Abstract
Objectives: Determination of the primary site of origin for mucinous neoplasms identified in the peritoneal and/or pelvic cavities may be challenging, with major differential diagnoses including appendiceal mucinous neoplasm (AMN) and ovarian mucinous neoplasm (OMN). Special AT-rich sequence binding protein 2 (SATB2) has been shown to be highly selectively expressed in the lower gastrointestinal tract, including the appendix.
Methods: We investigated the utility of a dual stain (DS) with SATB2 or caudal type homeobox 2 (CDX2) and cytokeratin 20 (CK20) or villin in distinguishing AMNs from OMNs. Tissue microarrays with 40 AMNs and 18 OMNs were stained with SATB2 or CDX2 paired with either CK20 or villin.
Results: SATB2 single stain showed a good sensitivity of 83% and the highest specificity of 78% for AMNs over OMNs among all four stains. DS with SATB2 and villin showed an identical sensitivity of 78% but specificity increased to 94%, while DS with SATB2 and CK20 showed a sensitivity of 80% and a specificity of 100%. In contrast, DS with CDX2 and CK20/villin showed slightly higher sensitivity but much lower specificity.
Conclusions: DS with SATB2/CK20 shows the greatest potential clinical utility in distinguishing AMNs from OMNs and is superior to DS with CDX2/CK20. Importantly, DS could be helpful for specimens with limited tissues.
Keywords: Dual immunostain, SATB2, CDX2, CK20, Villin, Appendiceal mucinous neoplasm, Ovarian mucinous neoplasm
Upon completion of this activity you will be able to:
• apply SATB2 and CK20 to assist in differentiating appendiceal mucinous neoplasms from ovarian mucinous neoplasms in real practice.
• compare the difference between SATB2 and CDX2 staining sensitivity and specificity in appendiceal mucinous neoplasms vs ovarian mucinous neoplasms.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™ per article. Physicians should claim only the credit commensurate with the extent of their participation in the activity. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Exam is located at www.ascp.org/ajcpcme.
Mucinous neoplasms (MNs) of various sites have a similar morphologic appearance to mucin-producing glandular epithelial cells and extracellular mucin. Although most cases of MNs arise from the gastrointestinal (GI) tract, they may also arise from the breast, lung, pancreas, and gynecologic tract. MNs frequently metastasize to the ovary, peritoneal surface, liver, or lung.1,2 Sometimes, the primary site of origin for MNs identified in the peritoneal and/or pelvic cavities is not easily discerned, and the major differential diagnosis includes appendiceal mucinous neoplasm (AMN) and ovarian mucinous neoplasm (OMN).
MNs express low-molecular-weight cytokeratins, such as cytokeratin (CK) 7, CK8, CK18, and CK20, with CK7 and CK20 having the most diagnostic value.1,3,4 In general, the lower GI tract mucinous neoplasms express CK20, whereas non-GI MNs express CK7.4 However, CK7 maybe expressed in up to 37% of appendiceal MNs, and CK20 may be expressed in 47% to 83% of ovarian MNs.5‐7 Similar results have been also found with another marker that has been used in the GI tract, villin.
Caudal type homeobox 2 (CDX2) is a transcription factor regulating intestinal epithelial cell differentiation and is strongly expressed in up to 100% of GI epithelial tumors, including AMNs.8,9 Previous studies have evaluated CDX2 expression in primary OMNs, and the reported range of expression of CDX2 in primary ovarian mucinous tumors is broad (0%-100%), with an average of 40%.7‐19
Besides CK20, CK7, and CDX2, other markers, including paired box gene 8 (PAX8) and estrogen receptor (ER), can be used to differentiate OMNs from AMNs. Although PAX8 is very specific for OMNs over AMNs that do not express PAX8, its sensitivity is low, since only 40% of OMNs express PAX8.20 Similar results have been found for ER expression in OMNs, with only 50% of OMNs expressing ER.21
Special AT-rich sequence binding protein 2 (SATB2) is a 733–amino acid human DNA-binding protein involved in transcriptional regulation and chromatin remodeling, and its expression is restricted to glandular cells lining the lower GI tract.22 A recent study demonstrated that SATB2 is a sensitive and highly specific marker for colorectal carcinoma (CRC) with distinct positivity in 85% of all CRCs, and SATB2 in combination with CK20 can identify 97% of CRCs.23 The same study also revealed 3.3% of ovarian cancers and 5.7% of lung adenocarcinomas were positive for SATB2, while all gastric and pancreatic cancers were negative for SATB2.23 Other studies showed that downregulated expression of SATB2 is associated with metastasis and poor prognosis in colorectal cancer.24,25 Besides expression in the lower GI tract, SATB2 is also a marker of osteoblastic differentiation in benign and malignant mesenchymal tumors.26,27 Two recent studies demonstrated that SATB2 was useful in differentiating OMN from AMN.28,29 Given the high sensitivity and specificity of SATB2 in CRC and the challenge in differentiating abdominal/pelvic mucinous neoplasms, we were interested in its utility compared with CDX2, especially in combination with CK20 or villin, in distinguishing AMNs from OMNs.
