Summary
Spontaneous resolution of chronic hepatitis C virus (HCV) infection is a rare event. We describe spontaneous resolution in 26.9% of a cohort of HCV-infected women by 12-months post-partum. Understanding the underlying immunological changes in similar cohorts could inform immune therapies and vaccine development.
Keywords: hepatitis C virus, pregnancy, viral clearance, spontaneous clearance, IL28B
Abstract
Background
Postpartum hepatitis C viral (HCV) load decline followed by spontaneous clearance has been previously described. Herein we identify predictors for viral decline in a cohort of HCV-infected postpartum women.
Methods
Pregnant women at Cairo University were screened for anti-HCV antibodies and HCV RNA, and viremic women were tested for quantitative HCV RNA at 3, 6, 9, and 12 months postpartum. Spontaneous clearance was defined as undetectable viremia twice at least 6-months apart. Associations between viral load and demographic, obstetrical, HCV risk factors, and interleukin-28B gene (IL28B) polymorphism (rs12979860) were assessed.
Results
Of 2514 women, 97 (3.9%) had anti-HCV antibodies, 54 (2.1%) were viremic and of those, 52 (2.1%) agreed to IL28B testing. From pregnancy until 12 months postpartum, IL28B-CC allele women had a significant viral decline (P = .009). After adjusting, the IL28B-CC allele had a near significant difference compared to the CT allele (odds ratio [OR], 0.75; 95% confidence interval [CI], 0.75,1.00; P = .05), but not the TT allele (OR, 0.91; 95% CI, 0.61,1.38; P = .64). All 14/52 (26.9%) women who subsequently cleared were among the 15 with undetectable viremia at 12 months, making that time point a strong predictor of subsequent clearance (sensitivity = 100%, specificity = 97.4%, positive predictive value = 93.3%, negative predictive value = 100%).
Conclusions
IL28B-CC genotype and 12-month postpartum undetectable viremia were the best predictors for viral decline and subsequent clearance. These 2 predictors should influence clinical decision making.
Hepatitis C virus (HCV) is a well-known cause of chronic hepatitis and liver disease. The majority of adults and many children progress to chronic infection with viremia. While children can spontaneously clear HCV early in life, once chronic infection is established in adults, spontaneous resolution is a very rare occurrence [1–7]. However, a few prior studies have shown that some chronically infected pregnant women spontaneously clear their infection in the postpartum period [8, 9]. Other studies have described intensive immune analysis of small cohorts of postpartum HCV-infected women, including 1 who spontaneously cleared her infection [10]. Larger studies are required to better define which women with chronic HCV have a higher likelihood of spontaneous resolution in the postpartum period.
While several predictors have been proposed for spontaneous clearance in other groups of patients, the interleukin-28B gene (IL28B) locus proved to be the best predictor in acute and chronic HCV infection and among those treated with interferon (IFN) and ribavirin [11–14]. While a broad and robust cellular immune response is known to be necessary for clearance of acute HCV infection, the underlying changes prior to clearance of chronic HCV remain largely unknown [7, 15]. A study that followed a single individual over a period of 2 years after infection demonstrated that a spike in anti-HCV antibodies preceded clearance of HCV, thereby demonstrating the importance of a humoral response in clearance [6]. However, conducting this level of follow-up in chronically HCV-infected patients requires following a large cohort of individuals over several years at an exorbitant cost. Hence, identifying specific cohorts likely to spontaneously clear their HCV infection in a predictable and time-limited manner could contribute significantly to the understanding of the underlying immunological changes.
Our research team has been studying HCV-infected pregnant women in the high-seroprevalence country of Egypt [16]. We designed this study to prospectively follow pregnant women with chronic HCV infection for 1 year postpartum to determine changes in their viral load over time and the frequency of spontaneous clearance. We evaluated several host and viral factors associated with clearance, including the IL28B allele.
