Cancer risk in 183542 older persons living with human immunodeficiency virus infection was evaluated using data from the HIV/AIDS Cancer Match Study. Relative risk of most cancers decreased with age, but absolute risks were higher for some cancers.
Keywords: HIV, cancer, aging, epidemiology
Abstract
Background
Cancer risk is increased in persons living with human immunodeficiency virus (HIV) (PLWH). Improved survival has led to an aging of PLWH. We evaluated the cancer risk in older PLWH (age ≥50 years).
Methods
We included data from the HIV/AIDS Cancer Match Study (1996–2012) and evaluated risks of Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, and cervical, anal, lung, liver, oral cavity/pharyngeal, breast, prostate, and colon cancers in older PLWH with risk in the general population by calculating standardized incidence ratios (SIRs) and excess absolute risks (EARs). Cancer risk by time since HIV diagnosis was estimated using Poisson regression.
Results
We identified 10371 cancers among 183542 older PLWH. Risk was significantly increased for KS (SIR, 103.34), NHL (3.05), Hodgkin lymphoma (7.61), and cervical (2.02), anal (14.00), lung (1.71), liver (2.91), and oral cavity/pharyngeal (1.66) cancers, and reduced for breast (0.61), prostate (0.47), and colon (0.63) cancers. SIRs declined with age for all cancers; however, EARs increased with age for anal, lung, liver, and oral cavity/pharyngeal cancers. Cancer risk was highest for most cancers within 5 years after HIV diagnosis; risk decreased with increasing time since HIV diagnosis for KS, NHL, lung cancer, and Hodgkin lymphoma.
Conclusions
Cancer risk is elevated among older PLWH. Although SIRs decrease with age, EARs are higher for some cancers, reflecting a greater absolute excess in cancer incidence among older PLWH. High risk in the first 5 years after HIV diagnosis for some cancers highlights the need for early HIV diagnosis and rapid treatment initiation.
Persons living with human immunodeficiency virus (HIV) (PLWH) infection have an increased cancer risk due to HIV-induced immunosuppression, coinfections with oncogenic viruses, and high prevalence of behavioral risk factors for cancer such as smoking [1–10]. Besides the AIDS-defining cancers (ADCs)—that is, Kaposi sarcoma (KS), certain non-Hodgkin lymphomas (NHLs), and cervical cancer—the risks of some non–AIDS-defining cancers (NADCs), including lung, liver, anal, and oral cavity/pharyngeal cancers, and Hodgkin lymphoma are also increased [2, 5–10].
The introduction of highly active antiretroviral therapy (HAART) has led to a steady increase in the life expectancy of PLWH [11]. Consequently, the number of older PLWH in the United States has also increased. In 2014, approximately 44% of PLWH in the United States were aged ≥50 years, and 17% of new HIV diagnoses were made in this age group [12, 13]. Because of improved immunity due to HAART, rates of ADCs have declined over the past 2 decades [7, 14, 15]. However, rates of many NADCs increase with age. It is unclear whether age-related immune senescence in the setting of HIV-related immunosuppression will result in particularly elevated cancer rates among older PLWH.
PLWH aged ≥50 years are a heterogeneous group including long-term survivors who have lived with HIV infection for many years and individuals with newly diagnosed infection; whether cancer risk varies based on the duration of HIV infection is uncertain. Prolonged immunosuppression in those with long-term HIV infection may increase cancer risk. Alternatively, persons with HIV infection newly diagnosed at an older age may have more severe immunosuppression and a consequent high risk for cancer. Such persons may present late with a lower CD4+ T-cell count, later HAART initiation, and more rapid progression to AIDS than young PLWH [16–19].
As the proportion of older PLWH continues to grow, a comprehensive evaluation of cancer risk in this population is needed. We therefore examined cancer risk in older PLWH compared with the general population, and we evaluated the effects on cancer risk of age and time since HIV diagnosis.
SUBJECTS AND METHODS
Study Population and Outcomes
The US HIV/AIDS Cancer Match study links data from population-based HIV and cancer registries using a probabilistic algorithm (https://hivmatch.cancer.gov) [14]. This analysis included data from 9 US states/territories (see footnote to Table 1). This study was approved by the institutional review boards at the National Cancer Institute and, as required, by participating cancer and HIV registries.
Table 1.
Person-Years Contributed by Older Persons Living With Human Immunodeficiency Virus Infection According to Age Categoriesa
| Characteristic | Proportion of Total PY, % | |||
|---|---|---|---|---|
| Total (928124 PY) |
Age 50–59 y (697455 PY) |
Age 60–69 y (189015 PY) |
Age ≥70 y (41724 PY) |
|
| Race/ethnicity | ||||
| Non-Hispanic white | 25.8 | 25.8 | 26.1 | 25.6 |
| Non-Hispanic black | 47.6 | 48.0 | 46.5 | 45.3 |
| Hispanic | 26.6 | 26.2 | 27.3 | 29.0 |
| Sex/risk group | ||||
| MSM | 27.0 | 27.0 | 27.1 | 25.9 |
| Male IDUs | 22.1 | 23.3 | 19.9 | 11.5 |
| MSM/IDUs | 3.9 | 4.4 | 2.8 | 1.2 |
| Male other/unknown | 21.3 | 19.4 | 25.4 | 34.6 |
| Female IDUs | 7.5 | 8.5 | 5.1 | 2.3 |
| Female other/unknown | 18.2 | 17.4 | 19.7 | 24.6 |
| Calendar year | ||||
| 1996–2000 | 2.9 | 3.1 | 2.3 | 2.3 |
| 2001–2005 | 23.3 | 24.4 | 20.0 | 19.6 |
| 2006–2012 | 73.8 | 72.5 | 77.7 | 78.2 |
| Prior AIDS diagnosis | ||||
| No | 28.1 | 28.5 | 26.9 | 26.1 |
| Yes | 71.9 | 71.5 | 73.1 | 73.9 |
| Time since HIV diagnosis | ||||
| Unknown | 22.2 | 22.0 | 22.5 | 22.7 |
| ≤5 y | 16.9 | 17.0 | 16.2 | 16.1 |
| 5.01–10 y | 24.0 | 24.0 | 23.6 | 24.6 |
| 10.01–15 y | 20.9 | 20.9 | 20.7 | 21.0 |
| ≥15.01 y | 16.1 | 16.0 | 16.9 | 15.5 |
Abbreviations: HIV, human immunodeficiency virus; IDUs, injection drug users; MSM, men who have sex with men; PY, person-years.
