Abstract
Streptococcus agalactiae (group B streptococcus [GBS]) infection in pregnant women is the leading cause of infectious neonatal morbidity and mortality in the United States. Although inflammation during infection has been associated with preterm birth, the contribution of GBS to preterm birth is less certain. Moreover, the early mechanisms by which GBS interacts with the gestational tissue to affect adverse pregnancy outcomes are poorly understood. We hypothesized that short-term GBS inoculation activates pathways related to inflammation and premature birth in human extraplacental membranes. We tested this hypothesis using GBS-inoculated human extraplacental membranes in vitro. In agreement with our hypothesis, a microarray-based transcriptomics analysis of gene expression changes in GBS-inoculated membranes revealed that GBS activated pathways related to inflammation and preterm birth with significant gene expression changes occurring as early as 4 h postinoculation. In addition, pathways related to DNA replication and repair were downregulated with GBS treatment. Conclusions based on our transcriptomics data were further supported by responses of prostaglandin E2 (PGE2), and matrix metalloproteinases 1 (MMP1) and 3 (MMP3), all of which are known to be involved in parturition and premature rupture of membranes. These results support our initial hypothesis and provide new information on molecular targets of GBS infection in human extraplacental membranes.
Keywords: premature birth, extraplacental membranes, maternal/fetal membranes, transcriptomics
Summary Sentence
Group B Streptococcus (GBS) activates premature birth and inflammation pathways in human extraplacental membranes in vitro, providing insight into early molecular responses underpinning GBS contributions to adverse birth outcomes.
Introduction
Intrauterine infection has been well established as a cause of preterm birth and other adverse birth outcomes. Bacteria colonize the vagina and cervix, migrate to the placenta and maternal–fetal membranes, cross the extraplacental membranes, and then colonize the amniotic cavity and fetus [1]. Infection increases inflammatory cytokines such as interleukin (IL) 1 beta (IL1B), IL6, C-X-C motif chemokine ligand 8 (CXCL8), and tumor necrosis factor (TNF) (IL1B, IL6, IL8, and TNF) in the amniotic fluid [2–4], and increased levels of these inflammatory cytokines in amniotic fluid have been associated with adverse outcomes such as preterm birth [3].
Streptococcus agalactiae (group B streptococcus [GBS]) is the leading cause of infectious neonatal morbidity and mortality in the United States [5]. GBS in the female reproductive tract is associated with neonatal sepsis and meningitis, and GBS-infected infants born preterm have increased odds of mortality compared to infants delivered at term [1, 6]. In addition, GBS is the most common microorganism isolated from maternal and fetal tissues of women with midgestation spontaneous abortion [7]. Although GBS infection is recognized as a major cause of some adverse birth outcomes, its role in preterm birth is less certain. Studies report genital GBS colonization of pregnant women associated with early term births and low birth weight [8] and with premature labor [9, 10]. Likewise, histological chorioamnionitis and preterm birth at less than 32 weeks gestation are associated with GBS isolation from extraplacental membranes [3, 11]. A review and meta-analysis of studies published between 1996 and 2008 reported increased odds of preterm birth in case control and cross sectional studies of women with genitourinary tract GBS detection at delivery but not in cohort studies of women with GBS detection during pregnancy. However, the latter analysis cited inconsistencies among the studies and lack of rigorous randomized controlled trials as significant limitations that hamper conclusions about the role of GBS infection in preterm birth, a conclusion reiterated in a more recent review [12]. Despite the limitations of clinical studies, recent studies using mouse models have provided insight into mechanisms by which GBS induces a preterm birth phenotype, demonstrating that GBS contributes to fetal injury and preterm birth via activation of the inflammatory responses in gestational tissue [13–17].
Healthy extraplacental membranes (also called gestational membranes or maternal–fetal membranes), composed primarily of decidual cells, chorionic trophoblasts, fibroblasts, and amnion epithelial cells, provide a barrier that protects the fetus from infection. Gestational tissues stimulated with GBS produce unique inflammatory cytokine profiles. We previously reported that GBS inoculation ex vivo stimulated secretion of proinflammatory cytokines such as IL1A, IL1B, TNF, and CXCL8 (IL8) from human extraplacental membranes in transwell cultures and from human extraplacental membranes dissected free of amnion [18, 19]. Other studies reported similar results, showing that GBS stimulated IL1B, IL6, IL10, and TNF release from human extraplacental membranes [20–22].
Although these recent studies have shed light on the mechanisms by which GBS infection leads to adverse birth outcomes, many of these studies were conducted in animal models and/or at time points at least 24 h after GBS inoculation. Early changes in molecular and cellular responses that underpin susceptibility of human extraplacental membranes to GBS infection are poorly understood [23]. Understanding early responses of the gestational tissues may allow for earlier interventions and treatment for possible adverse birth outcomes and could elucidate differences between acute and chronic responses. In the present study, we report for the first time genome-wide expression profiles in GBS-inoculated human extraplacental membranes in vitro. Using bioinformatics analysis of these expression profiles, we tested our hypothesis that GBS activates pathways related to inflammation and preterm birth in human extraplacental membranes early after inoculation. Conclusions based on our transcriptomics data were further supported by responses in key molecules involved in parturition and premature rupture of membranes (PPROM), including prostaglandin E2 (PGE2) and two matrix metalloproteinases (MMP 1 and 3).
Materials and methods
Reagents and materials
The GBS used in this study was strain A909, initially isolated from a septic newborn and transformed with plasmid encoding genes for green fluorescent protein and erythromycin resistance (construct RS020, a gift from Amanda Jones, University of Washington). GBS was grown at 37°C in planktonic culture using Todd Hewitt Broth (Becton-Dickinson, Franklin Lakes, NJ) or on sheep's blood agar plates (Blood Agar Base #2, Remel, Lenexa, KS and BBL defibrinated sheep blood, Franklin Lakes, NJ) with 5 μg/mL erythromycin (Acros Organics, Geel, Belgium). Media (DMEM catalog #21063 and DMEM:F12 catalog #11039), buffers, fetal bovine serum (FBS; catalog #10438), and penicillin/streptomycin (pen/strep; catalog #15140) were from GIBCO/ThermoFisher Scientific (Grand Island, NY).
Tissue collection
Human extraplacental membranes were collected from healthy pregnancies undergoing scheduled cesarean delivery at term or near term (36–40 weeks) prior to onset of active labor at the University of Michigan Birth Von Voigtlander Women's Hospital Birth Center. Only healthy, nonsmoking, singleton mothers were included. Women were excluded if they had spontaneous rupture of membranes, demonstrated more than six contractions per hour, significant obstetrical complications such as preterm labor or cervical cerclage, third trimester bleeding, multifetal pregnancy, suspicion for active vaginal or intrauterine infection, immunocompromised conditions, or major medical conditions (e.g., diabetes, hypertension, collagen vascular disease, chronic renal disease, sarcoidosis, hepatitis, HIV). Patients were also excluded if pathological evaluation of the placenta or membranes was warranted. Except for preoperatively administered antibiotics, women were excluded if prescription antibiotics were used during the 2 weeks preceding delivery. The University of Michigan Institutional Review Board approved this research (IRBMED#HUM0013915).