METHODS
Study Population
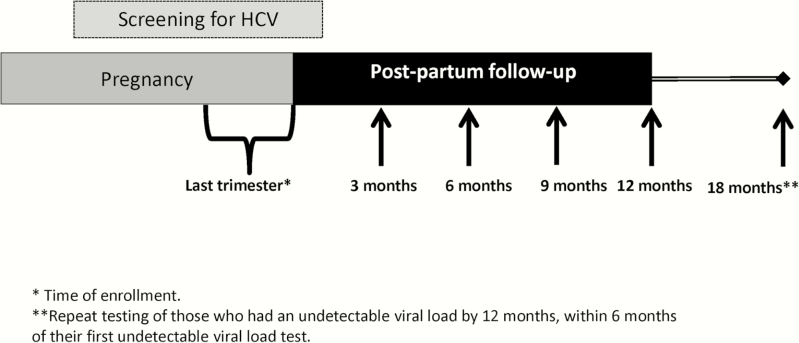
This prospective cohort study has been described in detail elsewhere [17, 18]. Briefly, all eligible pregnant women who attended the antenatal clinic of the Department of Obstetrics and Gynecology at Kasr Al-Aini Hospital, Faculty of Medicine, Cairo University, were invited to participate in the study. Inclusion criteria included healthy pregnant women aged 21–45 years attending the Cairo University antenatal clinic and in their last trimester of pregnancy. Women were excluded if they were diagnosed with placenta previa, eclampsia, uncontrolled diabetes, jaundice, advanced heart conditions, kidney failure, immunosuppressive disorders, or advanced psychiatric disorders or if they were unable to provide informed consent. All women invited to participate agreed to join the study (100%) and were screened for anti-HCV antibodies, and those who were anti-HCV positive were tested for viremia at baseline (third trimester) and every 3 months for the following 12 months after delivery (Figure 1). Women who had undetectable viral loads less than 6 months before their last (12 month) visit were recalled and tested again 6 months after their first undetectable viral load result. Women with 2 undetectable viral loads at least 6 months apart were considered to have spontaneously cleared their HCV infection. Viremic women were tested for IL28B polymorphism (rs12979860). Of the 54 viremic women, 52 agreed to IL28B testing (96.3%) and completed all follow-up visits (100% compliance), with no missing data.
Figure 1.
Schematic of follow-up of enrolled pregnant women. Pregnant women were screened for anti-hepatitis C virus (HCV) antibodies in their third trimester of pregnancy. Those who were anti-HCV positive were tested for viremia at baseline and then every 3 months for the following 12 months after delivery. Women who had undetectable viral loads less than 6 months before their last (12-month) visit were recalled and tested again 6 months after their first undetectable viral load result, up to 18 months after giving birth. Abbreviation: HCV, hepatitis C virus.
This study was conducted according to the principles of the Declaration of Helsinki (1964) and was independently approved by the institutional review boards (IRB) of Cairo University, University of Maryland at Baltimore, and University of North Carolina at Chapel Hill. Every pregnant woman signed an Arabic (local language) written informed consent form after the study was explained in detail in Arabic and all her questions were addressed. Women who were illiterate were accompanied by a literate companion who attended the consenting process and co-signed the informed consent after all their questions were answered.
Demographic and Obstetric History and Hepatitis C Virus-related Risk Factors
A detailed questionnaire was administered to women at enrollment in the local language (Arabic); for illiterate women, it was administered verbally by a member of the study group. Information collected included demographics (age, occupation, last menstrual period, and expected date of delivery), HCV-related risk factors (history of jaundice, surgery, liver disease, blood transfusion, needle-stick injury with a contaminated needle, tattoos, endoscopy, renal dialysis, dental care or tooth extraction, or if they ever injected drugs or other medications using shared needles), and obstetrical history (full-term and pre-term births, abortions, and living children)
Laboratory Testing
Maternal samples were drawn at the time of enrollment and tested for anti-HCV antibodies using a fully automated electrochemiluminescence immunoassay (Elecsys anti-HCV immunoassay; Cobas-Roche). Positive maternal samples were tested for quantitative HCV-RNA using real-time polymerase chain reaction (Roche LightCycler 2.0), with a lower limit of quantitation of 12 IU/mL, as described elsewhere [19]. Maternal IL28B genotyping was performed using the LightMix IL28B allelic discrimination kit (Roche Diagnostics, Mannheim, Germany) for detection of the human IL28B C/T polymorphism (rs no.12979860) in human nucleic acid extracts. This LightMix kit was tested on the LightCycler 2.0 instrument with the LightCycler FastStart DNA HybProbeMastermix.