aParticipating registries included the following: Colorado (years of coverage, 1996–2007), Connecticut (2005–2010), Georgia (2004–2012), Maryland (2008–2012), Michigan (1996–2010), New Jersey (1996–2012), New York (2001–2012), Puerto Rico (2003–2012), and Texas (1999–2009).
The HIV diagnosis date was identified from HIV registries as the date of the first available positive serological test for HIV infection. The follow-up for each person started at the later of the start of cancer registry coverage, 4 months after the HIV report date (or AIDS diagnosis date if earlier), or at age 50 years, and it ended at death or the end of cancer registry coverage, whichever came first.
We evaluated the risks of ADCs—KS, cervical cancer, and NHL (overall and by subtypes: diffuse large B-cell lymphoma, Burkitt lymphoma, primary central nervous system [CNS] lymphoma, and other/unspecified NHLs)—and certain NADCs with increased incidence among PLWH: cancers of the anus, lung, liver, and oral cavity/pharynx and Hodgkin lymphoma [5]. Female breast, colon, and prostate cancers were also included; although their rates increase with age, they are not elevated among PLWH [5]. The remaining cancers were grouped together in a separate category. Cancers were classified using the International Classification of Diseases for Oncology (third edition) topography and morphology codes for invasive cancers (Supplementary Table S1) [20].
Statistical Analyses
We compared cancer incidence rates between PLWH and the general population by calculating standardized incidence ratios (SIRs), which measure relative risk, and excess absolute risks (EARs), which measure absolute risk. SIRs were calculated as the observed divided by the expected number of cases; expected cases were estimated by applying general population cancer incidence rates to the HIV population by age, sex, race/ethnicity, calendar year, and cancer registry. Because the AIDS epidemic affected the contemporaneous rates of KS and primary CNS lymphoma in the general population, we calculated the expected number of cases for these cancers with pre-AIDS epidemic (1973–1979) general population cancer incidence rates obtained from the Surveillance, Epidemiology, and End Results (SEER) Program, stratified by race, sex, and age [21]. The EARs for each cancer were estimated as the difference between the observed and expected cancer rates for age groups 50–59 and ≥60 years. For comparison, we also evaluated the SIRs and EARs among PLWH <50 years of age.
Among PLWH, we estimated cancer risk according to age (50–59, 60–69, or ≥70 years), AIDS diagnosis (yes or no), and time since HIV diagnosis (≤5, 5.01–10, 10.01–15, or ≥15.01 years) by fitting Poisson regression models for each cancer type. Adjusted incidence rate ratios (aIRRs) were adjusted for race/ethnicity, sex/risk group, calendar year, region, prior AIDS diagnosis, and time since HIV diagnosis (in models for age) or age (in models for time since HIV diagnosis). Age, calendar year, prior AIDS diagnosis, and time since HIV diagnosis variables were updated over time.
We conducted some sensitivity analyses. Because outmigration of persons from registry areas may result in overestimating the person-time at risk, we decreased the person-time by 10% for PLWH after 10 years of follow-up and recalculated the SIRs [22]. It is possible that more cancer cases may be diagnosed shortly after HIV infection because there may be a period of increased medical care around that time. Hence, we excluded the first year after HIV diagnosis date and reestimated cancer risk according to time since HIV diagnosis. Because the risk of ADCs and NADCs have changed since the introduction of HAART, we restricted the follow-up time to calendar years 2001–2012 and recalculated the SIRs and aIRRs. Finally, we evaluated whether associations between age and cancer risk differed by time since HIV diagnosis, and whether associations between time since HIV diagnosis and cancer risk differed by age.
RESULTS
We studied 183542 PLWH aged ≥50 years old who contributed 928194 person-years of follow-up (Table 1). The person-time distribution was 75.1% in 50–59-year-olds, 20.4% in 60–69-year-olds, and 4.5% in those aged ≥70 years. A large fraction of person-time was contributed by non-Hispanic blacks (47.6%), men (74.3%), and those with a prior AIDS diagnosis (71.9%). A large proportion of person-time for the time since HIV diagnosis was unknown (22.2%). Among PLWH with a known date of HIV diagnosis, >60% were infected with HIV for ≥5 years, and approximately 20% had been living with HIV infection for >15 years.
