Nonoperative management of chronic granulomatous disease (CGD)–associated hepatic abscesses with concomitant corticosteroid and antimicrobial therapy is safe and effective, and may preserve liver health, ameliorating a major cause of late morbidity and mortality in CGD.
Keywords: chronic granulomatous disease, liver abscess, corticosteroids, baseline neutrophil function
Abstract
Background
Chronic granulomatous disease (CGD) is a rare genetic disorder causing recurrent infections. More than one-quarter of patients develop hepatic abscesses and liver dysfunction. Recent reports suggest that disease-modifying treatment with corticosteroids is effective for these abscesses. Comparison of corticosteroid therapy to traditional invasive treatments has not been performed.
Methods
Records of 268 patients with CGD treated at the National Institutes of Health from 1980 to 2014 were reviewed. Patients with liver involvement and complete records were included. We recorded residual reactive oxygen intermediate (ROI) production by neutrophils, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase germline mutation status, laboratory values, imaging characteristics, time to repeat hepatic interventions, and overall survival among 3 treatment cohorts: open liver surgery (OS), percutaneous liver-directed interventional radiology therapy (IR), and high-dose corticosteroid management (CM).
Results
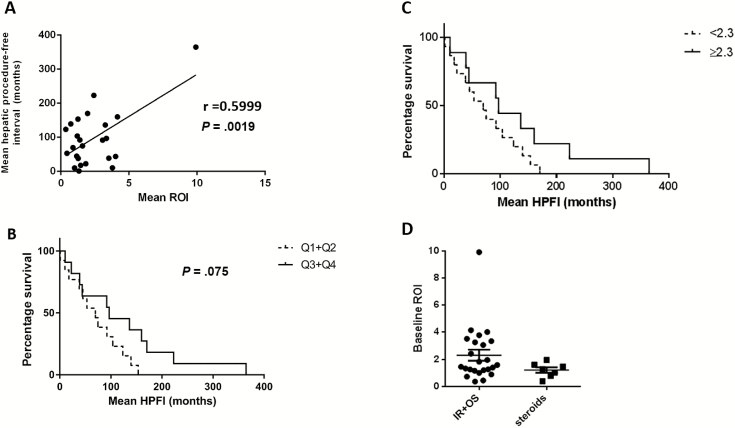
Eighty-eight of 268 patients with CGD suffered liver involvement. Twenty-six patients with a median follow-up of 15.5 years (8.5–32.9 years of follow-up) had complete records and underwent 100 standard interventions (42 IR and 58 OS). Eight patients received a treatment with high-dose corticosteroids only. There were no differences in NADPH genotype, size, or number of abscesses between patients treated with OS, IR, or CM. Time to repeat intervention was extended in OS compared with IR (18.8 vs 9.5 months, P = .04) and further increased in CM alone (median time to recurrence not met). Impaired macrophage and neutrophil function measured by ROI production correlated with shorter time to repeat intervention (r = 0.6, P = .0019).
Conclusions
Treatment of CGD-associated liver abscesses with corticosteroids was associated with fewer subsequent hepatic interventions and improved outcome compared to invasive treatments.
Chronic granulomatous disease (CGD) is a rare genetic disorder affecting approximately 1 in 250000 live births in the United States [1]. These patients suffer from recurrent bacterial and fungal infections secondary to impaired phagocyte respiratory burst resulting from inherited mutations in 1 of 5 phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase genes: gp91phox (CYBB, approximately 65% of patients), p22phox (CYBA, <5%), p47phox (NCF1, 30%), p67phox (NCF2, <5%), and p40phox (NCF4, 2 cases) [2]. Initially considered fatal, prophylactic antibiotics, antifungals, interferon-γ, and recently, bone marrow transplantation have significantly improved disease prognosis. Most patients now live into adulthood [3, 4].
Despite improved mortality, morbidity associated with CGD remains significant. While most infectious complications are pulmonary, almost a third of CGD patients will suffer from liver abscesses [4–6] and an associated worse overall survival [7]. Hepatic abscesses in CGD are phenotypically distinct from the pyogenic liver abscesses associated with other conditions. They present as septate masses surrounded by a thick pseudocapsule containing inspissated fluid and amorphous cell debris [6]. While surgical resection of abscesses refractory to multimodality antibiotic treatment is effective and associated with a low mortality, surgical morbidity may affect 56% of cases, leading some to favor debridement and percutaneous drainage when possible [6, 8–10]. Overall, the high incidence of, and the inability to accurately predict, recurrence has kept clinical management empiric.
