC migration [34,35]. Thus, we conclude that trophoblast-derived endogenous H2S stimulates placental angiogenesis in vitro at least partially mediated by AKT1-NOS3 pathway and MAPK3/1 pathways.
Materials and methods
Antibodies and chemicals
A cysteine-activated H2S donor 5a [36] was kindly provided by Dr Xian Min at the Washington State University. Mouse monoclonal antibodies (mAbs) against CBS and CTH and rabbit polyclonal antibody (pAb) against NOS3 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse mAb against beta-actin (ACTB) was from Life technologies (Carlsbad, CA). Rabbit pAbs against phospo-NOS3Ser1177 (pNOS3), phosphor-AKT1ser473 (pAKT1), phosphor-MAPK3/1Thr202/Tyr204 (pMAPK3/1), AKT1, and MAPK3/1 were from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit immunoglobins were obtained from Pierce (Rockford, IL). O-(Carboxymethyl) hydroxylamine hemihydrochloride (CHH, a CBS inhibitor) and PI3K inhibitor wortmannin were from Sigma-Aldrich (St. Louis, MO). β-cyano-L-alanine (BCA, a CTH inhibitor) was from Cayman Chemical (Ann Arbor, MI). Dynabeads CD31 EC beads and cell culture supplies were from Invitrogen (San Diego, CA). The mitogen-activated protein kinase kinase 1/2 inhibitor U0126 was from Cell Signaling Technology (Danvers, MA). Sodium hydrosulfide (NaSH) and all other chemicals were from Sigma (St Louis, MI), unless indicated.
Tissue collection
All human tissues were obtained at the University of California Irvine Medical Center. Written consent was obtained from all participants, and ethical approval was granted by the Institutional Review Board for Human Research at the University of California, Irvine. Uterine myometrium and endometrium tissues were collected from five pregnant subjects recruited with suspected placental accrete based on previous ultrasound findings in the event a hysterectomy was indicated. They were 35–44 year old and at 35–36 weeks gestation. The samples were collected immediately after Caesarean section hysterectomy and placed in chilled endothelial growth medium (ECM) and transported to the laboratory.
Trophoblast cell lines and primary cell isolation and culture
Human trophoblast-derived choriocarcinoma BeWo cells and JEG3 cells were purchased from American Type Culture Collection and grown in FK12 and DMEM, respectively, as described previously [37,38]. The first trimester trophoblast-derived HTR-8/SVneo cell line, kindly provided by Dr Peeyush K. Lala (Western University, Canada), was grown in RPMI 1640, as described previously [39]. All trophoblast cell line culture media contained 10% fetal bovine serum (FBS) and 1% antibiotics (100 U/ml penicillin and streptomycin). Primary human villous trophoblasts (HVT) were purchased from ScienCell (Carlsbad, CA). They were cultured in ScienCell Trophoblast Medium with 10% FBS and used without passage. The cells were authenticated by immunofluorescence staining with anti-cytokeratin-7 antibody (1 μg/ml, Dako) and time-dependent secretion of human chorionic gonadotropin (hCG, determined by using an Abcam enzyme-linked immunoassay kit according to the manufacturer’s instructions) in response to stimulation with 100 μM 8-bromo-cyclic adenosine monophosphate (cAMP). Human umbilical vein endothelial cells (HUVECs) were isolated from cords from healthy term placentas collected at the University of California Irvine Medical Center, with approval from the Institutional Review Boards and written consents from all subjects, as detailed previously [40]. Collagenase digestion was used to isolate primary human myometrial myocytes [41] and endometrial stromal cells (STCs) [42], as described previously. Primary human myometrial microvascular endothelial cells (hMMECs) and endometrial microvascular endothelial cells (hEMECs) were purified from the isolated myometrial myocyte and STC suspensions by using Dynabeads CD31 (platelet and EC adhesion molecule 1) EC beads according to the manufacturer's instruction. HUVECs, hMMECs, and hEMECs were cultured in ECM containing 10% FBS and 1% EC growth factors (ScienCell) and used at passage 2–4. Primary human myometrial myocytes and endometrial STCs were cultured in DMEM with 10% FBS and 1% antibiotics as described [41,42]. Ovine fetoplacental artery endothelial cells (oFPAECs) were isolated from secondary sheep placental arteries collected at the University of California San Diego with approval granted by the Animal Use Committee, and cultured in MCD131 containing 10% FBS and used at passages 7–11, as detailed previously [43].
Cell stimulation, sodium dodecyl sulfate (SDS) polyacrylamide gel electrophores (PAGE), and immunoblotting
Prior to each experiment, subconfluent (∼70%–80%) oFPAECs were serum starved in M-199 (Invitrogen) containing 1% FBS, 0.1% bovine serum albumin, and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid overnight. The cells were stimulated with or without vascular endothelial growth factor (VEGFA, 10 ng/ml), NaHS, (100 μM), or a cysteine-activated H2S donor 5a (1, 3, and 10 μM) for 10 min. Cells were washed four times with ice-cold phosphate-buffered saline. Total protein samples were extracted with a nondenaturing NP-40 lysis buffer [34] and quantified by using the Pierce BCA Protein Assay Kit. Equal amounts of total protein extracts (20 μg/lane) were separated on 10% SDS-PAGE and transferred to polyvinylidene difluoride membrane and immunoblotted as described previously [44]. Proteins of interest were measured by immunoblotting with specific antibodies against NOS3 (1:500), CBS (1:500), CTH (1:500), AKT1 (1:500), MAPK3/1 (1:1000), phosphorylated NOS1177 (1:500), phosphorylated AKT1 (1:500), and phosphorylated MAPK3/1 (1:1000). ACTB was measured by immunoblotting with an anti-ACTB mAb (1:20 000) to normalize changes in proteins. Normalized proteins were expressed as fold of untreated controls.
Methylene blue assay
The H2S synthesizing activity was measured by using the methylene blue assay [23], with minor modifications. Trophoblast cells (5 × 105/treatment in duplicate) were harvested and homogenized in ice-cold 50 mM potassium phosphate buffer, pH 8. The reaction mixture contained 50 mM potassium phosphate buffer pH 8.0, 10 mM L-cysteine, and 2 mM pyridoxal 5’-phosphate. Microtubes (2 ml) were used as the center wells; each contained 0.3 ml of 1% zinc acetate as trapping solution and a filter paper of 0.5 × 1.5 cm to increase the air/liquid contacting surface. The reaction was performed in 12 ml test tubes. The tubes containing the reaction mixture and center wells were flushed with N2 before being sealed with a double layer of Parafilm. The reaction was initiated by transferring the tubes from ice to a 37°C shaking water bath. After incubating at 37°C for 90 min, the reaction was stopped by adding 0.5 ml of 50% trichloroacetic acid. The tubes were sealed again and incubated at 37°C for another 60 min to ensure acomplete trapping of the H2S released from the mixture. Subsequently, 0.05 ml of 20 mM N, N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCl was added immediately after the addition of 0.05 ml 30 mM FeCl3 in 1.2 M HCl. The absorbance of the resulting solution at 670 nm was measured after 20 min. The H2S concentration was calculated based on a calibration curve generated from NaHS solutions. For CBS and CTH inhibition experiments, their respective inhibitors, CHH or BCA, were added separately or in combination (final concentration = 2 mM) to the reaction mixtures prior to initiating the methylene blue assay.