In this study, we explored the expression of SATB2, CDX2, CK20, and villin in both AMNs and OMNs by using dual stains (DSs) with one of the nuclear stains, SATB2 or CDX2, in combination with the cytoplasmic stain CK20 or villin and compared their sensitivities and specificities to determine whether any combination could provide improved diagnostic utility. DSs are particularly useful when limited material is available for staining.
Materials and Methods
Case Selection and Microarray Construction
After institutional review board approval from Ohio State University (OSU), an electronic pathology archive database search was performed for all primary AMNs and OMNs (including borderline tumors and carcinomas) diagnosed from 2000 to 2011 at OSU Wexner Medical Center. Forty AMNs and 18 OMNs were identified, and the diagnosis was confirmed after reviewing slides and correlating with patients’ clinical history. A tumor block representative of the primary tumor was collected from each case, and tissue microarrays (TMAs) were constructed with quadruplicate cores (AMNs) or duplicate cores (OMNs) of 1 mm for each tumor.
Dual Immunostains and Scoring
Double stains were performed on TMAs by pairing nuclear stain CDX2 (1:300; BioGenex, San Ramon, CA) or SATB2 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) with cytoplasmic stain CK20 (1:200; Dako, Carpenteria, CA) or villin (1:50; Cell Marque, Rocklin, CA). The 4-μm sections were dried at 55 °C for 3 hours and then subjected to heat-induced epitope retrieval for 20 minutes on a Leica-Bond Autostainer (Leica Biosystems, Buffalo Grove, IL). CDX2 and SATB2 were labeled as brown, and CK20 and villin were labeled as red. Bond Polymer Refine Detection Kits (Leica Biosystems) were used sequentially, and the sections were counterstained with hematoxylin. The Bond Polymer Refine Detection Kit was used as the brown chromogen, and the Bond Polymer Refine Red Detection Kit (Leica Biosystems) was used as the red chromogen. Stained slides were reviewed by three pathologists (R.R., J.R., and W.L.F.), and a consensus staining result was reached for each case. Staining results were scored as positive or negative, with a positive threshold of staining for 5% of tumor cells.
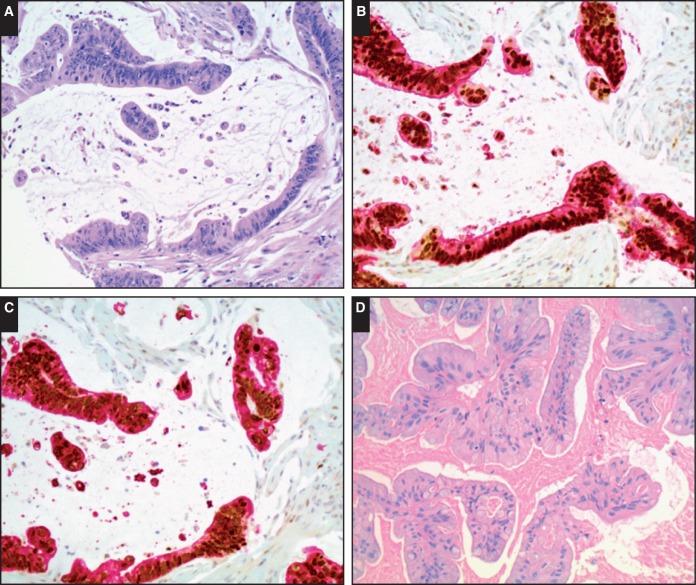
Representative images are demonstrated in Image 1 and Image 2.