During the follow-up, 10371 cancers were diagnosed, of which 1647 (15.9%) were ADCs and 8724 (84.1%) were NADCs (Table 2). As expected, rates were increased in older PLWH compared with the general population for KS (SIR, 103.34), total NHL (3.05), and cervical cancer (2.02). Among NHLs, rates were strongly elevated for CNS lymphoma (SIR, 47.39) and Burkitt lymphoma (13.75). Among the NADCs, a very high risk of anal cancer (SIR, 14.00) was observed, followed by Hodgkin lymphoma (7.61). Rates were moderately increased for liver (SIR, 2.91), lung (1.71), and oral cavity/pharyngeal cancers (1.66). Furthermore, rates of breast (SIR, 0.61), prostate (0.47), and colon cancer (0.63) were significantly lower among PLWH than in the general population. The SIRs did not differ appreciably after the person-time was adjusted for late follow-up, or when analyses were restricted to calendar years 2001–2012 (Supplementary Tables S2 and S4).
Table 2.
Rates and Standardized Incidence Ratios of Cancers in Older Persons Living With Human Immunodeficiency Virus Infection (Age ≥50 Years)
| Cancers | Observed Cases, No. | Expected Cases, No. | IR per 100000 PY | SIR (95% CI) |
|---|---|---|---|---|
| All cancers | 10371 | 8929 | 1117.3 | 1.16 (1.14–1.18) |
| ADCs | ||||
| Kaposi sarcoma | 338 | 3.3 | 36.4 | 103.34 (92.62–114.97) |
| NHL | 1222 | 401.2 | 131.7 | 3.05 (2.88–3.22) |
| Diffuse large B cell | 674 | 110.0 | 72.6 | 6.12 (5.67–6.61) |
| Burkitt | 103 | 7.5 | 11.1 | 13.75 (11.23–16.68) |
| CNS | 74 | 8.1 | 8.0 | 47.39 (37.21–59.49) |
| Other/unspecified | 371 | 282.1 | 39.9 | 1.32 (1.18–1.46) |
| Cervical cancer | 87 | 43.0 | 36.5 | 2.02(1.62–2.49) |
| NADCs | ||||
| Anal | 524 | 37.4 | 56.5 | 14.00 (12.82–15.25) |
| Lung | 1725 | 1012 | 185.8 | 1.71 (1.63–1.79) |
| Liver | 805 | 276.4 | 86.7 | 2.91 (2.71–3.12) |
| Hodgkin lymphoma | 253 | 33.3 | 27.3 | 7.61 (6.70–8.60) |
| Oral cavity/pharynx | 461 | 278.4 | 49.7 | 1.66 (1.51–1.81) |
| Female breast | 329 | 542.0 | 137.9 | 0.61 (.54–.68) |
| Prostate | 1341 | 2829 | 194.5 | 0.47 (.45–.50) |
| Colon (excluding rectum) | 360 | 571.3 | 38.8 | 0.63 (.57–.70) |
| Other cancers | 2926 | 5731 | 315.2 | 1.01 (.97–1.05) |
Abbreviations: ADCs, AIDS-defining cancers; CI, confidence intervals; CNS, central nervous system; IR, incidence rate; NADCs, non–AIDS-defining cancers; NHL, non-Hodgkin lymphoma; PY, person-years; SIR, standardized incidence ratio.
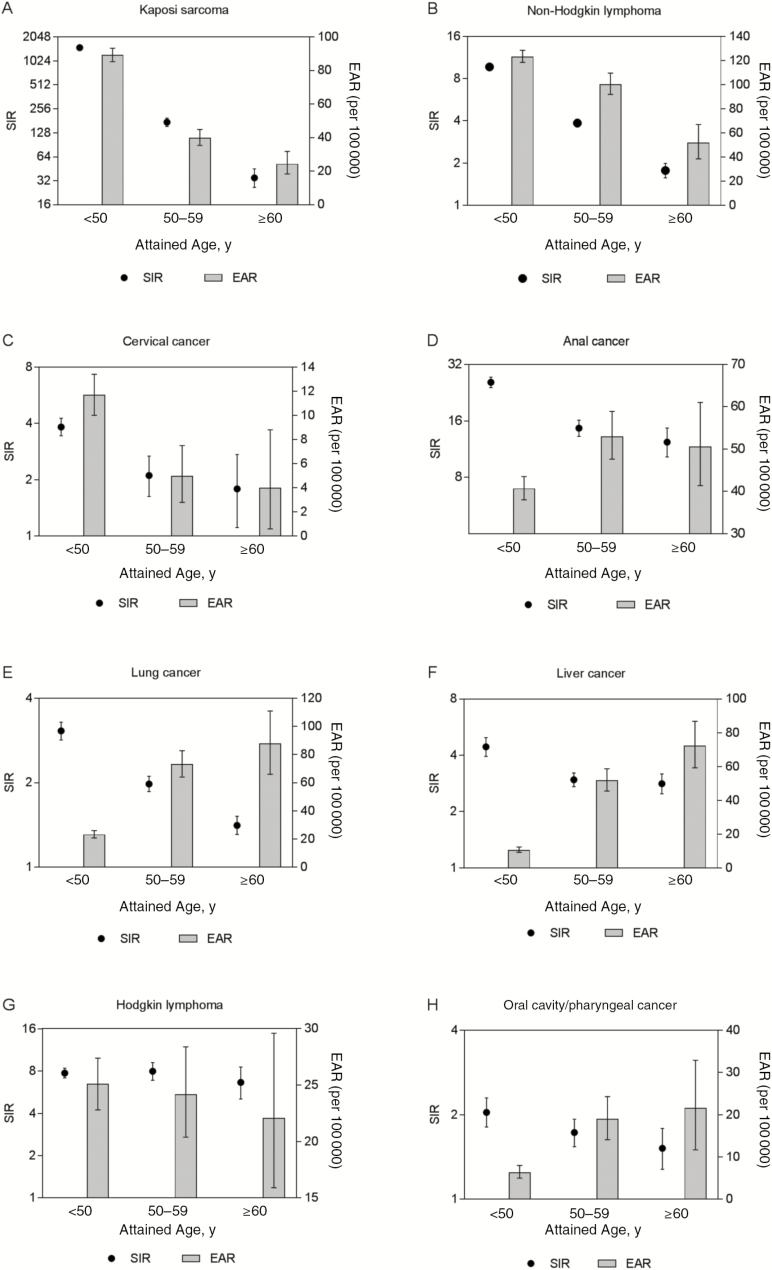
A comparison of SIRs and EARs by age for evaluated cancers is depicted in Figure 1. The SIRs were the highest for the youngest age group (<50 years) for all cancers and decreased with age, except for Hodgkin lymphoma, for which the SIRs were largely constant. The EARs also decreased with age for KS, NHL, cervical cancer, and Hodgkin lymphoma. In contrast, the EARs increased with age for cancers of the anus, lung, liver, and oral cavity/pharynx.