Recently, our group examined the heterogeneity in clinical phenotype and outcomes of CGD patients in correlation with residual neutrophil function. Survival was positively correlated with higher residual reactive oxygen intermediate (ROI) levels. Superoxide production by quartiles (Q1, Q2, Q3, Q4) ranged from 0.26 to 0.94, 0.95 to 1.67, 1.70 to 2.71, and 2.72 to 60.5, respectively [2]. p47phox mutations were associated with higher ROI levels and had commensurately better survival than severe gp91phox mutations [2]. Liver abnormalities such as increased alkaline phosphatase and thrombocytopenia were also major determinants of outcome [2]. Nodular regenerative hyperplasia, portal hypertension, liver fibrosis, and liver abscesses conspire to make liver dysfunction a strong predictor of mortality [2, 5–7]. These data suggest that liver inflammation and manipulation are likely modifiable factors influencing outcome.
Treatment of CGD-associated liver abscesses with high-dose corticosteroids and targeted intravenous antibiotics is an emerging approach that simultaneously modifies underlying inflammatory aberrancies while treating infection [11, 12]. Initial results in small case series have been promising, but have not compared corticosteroids to conventional management. Here, we compare biochemical and clinical outcomes between CGD patients with liver abscesses treated with open surgery (OS), interventional radiology (IR), or corticosteroid management (CM). We found improved liver function tests and reduced need for repeat surgical intervention in CM cases, suggesting that invasive therapy may not be optimal for all CGD liver abscesses.
METHODS
With approval from the institutional review board (IRB), review of prospectively maintained databases identified 268 patients with CGD treated at the National Institutes of Health Clinical Center (NIH/CC) from 1980 to 2014. Eighty-eight patients (32%) demonstrated liver involvement (Figure 1). Forty-eight of 88 patients lacked records of either prior liver abscesses or had management of liver abscesses carried out at an outside institution, and were excluded. Patients whose initial diagnosis was later changed or who died due to complications related to bone marrow transplant were also excluded. There was a small proportion of patients treated with multimodality antimicrobial therapy and supportive care only. Thirty-two (36%) with complete medical records and follow-up underwent at least 1 procedure or further intervention for liver involvement, either in the form of OS, IR, or CM (Figure 1).
Figure 1.
Patient inclusion schema. Thirty-two of 268 chronic granulomatous disease (CGD) patients were eligible for analysis based on the criteria of (1) having a history of CGD liver involvement and (2) having complete, uninterrupted follow-up of their CGD liver care available within National Institutes of Health medical records. Abbreviations: BMT, bone marrow transplant; CGD, chronic granulomatous disease; CM, high-dose corticosteroid management; IR, interventional radiology; NIH, National Institutes of Health; OS, open surgery.
Patients were admitted to the NIH/CC under the National Institute of Allergy and Infectious Diseases (NIAID). Patients with CGD-associated liver abscesses diagnosed from clinical history and appropriate imaging were evaluated by hepatology and surgical consultant services. After full records review, clinicopathological information captured during the initial multidisciplinary treatment of each patients’ liver abscess(es) included age at both CGD diagnosis and respective liver intervention, previously performed liver procedures, available computed tomography or magnetic resonance imaging (MRI), and laboratory studies including pre-and posttreatment liver function tests and pretreatment erythrocyte sedimentation rate (ESR). The pretreatment values were obtained at admission.
Measurement of NADPH oxidase activity and detection of ROI levels by myeloperoxidase-dependent hydrogen peroxide production or superoxide-dependent ferricytochrome-c reduction stimulated by phorbol esters was performed as previously described [2]. NADPH oxidase germline mutation analysis was performed according to standardized procedures and adherence to IRB guidelines, with all patients providing written consent.
Interventions for liver abscesses included open surgical procedures (OS) (liver abscess debridement/drainage, nonanatomical liver wedge resection, segmentectomy, or lobectomy), percutaneous interventional radiologic procedures (IR), and high-dose corticosteroid management (CM). CM as primary management included a median dose of 1 mg/kg/day (range, 0.6–1.8 mg/kg/day) of prednisone or methylprednisolone subsequently tapered over a median of 5 months (range, 1–7 months). CM included concurrent targeted antimicrobial therapy guided by mandatory aspiration cultures and directed by the treating infectious disease specialist.
Time to repeat hepatic intervention was defined as time from previous treatment (OS, IR, CM) to repeat intervention by either OS or IR. Of note, some patients received both IR and OS procedures at the NIH/CC and are included in each respective treatment arm based on the admission during which they received that treatment. All patients included in the CM cohort had received a prior intervention (either IR or OS) for liver involvement in the past at an outside institution. These patients are included in the respective treatment cohort based upon the treatment received during each admission.
Paired Student t, χ2, Kruskal-Wallis, and Mann-Whitney tests were utilized to compare variables between the individual groups. Patients with no further hepatic intervention were censored at date of last follow-up. The Kaplan-Meier method was used to calculate intervention-free and overall survival rates, then compared using log-rank (Mantel-Cox) analysis. Pearson r linear correlation coefficient test was used to measure the linear correlation between the variables ROI and time to repeat hepatic intervention. All statistical analyses were performed using Microsoft Excel 2016 or GraphPad Prism version 6.0 software.