Culture of extraplacental membranes
Extraplacental membranes were cultured as membrane punches or in a two-compartment transwell system as described previously [22, 24]. For membrane punches, membranes were dissected from placenta immediately following caesarian delivery and transported to the lab in Dulbecco phosphate-buffered saline. Membranes were rinsed with medium and blood clots were removed. Membranes were then blunt dissected to separate the choriodecidua from the amnion. After dissection, the choriodecidua was punched using a 12-mm biopsy punch. Tissue punches were placed in 12-well plates, a single punch per well, with 1 mL medium containing Dulbecco Modified Eagle Medium (DMEM) supplemented with 1% FBS and pen/strep. Cultures were incubated at 37°C and 5% CO2. After 4 h, the medium was exchanged for DMEM/1% FBS without antibiotics.
For transwell two-compartment systems, the full-thickness membranes were mounted on sterile transwell frames that had no synthetic membrane (gift from Corning, NY) and held in place with sterile latex elastic bands (ORMCO, Orange, CA). The membranes were affixed with the choriodecidua facing the inner chamber of the transwell insert and the amnion facing the outer chamber. The transwell inserts with membranes were placed in the wells of 12-well culture plates containing DMEM supplemented with 1% FBS and pen/strep. To maintain equal medium levels between the inner and outer transwell chambers, and thereby avoid potential confounding of results due to hydrostatic pressure, 0.5 mL medium was added to the smaller inner chamber and 1.5 mL medium was added to the larger outer chamber. Cultures were incubated at 37°C and 5% CO2. After 4 h, the medium was exchanged for DMEM/1% FBS without antibiotics.
Group B streptococcus coculture with extraplacental membranes
For GBS treatment of choriodecidual punches, GBS in early exponential growth phase was diluted with DMEM/1% FBS to an estimated 1 × 106 colony forming units/mL (CFU/mL). This concentration of GBS was used because it has been shown to increase inflammatory cytokines and antimicrobial peptides in previous experiments and was sufficient to induce inflammation and contractions in nonhuman primates [19, 22, 25, 26]. Inoculant concentrations were validated by overnight growth on 5% sheep blood agar with erythromycin. The medium of the choriodecidual punches was replaced with 1 mL GBS inoculant (∼1 × 106 CFU/mL) or fresh DMEM/1%FBS (control). Following 4, 8, and 24 h of incubation with GBS, the choriodecidual punches were collected in RNA stabilizing reagent and stored at –80°C before RNA extraction. RNA samples from three membrane punches per woman were pooled and n = 4 women per experimental condition (i.e., control and GBS treated at three different time points for a total of 24 RNA samples).
For transwell cultures, the medium of the inner transwell choriodecidual compartment was replaced with 0.5 mL GBS inoculant (1 × 106 CFU/mL) or fresh DMEM/1%FBS without GBS (controls). Amnion compartment medium was also replaced with DMEM/1%FBS. After coculturing with GBS for 24 h, medium from both amniotic and choriodecidual compartments was passed through a 0.2 μm pore filter and then stored at –80°C for subsequent enzyme-linked immunosorbant assay (ELISA). Coculture experiments were conducted in triplicate wells using extraplacental membranes from six to nine women.
Affymetrix microarray analysis
After culture with treatments, choriodecidual punches were homogenized in radioimmunoprecipitation assay (RIPA) buffer with Lysing Matrix D beads using 2–40 s pulses with a 5 min rest on ice on the FastPrep instrument (MP Biosciences). Total RNA from punches was then extracted using RNeasy Plus Mini kits (Qiagen, Valencia, CA). RNA samples from three replicate membrane punches per woman were pooled (n = 4 women) for the microarray analysis. RNA from three to four women was pooled for the RT-PCR analysis. TURBO DNA-free procedure was then conducted to remove possible genomic DNA contamination. After the clean-up, RNA samples were submitted to the University of Michigan MicroArray Core for Affymetrix microarray analysis (Human Gene ST 2.1 plate). A total of 24 arrays were performed, four each for control and GBS at time points 4, 8, and 24 h.
Data from 24 raw .CEL files were normalized using RMA with default parameters in R version 3.1.2. We checked Probe Density, Normalized Unscaled Standard Errors (boxplot), and principal components analysis graphics to assess data quality. We did not observe any significant outliers or differences in quality among the arrays. Tests for differential expression between GBS-infected and uninfected membranes after 4-, 8-, and 24-h infection were performed using limma [27] with arrayWeights, eBayes, and intensity-based moderated t-statistics (IBMT) [28] corrections. IBMT provides improved estimates of variance based on genes with similar expression levels using an empirical Bayesian model. P-values were corrected for multiple testing using the Benjamini and Hochberg False Discovery Rate (FDR) approach. Genes were selected as being differentially expressed if they changed ≥ ± 1.5 fold and had IBMT FDR ≤ 0.05. All Affymetrix data are available in Gene Expression Omnibus, accession ID GSE96557.
Real Time Quantitative Reverse Transcription PCR (qRT-PCR)
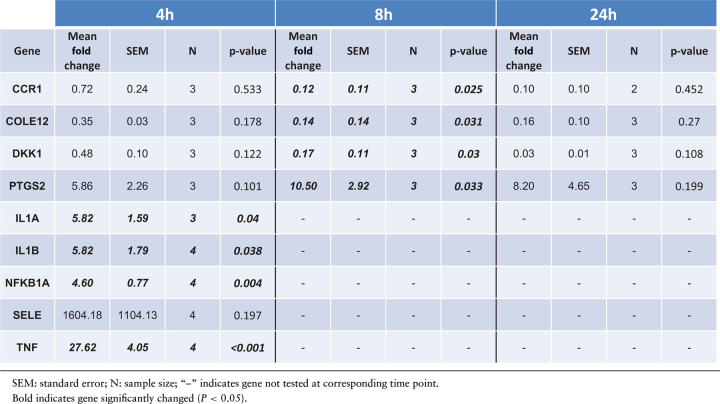
For qRT-PCR, the cDNA was synthesized with 1 μg total RNA using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA) according to manufacturer's instructions. We validated the findings of the arrays for selected genes with significant mRNA expression changes with GBS coculture at 4 h (FDR < 0.05, log2 (fold change) ≥2): tumor necrosis factor (TNF), selectin E (SELE), C-C motif chemokine ligand 3 like 3 (CCL3L3), C-C motif chemokine ligand 4 (CCL4), C-C motif chemokine ligand 20 (CCL20), interleukin 6 (IL6), G0/G1 switch 2 (G0S2), interleukin 1 beta (IL1B), NFKB inhibitor alpha (NFKBIA), interleukin 1 alpha (IL1A), and prostaglandin-endoperoxide synthase 2 (PTGS2). Although IL1A and PTGS2 did not meet the criteria, they were included for validation because of their importance in the process of parturition. In addition, the genes with low expression were excluded. CCL3L3 and CCL20 were excluded later due to low efficiency of the primers or lack of gene-specific primer pairs. Although there were not significantly downregulated genes at 4 h, collectin subfamily member 12 (COLEC12), dickkopf WNT signaling pathway inhibitor 1 (DKK1), and C-C motif chemokine receptor 1 (CCR1) were validated because they were significantly downregulated at 8 and 24 h. The qRT-PCR reactions were prepared with SsoAdvanced SYBR Green Supermix and primers, and run on a Bio-Rad CFX96 Real time C1000 thermal cycler following the manufacturer's recommended protocols. The following conditions were used for PCR: 95°C for 10 min, followed by 39 cycles of 15 s at 95°C, and then 5 s at 60°C. The mRNA levels of each gene of interest were normalized to β-2-micoglobulin mRNA levels and presented as fold change compared to solvent controls. All pooled RNA samples (from three replicate punches per woman) were run in triplicate. Data were analyzed using CFX software (BioRad, Hercules, CA).