Cell proliferation
Cell proliferation was measured by CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) exactly following the manufacturer's instructions as described previously [44].
Cell migration
A transwell migration assay using the 24-well Multiwell BD Falcon FluoroBlok Insert System (8.0 μm pores; BD Biosciences, San Jose, CA) was carried out as described previously [34], with modifications for measuring cell migration under the influence of trophoblast-derived H2S. Briefly, the top insert was first coated with 20 μg/ml of fibronectin (Sigma-Aldrich) overnight at 4°C. BeWo or HTR-8/SVneo cells were seeded into the bottom chamber with FK12 and DMEM, respectively, as described above and cultured until reaching ∼70% confluence. On the day of experiment, oFPAE cells were resuspended in MCDB131 medium containing 0.1% bovine serum albumin and 0.2% FBS and seeded in the top insert at a density of 1.5 × 104 cells/well. Then the inserts were assembled with the trophoblast cultures as a transwell system, with or without 2 mM CHH and 2 mM BCA that were added into the trophoblast cultures in the bottom chamber. The systems were cultured under humid air with 5% CO2 at 37°C to allow oFPAECs to migrate. After removing the medium in the top insert at 16 h after co-culture, the inserts were placed into wells of a new 24-well plate, containing 0.5 ml/well of Hanks’ buffer salt solution with a cell hydrolysable fluorescent dye calcein acetoxymethyl ester (Calcein-AM, 0.2 μg/ml, Invitrogen). After incubation at 37°C for 30 min, the inserts were examined with a 10× objective by using an inverted fluorescence microscopy. Cells migrated to the bottom side of the filter membrane of the insert were green fluorescence labeled by Calcein-AM. Digitalized images of green fluorescently labeled cells migrated to the bottom side of the filter membrane of the inserts from four randomly chosen fields were captured with excitation at 494 nm and emission at 517 nm by a Hamamatsu charge-coupled device camera using the SimplePCI image analysis software. The numbers of migrated cells in four fields of each treatment were counted offline using the same software and averaged.
Tube formation
Endothelial cell differentiation (in vitro tube formation) was determined as described previously [44].
Experimental replication and statistical analysis
All experiments were repeated at least four times. Data were presented as means ± SD, and analyzed by one-way analysis of variance using SigmaPlot (Systat Software Inc., San Jose, CA). Student paired t-test we used for comparison of data between two groups. Significance was defined as P < 0.05.
Results
H2S biosynthesis in immortalized human trophoblast cell lines and placental ECs
Immunoblotting revealed that baseline CBS protein was readily detectable at a comparable level among all the widely used trophoblast cell lines, while CTH protein was found to be highest in BeWo cells, modest in JEG3 cells, and very low in HTR-8/SVneo cells. Ovine FPAECs expressed both CBS and CTH proteins, while HUVECs only expressed CBS protein. As expected, both types of ECs expressed NOS3 protein. NOS3 protein was undetectable in all the trophoblast cell lines (Figure 1A).
Figure 1.
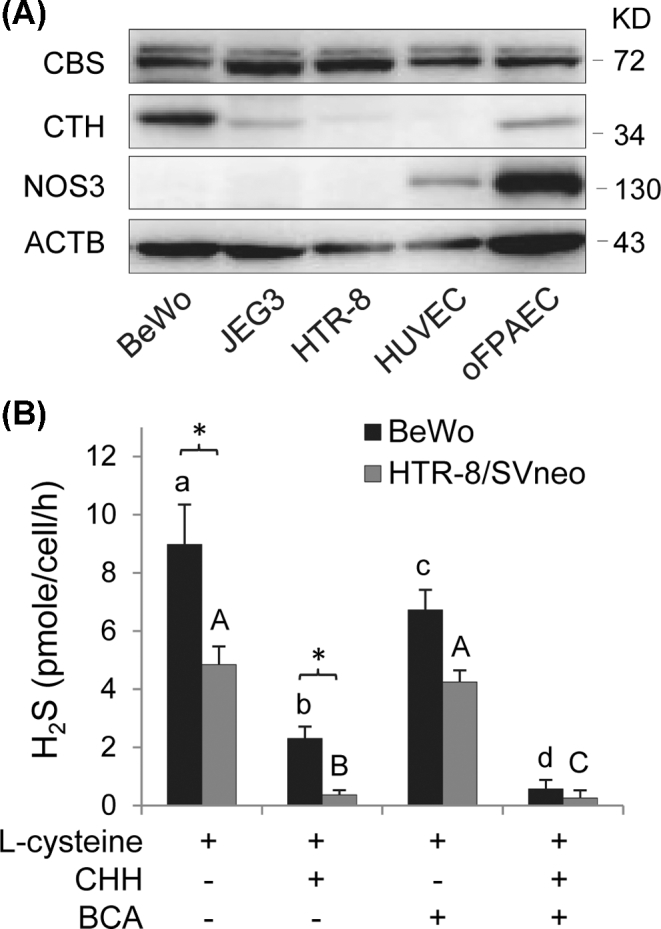
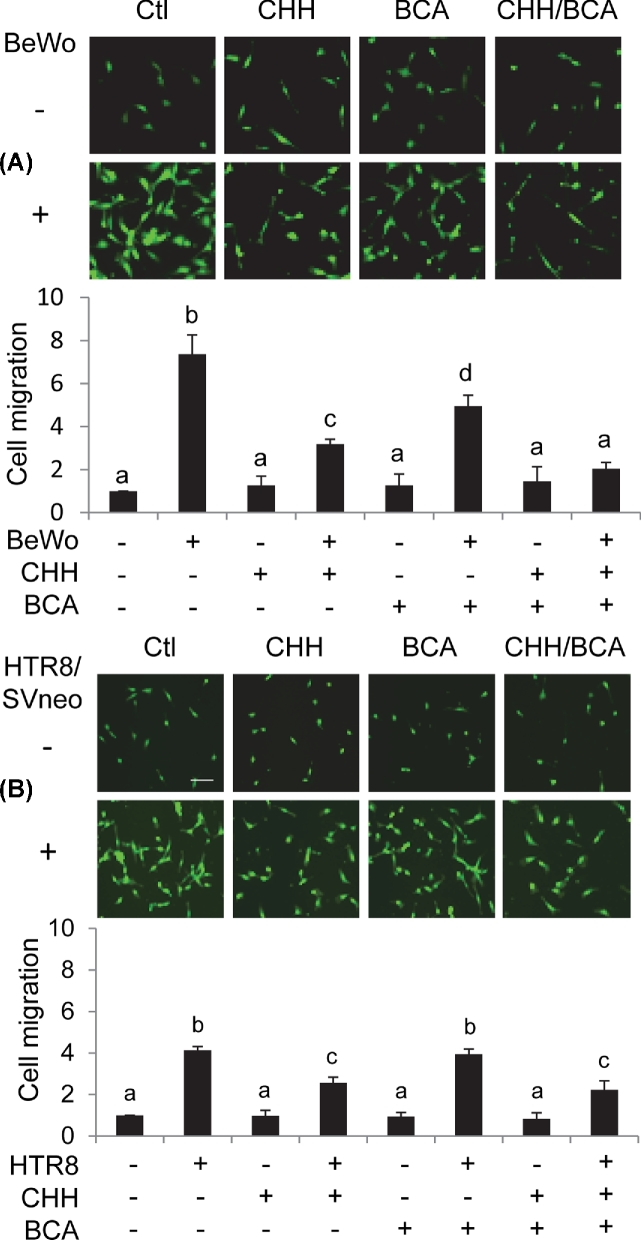
Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH) proteins in human placental trophoblast cell lines and placental ECs and H2S production in trophoblast cells. (A) Qualitative immunoblotting analysis of CBS and CTH and NOS3 protein expression in human trophoblast cell lines and placental ECs. Equal amount of proteins (20 μg/lane) of BeWo, JEG3, HTR-8/SVneo, HUVECs, and oFPAECs were resolved on SDS-PAGE and transferred to polyvinylidene difluoride membranes. Proteins of NOS3, CBS, and CTH were analyzed by immunoblotting with specific antibodies, respectively. Beta-actin was used as the sample loading control. (B) BeWo and HTR-8/SVneo cells (5 × 105/reaction) were used to determine the H2S synthesizing activity assay with or without addition of CHH (2 mM) and/or BCA (2 mM) by using the methylene blue assay. Data were expressed as mean ± SD from four independent experiments. Bars with different letters differ significantly (P < 0.05).