Figure 1.
Risk of cancers in persons living with human immunodeficiency virus (HIV) compared with the general population. The figure plots the standardized incidence ratios (SIRs) on the left y-axis, the excess absolute risks (EARs) per 100000 population on the right y-axis, and 3 age groups on the x-axis (<50, 50–59, and ≥60 years). A–C, Risks of 3 AIDS-defining cancers: Kaposi sarcoma (A), non-Hodgkin lymphoma (B), and cervical cancer (C). D–H, Risks of 5 non-AIDS-defining cancers: anal cancer (D), lung cancer (E), liver cancer (F), Hodgkin lymphoma (G), and oral cavity/pharynx cancer (H).
Examining cancer incidence rates among PLWH by age group (Table 3), we observed a significant decrease in KS risk with increasing age. Compared with 50–59-year-olds, PLWH aged 60–69 (aIRR, 0.68) or ≥70 ( 0.43) years had lower KS rates (Ptrend = .001). In contrast, rates for lung, prostate, and colon cancers increased with age (Ptrend < .001) while the increasing trend for breast cancer was borderline significant (Ptrend = .058). Liver and oral cavity/pharyngeal cancer rates were higher among 60–69-year-olds than in the 50–59-year age group (Ptrend < .05). No association between age and cancer risk was observed for NHL (total or subtypes), cervical cancer, anal cancer, or Hodgkin lymphoma (Ptrend > .05).
Table 3.
Risk of Cancers in Older Persons Living With Human Immunodeficiency Virus Infection According to Age and Prior AIDS Diagnosis
| Cancers | Age 50–59 y | Age 60–69 y | Age ≥70 y | P trend | AIDS Diagnosis (Yes vs No): aIRRb (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Cancers, No. | aIRRa (95% CI) | Cancers, No. | aIRRa (95% CI) |
Cancers, No. | aIRRa (95% CI) |
|||
| ADCs | ||||||||
| Kaposi sarcoma | 280 | Reference | 51 | 0.68 (.51–.92) | 7 | 0.43 (.20–.92) | .001 | 1.86 (1.40–2.47) |
| NHL | 946 | Reference | 227 | 0.90 (.78–1.04) | 49 | 0.88 (.66–1.18) | .13 | 1.74 (1.50–2.01) |
| Diffuse large B cell | 521 | Reference | 127 | 0.90 (.74–1.10) | 26 | 0.84 (.56–1.25) | .21 | 1.97 (1.61–2.41) |
| Burkittc | 82 | Reference | 21 | 0.80 (.49–1.30) | … | … | .37 | 1.22 (.77–1.92) |
| CNSc | 63 | Reference | 11 | 0.53 (.28–1.01) | … | … | .053 | 5.17 (2.25–12.21) |
| Other/unspecified | 280 | Reference | 71 | 0.94 (.73–1.21) | 20 | 1.12 (.71–1.77) | .33 | 1.50 (1.18–1.90) |
| Cervical cancerc | 66 | Reference | 21 | 1.10 (.67–1.82) | … | … | .70 | 1.50 (.91–2.49) |
| NADCs | ||||||||
| Anal | 397 | Reference | 112 | 1.05 (.85–1.29) | 15 | 0.66 (.40–1.11) | .45 | 2.66 (2.04–3.47) |
| Lung | 1030 | Reference | 552 | 2.11 (1.90–2.34) | 143 | 2.62 (2.20–3.13) | <.001 | 1.37 (1.22–1.53) |
| Liver | 546 | Reference | 226 | 1.61 (1.38–1.89) | 33 | 1.29 (.91–1.84) | <.001 | 0.92 (.78–1.09) |
| Hodgkin lymphoma | 193 | Reference | 53 | 1.00 (.74–1.36) | 7 | 0.58 (.27–1.23) | .32 | 2.19 (1.55–3.09) |
| Oral cavity/pharynx | 315 | Reference | 132 | 1.55 (1.26–1.90) | 14 | 0.76 (.44–1.30) | .03 | 1.67 (1.31–2.12) |
| Female breast | 232 | Reference | 77 | 1.26 (.97–1.63) | 20 | 1.32 (.83–2.09) | .058 | 1.03 (.82–1.31) |
| Prostate | 627 | Reference | 550 | 3.11 (2.77–3.49) | 164 | 4.12 (3.46–4.90) | <.001 | 0.84 (.74–.94) |
| Colon (excluding rectum) | 177 | Reference | 125 | 2.59 (2.06–3.26) | 58 | 5.28 (3.90–7.15) | <.001 | 0.89 (.71–1.13) |
| Other cancers | 1845 | Reference | 829 | 1.69 (1.55–1.83) | 252 | 2.34 (2.05–2.68) | <.001 | 1.25 (1.15–1.36) |
Abbreviations: ADCs, AIDS-defining cancers; aIRR, adjusted incidence rate ratio; CI, confidence intervals; CNS, central nervous system; NADCs, non–AIDS-defining cancers; NHL, non-Hodgkin lymphoma.