Pathway enrichment analysis
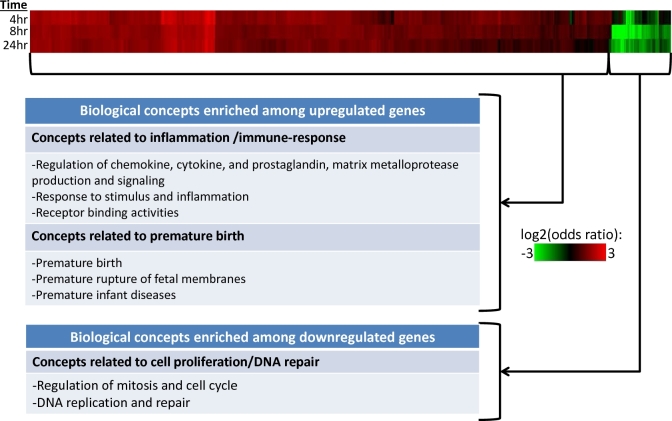
To identify biologically defined gene sets significantly enriched among genes upregulated or downregulated in GBS-inoculated membranes, we used LRpath [29]. LRpath tests for enrichment of biological processes, pathways, and relevant terms across several databases, including Gene Ontology (GO) terms, Medical Subject Headings (MeSH), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, Drug Bank, and Metabolite annotations. We used the directional method in LRpath for each of the three time points. This method allows for the discrimination between biological concepts enriched with either upregulated or downregulated genes. We checked for consistency of results across the three time points to identify biologically relevant trends over time. To visualize how the affected pathways change over time, we then clustered pathways using average linkage hierarchical clustering, then created a heatmap of enrichment testing results across the three time points using the resulting log2(odds ratio) for enrichment and including gene sets with P-value < 0.001 in at least two comparison(s). LRpath was used for pathway enrichment and clustering analysis while Java TreeView was used to create and visualize the heatmap.
Networks for inflammation and premature birth
Protein interaction networks provide a method of visualizing how a set of proteins may impact each other (binding to each other, regulating each other's expression or activity, etc.). Well-defined networks (e.g., Wnt signaling) are annotated as pathways in multiple databases, though many other networks are not yet defined. By developing and visualizing these novel protein interaction networks, we may improve our understanding of the biological relevance of a gene set. Based on the LRPath enrichment testing results, we used Metacore (Thomson Reuters) to develop novel protein interaction networks for the MeSH terms “inflammation” and “premature birth” using Metacore (Thomson Reuters). For each protein interaction network, we set Metacore parameters to build a parsimonious network—the simplest model that incorporates all of the data (shortest path algorithm, direct interactions only). A parsimonious network maximizes our understanding of the biological relevance of a gene set, with a minimum of extraneous interactions. In both cases, all but one of the genes were connected to the network by at least one interaction, consistent with a tightly connected set of genes.
Prostaglandin E2 and matrix metalloproteinase enzyme-linked immunosorbant assays
PGE2 concentrations in GBS choriodecidual and amnion conditioned medium from transwell cultures were measured using a commercially available ELISA kit according to manufacturer's instructions (R&D systems, Minneapolis, MN). The PGE2 ELISA detection range was 16–2000 pg/mL. MMP concentrations in GBS choriodecidual conditioned medium were measured by the University of Michigan Immunologic Monitoring Core using commercially available ELISA kits (R&D Systems) that detect pro and active forms of the MMPs analyzed. MMP detection ranges were as follows: 156.0–10,000 pg/mL (MMP1), 0.6–20 ng/mL (MMP2), 31.2–2000 pg/mL (MMP3), and 31.2–2000 pg/mL (MMP9). These samples were diluted as necessary. PGE2 and MMP concentrations are reported as pg/mL medium.
Statistical analysis
Data from MMP and PGE2 ELISAs are expressed as mean ±SEM and were analyzed using SigmaStat 3.5 software (SigmaStat Software, San Jose, CA). Mixed effects models and t-tests were used to compare differences in levels of MMPs and PGE2, respectively in control vs. GBS-treated media. Data were considered significant if the P-value was <0.05.
Results
Differentially regulated genes from group B streptococcus inoculation over time
Transcriptome profiling of human extraplacental membranes inoculated with GBS for 4, 8, and 24 h was conducted using the Affymetrix Human Gene ST 2.1 array. Gene expression of GBS-cocultured membrane punches at each time point was compared to time-matched nontreated (NT) controls. We identified 41, 127, and 635 genes differentially regulated by GBS infection compared to NT at 4, 8, and 24 h, respectively (Supplementary Table S1), suggesting that a few select genes change early (4 h) leading to more extensive changes at 8 h and even more at 24 h. Notable genes showing significant differential expression at all three time points include TNF, TNF alpha induced protein 3 (TNFAIP3), IL6, IL1B, interleukin 23 subunit alpha (IL23A), NFKBIA, NFKB inhibitor zeta (NFKBIZ), TNF receptor associated factor 1 (TRAF1), and PTGS2 (Supplementary Table S1). Consistent with the array results, qRT-PCR analysis showed that GBS treatment for 4 h significantly increased mRNA expression of IL1A, ILB, NFKB1A, and TNF by 5.8 fold, 5.8 fold, 4.6 fold, and 27.6 fold, respectively. Expression of PTGS2 was significantly upregulated at 8 h by 10.5 fold, although its expression was not significantly changed at 4 and 24 h possibly due to large variability. Also consistent with the array results, qRT-PCR showed that mRNA expression of DKK1, CCR1, and COLE12 was decreased with GBS at 8 h by 0.17 fold, 0.12 fold, and 0.14 fold, respectively (Table 1; P < 0.05).
Table 1.
Validation of microarray data for select genes using qRT-PCR.
|
Identification of biological concepts differentially regulated with group B streptococcus treatment
Functional enrichment analysis was conducted with the array results using LRpath to identify biological concepts differentially regulated with GBS stimulation across time. The complete LRpath results are available in Supplementary Table S2. We then performed clustering analysis using the LRpath application on concepts exhibiting significant enrichment (P < 0.001) for at least two of three time points (4, 8, 24 h). The clustering analysis results for GO terms and pathways revealed tightly clustered up- and downregulated concepts (Figure 1). Enriched biological pathways among upregulated genes include innate immune response-related pathways: regulation of chemokine, cytokine, and prostaglandin (PG) production and activity (IL1A, IL1B, C-X-C motif chemokine ligand 8 [CXCL8], TNF, and PTGS2), response to stimulus and inflammation (NFKBIA, NFKBZ) (see network analysis, below), differentiation and recruitment of immune cells, and receptor binding activities (IL1A, IL1B, CCR1, C-C motif chemokine receptor 5 [CCR5], and TRAF1). In addition, prematurity-related pathways such as premature birth (see network analysis, below), premature rupture of fetal membranes, and premature infant diseases related to prematurity were enriched among upregulated genes. Pathways related to cell cycle regulation, mitosis, DNA replication, DNA repair were enriched among downregulated genes, suggesting that GBS may suppress cell proliferation and DNA repair in gestational membranes. The complete list of enriched pathways from the cluster analysis is available in Supplementary Table S3.
Figure 1.
Significantly enriched biological concepts among differentially expressed genes in GBS-treated extraplacental membrane cultures. Extraplacental membranes were obtained from pregnant donors and inoculated with GBS for 4, 8, or 24 h followed by RNA extraction. Microarray analysis was used to identify differentially expressed genes. LRPath software was then used to identify significantly enriched biological concepts (e.g., KEGG pathways, GO terms) among differentially expressed genes. Enriched biological concepts were identified by an enrichment P-value < 0.001 for at least two time points. Color intensity indicates log2(odds ratio) for enrichment (green = downregulated concepts, red = upregulated concepts).