We determined the enzymatic sources of H2S in BeWo and HTR-8/SVneo cells. Both had the ability of synthesizing H2S; however, the H2S synthesizing activity in BeWo cells nearly doubled that of HTR-8/SVneo cells (P < 0.05). Incubation with the CTH inhibitor, BCA inhibited ∼25% (P < 0.05) of the H2S synthesizing activity in BeWo cells; incubation with the CBS inhibitor CHH was much more potent as it inhibited ∼75% (P < 0.001) of the H2S synthesizing activity in BeWo cells. The combination of BCA and CHH completely inhibited the H2S synthesizing activity in BeWo cells. These data show that both CBS and CTH are involved in H2S biosynthesis in BeWo cells. In contrast, incubation with CHH alone was able to inhibit ∼92% of the H2S synthesizing activity in HTR-8/SVneo cells, while BCA alone was ineffective and had no additive effects to that of CHH (Figure 1B). These data show that only CBS is involved in H2S biosynthesis in HTR-8/SVneo cells.
Effects of exogenous H2S on ovine placental artery endothelial cell angiogenesis in vitro
We investigated if exogenous H2S donors stimulate proliferation, migration, and tube formation of oFPAECs (i.e. in vitro angiogenesis). As a positive control, incubation with VEGFA (10 ng/ml) significantly (P < 0.05) stimulated oFPAEC proliferation (Figure 2A), migration (Figure 2B), and tube formation (Figure 2C), similar to our previous reports [34]. Incubation with two different H2S donors (i.e. 100 μM NaHS and 10 μM 5a) also significantly stimulated in vitro angiogenesis of oFPAECs. Treatment with 100 μM NaHS stimulated cell proliferation, migration, and tube formation with comparable effects to that of VEGFA. Treatment with 10 μM 5a induced similar significant effects on cell proliferation and migration and lower but still significant tube formation response compared to VEGFA and NaSH in oFPAECs (Figure 2).
Figure 2.
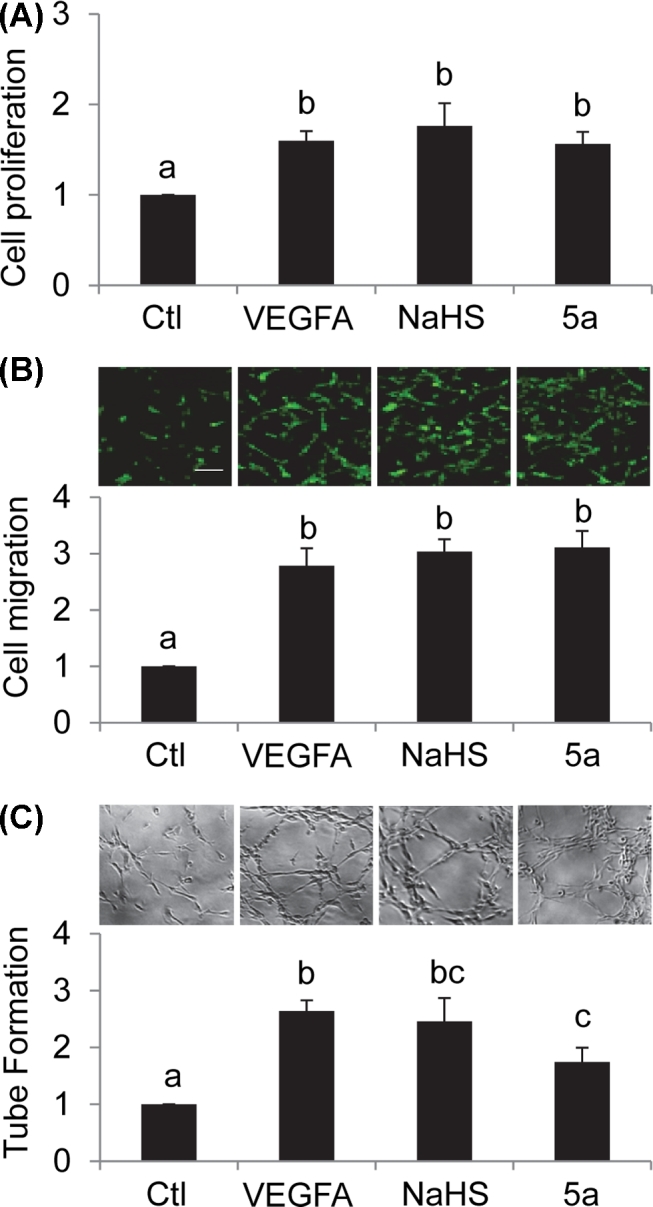
Effects of exogenous H2S donors on in vitro angiogenesis in ovine placental artery ECs (oFPAECs). (A) Cells were treated with or without VEGFA (10/ng/ml) or H2S donors NaHS (100 μM) or 5a (10 μM) for 24 h. Cell proliferation was measured by using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit. Data were expressed as mean ± SD from four independent experiments using oFPAECs from four different ewes and calculated as fold of control. Bars with different letters differ significantly (P < 0.05). (B) Cell migration was determined by a transwell migration assay after treatment with or without VEGFA (10/ng/ml) or H2S donors NaHS (100 μM) or 5a (10 μM) for 16 h. The migrated cells on the bottom of the insert were stained with calcein AM (0.2 μg/ml) and fluorescence images were captured by a fluorescent microscope. The migrated cells were quantified. Data were expressed as mean ± SD from four independent experiments using oFPAECs from four different ewes and calculated as fold of control. Bars with different letters differ significantly (P < 0.05). Bar = 100 μm. (C) Tube formation was determined by using a matrigel tube formation assay after treatment with or without VEGFA (10/ng/ml) or H2S donors NaHS (100 μM) or 5a (10 μM) for 16 h. Data were expressed as mean ± SD from four independent experiments using oFPAECs from four different ewes and calculated as fold of control. Bars with different letters differ significantly (P < 0.05).