Image 1.
Representative images of dual stains with special AT-rich sequence binding protein 2 (SATB2) and cytokeratin 20 (CK20) or villin in appendiceal and ovarian mucinous neoplasms. A, Invasive appendiceal mucinous carcinoma (H&E, ×200). B, Dual stain with both positive SATB2 (brown nuclear staining) and positive CK20 (red cytoplasmic staining) in invasive appendiceal mucinous carcinoma (×200). C, Dual stain with positive SATB2 and positive villin (red cytoplasmic staining) in invasive appendiceal mucinous carcinoma (×200). D, Ovarian mucinous borderline tumor (H&E, ×200). E, Dual stain with negative SATB2 and positive CK20 in ovarian mucinous borderline tumor (×200). F, Dual stain with negative SATB2 and positive villin in ovarian mucinous borderline tumor (×200).
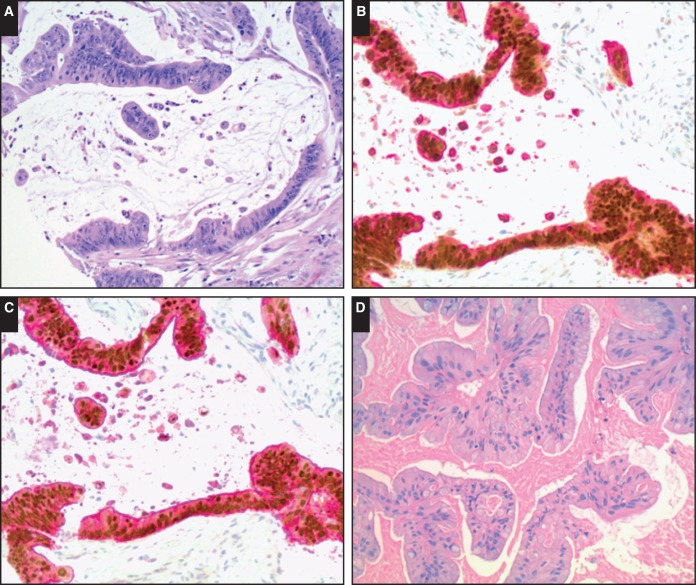
Image 2.
Representative images of dual stains with caudal type homeobox 2 (CDX2) and cytokeratin 20 (CK20) or villin in appendiceal and ovarian mucinous neoplasms. A, Invasive appendiceal mucinous carcinoma (H&E, ×200). B, Dual stain with positive CDX2 (brown nuclear staining) and positive CK20 in invasive appendiceal mucinous carcinoma (×200). C, Dual stain with positive CDX2 and positive villin in invasive appendiceal mucinous carcinoma (×200). D, Ovarian mucinous borderline tumor (H&E, ×200). E, Dual stain with scattered CDX2 positivity (arrows) and positive CK20 in ovarian mucinous borderline tumor (×200). F, Dual stain with scattered CDX2 positivity (arrows) and positive villin in ovarian mucinous borderline tumor (×200).
Statistical Analysis
The sensitivity, specificity, and overall percentage correct (in differentiating AMNs from OMNs) were calculated with exact binomial 95% confidence intervals (CIs) for each stain and DS combination separately. Next, sensitivity and specificity were directly compared between selected stains using McNemar’s test for paired comparisons; exact P values were calculated. The 95% CI for the differences between sensitivities and specificities were calculated using methods based on the score interval as previously described.30,31 All analyses were performed using SAS/STAT software, version 9.4 of the SAS System for Windows (SAS Institute, Cary, NC).
Results
We first evaluated the staining results in AMNs and OMNs for each marker individually. As shown in Table 1, SATB2 nuclear staining was present in 33 (83%) AMNs but only in four (22%) OMNs. CK20 cytoplasmic staining was present in 39 (98%) AMNs and nine (50%) OMNs. CDX2 nuclear staining was seen in 37 (93%) AMNs and eight (44%) OMNs. Villin cytoplasmic staining was identified in 38 (95%) AMNs and 14 (78%) OMNs.