Network analysis for inflammation and premature birth pathways
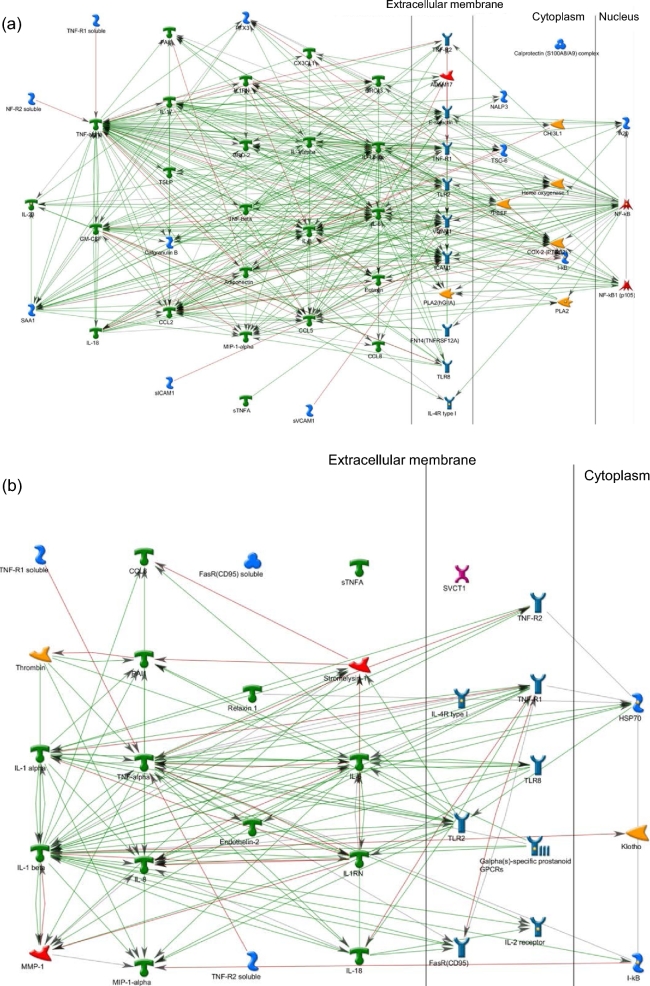
In the LRPath analysis, the MeSH terms “inflammation” and “premature birth” were highly enriched among differentially expressed genes (FDRs of 2.7 × 10−24 and 9.8 × 10−14, respectively), consistent with our hypothesis that GBS activates inflammatory pathways important in the etiology of preterm birth. To help us understand the biological relevance of these results, we used network building to visualize interactions among the differentially expressed genes annotated for “inflammation” (MeSH) (Figure 2a) and “premature birth” (Figure 2b). Green arrows indicate positive interactions between objects (activation), while red arrows show negative interactions (inhibition). Gray arrows represent unspecified interactions. For both networks, we observe very highly connected gene sets, suggesting that these genes work very closely together. For the inflammation network, the major hubs include TNF (82 edges), IL1B (61 edges), IL6 (52 edges), CCL2 (40 edges), and NFKB (40 edges) (Figure 2a; Supplementary Table S5). For the premature birth network, TNF (39 edges), IL1B (33 edges), IL6 (23 edges), IL1A (17 edges), and TLR2 (16 edges) were the top hubs (Figure 2b; Supplementary Table S5). For both networks, TNF was a convergence hub, suggesting a central role of TNF in the regulation of both networks. A large overlap in the gene lists (14 genes) for the two networks suggests extensive interplay between the pathways. For both networks, placenta is one of the tissues annotated for enriched expression in the relevant gene set (Supplementary Table S4 and S5). Enriched disease-related annotations for the inflammation network include rheumatoid arthritis, rheumatic diseases, arthritis, joint diseases and inflammation, consistent with the inflammation phenotype (Supplementary Table S4). Enriched disease-related annotations for the premature birth network include connective tissue diseases, obstructive lung disease, immediate hypersensitivity, and asthma but does not include birth or reproduction-related diseases (Supplementary Table S4), suggesting that the network with this particular set of gene interactions is novel for the premature birth phenotype. Additional disease annotations for the preterm birth network were infection and bacterial infections and mycoses, relevant to the GBS treatment used in this study.
Figure 2.
Networks for inflammation and premature birth. Significantly enriched biological concepts among genes impacted by GBS treatment were identified with LRPath software. Networks were constructed for the union of gene sets that LRPath found to be significant at 4, 8, and 24 h for the biological concepts (a) “inflammation” and (b) “preterm birth,” as defined in the MeSH database. Green arrows indicate positive interactions between objects (activation), while red arrows show negative interactions (inhibition). Gray arrows represent unspecified interactions.
Effect of group B streptococcus on matrix metalloproteinase release from extraplacental membranes
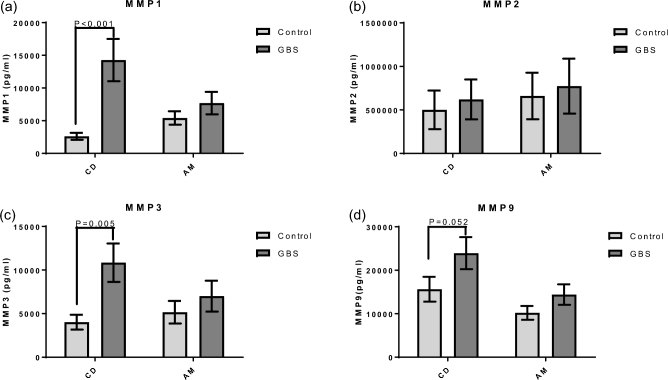
Because MMP-related pathways were enriched in the LRPath and cluster analysis, and MMPs play critical roles remodeling the cervix and extraplacental membranes during labor [23], MMP release from GBS-stimulated extraplacental membranes was measured using ELISA. Extraplacental membranes in transwell culture released increased amounts of MMP1 (5.5 fold) and MMP3 (2.7 fold) into the medium of the choriodecidual compartment after 24-h exposure to GBS (Figure 3a and c, P < 0.05). MMP9 secretion into the choriodecidual compartment was increased with GBS at 24 h, though it was not statistically significant (Figure 3d, P = 0.052). The levels of MMP1, MMP3, MMP9 in the amniotic compartment were not significantly changed with GBS at 24 h. MMP2 release did not change significantly either in the choriodecidual or in the amniotic compartments with GBS stimulation for 24 h (Figure 4B).
Figure 3.
GBS-stimulated MMP release from gestational membranes. Extraplacental membranes were obtained from pregnant donors and cultured in a transwell system with choriodecidua (CD) and amnion (AM) facing the inner and outer chambers, respectively. Cultures were inoculated with GBS (1 × 106 CFU/mL) for 24 h followed by collection of media from CD and AM compartments. Levels of MMPs 1, 2, 3, and 9 (a–d, respectively) were then detected in media via ELISA assay. Mixed effects models were used to identify significant difference in MMP levels between GBS-treated and non-treated control samples (P < 0.05). N = 9 women (2–4 replicates/woman).
Figure 4.