Effects of co-culture with trophoblast cell lines on ovine placental artery endothelial cell migration
Next, we determined the effects of endogenous H2S derived from trophoblast cells on the migration of oFPAECs by using a transwell assay. In these experiments, BeWo and HTR-8/SVneo cells were chosen because both have H2S synthesizing ability but with different CBS and CTH expression patterns, BeWo expressing both but HTR-8/SVneo only expressing CTH (Figure 1A). Thus, they can be used to differentiate the role of H2S derived from CBS/CTH or CTH only, respectively.
Co-culture with BeWo and HTR-8/SVneo cells stimulated oFPAEC migration by 7.64 ± 0.35 fold (P < 0.001) and 4.06 ± 0.12 fold (P < 0.01), respectively. The stimulatory effect of BeWo cells was significantly greater than that of HTR-8/SVneo cells (P < 0.05). H2S inhibition by both CHH and BCA effectively inhibited oFPAEC migration induced by co-culture with BeWo cells (P < 0.05); the inhibitory effect of CHH was slightly greater than BCA but the difference did not reach statistical significance. The combination of CHH and BCA completely blocked the stimulatory effect of BeWo co-culture (Figure 3A). In contrast, H2S inhibition by CHH alone was able to inhibit oFPAEC migration induced by co-culture with HTR-8/SVneo cells and this was as effective as the combination of CHH and BCA, while BCA alone was ineffective (Figure 3B).
Figure 3.
Effects of co-culture with trophoblast cell lines on migration of ovine placental artery ECs (oFPAECs). Ovine FPAECs (1.5 × 104/well) were seeded on the top of the inserts in a six-well transwell plate. BeWo (A) or HTR-8/SVneo cells (B) were cultured in the bottom chamber with approximately 90% confluence with or without CHH (2 mM) and/or BCA (2 mM). The assembled co-culture system was cultured to allow oFPAEC cell to migrate for 16 h. The migrated cells were quantified as Figure 2B. Data were expressed as mean ± SD from four independent experiments using oFPAECs from four different ewes and calculated as fold of control. Bars with different letters differ significantly (P < 0.05).
Effects of exogenous H2S on cell signaling pathways in ovine placental artery endothelial cells
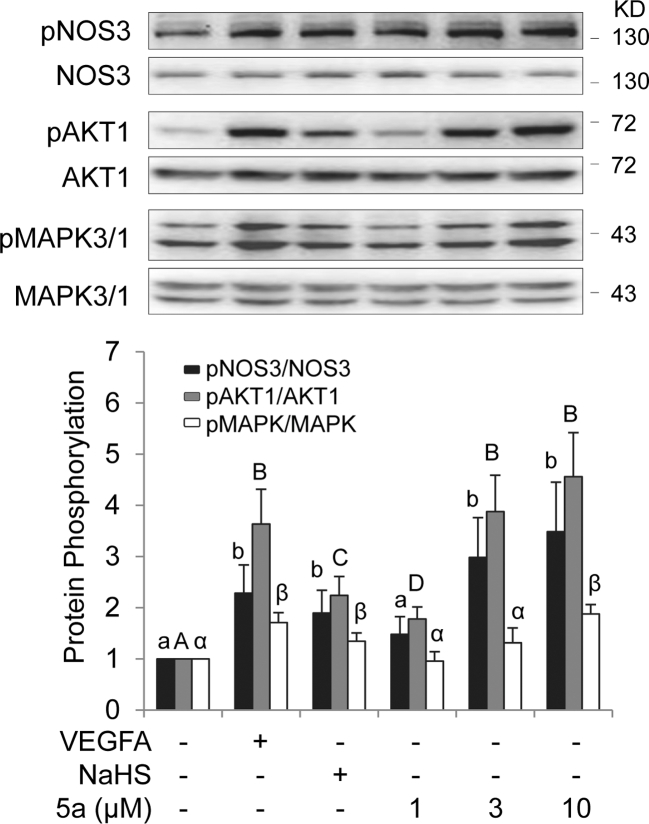
Our previous studies have shown that activation of cell signaling pathways, including MAPK3/1, AKT1, and NOS3/NO, plays a critical role in mediating in vitro angiogenesis of oFPAECs in response to stimulation with angiogenic growth factors such as VEGFA [34,35] and basic fibroblast growth factor [44,45]. We then investigated whether exogenous H2S donors activate these pathways in oFPAECs. Treatment with 10 ng/ml VEGFA for 10 min significantly stimulated phosphorylation of NOS3, MAPK3/1, AKT1, and NOS3, similarly as shown previously [34,35]. Treatment with relative low concentration of NaSH at 100 μM also stimulated activation of MAPK1/3, AKT1, and NOS3; however, the response in AKT1 activation was slightly weaker than that to VEGFA. Treatment with increasing concentrations (1, 3, and 10 μM) of 5a stimulated concentration-dependent activation of AKT1 and NOS3, with significant activation at 3 or 10 μM. However, only at a higher concentration (10 μM), 5a activated MAPK3/1 (Figure 4).
Figure 4.
(A) Effects of exogenous H2S donors on activation of NOS3, AKT1, and MAPK3/1 ovine placental artery ECs (oFPAECs). Cells were seeded in a six-well plate. After serum starvation, subconfluent cells were treated with VEGFA (10 ng/ml), H2S donor NaHS (100 μM) and H2S donor 5a (1, 3, and 10 μM) for 10 min. Equal amounts of proteins (20 μg/lane) were subjected to immunoblotting with specific antibodies against phosphorylated NOS3, AKT1, and pMAPK3/1. Phosphorylation of NOS3, AKT1, and pMAPK3/1 was quantified as a ratio to their total protein levels, respectively. Data were expressed as mean ± SD from four independent experiments using oFPAECs from four different ewes and presented as fold of control. Bars with different letters differ significantly (P < 0.05).