aAdjusted for race, sex/risk group, calendar year, region, prior AIDS diagnosis, and time since HIV diagnosis
bAdjusted for age, race, sex/risk group, calendar year, region, and time since HIV diagnosis
caIRRs and Ptrend values for these cancers are presented for the 50–59- and ≥60-year age groups because too few cancers occurred in the ≥70-year category (1 Burkitt lymphoma, 2 CNS lymphomas, and 2 cervical cancers).
PLWH with a prior AIDS diagnosis had increased rates of KS, NHL (particularly diffuse large B-cell lymphoma [aIRR, 1.97] and CNS lymphomas [5.17]), anal, lung, and oral cavity/pharyngeal cancer, and Hodgkin lymphoma than those without a previous AIDS diagnosis (aIRRs range, 1.37–2.66; Table 3). Prostate cancer rates were lower among those with a prior AIDS diagnosis (aIRR, 0.84), but rates of cervical, liver, breast, and colon cancer did not differ significantly .
The risk of most cancers was highest within the first 5 years after HIV diagnosis, gradually decreasing over time (Table 4). Significant decreasing trends were observed for KS (Ptrend < .001), total NHL (Ptrend < .001), lung cancer (Ptrend < .001), breast cancer (Ptrend = .02), and Hodgkin lymphoma (Ptrend = .04), with a marginally significant trend for prostate cancer (Ptrend = .07). In contrast, there was an indication that risk increased with increasing time since HIV diagnosis for anal (Ptrend = .07) and liver (Ptrend = .08) cancers. Excluding the first year after HIV diagnosis did not affect our results (Supplementary Table S3). Restricting the analyses to calendar years 2001–2012 did not affect the associations between age or time since HIV diagnosis and cancer risk (Supplementary Tables S5 and S6).
Table 4.
Risk of Cancers in Older Human Immunodeficiency Virus-Infected Individuals According to Time Since Human Immunodeficiency Virus Diagnosis
| Cancers | ≤5 y Since Diagnosis | 5.01–10 y Since Diagnosis | 10.01–15 y Since Diagnosis | ≥15.01 y Since Diagnosis | P trend | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cancers, No. | aIRRa (95% CI) |
Cancers, No. | aIRRa (95% CI) |
Cancers, No. | aIRRa (95% CI) |
Cancers, No. | aIRRa (95% CI) |
||
| ADCs | |||||||||
| Kaposi sarcoma | 95 | Reference | 81 | 0.58 (.43–.79) | 55 | 0.44 (.31–.63) | 40 | 0.43 (.29–.65) | <.001 |
| NHL | 355 | Reference | 308 | 0.60 (.51–.70) | 204 | 0.45 (.38–.54) | 123 | 0.36 (.29–.45) | <.001 |
| Diffuse large B cell | 183 | Reference | 164 | 0.60 (.49–.75) | 122 | 0.50 (.39–.64) | 66 | 0.36 (.26–.49) | <.001 |
| Burkitt | 33 | Reference | 22 | 0.44 (.25–.76) | 18 | 0.39 (.21–.72) | 9 | 0.24 (.11–.53) | <.001 |
| CNS | 24 | Reference | 19 | 0.54 (.29–1.00) | 10 | 0.33 (.15–.71) | 10 | 0.46 (.20–1.05) | .02 |
| Other/unspecified | 115 | Reference | 103 | 0.63 (.48–.82) | 54 | 0.39 (.28–.54) | 38 | 0.38 (.26–.55) | <.001 |
| Cervical cancer | 21 | Reference | 22 | 0.75 (.40–1.37) | 16 | 0.61 (.31–1.23) | 12 | 0.64 (.29–1.43) | .20 |
| NADCs | |||||||||
| Anal | 59 | Reference | 99 | 1.03 (.74–1.43) | 140 | 1.50 (1.09–2.06) | 95 | 1.21 (.86–1.72) | .07 |
| Lung | 355 | Reference | 459 | 0.92 (.80–1.06) | 367 | 0.87 (.74–1.02) | 213 | 0.70 (.58–.84) | <.001 |
| Liver | 98 | Reference | 155 | 0.97 (.75–1.26) | 180 | 1.17 (.90–1.51) | 157 | 1.20 (.91–1.59) | .08 |
| Hodgkin lymphoma | 64 | Reference | 61 | 0.69 (.48–.99) | 41 | 0.54 (.36–.82) | 39 | 0.70 (.45–1.09) | .04 |
| Oral cavity/pharynx | 79 | Reference | 117 | 1.02 (0.76–1.36) | 96 | 0.93 (.68–1.27) | 66 | 0.82 (.57–1.17) | .22 |
| Female breast | 75 | Reference | 90 | 0.85 (.62–1.17) | 63 | 0.72 (.50–1.02) | 38 | 0.64 (.42–.98) | .02 |
| Prostate | 231 | Reference | 363 | 1.08 (.91–1.27) | 272 | 0.94 (.78–1.13) | 191 | 0.86 (.70–1.07) | .07 |
| Colon (excluding rectum) | 68 | Reference | 90 | 0.94 (.68–1.30) | 82 | 1.03 (.73–1.45) | 43 | 0.75 (.49–1.14) | .32 |
| Other cancers | 568 | Reference | 741 | 0.91 (.82–1.02) | 643 | 0.91 (.80–1.02) | 429 | 0.79 (.69–.91) | <.001 |
Abbreviations: ADCs, AIDS-defining cancers; aIRR, adjusted incidence rate ratio; CI, confidence intervals; CNS, central nervous system; NADCs, non–AIDS-defining cancers; NHL, non- Hodgkin lymphoma.