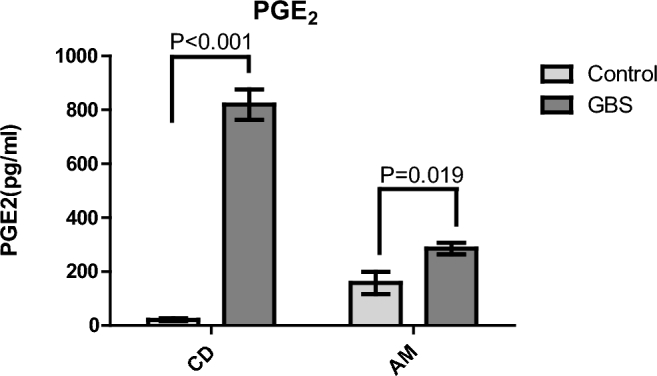
GBS-stimulated PGE2 release from gestational membranes. Extraplacental membranes were obtained from pregnant donors and cultured in a transwell system with choriodecidua (CD) and amnion (AM) facing the inner and outer chambers, respectively. Twenty-four hours after inoculation with GBS (1 × 106 CFU/mL) media was collected from CD and AM compartments. Levels of PGE2 in media were then detected via ELISA assay. T-tests (P < 0.05) were used to identify significant differences in PGE2 levels between GBS-treated and control samples. N = 6 women (3 replicates/woman).
Effects of group B streptococcus on prostaglandin E2 release from extraplacental membranes
PGE2 is known to initiate the process of labor, and increased PGE2 release from gestational compartments has been associated with preterm birth [30]. We observed that mRNA expression of PTGS2 was significantly increased with GBS in the present study (Table 1). Added to this, PG-related pathways were highly enriched in the LRpath analysis, and PTGS2 plays role in the network for premature birth pathway. GBS-mediated PGE2 release from extraplacental membranes was measured in transwell culture medium at 24 h. Secretion of PGE2 into the medium significantly increased with GBS compared to tissue not exposed to GBS in the choriodecidual (38.7 fold) and amniotic compartments (1.8 fold), consistent with the array and network analysis (Figure 4).
Discussion
GBS remains a serious public health issue, though relatively few studies have examined mechanisms by which GBS promotes host responses in relevant human tissues. Furthermore, much remains unknown about how GBS impacts cellular pathways over time in human gestational tissue, particularly at early time points. To our knowledge, this is the first time transcriptomic profiles of extraplacental membranes have been analyzed under conditions of GBS inoculation. This transcriptomic analysis demonstrates that GBS activates genes involved in innate immune response and preterm birth pathways in human extraplacental membranes as early as 4 h after inoculation. Our pathway-based analysis was further supported by PCR validation of significantly changed genes (IL1A, IL1B, NFKB1A, TNF, and PTGS2). Responses in pathways were linked to increased release of molecules with direct functional relevance to parturition and are strongly associated with both inflammation and adverse birth outcomes, i.e. MMPs and PGE2 [31, 32].
To determine the molecular pathways activated within human fetal membranes upon GBS infection, we exposed human extraplacental membranes to GBS and analyzed gene expression microarray results over at three time points over a 24-h period using functional enrichment, clustering, and network analyses. Our results demonstrate a large-scale upregulation of innate-immune-related and prematurity-related pathways that was observed at 4 h after GBS inoculation, consistent with our hypothesis that GBS activates these pathways leading to preterm birth. Network analysis of the genes contained in the MeSH defined biological concepts “inflammation” and “premature birth” (both significantly enriched after GBS inoculation) revealed a highly coordinated set of genes with 24 genes overlapping between the two networks.
The enrichment of the biological concept “inflammation” has direct implications for GBS-induced risks for adverse birth outcomes and is consistent with studies showing that GBS induces inflammation in gestational tissues. Specifically, during intrauterine infection there is stimulation in the production of inflammatory cytokines such as interleukins, tumor necrosis factor, and granulocyte colony-stimulating factors [33]. Indeed, multiple studies have reported that GBS stimulates the release of inflammatory cytokines from human extraplacental membranes [18, 19, 21, 22, 34]. These cytokines stimulate gestational tissues to produce PGs such as PGE2 and PGF2A [35]. In extrauterine sites, PGE2 regulates the release of inflammatory cytokines, acts synergistically with CXCL8 to augment neutrophil chemotaxis, and stimulates the release of MMPs [36–38].
Enrichment of the biological concept “premature birth” in the analysis of GBS-stimulated gene expression changes in extraplacental membranes provides new support for a potential role of GBS in preterm birth. This finding is particularly interesting because of the lack of clear clinical human data on the potential association of preterm birth with GBS infection of the gravid reproductive tract [12, 39]. Clinical studies supporting a link between GBS infection and preterm birth include studies that show genital GBS colonization of pregnant women associated with early term births and low birth weight [8] and with premature labor [9, 10]. Adding further support, GBS was the most common microorganism isolated from maternal and fetal tissues of women with midgestation spontaneous abortion [40], and histological chorioamnionitis and preterm birth at less than 32 weeks gestation were associated with GBS isolation from extraplacental membranes [3, 11]. In a review of studies published between 1996 and 2008, Valkenburg-van den Berg et al. [39] identified 20 studies that met the review criterion of reported preterm birth rates in women with and without GBS colonization who did not receive antibiotic treatment during pregnancy, and conducted meta-analyses of these studies. In the latter review, a random effect meta-analysis of 11 cohort studies failed to detect significantly increased relative risk for preterm birth in women with GBS colonization; however, pooled estimates from five cross sectional studies and three case control studies that measured colonization at delivery showed elevated odds ratios for preterm birth of 1.75 (95% CI 1.43–2.14) and 1.59 (95% CI 1.03–2.44), respectively, and pooling the cross sectional and case control studies yielded odds of 1.76 (95% CI 1.44–2.15) [39]. The authors of the latter review noted significant inconsistencies among the studies, including differences in methods (especially for GBS detection), definitions (e.g., preterm delivery and GBS colonization), follow-up, and statistical adjustment for known preterm birth risk factors, among other concerns. A more recent but general review of gravid genitourinary tract infections in relation to preterm birth concluded that “the few studies published since 2008 have failed to support an association between colonization with GBS and preterm birth,” yet restated concerns of Valkenburg-van den Berg et al. [39] about the rigor of the studies and called for randomized controlled trials to evaluate the effect of GBS screening earlier in pregnancy on adverse pregnancy outcomes including preterm birth [12]. Despite the state of clinical findings, recent studies have elucidated mechanisms by which GBS induces a preterm birth phenotype in mouse models, demonstrating that a GBS-produced toxin (ornithine rhamnopolyene lipid/pigment) contributes to fetal injury and preterm birth via activation of the inflammasome as well as by promoting bacterial invasion of the placenta [13–17]. Our data, together with compelling animal model findings, lend support to the need for more rigorous examination of the role of GBS in preterm birth in human populations.
Our results indicate that GBS strongly stimulated secretion of PGE2 in response to GBS, and this increase was transcriptionally regulated, with highly induced expression of PTGS2, the gene for cyclooxygenase 2 (COX2). COX2 is a rate-limiting enzyme in the synthesis of PGs [41]. Increased PTGS2 mRNA expression and PGs in gestational compartments are associated with preterm birth [42, 43], and PGE2 and PGF2A stimulate uterine contraction during parturition [31, 44]. It has been shown that chorion cells isolated from human placenta produced PGE2 in response to heat-killed strains of GBS [45]. In pregnant rhesus monkeys, inoculation of GBS led to increased uterine contractility and release of IL1, IL6, PGE2, and PGF2A [26, 46]. The current study shows that a similar response is observed in human gestational tissue. These increased PGE2 levels lend further support to an additional mechanism by which GBS may contribute to risk of preterm birth, through PGE2-mediated increases in uterine contractibility.