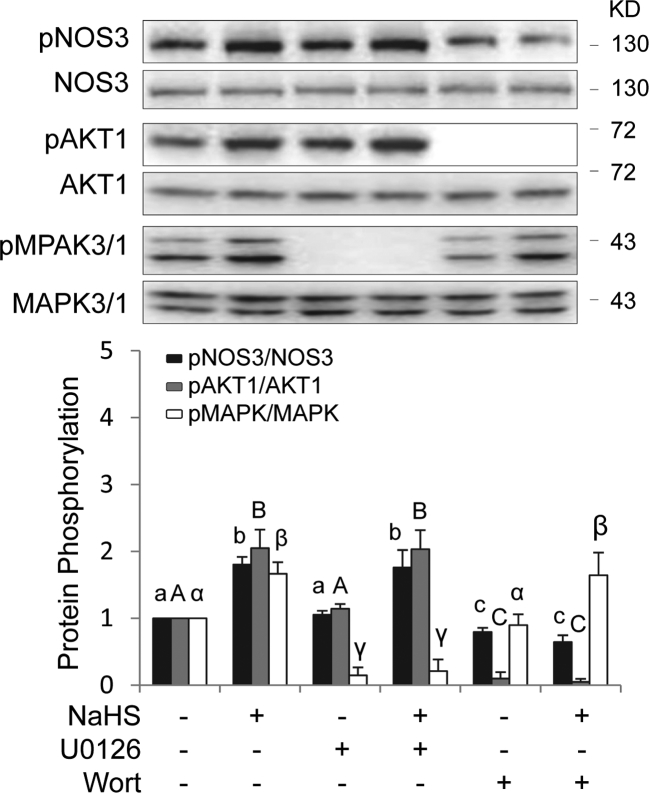
Exogenous H2S activates nitric oxide synthase via AKT1 but not mitogen-activated protein kinase 3/1 in ovine placental artery endothelial cells
As NOS3 can be directly activated through MAPK3/1 and AKT1-dependent phosphorylation [46–48], we determined the role of MAPK3/1 and AKT1 in NOS3 activation by exogenous H2S donors. Treatment with 100 μM NaSH for 10 min significantly stimulated NOS3 phosphorylation in oFPAECs. Pretreatment with the specific MAPK3/1 inhibitor U0126 (10 μM) for 1 h effectively blocked NaSH-stimulated MAPK3/1 phosphorylation, but did not alter NaSH-stimulated phosphorylation of AKT1 and NOS3. Pretreatment with the specific PI3K/AKT1 inhibitor wortmannin (100 nM) for 1 h effectively blocked NaSH-stimulated AKT1 and NOS3 phosphorylation, but did not alter NaSH-stimulated MAPK3/1 phosphorylation (Figure 5).
Figure 5.
Role of AKT1 and pMAPK3/1 in exogenous H2S donor NaHS-induced NOS3 activation in ovine placental artery ECs (oFPAECs). After serum starvation, subconfluent cells in a six-well plate were pretreated with or without U0126 (10 μM) or wortmannin (Wort, 100 nM) for 30 min, followed by treatment with or without a H2S donor NaHS (100 μM) for 10 min. Equal amounts (20 μg/ml) protein extracts were subjected to immunoblotting with specific antibodies against phosphorylated NOS3, AKT1, and pMAPK3/1. Phosphorylation of NOS3, AKT1, and pMAPK3/1 was quantified as a ratio to their total protein levels, respectively. Data were expressed as mean ± SD from four independent experiments using oFPAECs from four different ewes and presented as fold of control. Bars with different letters differ significantly (P < 0.05).
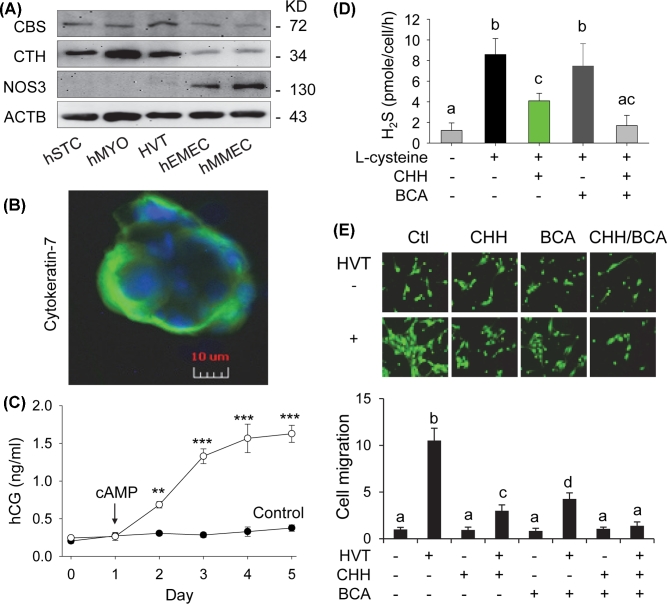
Effects of co-culture with primary human villous trophoblasts on ovine placental artery endothelial cell migration
Finally, we tested if H2S derived from primary HVT regulates oFPAEC cell migration to verify the data obtained by using immortalized human trophoblast cell lines. Immunoblotting revealed that primary HVT expressed both CBS and CTH proteins, which were also found in primary myometrial myocytes and hMMECs, and endometrial STCs and hEMECs. NOS3 protein was also not found in primary myometrial myocytes and endometrial STCs. The ScienCell HVT cells expressed trace amount of NOS3 protein (Figure 6A), similar to the trophoblast cell lines and likely contradicting to previous reports showing NOS3 protein in human extravillous trophoblasts in vivo and in vitro [49,50]. These cells were trophoblast cells as they stained positively with cytokeratin-7 (Figure 6B) and secreted hCG in response to stimulation with cAMP in a time-dependent fashion in culture (Figure 6C). L-cysteine stimulated the H2S synthesizing activity of HVT (P < 0.001), similar to BeWo cells (Figure 1B). Co-incubation with CHH and CHH in combination with BCA, but not CHH alone, significantly inhibited the H2S synthesizing activity of HVT (P < 0.001) (Figure 6D). Co-culture with HVT strongly stimulated oFPAEC migration by 10.52 ± 1.32 fold (P < 0.001). The stimulatory effect of HVT was significantly greater than that of BeWo (P < 0.05) and HTR-8/SVneo (P < 0.01) cells. H2S inhibition by either CHH or BCA effectively attenuated HVT co-culture stimulated oFPAEC migration (P < 0.001); however, the inhibitory effect of CHH was greater than that of BCA (P < 0.05). The combination of CHH and BCA completely blocked the stimulatory effect of HVT co-culture (Figure 6E).
Figure 6.