aAdjusted for age, race, sex/risk group, calendar year, region, and prior AIDS diagnosis.
The associations between age and cancer risk did not differ by time since HIV diagnosis for all cancers (all Pinteraction > .05). However, there was significant heterogeneity in the association between time since HIV diagnosis and cancer risk by age for NHL (Pinteraction = .003) and Hodgkin lymphoma (Pinteraction = .02). The aIRRs for NHL decreased with time since HIV diagnosis for both age categories (Ptrend < .001 for ages 50–59 and ≥60 years). For Hodgkin lymphoma, the trend for decreasing rates with time since HIV diagnosis was observed only for ≥60-year-olds (Ptrend = .006) and not among those aged 50–59 years (Ptrend = .41).
DISCUSSION
With the success of HAART, HIV infection has been transformed from a terminal illness to a more manageable chronic disease. Consequently, more PLWH are reaching older ages. In our analyses that included >180000 older PLWH, we observed that cancer risk was elevated in older PLWH compared with the general population, although the relative risk for most cancers declined with age. However, EARs, which measure absolute risk and thus reflect the number of excess cancers occurring among PLWH, increased with age for some NADCs. We also observed that for many cancers, cancer risk was highest within the first 5 years after HIV diagnosis.
In the past 20 years, there has been a steady shift in the demographic profile of the HIV/AIDS epidemic in the United States toward older age groups. In 2002, the Centers for Disease Control and Prevention estimated that approximately 8.5% of PLWH were >50 years of age, and that proportion had increased to 44% in 2014 [12]. With aging, there is a complex interaction between various biological processes which may modulate cancer risk among PLWH: (1) factors related to aging, including age-related immune senescence; (2) factors associated with HIV infection and its related immune dysfunction, such as HIV viral load, CD4+ T-cell count, and effectiveness of HAART; and (3) various host factors that may affect cancer risk, such as viral coinfections and smoking. Furthermore, the immunosuppressive state in PLWH changes over time. The natural course of HIV infection is characterized by progressive decline in immune function from primary HIV infection to symptomatic disease with onset of AIDS-defining conditions [23]. HAART alters the natural history of HIV infection with gradual improvement of immunosuppressive state and restoration of CD4+ T-cell count over time. In addition, the duration of immunosuppression in a person infected with HIV also increases with time.
Few previous studies have been able to evaluate cancer risk in older PLWH because this population has historically been too small to target [24, 25]. In our study, we observed that the risk of ADCs and certain NADCs were elevated in older PLWH compared with the general population, as has been previously reported in mostly young PLWH [3, 7, 26]. Because cancer risk increases with age, and PLWH are at a high risk for cancer, the aging of PLWH raises the question of whether the combined effect of age and HIV infection will further increase cancer risk in PLWH. As captured by SIRs, we did not observe an amplified cancer risk associated with aging among PLWH. In fact, the SIRs were highest for the young PLWH and declined with age, except for Hodgkin lymphoma, suggesting that HIV-related immunosuppression may play a larger role in cancer development in younger groups.
Although the risk of anal, lung, liver, and oral cavity/pharyngeal cancers in PLWH relative to the general population decreased with age, the excess cancer rates increased in absolute terms, as reflected in the EARs. Owing to increasing cancer incidence in the general population with age, even a modest decrease in SIRs with advancing age could lead to an increase in EARs and thus number of cancer cases. Thus, as PLWH continue to age, we may continue to observe an increase in the absolute number of these NADCs.
Risks for KS, NHLs, Hodgkin lymphoma, and lung and breast cancer were highest within the first 5 years after HIV diagnosis. The intensity of immunosuppression may be highest in the initial period, when PLWH with newly diagnosed infection are untreated and may be especially immunosuppressed [27, 28]. Alternatively, it is possible that persons who survived for several years with HIV infection may represent a healthy subset of PLWH, whereas those with newly acquired and diagnosed HIV infection may have a higher prevalence of cancer risk factors. For example, a diagnosis of HIV infection may lead to changes in lifestyle habits, such as reducing cigarette smoking [29]. Increased medical surveillance shortly after HIV diagnosis may also increase the possibility of detecting cancers, but excluding the first year of follow-up after HIV diagnosis did not eliminate the trend that we observed. In contrast, anal and liver cancer rates marginally increased with time since HIV diagnosis, which may represent the role of prolonged immunosuppression in increasing cancer rates due to human papillomavirus or hepatitis virus coinfections.