RESULTS
Chronic Granulomatous Disease Patients With Liver Abscesses Undergo a Variable Number of Liver-Directed Procedures
Of 268 patients captured between 1980 and 2014 at our institution, 32 were reported with liver involvement and had complete clinicopathological information with continuous follow-up (Figure 1). Table 1 summarizes baseline clinicopathological variables including ROI levels and NADPH genotype of patients who underwent either IR, OS, or CM. The number of liver procedures ranged from 1 to 13 with an average of 3.7 procedures per patient. At any given intervention, patients had a mean number of 2.5 liver abscesses (range, 1–13) with a mean diameter of 3.5 cm (range, 1–8). ESR, as a marker of severity of inflammation, was recorded at admission with mean preintervention ESR of 69 mm/hour (range, 10–145 mm/hour; normal range, 0–25 mm/hour). Median follow-up was 15.5 years (8.5–32.9 years of follow-up), with 18 patients (69%) alive at final analysis. Seven patients underwent ≥4 procedures. In patients who underwent <4 procedures compared with patients who underwent 4 or more liver interventions, neither abscess number (average, 2 in both groups, P = .28), abscess size (3.5 vs 3 cm, P = .56), nor ESR level (78 vs 66 mm/hour, P = .17) differed.
Table 1.
Patient demographics and baseline characteristics, interventional radiology & open surgery compared to high-dose corticosteroid management
| IR & OS | CM | |
|---|---|---|
| Number of patients | 26 | 8 |
| Male/Female | 22/4 | 8/0 |
| Caucasian | 21 (81%) | 5 (63%) |
| African American | 4 (15%) | 1 (13%) |
| Asian | 1 (4%) | 1 (13%) |
| Other | 0 (0%) | 1 (13%) |
| Median age at CGD diagnosis (years) | 2 (0–22) | 2 (1 month-31 years) |
| NAPDH oxidase genotype | gp91phox: 16 (61%) | gp91phox: 8 (100%) |
| p47phox: 7 (27%) | ||
| p22phox: 2 (8%) | ||
| p67phox: 1 (4%) | ||
| Residual ROI (superoxide nmol/hr) (n=24, n=7)a | <2.3: 9 (38%)≥2.3: 15 (62%) | <2.3: 7 (100%) |
| ≥2.3: 0 (0%) | ||
| Quartilesb | Q1: 4 (16%) | Q1: 2 (28.5%) |
| Q2: 9 (38%) | ||
| Q3: 3 (13%) | ||
| Q4: 8 (33%) | ||
| Median overall follow-up (years) | 15.5 (8.5–32.9) | 4.8 (2.9–5.8) |
| Number alive at final analysis | 18 (69)% | 6 (75%) |
| 90-day mortality rate | 0% | 0% |
| Mean number of liver abscesses | 2.5 (1–13) | 4.9 (2–14) |
| Mean size of largest liver abscess (cm) | 3.5 (1–8) | 4.7 (3–8) |
| Mean ESR prior to intervention (mm/hr)c | 69 (10–145) | 73.6 (34–140) |
| Median age at first liver procedure at NIH (years) | 18.14 (2.8–32.7) | |
| Number of total liver procedures per patient | 3.7 (1–13) | |
| Median age at high-dose steroid treatment (years) | 19 (0.5–46) | |
| Median dose of high-dose steroid treatment (mg/kg/day) | 1 (0.6–1.8) | |
| Median duration of steroid taper (months) | 5 (1–7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CGD, chronic granulomatous disease; CM, corticosteroid management; ESR, erythrocyte sedimentation rate; IR, interventional radiology; NADPH, nicotinamide adenine dinucleotide phosphate; NIH, National Institutes of Health; OS, open surgery; ROI, reactive oxygen intermediate.
an = 2 and n = 1 without data , refers to the number of represented patients from the IR and OS cohorts, respectively.
bAs defined per reference [2], ranges of superoxide production for Q1, Q2, Q3, and Q4 are0.26–0.94, 0.95–1.67, 1.70–2.71, and 2.72–60.5, respectively.
cMeasured at time of admission.
Prolonged Time to Repeat Hepatic Intervention in Patients Who Underwent Open Surgery Compared to Percutaneous Procedures
One hundred liver procedures were performed on 26 patients with CGD-associated liver abscesses, including 42 IR (15 patients) and 58 OS (22 patients) (Table 2). Twenty patients underwent multiple procedures (mean, 3.7 procedures per patient [range, 1–13]), and 10 patients underwent both IR and OS operations at separate times during their management. Baseline clinicopathological characteristics did not differ excepting median age of IR patients of 23.2 years (9.6–42.6 years) compared to OS patients at 18.2 years (2.7–35.6 years) (P = .007). Of IR procedures, the majority were percutaneous aspiration and drain placement (90%).
Table 2.