H2S biosynthesis in primary villous trophoblasts (HVT) and placental artery EC migration. (A) Qualitative immunoblotting analysis of CBS and CTH and NOS3 protein expression in primary human villous trophoblasts (HVT), human endometrial stromal cells (STC), and microvascular ECs (hEMEC), and human myometrial myocytes (hMYO) and microvascular ECs (hMMEC). Beta-actin was used as a loading control. (B) Immunofluorescence microscopy: Primary HVT cells cultured on glass coverslips were fixed with methanol and stained with anti-human cytokeratin-7 antibody (1 μg/ml), followed by Alex486-labeled mouse anti-human IgG (2 μg/ml). Coverslips were mounted with Prolong Gold antifade reagent containing 4΄,6-diamidino-2-phenylindole (DAPI) for labeling cell nuclei. Images were acquired using a fluorescence microscopy. Cells incubated with no first antibody displayed no immunofluorescence staining (data not shown). (C) Human chorionic gonadotropin (hCG) secretion: Primary HVT cells (5 × 105/well) were cultured in six-well plate and stimulated with or without out 8-bromo cyclic adenosine monophosphate (100 μM, cAMP) from day 1 for 5 days in culture. Human chorionic gonadotropin content was determined by an enzyme-linked immunoassay kit in conditioned media sampled daily. Data were expressed as mean ± SD from four independent experiments. **P < 0.01; ***P < 0.001. (D) Primary HVT cells (5 × 105/reaction) were used to determine the H2S synthesizing activity assay with or without addition of CHH (2 mM) and/or BCA (2 mM) by using the methylene blue assay. Data were expressed as mean ± SD from four independent experiments. Bars with different letters differ significantly (P < 0.05). (E) Ovine fetoplacental artery endothelial cells (oFPAECs, 1.5 × 104/well) were seeded on the top of the inserts in a six-well transwell plate. HVT were cultured in the bottom chamber with approximately 90% confluence with or without CHH (2 mM) and/or BCA (2 mM). The assembled co-culture system was cultured to allow oFPAEC cell to migrate for 24 h. The migrated cells were quantified as Figure 2B. Data were expressed as mean ± SD from four independent experiments using oFPAECs from four different ewes and calculated as fold of control. Bars with different letters differ significantly (P < 0.05).
Discussion
We have investigated herein whether trophoblast cells synthesize H2S and whether trophoblast-derived H2S stimulates placental EC angiogenesis in vitro by using the widely used human placenta trophoblast-derived cell lines (i.e. BeWo, JEG3, and HTR-8/SVneo cells), primary HVT, and a widely used EC model (oFPAECs) for placental angiogenesis. We report for the first time that exogenous H2S stimulates in vitro angiogenesis in placental ECs and that endogenous H2S derived from human trophoblasts potently stimulates placental EC migration, which is at least partially mediated by interacting with the endothelial NOS3-NO pathway via AKT1-dependent mechanisms.
Our first series of experiments conducted using oFPAECs show that exogenous H2S from donors NaSH and 5a, at the most effective concentrations tested in other EC types [13,51], stimulate cell proliferation, migration, and tube formation of oFPAECs in vitro, with comparable potency to the dominant angiogenesis promoter VEGFA. These results are consistent with previous studies using HUVECs and other EC models [13,51]. These findings implicate a potential role of H2S in placental angiogenesis and raise an important question whether endogenous H2S stimulates placental angiogenesis. Endogenous H2S in mammalian tissues is mainly synthesized from L-cysteine by two pyridoxal 5’-phosphate-dependent enzymes, including CBS and CTH [18–20]. Our data show that all three trophoblast cell lines express comparable strong baseline CBS protein expression, with differentially expressed CTH protein with highest levels in BeWo, low in JEG3, and nearly undetectable in HTR-8/SVneo cells. Consistent with their distinct CBS and CTH protein expression patterns, we have observed that the H2S producing capacity of BeWo cells doubles that of HTR-8/SVneo cells. Co-incubation with the CBS inhibitor CHH significantly inhibits H2S production in both BeWo and HTR-8/SVneo cells, while the CTH inhibitor BCA inhibits H2S production in BeWo cells only. Although it is unknown why these trophoblast cell lines have distinct CBS and CTH expression patterns, our current results show that both CBS and CTH contribute to H2S biosynthesis in BeWo cells and only CBS is required for H2S biosynthesis in HTR-8/SVneo cells.
Next, we have used a co-culture model with trophoblasts and oFPAECs to determine if endogenous H2S derived from trophoblasts regulates oPAEC migration. In these studies, BeWo and HTR-8/SVneo cells are chosen as the trophoblast cell models for determining the enzymatic sources of H2S in trophoblasts to stimulate oFPAEC angiogenesis. Due to their distinct CBS and CTH protein expression patterns, BeWo cells offer a natural model for endogenous H2S from both CBS and CTH, and HTR-8/SVneo cells offer a natural model for endogenous H2S from CBS only. Our data show that co-culture with HTR-8/SVneo cells is as effective as H2S donors and VEGFA in stimulating oFPAEC migration. The stimulatory effect of co-culture with HTR-8/SVneo cells on oFPAEC migration is significantly inhibited by CHH but not BCA, showing that CBS is the enzymatic source for H2S derived from HTR-8/SVneo cells, consistently with only CBS but not CTH protein expression in this cell line. Co-culture with BeWo cells is more potent in stimulating oFPAEC migration and nearly doubles that of HTR-8/SVneo cells, which is significantly inhibited by incubation with either CHH or BCA. These findings show that both CBS and CTH are involved in endogenous H2S generation in BeWo cells, consistent with both CBS and CTH protein expressions in this cell line. We are able to confirm the findings obtained with immortalized trophoblast cells lines by using primary HVT. The H2S synthesizing activity of HVT is originated from both CBS and CTH in accordance with their protein expression patterns. Co-culture with HVT is even more potent than BeWo cells in stimulating oFPAEC cell migration, which is significantly inhibited by CHH and CHH in combination with BCA, but not BCA alone. These findings show that both CBS and CTH are involved in baseline endogenous H2S production in HVT; however, CBS/H2S seems to play a major role stimulating placental endothelial angiogenesis.
Surprisingly, endogenous H2S from trophoblast cells is more potent than exogenous H2S from donors in stimulating oFPAEC migration. H2S elicits its biological functions in a similar way to the other members (i.e. NO and CO) of the gasotransmitter family. They can rapidly diffuse into cells through cell membranes without the need of a specific transporter to carry out its biological function in a bell-shape dose-dependent manner, i.e. cytoprotective effects at low concentrations and cytotoxic effects at higher concentrations. It is possible that trophoblast-derived H2S could fail in more physiologically relevant concentrations to stimulate EC migration. Of note, addition of both CHH and BCA is able to completely block oFPAEC cell migration stimulated by co-culture with HVT and BeWo cells but not HTR8-SVneo cells, even though the combination of CHH and BCA completely inhibits the H2S synthesizing activity in these cells. These findings, together with differential CBS and CTH protein expression patterns in HVT vs. trophoblast cell lines, enforce the need of using primary trophoblasts for mechanistic understanding of human placental biology.
Activation of PI3K/AKT1 and MAPK3/1 as well as NOS3–NO has been previously reported to at least partially mediate the effects of H2S on angiogenesis in vitro and in vivo. For instance, the angiogenic role of H2S is initially reported in retinal RF/6A ECs, which is dependent on AKT1 and MAPK3/1 phosphorylation [12]. In bovine aortic arterial ECs, H2S activates NOS3 to produce NO via activation of PI3K/AKT1 [52]. Our current data show that H2S donors rapidly phosphorylate AKT1, MAPK3/1, and NOS3 in oFPAECs. Furthermore, H2S-stimulated NOS3 activation could be blocked by a specific PI3K inhibitor wortmannin [53], but not by a highly selective inhibitor for the MAPK3/1 pathway U0126 [54]. Together with our previous studies using oFPAEC model showing that activation of these pathways are important for placental angiogenesis [5,34,35,44,45], our current data show that H2S stimulates angiogenesis, at least in part, through activation of these cell signaling pathways in oFPAEC cells.