We observed lower risk of breast, prostate, and colon cancer rates in older PLWH compared with the general population, as reported elsewhere [26, 30–33]. As in the general population, prostate and colon cancer rates increased with age, and the trend for breast cancer was borderline significant. Because these are common screen-detectable cancers, it has been speculated that their reduced rates may reflect lower rates of cancer screening among PLWH compared with the general population. However, recent studies suggest that the deficit of breast, colon, and prostate cancers among PLWH cannot be completely explained by lower rates of cancer screening [34, 35]. Biological explanations for these deficits need to be explored further.
Given the aging of PLWH and the increase in the burden of NADCs, it is essential to target available cancer prevention and screening strategies toward this population. Cancer prevention among PLWH is largely based on restoring immunity with HAART and reducing established cancer risk factors [36]. Timely linkage of care with HAART initiation and uninterrupted treatment for persons in whom HIV infection has been newly diagnosed is essential to restore immunity. This also gives an opportunity for healthcare providers to diagnose and treat coinfections and to initiate counseling on modification of lifestyle behaviors, such as smoking. General population screening guidelines for breast, prostate, and colon cancers should be followed. Cervical cancer screening is recommended at more frequent intervals than for HIV-uninfected women [37, 38], and the benefits of anal cancer screening are still being evaluated [37, 38]. Finally, screening for lung cancers with low-dose computed tomography may be beneficial, but more data are needed among PLWH [37, 38].
Strength of our study included the use of a large population-based cohort of >180000 PLWH aged ≥50 years with systematic cancer ascertainment and the evaluation of cancer risk by time since HIV diagnosis. However, we were unable to include information on HAART use, CD4+ T-cell count, or HIV viral load in our analyses. Therefore, we cannot definitively interpret trends with respect to time since HIV diagnosis. In addition, our data do not include information about cancer risk factors, such as cigarette smoking and viral coinfection. Because the timing of HIV acquisition was not known, the duration of HIV infection could be calculated only from the first available positive serological test date, and we were missing this information for 22.2% of person-time contributed by PLWH in our study. We were unable to adjust for competing risks due to coexisting comorbid conditions in our analyses. We used the general population as our control group because we did not have access to an ideal HIV-uninfected population that may have similar characteristics to PLWH [39]. Finally, our analysis includes only PLWH in whom HIV infection has been diagnosed, and an estimated 13% of PLWH are unaware that they are infected, >7% of whom may be >50 years of age [40].
In conclusion, rates of several cancer types are elevated in older PLWH compared with the general population, albeit in the absence of information on important cancer risk factors. However, the relative risk of cancers is lower than for younger PLWH, suggesting that the combined effect of aging and HIV does not further amplify the relative risk of cancers. Despite lower relative risks, the absolute risk difference is higher for some NADCs among older PLWH, leading to a greater incidence of excess cancers in this group. Thus, there is a continued need for cancer prevention and early detection among older PLWH. Cancer risk was also highest within the first 5 years after HIV diagnosis for most cancers, underscoring the importance of early HIV diagnosis, rapid initiation of HAART after HIV diagnosis, and interventions to reduce known risk factors in older PLWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge the support and assistance provided by individuals at the following state HIV/AIDS and cancer registries: Colorado, Connecticut, Georgia, Maryland, Michigan, New Jersey, New York, Puerto Rico, and Texas. We also thank Timothy McNeel at Information Management Services for programming support.
Disclaimer. The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute (NCI), HIV/AIDS or cancer registries, or their contractors.
Financial support. This research was supported in part by the Intramural Research Program of the NCI. The following cancer registries were supported by the SEER Program of the NCI: Connecticut (grant HHSN261201300019I) and New Jersey (grants HHSN261201300021I and N01-PC-2013-00021). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: Colorado (grant NU58DP006347-01), Georgia (grant 5U58DP003875-01), Maryland (grant 5NU58DP003919-05-00), Michigan (grant 5U58DP003921-03), New Jersey (grant NU58/DP003931-05-00), New York (grant U58/DP003879), and Texas (grant 5U58DP000824-04). The New Jersey State Cancer Registry was also supported by the state of New Jersey, the Maryland Cancer Registry by the State of Maryland and the Maryland Cigarette Restitution Fund, and the New York State Cancer Registry by the state of New York. The following HIV registries were supported by HIV Incidence and Case Surveillance Branch of the Centers for Disease Control and Prevention, National HIV Surveillance Systems: Colorado (grant NU62PS003960), Connecticut (grant 5U62PS001005-05), Michigan (grant U62PS004011-02), and New Jersey (grant U62PS004001-2).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 50th Annual Meeting of the Society for Epidemiologic Research, Seattle, Washington, 20–23 June 2017; 16th International Conference on Malignancies in HIV/AIDS, Bethesda, Maryland, 23–24 October 2017.