Clinical, Radiological, and Infectious Disease Baseline Characteristics of Chronic Granulomatous Disease Patients With Liver Abscesses Treated by Either Interventional Radiology or Open Surgical Management
| Characteristic | IR | OS | P Value |
|---|---|---|---|
| No. of procedures | 42 | 58 | |
| No. of patients | 15 | 22 | |
| Median age at procedure, y | 23.2 (9.6–42.6) | 18.2 (2.7–35.6) | .007 |
| Type of procedure | Percutaneous aspiration and drain placement: 38 (90%) Other: 4 (10%) |
Anatomical liver lobectomy or segmentectomy: 7 (12%) Nonanatomical wedge resection: 35 (60%) Open drainage and debridement: 16 (27%) |
|
| Residual ROI (superoxide nmol/h) | 1.64 (0.39–9.91) | 1.59 (0.36–4.15) | .794 |
| Mean No. of liver abscesses at time of procedure | 2.1 (1–8) | 2.7 (1–13) | .26 |
| Mean size of largest liver abscess at time of procedure, cm | 3.0 (1–6) | 3.8 (0.9–6.4) | .10 |
| Mean ESR prior to interventiona | 66.3 (12–132) | 70.9 (10–145) | .61 |
| Median overall follow-up, y | 14.8 (8.5–29.4) | 15.6 (10.9–32.9) | .89 |
| Median time to repeat hepatic interventionb, mo | 9.5 (0.1–274.1) | 18.8 (0.3–245.77) | .036 |
| 90-d mortality rate following procedure | 4.7% | 1.7% | .56 |
Abbreviations: ESR, erythrocyte sedimentation rate; IR, interventional radiology; OS, open surgery; ROI, reactive oxygen intermediate.
aMeasured at time of admission.
bAs either OS or IR-guided repeat hepatic intervention.
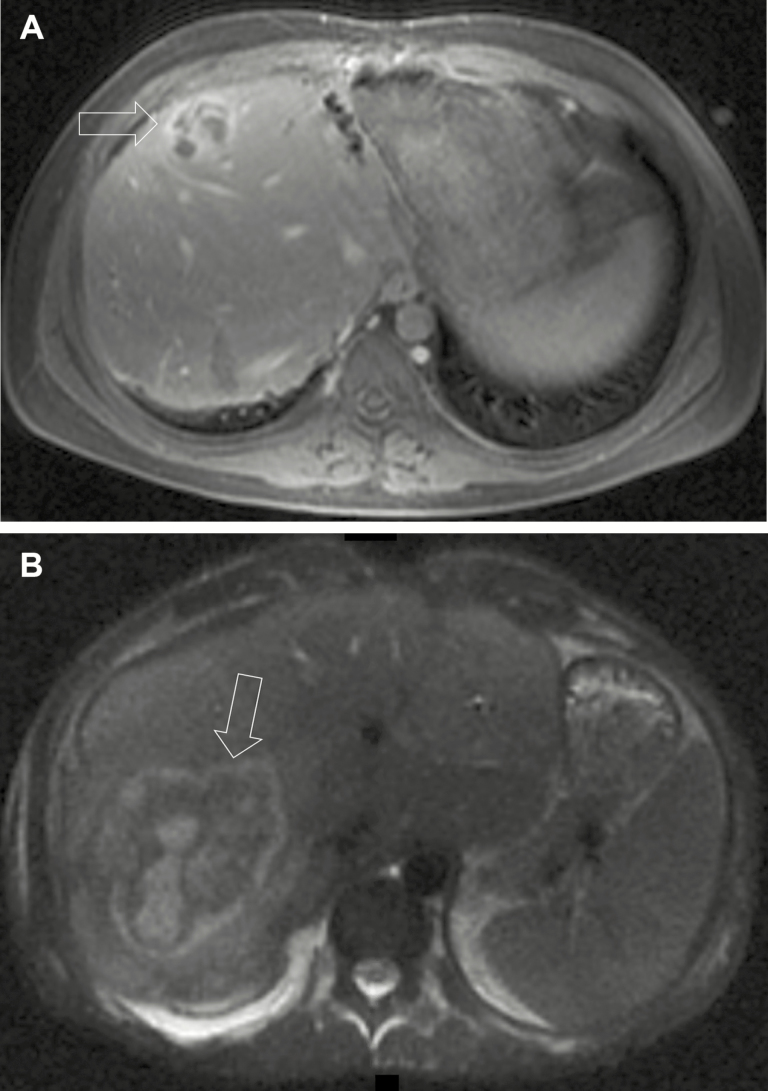
The surgical procedures performed varied. There were 35 (60%) nonanatomical liver wedge resections. Anatomical liver resections included 5 (9%) segmentectomies and 2 (3%) lobectomies. In addition to resection, 13 (22%) open drainage procedures and 3 (5%) open debridements were performed. Representative imaging of 1 patient treated by OS is shown in Figure 2. There was no difference in preintervention number of abscesses (2.09 vs 2.68, P = .26), diameter of largest abscess (2.95 vs 3.81cm, P = .10), ESR values (65.5 vs 73 mm/hour, P = .61), or ROI values (1.64 nmol superoxide/hour vs 1.59 nmol superoxide/hour, P = .7944) between IR and OS groups, respectively.