Our transcriptomic results indicate that GBS-stimulated cytokine responses in extraplacental membranes were transcriptionally regulated. Of particular note, we found that TNF was a common factor in both inflammation and preterm birth transcriptional networks. This observation is consistent with various studies that have found that TNF plays a prominent role in the etiology of preterm birth [47–49]. In addition to its central role as a transcriptional regulator for the inflammation and preterm birth networks, upregulation of TNF could contribute to the preterm premature rupture of the membranes in cases of GBS infection by promoting apoptosis and could play a role in membrane rupture through this mechanism [50, 51].
Pathways related to cell cycle regulation, mitosis, DNA replication, and DNA repair were enriched among downregulated genes, suggesting that GBS may suppress cell proliferation and DNA repair in extraplacental membranes. Alterations in these processes could have implications for preterm birth because DNA damage associated with cell death is thought to play a role in membrane rupture during labor. Decreased capacity for DNA repair during GBS infection could lead to fragility of extraplacental membranes and increase the risk of preterm birth, as GBS has been found to induce pathways such as apoptotic DNA fragmentation in choriodecidua in a nonhuman primate model [52, 53]. In addition, Vanderhoeven et al. [53] found that GBS inoculation in nonhuman primates caused a downregulation of cytokeratin and cytoskeletal-related genes, suggesting that GBS infection weakens extraplacental membrane integrity and contributes to PPROM. The results of the current study provide further evidence that GBS infection contributes to weakening of extraplacental membrane integrity via the downregulation of DNA repair and cell proliferation.
The mRNA expression of DKK1, CCR1, and COLE12 were all significantly decreased in the choriodecidua by GBS 8 h after inoculation, suggesting that that changes in these genes are part of an early response to GBS infection. Although these genes are involved in various functions in female gestational tissues such as the placenta (see below), the roles of these genes in extraplacental membranes have not been extensively explored. Dickkopf-1 (product of DKK1) is a secreted glycoprotein that can antagonize the canonical Wnt signaling pathway. Accumulating evidence suggests that Wnt signaling is a pivotal pathway that promotes endometrial function, decidualization, trophoblast differentiation and invasion, and inappropriate activation of Wnt signaling is often associated with infertility, endometriosis, endometrial cancer, and gestational diseases [54, 55]. DKK1 is prominently expressed in human cytotrophoblast cell, but absent in the human placental choriocarcinoma cell lines JAR and JEG3, suggesting a correlation between DKK1 and carcinogenesis of placental choriocarcinoma. Exogenous introduction of DKK1 repressed proliferation in JAR and JEG3, induced apoptosis in JAR, and revealed significant tumor suppression effects of DKK1 in placental choriocarcinoma [56]. DKK1 has also been expressed in uterine tissue, with significantly lower expression in endometrial carcinoma than in benign endometrium [57]. In the placenta, DKK1 is expressed predominantly in the syncytiotrophoblast and the extravillous trophoblast, and its expression significantly increased in pre-eclamptic placental tissues compared to normal placental controls, suggesting that DKK1 overexpression might be associated with the process of the pathogenesis of pre-eclampsia [55].
Chemokine (C-C motif) receptor 1 (CCR1) was also decreased after GBS inoculation. Placental trophoblasts acquire CCR1 as they differentiate to an invasive phenotype at villous-anchoring sites, consistent with a novel role for the chemokine-CCR1 system in the initial step of trophoblastic invasion towards the maternal tissue [58]. Expression of CCR1 in placentas of patients with hypertensive disorder complicated pregnancies has been found to be significantly lower compared to controls [59].
Collectin subfamily member 12 (COLEC12) is a scavenger receptor that displays several functions associated with host defense. It can bind to carbohydrate antigens on microorganisms, facilitating their recognition and removal [60]. It promotes binding and phagocytosis of gram-positive, gram-negative bacteria, and yeast. COLE12 is expressed strongly in the placenta [61]. Scavenger receptor gram-positive bacteria (lipoteichoic acid), gram-negative bacteria (lipopolysaccharide), intracellular bacteria, and CpG DNA SR can alter cell morphology, and their expression is affected by various cytokines [62]. Thus, although DKK1, CCR1, and COLE12 clearly play important roles in gestational tissues such as placenta and decidua, much less is known about their roles in extraplacental membranes and how they influence responses to microbial infection. Given that these genes responded soon after GBS inoculation (significant decreases were observed after 8 h of GBS treatment but trends started as early as 4 h), their potential role as early molecular mediators of extraplacental membrane responses to GBS should be explored further.
Secretion of MMPs 1 and 3, increased by GBS in the current study, is thought to play a role in the rupture of membranes for term and preterm labor [63, 64]. MMPs are a family of proteases expressed initially as proenzymes/zymogens and whose activities are regulated by zymogen activation and endogenous tissue inhibitors of MMPs (TIMPs), in addition to regulation at the gene transcription level [65]. The current study did not distinguish between pro and active forms the MMPs, using an ELISA assay that detected both. MMPs can degrade connective tissue such as extraplacental membranes leading to rupture [66] and can also remodel the collagen in the cervix and soften it [67]. These two phenotypic changes are necessary for normal parturition and represent plausible mechanisms by which GBS may contribute to preterm labor. For example, GBS- and interleukin-1β-induced preterm labor and spontaneous term labor are accompanied by progressive increases in amniotic fluid MMP9 in rhesus monkeys [68, 69]. Researchers have also found that human extraplacental membranes mounted in transwell devices and treated with GBS showed an increased release of MMP9 and MMP2 [68, 69]. In our study, GBS significantly increased MMPs 1 and 3 in choriodecidual compartment but not in amniotic compartment. This result may be due in part to the fact that GBS was added to the inner transwell choriodecidual compartment but not to the outer amniotic compartment. Therefore, the direct contact of GBS with choriodecidua may contribute to the augmented MMP release in choriodecidua. The lack of significantly increased MMP release from amnion may indicate that cellular signaling from choriodecidua to amnion takes time, and that longer incubation with GBS may lead to higher MMP levels in amnion than those at 24 h. Zaga-Clavellina et al. (2011) compared MMPs 2 and 9 secretion in three different conditions with Escherichia coli in transwell cultures: choriodecidual infection, amniotic infection, and chorioamniotic infection [70]. They found that Escherichia coli induced an increase of pro-MMP2 and pro-MMP9 levels secreted to the culture medium mainly by the choriodecidual region regardless of which side of the membrane was the first zone of contact. However, when the stimulus was applied simultaneously on both sides of the membrane, the secretion of both zymogens was significant in both regions. This suggests that the choriodecidua may play a critical role in initial MMP release in response to bacterial infection and in the regulation of amniotic MMP response. Further study will be needed to confirm this differential response from two compartments and to elucidate potential mechanisms for it. In addition, further studies will be needed to fully characterize the responses of MMP3 in GBS-inoculated fetal membranes. While our microarray analysis showed that MMP1 gene expression was significantly upregulated after 24 h of treatment with GBS, MMP3 gene expression was not (Supplementary Table S1). Reasons for this discrepancy may include factors such as degree of variability of MMP3 expression in both treated and control samples as well as temporal differences between responses in gene and protein expression. Moreover, additional endpoints that impact on MMP activity, including assessment of TIMPs and assays to distinguish pro and active MMPs, would allow for a more complete assessment of GBS effects on this pathway.
There are several limitations that should be noted in this study. First, because these were ex vivo experiments using isolated extraplacental membranes, adaptive immune responses were not represented. In addition, despite robust and consistent responses to GBS inoculation, it should be noted that membranes were obtained from a limited sample size of women (n = 4 women per experimental condition). In order to determine the range of responses observed across a larger set of human subjects and how adaptive immune responses may alter the effect of GBS inoculation on extraplacental membranes, further experimentation is needed.