Table 1.
Single-Stain Results in AMNs and OMNs for Each Marker
| Neoplasm | CDX2+, No. (%) | SATB2+, No. (%) | CK20+, No. (%) | Villin+, No. (%) |
|---|---|---|---|---|
| AMN (n = 40) | 37 (93) | 33 (83) | 39 (98) | 38 (95) |
| OMN (n = 18) | 8 (44) | 4 (22) | 9 (50) | 14 (78) |
AMN, appendiceal mucinous neoplasm; CDX2, caudal type homeobox 2; CK20, cytokeratin 20; OMN, ovarian mucinous neoplasm; SATB2, special AT-rich sequence binding protein 2; +, positive.
Next we evaluated the dual staining results. DS with SATB2 and CK20 showed positivity for either marker in all 40 AMNs (100%). Thirteen OMNs (72%) showed positive staining for either SATB2 or CK20. None of OMNs was positively stained for both SATB2 and CK20 simultaneously. In contrast, 32 AMNs (80%) showed positive staining for both SATB2 and CK20 simultaneously. For DS with CDX2 and CK20, 36 AMNs (90%) were positively stained for both markers, while eight OMNs (44%) also showed positive staining for both markers. DS with SATB2/villin or CDX2/villin showed positive staining for both markers in most AMNs. One OMN (6%) was positive for both SATB2 and villin; however, eight OMNs (44%) showed positive staining for CDX2 and villin simultaneously Table 2.
Table 2.
Dual-Stain Results in AMNs and OMNs for Each Combination
| Neoplasm | CDX2/CK20, No. (%) | CDX2/Villin, No. (%) | SATB2/CK20, No. (%) | SATB2/Villin, No. (%) |
|---|---|---|---|---|
| AMN (n = 40) | ||||
| Both (+) | 36 (90) | 35 (85) | 32 (80) | 31 (78) |
| Either (+) | 40 (100) | 40 (100) | 40 (100) | 40 (100) |
| Both (−) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| OMN (n = 18) | ||||
| Both (+) | 8 (44) | 8 (44) | 0 (0) | 1 (6) |
| Either (+) | 9 (50) | 14 (78) | 13 (72) | 17 (94) |
| Both (−) | 9 (50) | 4 (22) | 5 (28) | 1 (11) |
AMN, appendiceal mucinous neoplasm; CDX2, caudal type homeobox 2; CK20, cytokeratin 20; OMN, ovarian mucinous neoplasm; SATB2, special AT-rich sequence binding protein 2; +, positive; –, negative.
The sensitivity and specificity for each marker or each DS combination were determined. SATB2 single stain showed a sensitivity of 83% and a specificity of 78% for AMNs over OMNs while CDX2 single stain showed a sensitivity of 93% and a specificity of 56% for AMNs. CK20 single stain showed a sensitivity of 98% and a specificity of 50% for AMNs, while villin single stain showed a sensitivity of 95% and a specificity of 22% for AMNs. For DSs with both positive, DS with SATB2 and villin showed a sensitivity of 78% and a specificity of 94% for AMNs, while DS with SATB2 and CK20 showed a sensitivity of 80% and a specificity of 100% for AMNs. In contrast, DS with CDX2 and CK20 showed a slightly higher sensitivity (90%) but much lower specificity (56%) Table 3. DS with CDX2 and villin showed similar sensitivity (88%) and specificity (56%) to DS with CDX2 and CK20.
Table 3.