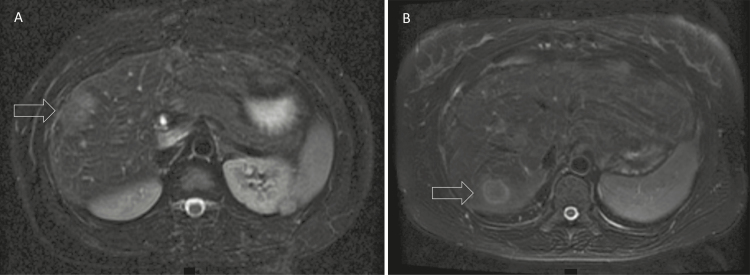
Figure 2.
A, Magnetic resonance image showing a 2-cm abscess (arrow) in the right anterior sector of the liver of a 22-year old male patient with chronic granulomatous disease (CGD) treated with multiple courses of antibiotics. Aspirate grew Staphylococcus aureus and he eventually underwent nonanatomical wedge resection. B, New 2-cm abscess (arrow) in the right posterior sector in the same patient 13 months later.
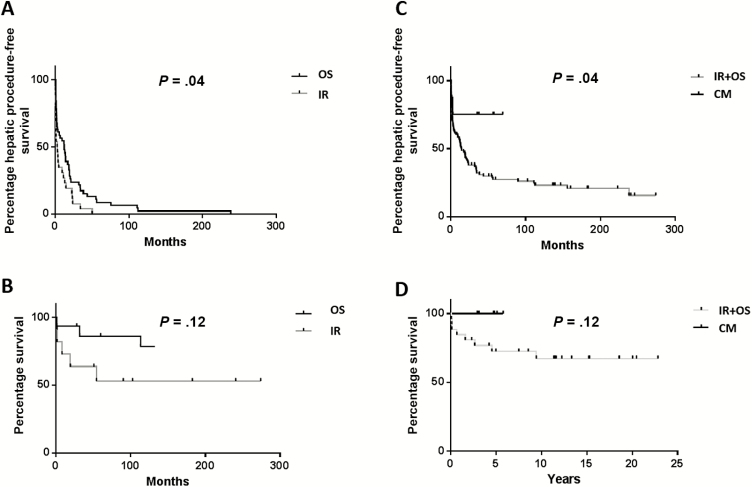
The median overall follow-up for the IR group was 14.8 years (range, 8.46–29.44) compared to 15.6 years (range, 10.85–32.89) for the OS group (P = .89). Time interval from initial liver treatment (IR or OS) until next intervention was longer in the OS group than in the IR group: 18.8 months (range, 0.27–245.8 months) vs 9.5 months (range, 0.07–274.1 months) (P = .036; Figure 3A). Overall survival was calculated from last liver treatment. Median overall survival was not met in either cohort and there was no difference in overall survival between the groups (P = .12; Figure 3B). Ninety-day mortality rate following IR procedure was 4.7%. One patient suffered intracerebral hemorrhage secondary to disseminated intravascular coagulopathy and another died of overwhelming sepsis 1.37 months following percutaneous drainage. Ninety-day mortality following OS was 1.7%. One patient died 0.9 months following open debridement from overwhelming sepsis.
Figure 3.
Kaplan-Meier curves showing time to repeat hepatic intervention (A) for the 2 standard interventions, interventional radiology (IR) or open surgery (OS). B, Overall survival rates from time of last intervention; comparisons of survival curves by log-rank (Mantel-Cox) test, 2-tailed P values. C, Time to repeat hepatic intervention (in months) of chronic granulomatous disease patients with liver abscesses managed with IR or OS vs patients managed with high-dose corticosteroids (CM). Patients who underwent operative (OS) or percutaneous (IR) management of liver abscesses are combined. D, Overall survival rates of patients who last underwent OS or IR procedures compared with patients who received CM as last intervention; log-rank (Mantel-Cox) test, 2-tailed P values.