Conclusion
In conclusion, our analysis shows that GBS activates pathways related to inflammation and premature birth in human extraplacental membranes and downregulates pathways related to DNA replication and repair. These changes were observed as early as 4 h after treatment inoculation with GBS. These results are supported further by responses in key molecules involved in parturition including PGE2 as well as MMP1 and MMP3. To our knowledge, this is the first time responses to GBS treatment have been investigated in this tissue as early as 4 h postinoculation. Taken together, our results are consistent with our initial hypothesis and provide new information on molecular targets of GBS infection in this critical gestational tissue.
Supplementary data
Supplementary data are available at BIOLRE online.
Table S1. Table showing differentially expressed genes 4, 8 and 24 hours after GBS treatment
Table S2. Table showing all pathways identified by LRpath at 4, 8 and 24 hours after GBS treatment
Table S3. List of significantly enriched pathways identified by LRpath
Table S4. Genes included in Inflammation network identified using Metacore software
Table S5. Genes included in Premature Birth network identified using Metacore software
Footnotes
Grant Support: This work was supported by a research project to RL-C from the Global Alliance to Prevent Prematurity and Stillbirth, an initiative of Seattle Children's and the Bill & Melinda Gates Foundation (912001), and through the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), Superfund Research Program PROTECT Center (P42ES017198). Fellowship support to SMH was from the NIEHS, NIH (T32ES007062). Additional support was provided by the Omics and Bioinformatics Core of the Michigan Center for Lifestage Environmental Exposure and Disease through a center grant from the NIEHS, NIH (P30ES017885).
Accession Number: GSE96557
References
- 1. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000; 342:1500–1507. [DOI] [PubMed] [Google Scholar]
- 2. Basso B, Gimenez F, Lopez C. IL-1beta, IL-6 and IL-8 levels in gyneco-obstetric infections. Infect Dis Obstet Gynecol 2005; 13:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993; 81:941–948. [PubMed] [Google Scholar]
- 4. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009; 16:206–215. [DOI] [PubMed] [Google Scholar]
- 5. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36. [PubMed] [Google Scholar]
- 6. Jordan HT, Farley MM, Craig A, Mohle-Boetani J, Harrison LH, Petit S, Lynfield R, Thomas A, Zansky S, Gershman K, Albanese BA, Schaffner W et al. . Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr Infect Dis J 2008; 27:1057–1064. [DOI] [PubMed] [Google Scholar]
- 7. Hooper K, McDonald TA. The PBDEs: an emerging environmental challenge and another reason for breast-milk monitoring programs. Environ Health Perspect 2000; 108:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell K, Brou L, Bhat G, Drobek CO, Kramer M, Hill A, Fortunato SJ, Menon R. Group B Streptococcus colonization and higher maternal IL-1beta concentrations are associated with early term births. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 2013; 26:56–61. [DOI] [PubMed] [Google Scholar]
- 9. Allen U, Nimrod C, MacDonald N, Toye B, Stephens D, Marchessault V. Relationship between antenatal group B streptococcal vaginal colonization and premature labour. Paediatrics & Child Health 1999; 4:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seyyed EZ, Toossi E, Jalalvand A, Sajadi M. Group B Streptococci investigation in pre-term labors. Med Arch 2013; 67:124–125. [DOI] [PubMed] [Google Scholar]
- 11. Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol 1991; 165:955–961. [DOI] [PubMed] [Google Scholar]
- 12. Cunnington M, Kortsalioudaki C, Heath P. Genitourinary pathogens and preterm birth. Curr Opin Infect Dis 2013; 26:219–230. [DOI] [PubMed] [Google Scholar]
- 13. Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Adams Waldorf KM, Rajagopal L. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med 2013; 210:1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whidbey C, Vornhagen J, Gendrin C, Boldenow E, Samson JM, Doering K, Ngo L, Ezekwe EA Jr., Gundlach JH, Elovitz MA, Liggitt D, Duncan JA et al. . A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Mol Med 2015; 7:488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Equils O, Moffatt-Blue C, Ishikawa TO, Simmons CF, Ilievski V, Hirsch E. Pretreatment with pancaspase inhibitor (Z-VAD-FMK) delays but does not prevent intraperitoneal heat-killed group B Streptococcus-induced preterm delivery in a pregnant mouse model. Infect Dis Obstet Gynecol 2009; 2009:749432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boldenow E, Gendrin C, Ngo L, Bierle C, Vornhagen J, Coleman M, Merillat S, Armistead B, Whidbey C, Alishetti V, Santana-Ufret V, Ogle J et al. . Group B Streptococcus Circumvents Neutrophils and Neutrophil Extracellular Traps during Amniotic Cavity Invasion and Preterm Labor. Sci Immunol 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, Adams Waldorf KM, Rajagopal L. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. MBio 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boldenow E, Hassan I, Chames MC, Xi C, Loch-Caruso R. The trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine but not trichloroacetate inhibits pathogen-stimulated TNF-alpha in human extraplacental membranes in vitro. Reprod Toxicol 2015; 52:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boldenow E, Hogan KA, Chames MC, Aronoff DM, Xi C, Loch-Caruso R. Role of cytokine signaling in group B streptococcus-stimulated expression of human Beta defensin-2 in human extraplacental membranes. Am J Reprod Immunol 2015; 73:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menon R, Peltier MR, Eckardt J, Fortunato SJ. Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. American journal of obstetrics and gynecology 2009; 201:306e301–306. [DOI] [PubMed] [Google Scholar]
- 21. Peltier MR, Drobek CO, Bhat G, Saade G, Fortunato SJ, Menon R. Amniotic fluid and maternal race influence responsiveness of fetal membranes to bacteria. J Reprod Immunol 2012; 96:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod 2004; 71:1296–1302. [DOI] [PubMed] [Google Scholar]
- 23. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thiex N, Chames M, Loch-Caruso R. Tissue-specific cytokine release from human extra-placental membranes stimulated by lipopolysaccharide in a two-compartment tissue culture system. Reproductive Biology and Endocrinology 2009; 7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boldenow E, Jones S, Lieberman RW, Chames MC, Aronoff DM, Xi C, Loch-Caruso R. Antimicrobial peptide response to group B Streptococcus in human extraplacental membranes in culture. Placenta 2013; 34:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994; 171:1660–1667. [DOI] [PubMed] [Google Scholar]
- 27. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sartor MA, Tomlinson CR, Wesselkamper SC, Sivaganesan S, Leikauf GD, Medvedovic M. Intensity-based hierarchical Bayes method improves testing for differentially expressed genes in microarray experiments. BMC Bioinformatics 2006; 7:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim JH, Karnovsky A, Mahavisno V, Weymouth T, Pande M, Dolinoy DC, Rozek LS, Sartor MA. LRpath analysis reveals common pathways dysregulated via DNA methylation across cancer types. BMC Genomics 2012; 13:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazor M, Wiznitzer A, Maymon E, Leiberman JR, Cohen A. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci 1990; 26:425–428. [PubMed] [Google Scholar]