Sensitivity and Specificity for All Stainsa
| Type/Stain | Sensitivity (95% CI) | Specificity (95% CI) | Overall % Correct (95% CI) |
|---|---|---|---|
| Dual stains (both positive) | |||
| CDX2/CK20 | 0.90 (0.81-0.99) | 0.56 (0.31-0.78) | 0.79 (0.69-0.90) |
| CDX2/villin | 0.88 (0.77-0.98) | 0.56 (0.31-0.78) | 0.78 (0.65-0.87) |
| SATB2/CK20 | 0.80 (0.64-0.91) | 1.00 (0.81-1.00) | 0.86 (0.75-0.94) |
| SATB2/villin | 0.78 (0.62-0.89) | 0.94 (0.73-1.00) | 0.83 (0.71-0.91) |
| Single stain | |||
| CDX2 | 0.93 (0.80-0.98) | 0.56 (0.31-0.78) | 0.81 (0.69-0.90) |
| SATB2 | 0.83 (0.67-0.93) | 0.78 (0.52-0.94) | 0.81 (0.69-0.90) |
| Villin | 0.95 (0.83-0.99) | 0.22 (0.06-0.48) | 0.72 (0.59-0.83) |
| CK20 | 0.98 (0.97-1.00) | 0.50 (0.26-0.74) | 0.83 (0.71-0.91) |
CDX2, caudal type homeobox 2; CI, confidence interval; CK20, cytokeratin 20; SATB2, special AT-rich sequence binding protein 2.
Sensitivity and specificity are relative to differentiating appendiceal mucinous neoplasms vs ovarian mucinous neoplasms.
Finally, we directly compared the sensitivity and specificity among different single stains or DSs. No statistically significant differences were found in either sensitivity or specificity between SATB2 and CDX2 single stain (P = .289 for sensitivity and P = .388 for specificity). Furthermore, there were no statistically significant improvements in sensitivity for any DS compared with CDX2 single stain alone. However, both SATB2/CD20 and SATB2/villin DSs (both positive) had higher specificities compared with CDX2 alone (difference in specificity for SATB2/CD20 vs CDX2 alone = 0.44 [95% CI, 0.13-0.69]; difference in specificity for SATB2/villin vs CDX2 alone = 0.39 [95% CI, 0.06-0.64]; P < .05 for both comparisons). In addition, SATB2/CK20 DS (both positive) did show a significantly higher specificity in identifying AMNs than CDX2/CK20 DS (difference in specificity = 0.12; 95% CI, 0.09-0.17; P = .001).
Discussion
The appendix and ovary are the two main sites of origin for MNs presenting in the pelvis and/or peritoneum. Differentiation between these two sites of origin can, at times, be very challenging because MNs from either site can appear morphologically identical.32 Approximately 37% of all malignant appendiceal neoplasms are mucinous cystic neoplasms, and there is a female predominance.33,34 The female predominance in AMNs can lead to challenges when discerning the site of origin from the ovary. OMNs constitute 15% of all ovarian neoplasms, and most OMNs are unilateral.35 In addition, pathologists may have very little epithelium available for diagnosis, and if a panel of immunohistochemical (IHC) stains is necessary, tissue depletion can limit the number of stains that may be performed. Therefore, it is important to use IHC stains efficiently to determine site of origin without doing unnecessary stains to increase hospital costs for the patient and wasting tissue. Although multiple immunostains or different combinations of immunostains, including CK7, CK20, villin, CDX2, PAX8, and ER, have been previously investigated, none is able to effectively differentiate AMNs from OMNs with a high sensitivity and specificity. The IHC nuclear stain for SATB2 has recently been proven as a reliable marker for the lower GI tract, including the appendix.23 Two recent studies investigated the utility of SATB2 staining in differentiating OMN from AMN.28,29 Strickland et al28 found SATB2 was positive in 93.8% (30/32) of appendiceal tumors and in only one of 40 ovarian tumors. Moh et al29 found SATB2 was positive in 20 of 22 primary and metastatic AMNs but positive in only four of 12 mucinous cystadenomas with mature teratoma, and it was negative in 22 mucinous cystadenomas that lacked a component of mature teratoma, 60 mucinous borderline tumors, and 17 mucinous adenocarcinomas.
Consistent with the findings from these two recent studies, our data showed SATB2 stained most AMNs while showing almost no expression in OMNs with a sensitivity of 83% and a specificity of 78% for AMNs. Furthermore, our study demonstrated that the DS with SATB2 and CK20 showed positivity for either marker in 100% of AMNs, while none of OMNs was positively stained for both SATB2 and CK20 simultaneously, with a sensitivity of 80% and a specificity of 100% for AMNs. Our data demonstrated that SATB2/CK20 DS (both positive) had a significantly higher specificity in identifying AMNs than CDX2/CK20 DS (both positive).