Corticosteroid Management of Liver Abscesses in Chronic Granulomatous Disease Patients Is Associated With Fewer Subsequent Hepatic Interventions
Identification of patients with liver abscesses not amenable to OS or IR motivated therapy with high-dose corticosteroids and targeted antimicrobial therapy [11, 12]. Representative hepatic imaging of successful CM is shown in Figure 4. Characteristics of patients treated with corticosteroids and targeted antimicrobial therapy are listed in Table 1. The CM cohort had a mean of 4.86 abscesses (range, 2–14), higher than the IR (P = .02) but not different than the OS (P = .14) cohort. There was no difference in CM compared to IR or OS regarding size of the largest abscess, admission ESR levels, or baseline antimicrobial prophylaxis. Of patients in the OS, IR, and CM cohorts with available record, 89%, 100%, and 100% were on trimethoprim-sulfamethoxazole, and 41%, 70%, and 86% were on antifungal prophylaxis. The number of patients receiving prophylactic interferon-γ therapy at abscess occurrence was small and not different among the groups. Microbiology from liver abscesses most commonly grew Staphylococcus aureus (OS, 64%; IR, 67%; and CM, 71%, in patients with available culture data). Recurrent abscesses re-grew the prior organism in the majority. Less frequently identified microorganisms included Lactobacillus, Actinomyces, Candida, and Aspergillus and were commonly part of systemic infections. There were no mortalities within the CM cohort, with median follow-up of 4.75 years (range, 2.93–5.78), with shorter observation time secondary to recent institution of this therapy. Within the CM group, there were significantly fewer subsequent hepatic interventions than either IR or OS cohorts (P = .04). Only 2 of 8 patients required subsequent intervention (Figure 3C). Overall survival between the OS/IR and CM groups was similar (P = .12) (Figure 3D).
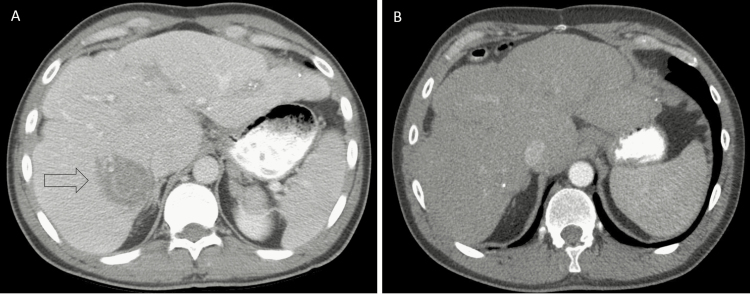
Figure 4.
Computed tomography image revealing a 4.6-cm abscess in the right posterior sector (arrow) of the liver (A), and resolution of this abscess 20 months after high-dose corticosteroid therapy with antibiotics was commenced (B).
Improved Biochemical Response in Chronic Granulomatous Disease Patients With Liver Disease Treated With Corticosteroids
We next examined liver function across the 3 treatment modalities, hypothesizing that either steroidal inflammatory modulation or removal of diseased liver volume would improve liver function laboratory values. Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase (ALP), and total bilirubin levels were analyzed.
Table 3 lists pre- and postintervention liver functions for the 3 treatment groups. The median ALP levels in the OS group fell from 169 U/L (range, 37–1570 U/L) presurgery to 137 U/L (range, 34–835 U/L) postsurgery (P = .0001). To assess whether this effect was secondary to removal of abnormal liver associated with major liver resections, we performed a subanalysis on the 7 patients who underwent liver lobectomy or segmentectomy. There was no difference between presurgical and postsurgical liver functions, including ALP. Furthermore, significant improvement in posttreatment ALP was also seen in the CM cohort (209 U/L [range, 69–519 U/L] vs 129 U/L [range, 81–359 U/L], P = .04). There was no significant change in liver function in the IR group. Thus, improvement in time to repeat hepatic intervention correlated to improved liver function in OS and CM patients.
Table 3.
Liver Function Tests Before and After the 3 Intervention Groups
| Test | Pre-IR | Post-IR | P Value | Pre-OS | Post-OS | P Value | Pre-CM | Post-CM | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Median ALT, U/L | 28 (7–965) | 41 (10–1351) | .28 | 26 (2–390) | 35 (7–351) | .31 | 27.5 (20–53) | 31 (15–100) | .31 |
| Median AST, U/L | 29 (8–464) | 38 (9–704) | .32 | 26 (8–740) | 27 (7–138) | .21 | 16 (8–52) | 11 (7–67) | .55 |
| Median ALP, U/L | 195 (59–2500) | 217 (52–2304) | .50 | 169 (37–1570) | 137 (34–835) | .0001 | 209 (69–519) | 129 (81–359) | .04 |
| Median TB, mg/dL | 0.6 (0.2–16.7) | 0.8 (0.2–19.4) | .29 | 0.4 (0.1 (9.7) | 0.6 (0.1–6) | .10 | 0.5 (0.1–0.7) | 0.3 (0.1–0.8) | .47 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CM, high dose corticosteroid group; IR, interventional radiology group; OS, open surgery group; TB, total bilirubin.
Neutrophil Function Is Associated With Surgical Outcome Time to Repeat Hepatic Intervention in Chronic Granulomatous Disease Patients With Liver Abscesses
Previous work established residual neutrophil superoxide production as prognostic for overall survival in CGD [2]. A cutoff of 2.3 nmol superoxide/106 cells/hour in conjunction with mean fluorescence intensity of neutrophil dihydrorhodamine oxidation of 225 arbitrary units discriminated between survival outcomes. Additionally, each quartile of increased residual superoxide production correlated with improved survival [2]. To determine whether the intervention-free interval correlated with neutrophil function, we examined the relationship between residual ROI levels and time to repeat hepatic intervention (Figure 5A). There was a linear correlation between these 2 variables (r = 0.6, P = .0019), and the association between elevated ROI levels and prolonged intervention-free interval was noted as a trend in Kaplan-Meier analysis (Figure 5B and 5C). NADPH oxidase genotype was not associated with time to repeat hepatic intervention (p47phox [69.75 months] vs gp91 phox [96.83 months], P = .4305). Importantly, the improved outcome observed in patients treated with CM compared to patients managed by IR or OS was not due to different baseline neutrophil function measured as ROI (mean ROI, 2.12 nmol superoxide/106 cells for OS and IR vs 1.64 nmol superoxide/106 cells for CM) (P = .202; Figure 5D).