The human placenta is composed of heterogeneous cell types; trophoblasts and ECs are the major ones in close contact. Our current data obtained with a co-culture model of human trophoblasts (i.e. immortalized human trophoblast cell lines and primary HVT) and placental ECs show a following scenario of trophoblast–EC interaction in human placenta (Figure 7). Once H2S is synthesized by trophoblasts, it diffuses into placental ECs to rapidly stimulate AKT1-dependent NOS3 activation and NO production. H2S and NO play a mutual role in regulating angiogenesis and dilation/relaxation in the microvasculature [16]. H2S also activates MAPK3/1 that is involved in H2S stimulation of angiogenesis in ECs [12]. Collectively, endogenous H2S derived from trophoblasts stimulates placental EC angiogenesis through AKT1-NOS3 and MAPK3/1 pathways. It is speculating that the H2S-mediated trophoblast-EC interaction may be disturbed in preeclampsia due to altered trophoblast H2S biosynthesis [29–31]. Also, perturbation of this cell–cell interaction may play a role in mediating the impaired placental angiogenesis, shallow trophoblast invasion, and impaired uterine spiral remodeling as consequences of dysregulated H2S signaling in preeclampsia [33]. Moreover, our data show that CBS and CTH proteins are expressed in a variety of cell types in uterine myometrium and endometrium, including ECs, myocytes, STCs, and placental ECs. Thus, endogenous H2S may also be synthesized from these cells to add onto that from trophoblasts to regulate angiogenesis in the uterine and placental circulations.
Figure 7.
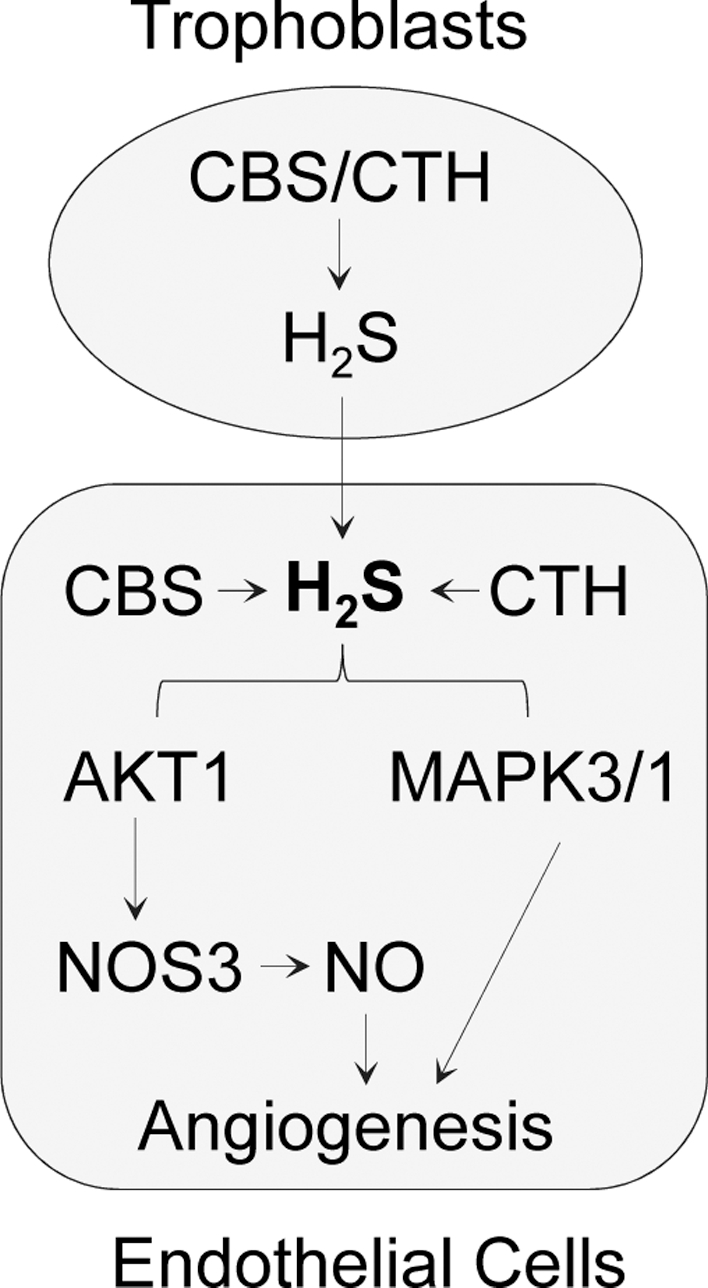
Trophoblast and endothelial cell–cell interaction via endogenous H2S-derived from trophoblasts stimulates placental endothelial EC angiogenesis through a PI3K/AKT1-NOS3/NO and MAPK3/1 pathway in human placenta. Once H2S is synthesized by trophoblast cells, it diffuses into placental ECs to activate the PI3K/AKT1/NOS3 and MAPK3/1 pathways, which in turn stimulate placental EC angiogenesis.