The current study is limited by the small cohort size, as well as the fact that the sensitivity of the single stains was very high (>90% for all stains), making improvements in sensitivity with DSs difficult to detect. In addition, we used TMA rather than whole sections in this study to demonstrate the utility of these stains in a small biopsy specimen. Additional studies with larger cohorts are necessary to validate our findings.
In summary, the current study is one the few showing the utility of SATB2 to help distinguish AMN from OMN. Specifically, it demonstrates the utility even with small samples. In addition, to our knowledge, it is the first immunohistochemical analysis of these neoplasms with the use DSs of SATB2 or CDX2 with CK20 or villin, and it directly compared DSs of SATB2 and CK20/villin with DSs of CDX2 and CK20/villin. Our data suggest that DS with SATB2/CK20 shows potential clinical utility in distinguishing AMNs from OMNs and is superior to any single stain or CDX2/CK20 DS investigated in our study. In addition, our results suggest that DSs could be helpful for classification of specimens with limited tissues.
References
- 1. Chou YY, Jeng YM, Kao HL, et al. Differentiation of ovarian mucinous carcinoma and metastatic colorectal adenocarcinoma by immunostaining with beta-catenin. Histopathology. 2003;43:151-156. [DOI] [PubMed] [Google Scholar]
- 2. Seidman JD, Elsayed AM, Sobin LH, et al. Association of mucinous tumors of the ovary and appendix: a clinicopathologic study of 25 cases. Am J Surg Pathol. 1993;17:22-34. [DOI] [PubMed] [Google Scholar]
- 3. Prayson RA. Clinicopathologic study of 61 patients with ependymoma including MIB-1 immunohistochemistry. Ann Diagn Pathol. 1999;3:11-18. [DOI] [PubMed] [Google Scholar]
- 4. Vang R, Gown AM, Barry TS, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006;30:1130-1139. [DOI] [PubMed] [Google Scholar]
- 5. Chu PG, Chung L, Weiss LM, et al. Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am J Surg Pathol. 2011;35:1830-1836. [DOI] [PubMed] [Google Scholar]
- 6. McCluggage WG, Wilkinson N.. Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005;47:231-247. [DOI] [PubMed] [Google Scholar]
- 7. Vang R, Gown AM, Barry TS, et al. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod Pathol. 2006; 19:1421-1428. [DOI] [PubMed] [Google Scholar]
- 8. Kaimaktchiev V, Terracciano L, Tornillo L, et al. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol. 2004;17:1392-1399. [DOI] [PubMed] [Google Scholar]
- 9. Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310. [DOI] [PubMed] [Google Scholar]
- 10. Barbareschi M, Murer B, Colby TV, et al. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol. 2003;27:141-149. [DOI] [PubMed] [Google Scholar]
- 11. Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766-3772. [DOI] [PubMed] [Google Scholar]
- 12. Fraggetta F, Pelosi G, Cafici A, et al. CDX2 immunoreactivity in primary and metastatic ovarian mucinous tumours. Virchows Arch. 2003;443:782-786. [DOI] [PubMed] [Google Scholar]
- 13. Groisman GM, Meir A, Sabo E.. The value of Cdx2 immunostaining in differentiating primary ovarian carcinomas from colonic carcinomas metastatic to the ovaries. Int J Gynecol Pathol. 2004;23:52-57. [DOI] [PubMed] [Google Scholar]
- 14. Kim MJ. The usefulness of CDX-2 for differentiating primary and metastatic ovarian carcinoma: an immunohistochemical study using a tissue microarray. J Korean Med Sci. 2005;20:643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Logani S, Oliva E, Arnell PM, et al. Use of novel immunohistochemical markers expressed in colonic adenocarcinoma to distinguish primary ovarian tumors from metastatic colorectal carcinoma. Mod Pathol. 2005;18:19-25. [DOI] [PubMed] [Google Scholar]