Figure 5.
A, Baseline residual reactive oxygen intermediate (ROI) levels (superoxide nmol/hour) are correlated with time to repeat hepatic intervention in patients with chronic granulomatous disease (CGD) with liver abscesses (Pearson product-moment correlation coefficient, r = 0.5999, 2-tailed P value). Surgical outcome measured by time to repeat hepatic intervention in CGD patients with liver abscesses by previously defined ROI quartiles (in superoxide nmol/hour) predictive of poor outcome (Q1 and Q2 [0.26–0.94 and 0.95–1.67, respectively] vs Q3 and Q4 [1.70–2.71 and 2.72–60.5, respectively]) (B), or by low vs high preoperative residual neutrophil function (ROI <2.3 nmol superoxide/hour [dashed line] vs ≥2.3 nmol superoxide/hour ROI levels) (C). Hepatic procedure-free interval (HPFI) mean of any hepatic intervention for each patient. Patients who received corticosteroids had similar baseline neutrophil function compared to patients who underwent interventional radiology (IR) or open surgery (OS) (D) (mean baseline ROI in neutrophils and monocytes, 2.12 nmol superoxide/106 cells vs steroids, 1.64 nmol superoxide/106; Mann-Whitney U test, 2-tailed P = .202).
DISCUSSION
Great strides have been made in understanding the pathophysiology and underlying genetics of CGD, and elucidation of causative germline defects has allowed early detection, prognostication, and risk stratification. Recently, understanding the prognostic significance of neutrophil function and the end organ damage from dysfunctional immune cell activation has led to a more rational approach to treatment [2, 5–7, 12, 13]. This is particularly true of hepatic involvement, and degree of liver dysfunction is a strong predictor of adverse outcomes in CGD [5–7]. Histopathological correlates of liver dysfunction in CGD patients are portal hypertension, nodular regeneration, and fibrosis [5], with portal hypertension being a known cause of decreased bacterial clearance [14]. In CGD, increased bacterial translocation from impaired intestinal barrier function and lowered hepatic immune surveillance may predispose to the development of liver abscesses [7].
We demonstrate that medical management of CGD-associated liver abscesses with corticosteroids and antibiotics is associated with prolonged intervention-free interval compared with procedural intervention. We hypothesize that steroid-induced reduction of systemic inflammation reduces immune infiltration and capillary leak of the central venules, improving portal hypertension and liver function with decreasing portovenous shunting, thereby restoring the preabscess immune milieu. The use of pharmacotherapies against deranged inflammatory responses in conditions other than CGD have reduced the need for procedural intervention and improved survival. For example, in ulcerative colitis, caused by abnormal T helper cell type 2 (Th2) response, timely treatment with high-dose corticosteroids followed by maintenance immunosuppression has been shown to reduce mortality compared to placebo [15]. Also, reduction in need for surgery and complications such as toxic megacolon has been noted with immune-modulating therapy in ulcerative colitis [16, 17]. The improved outcome in patients treated with corticosteroids in combination with antimicrobials might also be explained by better tissue penetration of the antimicrobial agents due to a less inflamed environment, in particular in the surrounding pseudocapsule of the liver abscess.
We studied 26 CGD patients suffering liver involvement with a median complete follow-up of 15.5 years and an average of 3.7 liver-directed treatments (1–13) in the form of OS, IR, or CM. OS management of liver abscesses was associated with a longer intervention-free interval than IR procedures. Unlike pyogenic abscesses, hepatic abscesses in CGD are frequently multiloculated (Figure 6A), with viscous contents, and a thickened pseudocapsule (Figure 6B). While MRI may help characterize the dense granulation associated with these abscesses [10], their unique characteristics appear to be difficult to treat by an IR approach [6].
Figure 6.
Multiloculation (A) and thickened pseudocapsule (B) (arrows) of liver abscesses observed in patients with chronic granulomatous disease (CGD) by contrast-enhanced magnetic resonance imaging.