Acknowledgments
The authors thank all the participants for donating the tissue samples, the attending physicians of the Department of Obstetrics & Gynecology at the University of California, Irvine for their assistance in tissue collection, Dr Min Xian (Washington State University) for the H2S donor 5a, and Dr Peeyush K. Lala (Western University, Canada) for the HTR-8/SVneo cell line.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1. Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267:10931–10934. [PubMed] [Google Scholar]
- 2. Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9:653–660. [DOI] [PubMed] [Google Scholar]
- 3. Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod 2001; 64:1033–1040. [DOI] [PubMed] [Google Scholar]
- 4. Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta 2004; 25:114–126. [DOI] [PubMed] [Google Scholar]
- 5. Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation 2014; 21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol 2006; 572:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, Lyall F, Greer IA. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta 1996; 17:37–48. [DOI] [PubMed] [Google Scholar]
- 8. Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta 2004; 25:127–139. [DOI] [PubMed] [Google Scholar]
- 9. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308:1592–1594. [DOI] [PubMed] [Google Scholar]
- 10. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 2012; 92:791–896. [DOI] [PubMed] [Google Scholar]
- 11. Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008; 322:587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 2007; 76:29–40. [DOI] [PubMed] [Google Scholar]
- 13. Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 2009; 106:21972–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 2001; 20:6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paul BD, Snyder SH. H(2)S signaling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol; 13:499–507. [DOI] [PubMed] [Google Scholar]
- 16. Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 2012; 109:9161–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol (1985) 2006; 100:1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bukovska G, Kery V, Kraus JP. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein Expr Purif 1994; 5:442–448. [DOI] [PubMed] [Google Scholar]
- 19. Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J 1990; 269:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kredich NM, Foote LJ, Keenan BS. The stoichiometry and kinetics of the inducible cysteine desulfhydrase from Salmonella typhimurium. J Biol Chem 1973; 248:6187–6196. [PubMed] [Google Scholar]
- 21. Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem 2010; 113:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal 2009; 2:re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lechuga TJ, Zhang HH, Sheibani L, Karim M, Jia J, Magness RR, Rosenfeld CR, Chen DB. Estrogen replacement therapy in ovariectomized nonpregnant ewes stimulates uterine artery hydrogen sulfide biosynthesis by selectively up-regulating cystathionine beta-synthase expression. Endocrinology 2015; 156:2288–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheibani L, Lechuga TJ, Zhang HH, Hameed A, Wing DA, Kumar S, Rosenfeld CR, Chen DB. Augmented H2S production via CBS upregulation plays a role in pregnancy-associated uterine vasodilation. Biol Reprod 2017; 96:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ning N, Zhu J, Du Y, Gao X, Liu C, Li J. Dysregulation of hydrogen sulphide metabolism impairs oviductal transport of embryos. Nat Commun 2014; 5:4107. [DOI] [PubMed] [Google Scholar]
- 26. Nuno-Ayala M, Guillen N, Arnal C, Lou-Bonafonte JM, de Martino A, Garcia-de-Jalon JA, Gascon S, Osaba L, Osada J, Navarro MA. Cystathionine beta-synthase deficiency causes infertility by impairing decidualization and gene expression networks in uterus implantation sites. Physiol Genomics 2012; 44:702–716. [DOI] [PubMed] [Google Scholar]
- 27. You XJ, Xu C, Lu JQ, Zhu XY, Gao L, Cui XR, Li Y, Gu H, Ni X. Expression of cystathionine beta-synthase and cystathionine gamma-lyase in human pregnant myometrium and their roles in the control of uterine contractility. PLoS One 2011; 6:e23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. You X, Chen Z, Zhao H, Xu C, Liu W, Sun Q, He P, Gu H, Ni X. Endogenous hydrogen sulfide contributes to uterine 1 quiescence during pregnancy. Reproduction 2017; 153:143–150. [DOI] [PubMed] [Google Scholar]
- 29. Hodges J, Kim T, Wang W, Liao W, Chen D. Hydrogen sulfide synthesizing enzymes are upregulated in human placentas in preeclampsia. Reprod Sci 2011; 18:76A. [Google Scholar]
- 30. Holwerda KM, Bos EM, Rajakumar A, Ris-Stalpers C, van Pampus MG, Timmer A, Erwich JJ, Faas MM, van Goor H, Lely AT. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta; 33:518–521. [DOI] [PubMed] [Google Scholar]
- 31. Cindrova-Davies T, Herrera EA, Niu Y, Kingdom J, Giussani DA, Burton GJ. Reduced cystathionine gamma-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am J Pathol; 182:1448–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel P, Vatish M, Heptinstall J, Wang R, Carson RJ. The endogenous production of hydrogen sulphide in intrauterine tissues. Reprod Biol Endocrinol 2009; 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, Baily J, Miller MR, Cudmore M, Hadoke PW, Wang R, Gratacos E et al. . Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 2013; 127:2514–2522. [DOI] [PubMed] [Google Scholar]
- 34. Liao WX, Feng L, Zhang H, Zheng J, Moore TR, Chen DB. Compartmentalizing VEGF-induced ERK2/1 signaling in placental artery endothelial cell caveolae: a paradoxical role of caveolin-1 in placental angiogenesis in vitro. Mol Endocrinol 2009; 23:1428–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao WX, Feng L, Zheng J, Chen DB. Deciphering mechanisms controlling placental artery endothelial cell migration stimulated by vascular endothelial growth factor. Endocrinology 2010; 151:3432–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao Y, Wang H, Xian M. Cysteine-activated hydrogen sulfide (H2S) donors. J Am Chem Soc 133:15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pattillo RA, Gey GO, Delfs E, Mattingly RF. Human hormone production in vitro. Science 1968; 159:1467–1469. [DOI] [PubMed] [Google Scholar]
- 38. Blanchon L, Sauvant P, Bavik C, Gallot D, Charbonne F, Alexandre-Gouabau MC, Lemery D, Jacquetin B, Dastugue B, Ward S, Sapin V. Human choriocarcinoma cell line JEG-3 produces and secretes active retinoids from retinol. Mol Hum Reprod 2002; 8:485–493. [DOI] [PubMed] [Google Scholar]
- 39. Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993; 206:204–211. [DOI] [PubMed] [Google Scholar]
- 40. Zhang HH, Feng L, Livnat I, Hoh JK, Shim JY, Liao WX, Chen DB. Estradiol-17beta stimulates specific receptor and endogenous nitric oxide-dependent dynamic endothelial protein S-nitrosylation: analysis of endothelial nitrosyl-proteome. Endocrinology 2010; 151:3874–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. Telomerase immortalization of human myometrial cells. Biol Reprod 2002; 67:506–514. [DOI] [PubMed] [Google Scholar]
- 42. Chen DB, Hilsenrath R, Yang ZM, Le SP, Kim SR, Chuong CJ, Poindexter AN 3rd, Harper MJ. Leukaemia inhibitory factor in human endometrium during the menstrual cycle: cellular origin and action on production of glandular epithelial cell prostaglandin in vitro. Hum Reprod 1995; 10:911–918. [DOI] [PubMed] [Google Scholar]
- 43. Chen DB, Li SM, Qian XX, Moon C, Zheng J. Tyrosine phosphorylation of caveolin 1 by oxidative stress is reversible and dependent on the c-src tyrosine kinase but not mitogen-activated protein kinase pathways in placental artery endothelial cells. Biol Reprod 2005; 73:761–772. [DOI] [PubMed] [Google Scholar]
- 44. Feng L, Zhang HH, Wang W, Zheng J, Chen DB. Compartmentalizing proximal FGFR1 signaling in ovine placental artery endothelial cell caveolae. Biol Reprod 2012; 87:40:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng L, Liao WX, Luo Q, Zhang HH, Wang W, Zheng J, Chen DB. Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae. J Cell Physiol 2012; 227:2480–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 1999; 103:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology 2004; 145:113–125. [DOI] [PubMed] [Google Scholar]
- 48. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999; 399:601–605. [DOI] [PubMed] [Google Scholar]
- 49. Eis AL, Brockman DE, Pollock JS, Myatt L. Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta 1995; 16:113–126. [DOI] [PubMed] [Google Scholar]
- 50. Martin D, Conrad KP. Expression of endothelial nitric oxide synthase by extravillous trophoblast cells in the human placenta. Placenta 2000; 21:23–31. [DOI] [PubMed] [Google Scholar]
- 51. Zhou Y, Li XH, Zhang CC, Wang MJ, Xue WL, Wu DD, Ma FF, Li WW, Tao BB, Zhu YC. Hydrogen sulfide promotes angiogenesis by downregulating miR-640 via the VEGFR2/mTOR pathway. Am J Physiol Cell Physiol 2016; 310: C305–C317. [DOI] [PubMed] [Google Scholar]
- 52. Predmore BL, Julian D, Cardounel AJ. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front Physiol; 2:104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 2001; 70:535–602. [DOI] [PubMed] [Google Scholar]
- 54. Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL et al. . Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 1998; 273:18623–18632. [DOI] [PubMed] [Google Scholar]