- 31. Kota SK, Gayatri K, Jammula S, Kota SK, Krishna SV, Meher LK, Modi KD. Endocrinology of parturition. Indian J Endocrinol Metab 2013; 17:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007; 25:21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keelan JA, Coleman M, Mitchell MD. The molecular mechanisms of term and preterm labor: recent progress and clinical implications. Clin Obstet Gynecol 1997; 40:460–478. [DOI] [PubMed] [Google Scholar]
- 34. Menon R, Pearce B, Velez D, Merialdi M, Williams S, Fortunato S, Thorsen P. Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Reproductive Biology and Endocrinology 2009; 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta 2003; 24Suppl A:S33–46. [DOI] [PubMed] [Google Scholar]
- 36. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol 2012; 188:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colditz IG. Effect of exogenous prostaglandin E2 and actinomycin D on plasma leakage induced by neutrophil-activating peptide-1/interleukin-8. Immunol Cell Biol 1990; 68 (Pt 6):397–403. [DOI] [PubMed] [Google Scholar]
- 38. Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood 2008; 111:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valkenburg-van den Berg AW, Sprij AJ, Dekker FW, Dorr PJ, Kanhai HH. Association between colonization with Group B Streptococcus and preterm delivery: a systematic review. Acta Obstet Gynecol Scand 2009; 88:958–967. [DOI] [PubMed] [Google Scholar]
- 40. McDonald HM, Chambers HM. Intrauterine infection and spontaneous midgestation abortion: is the spectrum of microorganisms similar to that in preterm labor? Infect Dis Obstet Gynecol 2000; 8:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shanmugam N, Todorov IT, Nair I, Omori K, Reddy MA, Natarajan R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (AGER), and in islets from diabetic mice. Diabetologia 2006; 49:100–107. [DOI] [PubMed] [Google Scholar]
- 42. Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta, -1 alpha, and -6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab 1993; 77:805–815. [DOI] [PubMed] [Google Scholar]
- 43. Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin endoperoxide H synthase (PGHS) activity and PGHS-1 and -2 messenger ribonucleic acid abundance in human chorion throughout gestation and with preterm labor. J Clin Endocrinol Metab 1998; 83:1358–1367. [DOI] [PubMed] [Google Scholar]
- 44. Behrman RE, Butler AS. Preterm Birth: Causes, Consequences, and Prevention. In: Behrman RE, Butler AS (eds.). Washington (DC); 2007. [PubMed] [Google Scholar]
- 45. Dudley DJ, Edwin SS, Dangerfield A, Van Waggoner J, Mitchell MD. Regulation of cultured human chorion cell chemokine production by group B streptococci and purified bacterial products. Am J Reprod Immunol 1996; 36:264–268. [DOI] [PubMed] [Google Scholar]
- 46. Gravett MG, Novy MJ. Endocrine-immune interactions in pregnant non-human primates with intrauterine infection. Infect Dis Obstet Gynecol 1997; 5:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 2006; 195:1578–1589. [DOI] [PubMed] [Google Scholar]
- 48. Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF 3rd. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol 2004; 190:1504–1508; discussion 1503A. [DOI] [PubMed] [Google Scholar]
- 49. Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, Bardy P, McDonald HM. Interleukins-1, -4, -6, -10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women: Risk of preterm birth. Am J Obstet Gynecol 2004; 191:2056–2067. [DOI] [PubMed] [Google Scholar]
- 50. Menon R, Lombardi SJ, Fortunato SJ. TNF-alpha promotes caspase activation and apoptosis in human fetal membranes. J Assist Reprod Genet 2002; 19:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kataoka S, Furuta I, Yamada H, Kato EH, Ebina Y, Kishida T, Kobayashi N, Fujimoto S. Increased apoptosis of human fetal membranes in rupture of the membranes and chorioamnionitis. Placenta 2002; 23:224–231. [DOI] [PubMed] [Google Scholar]
- 52. Kumagai K, Otsuki Y, Ito Y, Shibata MA, Abe H, Ueki M. Apoptosis in the normal human amnion at term, independent of Bcl-2 regulation and onset of labour. Mol Hum Reprod 2001; 7:681–689. [DOI] [PubMed] [Google Scholar]
- 53. Vanderhoeven JP, Bierle CJ, Kapur RP, McAdams RM, Beyer RP, Bammler TK, Farin FM, Bansal A, Spencer M, Deng M, Gravett MG, Rubens CE et al. . Group B streptococcal infection of the choriodecidua induces dysfunction of the cytokeratin network in amniotic epithelium: a pathway to membrane weakening. PLoS Pathog 2014; 10:e1003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation–review. Placenta 2010; 31:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Z, Li H, Zhang L, Jia L, Wang P. Differential expression of beta-catenin and Dickkopf-1 in the third trimester placentas from normal and preeclamptic pregnancies: a comparative study. Reprod Biol Endocrinol 2013; 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peng S, Miao C, Li J, Fan X, Cao Y, Duan E. Dickkopf-1 induced apoptosis in human placental choriocarcinoma is independent of canonical Wnt signaling. Biochem Biophys Res Commun 2006; 350:641–647. [DOI] [PubMed] [Google Scholar]
- 57. Yi N, Liao QP, Li T, Xiong Y. Novel expression profiles and invasiveness-related biology function of DKK1 in endometrial carcinoma. Oncol Rep 2009; 21:1421–1427. [DOI] [PubMed] [Google Scholar]
- 58. Sato Y, Higuchi T, Yoshioka S, Tatsumi K, Fujiwara H, Fujii S. Trophoblasts acquire a chemokine receptor, CCR1, as they differentiate towards invasive phenotype. Development 2003; 130:5519–5532. [DOI] [PubMed] [Google Scholar]
- 59. Li N, Liu Y, Wang X. Expressions of LKN-1,CCR1 and CCR3 in Placentas of Pregnant Women with Hypertensive Disorder. Journal of Practical Obstetrics and Gynecology 2010; 1. [Google Scholar]
- 60. Nakamura K, Funakoshi H, Miyamoto K, Tokunaga F, Nakamura T. Molecular cloning and functional characterization of a human scavenger receptor with C-type lectin (SRCL), a novel member of a scavenger receptor family. Biochem Biophys Res Commun 2001; 280:1028–1035. [DOI] [PubMed] [Google Scholar]
- 61. Ohtani K, Suzuki Y, Eda S, Kawai T, Kase T, Keshi H, Sakai Y, Fukuoh A, Sakamoto T, Itabe H, Suzutani T, Ogasawara M et al. . The membrane-type collectin CL-P1 is a scavenger receptor on vascular endothelial cells. J Biol Chem 2001; 276:44222–44228. [DOI] [PubMed] [Google Scholar]
- 62. Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol 2002; 14:123–128. [DOI] [PubMed] [Google Scholar]
- 63. Lockwood CJ, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikun G, Schatz F. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol 2005; 167:1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner S, Lockwood CJ. Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol 2004; 191:1996–2001. [DOI] [PubMed] [Google Scholar]
- 65. Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors — Novel strategies bring new prospects. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2017; 1864:1927–1939. [DOI] [PubMed] [Google Scholar]
- 66. Strauss JF., 3rd. Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci 2013; 20:140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gonzalez JM, Romero R, Girardi G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. J Reprod Immunol 2013; 97:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, Novy MJ. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol 2007; 197:518e511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vadillo-Ortega F, Sadowsky DW, Haluska GJ, Hernandez-Guerrero C, Guevara-Silva R, Gravett MG, Novy MJ. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol 2002; 186:128–138. [DOI] [PubMed] [Google Scholar]
- 70. Zaga-Clavellina V, Garcia-Lopez G, Flores-Pliego A, Merchant-Larios H, Vadillo-Ortega F. In vitro secretion and activity profiles of matrix metalloproteinases, MMP-9 and MMP-2, in human term extra-placental membranes after exposure to Escherichia coli. Reprod Biol Endocrinol 2011; 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.