- 16. Mazziotta RM, Borczuk AC, Powell CA, et al. CDX2 immunostaining as a gastrointestinal marker: expression in lung carcinomas is a potential pitfall. Appl Immunohistochem Mol Morphol. 2005;13:55-60. [DOI] [PubMed] [Google Scholar]
- 17. Moskaluk CA, Zhang H, Powell SM, et al. Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol. 2003;16:913-919. [DOI] [PubMed] [Google Scholar]
- 18. Raspollini MR, Amunni G, Villanucci A, et al. Utility of CDX-2 in distinguishing between primary and secondary (intestinal) mucinous ovarian carcinoma: an immunohistochemical comparison of 43 cases. Appl Immunohistochem Mol Morphol. 2004;12:127-131. [DOI] [PubMed] [Google Scholar]
- 19. Tornillo L, Moch H, Diener PA, et al. CDX-2 immunostaining in primary and secondary ovarian carcinomas. J Clin Pathol. 2004;57:641-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816-826. [DOI] [PubMed] [Google Scholar]
- 21. Vang R, Gown AM, Barry TS, et al. Immunohistochemistry for estrogen and progesterone receptors in the distinction of primary and metastatic mucinous tumors in the ovary: an analysis of 124 cases. Mod Pathol. 2006;19:97-105. [DOI] [PubMed] [Google Scholar]
- 22. FitzPatrick DR, Carr IM, McLaren L, et al. Identification of SATB2 as the cleft palate gene on 2q32-q33. Hum Mol Genet. 2003; 12:2491-2501. [DOI] [PubMed] [Google Scholar]
- 23. Magnusson K, de Wit M, Brennan DJ, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011;35:937-948. [DOI] [PubMed] [Google Scholar]
- 24. Wang S, Zhou J, Wang XY, et al. Down-regulated expression of SATB2 is associated with metastasis and poor prognosis in colorectal cancer. J Pathol. 2009;219:114-122. [DOI] [PubMed] [Google Scholar]
- 25. Eberhard J, Gaber A, Wangefjord S, et al. A cohort study of the prognostic and treatment predictive value of SATB2 expression in colorectal cancer. Br J Cancer. 2012;106:931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conner JR, Hornick JL.. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63:36-49. [DOI] [PubMed] [Google Scholar]
- 27. Righi A, Gambarotti M, Longo S, et al. Small cell osteosarcoma: clinicopathologic, immunohistochemical, and molecular analysis of 36 cases. Am J Surg Pathol. 2015;39:691-699. [DOI] [PubMed] [Google Scholar]
- 28. Strickland S, Parra-Herran C.. Immunohistochemical characterization of appendiceal mucinous neoplasms and the value of special AT-rich sequence-binding protein 2 in their distinction from primary ovarian mucinous tumours. Histopathology. 2016;68:977-987. [DOI] [PubMed] [Google Scholar]
- 29. Moh M, Krings G, Ates D, et al. SATB2 expression distinguishes ovarian metastases of colorectal and appendiceal origin from primary ovarian tumors of mucinous or endometrioid type. Am J Surg Pathol. 2016;40:419-432. [DOI] [PubMed] [Google Scholar]
- 30. Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17:2635-2650. [PubMed] [Google Scholar]
- 31. Newcombe RG. Simultaneous comparison of sensitivity and specificity of two tests in the paired design: a straightforward graphical approach. Stat Med. 2001;20:907-915. [DOI] [PubMed] [Google Scholar]
- 32. Young RH, Gilks CB, Scully RE.. Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei: a clinicopathologic analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol. 1991;15:415-429. [DOI] [PubMed] [Google Scholar]
- 33. Aho AJ, Heinonen R, Laurén P.. Benign and malignant mucocele of the appendix: histological types and prognosis. Acta Chir Scand. 1973;139:392.. [PubMed] [Google Scholar]
- 34. Higa E, Rosai J, Pizzimbono CA, et al. Mucosal hyperplasia, mucinous cystadenoma, and mucinous cystadenocarcinoma of the appendix: a re-evaluation of appendiceal "“mucocele.” Cancer. 1973;32:1525.. [DOI] [PubMed] [Google Scholar]
- 35. Lee KR, Scully RE.. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with pseudomyxoma peritonei. Am J Surg Pathol. 2000;24:1447-1464. [DOI] [PubMed] [Google Scholar]