In our study, the intervention-free interval was associated with improved postprocedure ALP levels in OS and CM patients, though ALP in the IR cohort did not improve. This is significant, as elevated ALP has been associated with increased mortality in patients with CGD [7]. While it is conceivable that the removal of additional “at-risk” liver tissue during surgical resection could explain the observed improvements in ALP in OS compared to IR, we saw no difference in intervention-free interval between major liver resections vs limited debridements/nonanatomical wedge resections (1.17 vs 1.6 years, P = .99). While the relatively small number of patients undergoing major liver surgery (n = 7) could have limited detection of a difference, the improvement in ALP in both CM and OS adds biochemical support to the hypothesis that intervention-free interval (improved in both OS and CM cohorts compared with IR) is an important measure of disease course and clinical outcome. It is interesting to note that neutrophil function as assessed by ROI in CGD patients with liver involvement who underwent additional liver-directed therapy was lower than in those managed medically [2]. This observation is in line with later findings showing neutrophil function to be correlated with outcome of liver-directed therapy, with patients with higher ROI levels having prolonged intervals to repeat hepatic interventions (r = 0.6, P < .05).
Our study is limited by being performed in a single center, its retrospective nature, and the empiric determination of need for hepatic intervention. Management was directed by a multidisciplinary team of CGD experts, surgeons, hepatologists, and interventional radiologists. The OS and IR groups were balanced, including baseline neutrophil function and severity. In contrast, the corticosteroid group had higher preoperative ALP levels and more abscesses, which was in part what necessitated nonoperative interventions. These increased markers of severity affirm the favorable results of corticosteroid intervention. Similarly, a trend of worse neutrophil function was noted in the steroid group than in the interventional groups (mean ROI levels, 2.12 for OS and IR vs 1.64 for CM, P = .202). The high rate of patients with incomplete records and follow-up is a concern for the introduction for potential bias. However, to provide well-documented, uninterrupted follow-up and accurately calculate intervention-free intervals, we included only patients with complete records.
CONCLUSIONS
This retrospective study of patients with CGD liver abscesses demonstrates that patients treated with corticosteroids and targeted antimicrobial therapy had longer times to subsequent hepatic intervention than those managed by open surgical or percutaneous drainage. Steroid therapy was also associated with improvement in liver function abnormalities and unassociated with mortality. These observational data, derived from a large prospective cohort, suggest that nonoperative approaches to CGD liver abscess are not only safe and effective, but may preserve liver health, ameliorating a major cause of late morbidity and mortality in CGD.
Notes
Financial support. This project was supported by an intramural grant from the National Institutes of Health (NIH) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Winkelstein JA, Marino MC, Johnston RB Jr et al. . Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000; 79:155–69. [DOI] [PubMed] [Google Scholar]
- 2. Kuhns DB, Alvord WG, Heller T et al. . Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 2010; 363:2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000; 79:170–200. [DOI] [PubMed] [Google Scholar]
- 4. Marciano BE, Spalding C, Fitzgerald A et al. . Common severe infections in chronic granulomatous disease. Clin Infect Dis 2015; 60:1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hussain N, Feld JJ, Kleiner DE et al. . Hepatic abnormalities in patients with chronic granulomatous disease. Hepatology 2007; 45:675–83. [DOI] [PubMed] [Google Scholar]
- 6. Lublin M, Bartlett DL, Danforth DN et al. . Hepatic abscess in patients with chronic granulomatous disease. Ann Surg 2002; 235:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feld JJ, Hussain N, Wright EC et al. . Hepatic involvement and portal hypertension predict mortality in chronic granulomatous disease. Gastroenterology 2008; 134:1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen LE, Minkes RK, Shackelford PG, Strasberg SM, Kuo EY, Langer JC. Cut it out: managing hepatic abscesses in patients with chronic granulomatous disease. J Pediatr Surg 2003; 38:709–13. [DOI] [PubMed] [Google Scholar]
- 9. van den Berg JM, van Koppen E, Ahlin A et al. . Chronic granulomatous disease: the European experience. PLoS One 2009; 4:e5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Eulate R, Hussain N, Heller T et al. . CT and MRI of hepatic abscess in patients with chronic granulomatous disease. AJR Am J Roentgenol 2006; 187:482–90. [DOI] [PubMed] [Google Scholar]
- 11. Yamazaki-Nakashimada MA, Stiehm ER, Pietropaolo-Cienfuegos D, Hernandez-Bautista V, Espinosa-Rosales F. Corticosteroid therapy for refractory infections in chronic granulomatous disease: case reports and review of the literature. Ann Allergy Asthma Immunol 2006; 97:257–61. [DOI] [PubMed] [Google Scholar]
- 12. Leiding JW, Freeman AF, Marciano BE et al. . Corticosteroid therapy for liver abscess in chronic granulomatous disease. Clin Infect Dis 2012; 54:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am 2013; 27:89–99, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandler NG, Koh C, Roque A et al. . Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141:1220–30, 1230.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955; 2:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Järnerot G, Hertervig E, Friis-Liby I et al. . Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005; 128:1805–11. [DOI] [PubMed] [Google Scholar]
- 17. Aratari A, Papi C, Clemente V et al. . Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis 2008; 40:821–6. [DOI] [PubMed] [Google Scholar]