References
- 1. Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS 2016; 30:273–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Althoff KN, McGinnis KA, Wyatt CM, et al. ; Veterans Aging Cohort Study (VACS) Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015; 60:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clifford GM, Polesel J, Rickenbach M, et al. ; Swiss HIV Cohort Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005; 97:425–32. [DOI] [PubMed] [Google Scholar]
- 4. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370:59–67. [DOI] [PubMed] [Google Scholar]
- 5. Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017; 4:e495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Massad LS, Hessol NA, Darragh TM, et al. . Cervical cancer incidence after up to 20 years of observation among women with HIV. Int J Cancer 2017; 141:1561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel P, Hanson DL, Sullivan PS, et al. . Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148:728–36. [DOI] [PubMed] [Google Scholar]
- 8. Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst 2015; 107:dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 2009; 52:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiels MS, Pfeiffer RM, Gail MH, et al. . Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Diagnoses of HIV infection among adults aged 50 years and older in the United States and dependent areas, 2010–2014. HIV Surveillance Suppl Rep 2016; 21:1–69. [Google Scholar]
- 13. Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2014. HIV Surveillance Rep 2014; 26:1–123. [Google Scholar]
- 14. Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA; HIV/AIDS Cancer Match Study AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst 2007; 99:962–72. [DOI] [PubMed] [Google Scholar]
- 15. Engels EA, Pfeiffer RM, Goedert JJ, et al. . Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids 2006; 20:1645–54. [DOI] [PubMed] [Google Scholar]
- 16. Smith RD, Delpech VC, Brown AE, Rice BD. HIV transmission and high rates of late diagnoses among adults aged 50 years and over. AIDS 2010; 24:2109–15. [DOI] [PubMed] [Google Scholar]
- 17. Althoff KN, Gebo KA, Gange SJ, et al. ; North American AIDS Cohort Collaboration on Research and Design CD4 count at presentation for HIV care in the United States and Canada: are those over 50 years more likely to have a delayed presentation?AIDS Res Ther 2010; 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV infection and older Americans: the public health perspective. Am J Public Health 2012; 102:1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He L, Pan X, Dou Z, et al. . The factors related to CD4+ T-cell recovery and viral suppression in patients who have low CD4+ T cell counts at the initiation of HAART: a retrospective study of the national HIV treatment sub-database of Zhejiang Province, China, 2014. PLoS One 2016; 11:e0148915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fritz A, Percy C, Jack A, et al. . International classification of diseases for oncology. 1st revision. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 21. Chaturvedi AK, Mbulaiteye SM, Engels EA. Underestimation of relative risks by standardized incidence ratios for AIDS-related cancers. Ann Epidemiol 2008; 18:230–4. [DOI] [PubMed] [Google Scholar]
- 22. Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med 2010; 170:1337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mindel A, Tenant-Flowers M. ABC of AIDS: Natural history and management of early HIV infection. BMJ 2001; 322:1290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biggar RJ, Kirby KA, Atkinson J, McNeel TS, Engels E; AIDS Cancer Match Study Group Cancer risk in elderly persons with HIV/AIDS. J Acquir Immune Defic Syndr 2004; 36:861–8. [DOI] [PubMed] [Google Scholar]
- 25. Yanik EL, Katki HA, Engels EA. Cancer risk among the HIV-infected elderly in the United States. AIDS 2016; 30:1663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engels EA, Biggar RJ, Hall HI, et al. . Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008; 123:187–94. [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg ES, Billingsley JM, Caliendo AM, et al. . Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997; 278:1447–50. [DOI] [PubMed] [Google Scholar]
- 28. Fleury S, Rizzardi GP, Chapuis A, et al. . Long-term kinetics of T cell production in HIV-infected subjects treated with highly active antiretroviral therapy. Proc Natl Acad Sci U S A 2000; 97:5393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Chen X, Li X, et al. . Cigarette smoking among Chinese PLWHA: An exploration of changes in smoking after being tested HIV positive. AIDS Care 2016; 28:365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herida M, Mary-Krause M, Kaphan R, et al. . Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol 2003; 21:3447–53. [DOI] [PubMed] [Google Scholar]
- 31. Pantanowitz L, Dezube BJ. Reasons for a deficit of breast cancer among HIV-infected patients. J Clin Oncol 2004; 22:1347–50. [DOI] [PubMed] [Google Scholar]
- 32. Frisch M, Biggar RJ, Engels EA, Goedert JJ; AIDS-Cancer Match Registry Study Group Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001; 285:1736–45. [DOI] [PubMed] [Google Scholar]
- 33. Shiels MS, Goedert JJ, Moore RD, Platz EA, Engels EA. Reduced risk of prostate cancer in U.S. Men with AIDS. Cancer Epidemiol Biomarkers Prev 2010; 19:2910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marcus JL, Chao CR, Leyden WA, et al. . Prostate cancer incidence and prostate-specific antigen testing among HIV-positive and HIV-negative men. J Acquir Immune Defic Syndr 2014; 66:495–502. [DOI] [PubMed] [Google Scholar]
- 35. Coghill AE, Engels EA, Schymura M, Mahale P, Shiels MS. Lower risks of breast, prostate, and colorectal cancers among HIV-infected individuals in the United States. J Natl Cancer Inst 2017; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol 2012; 24:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gopal S, Achenbach CJ, Yanik EL, Dittmer DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol 2014; 32:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goncalves PH, Montezuma-Rusca JM, Yarchoan R, Uldrick TS. Cancer prevention in HIV-infected populations. Semin Oncol 2016; 43:173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong C, Althoff K, Gange SJ. Identifying the appropriate comparison group for HIV-infected individuals. Curr Opin HIV AIDS 2014; 9:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2014. HIV Surveillance Suppl Rep 21(4). Available at: http://www.cdc.gov/hiv/library/reports/surveillance. Accessed 22 December 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.