Data Analysis
Women who spontaneously cleared their viremia were compared to those who remained infected after completion of follow-up. The associations between maternal risk factors and clearance were assessed and described. Given the small number of women with spontaneous clearance, the following nonparametric tests were used to compare between them: the Fisher exact test was used for categorical variables, the Mann-Whitney U test was used for continuous variables, and an exact test was used to test for Hardy-Weinberg equilibrium in the IL28B allele frequencies [20, 21]. Logistic regression was used for unadjusted and adjusted analyses; P values were computed using permutation tests; and 95% confidence intervals (CIs) were computed using the bootstrap. Generalized estimating equations (GEEs) were fit to compare average viral load (log transformed) over time between the IL28B genotypes after adjustment for age and number of full-term births. Analyses were performed using the SPSS statistical software, version 23.0 (IBM SPSS Statistics for Windows, version 23.0; IBM Corp, Armonk, New York) and R statistical software (version 3.2).
RESULTS
A total of 2514 pregnant women were tested for anti-HCV antibodies in their third trimester from December 2012 through March 2014. All agreed to participate and were followed after delivery until September 2015, with some additional follow-up testing in some individuals completed by May 2016. Of those, 97 (3.9%) were anti-HCV seropositive and 54 (2.1%) were also positive for HCV RNA. Of the 54 women with viremia, 52 agreed to be tested for IL28B polymorphism (rs12979860) and consisted of 28/52 (53.8%) with the CC allele, 17/52 (32.7%) with the CT allele, and 7/52 (13.5%) with the TT allele. We found little evidence of departure from Hardy-Weinberg equilibrium (P value = .18) in the IL28B allele frequencies compared to frequencies in other Middle Eastern cohorts [22–25].
Of those 52 women, 37 (71.2%) infected pregnant women had a significant drop in the HCV viral load (by more than one-half log decline) within the first 3 months after delivery. The steepest drop in HCV RNA was observed in the interval between 9 months and 12 months postpartum, with 25/52 women (48.1%) having a >2.0-log decline by 12 months. By 12 months after delivery, 15 women had dropped their viral load to an undetectable level (below the lower limit of quantitation of 12 IU/mL). Of these women, 14/15 (93.3%) continued to have an undetectable level of viremia at a subsequent visit 6 months later and were considered to have spontaneously cleared their viremia. This represents 26.9% of the 52 women with chronic HCV viremia. These 14 women were distributed as follows: 10/14 (71.4%) had the IL28B CC allele, 2/14 (14.2%) the CT allele, and 2/14 (14.2%) the TT allele (P = .215).
Association of Interleukin-28B Gene and Other Maternal Factors With Viral Load Drop and Spontaneous Clearance
Table 1 summarizes demographic, host, and viral factors among the women with spontaneous clearance and those who did not clear. Among the risk factors examined, only history of liver disease was associated with spontaneous clearance (P = .02; Table 1). Participants with the CT genotype of IL28B had the lowest proportion of clearance (11.8%), followed by the TT (28.6%) and CC (35.7%) genotypes; however, the result was not statistically significant (P = .21).
Table 1.
Unadjusted Comparison of Postpartum Women Tested for the Interleukin-28B Gene Allele Who Spontaneously Cleared Their Chronic Hepatitis C Virus Infection and Those Who Remained Persistently Infected
| Characteristic | Total, N = 52 | Spontaneously Cleared, N = 14 (%) | Persistently Infected, N = 38 (%) | P Value |
|---|---|---|---|---|
| Number (n = 52) | 14/52 (26.9) | 38/52 (73.1) | ||
| Mean age, y (standard deviation) | 31.7 (5.2) | 30.7 (5.2) | 32.0 (5.3) | .43 |
| Interleukin-28B gene (rs12979860) | ||||
| CC allele | 28/52 (53.8) | 10/28 (35.7) | 18/28 (64.3) | .21 |
| CT allele | 17/52 (32.7) | 2/17 (11.8) | 15/17 (88.2) | |
| TT allele | 7/52 (13.5) | 2/7 (28.6) | 5/7 (71.4) | |
| Maternal HCV viral load at baseline | ||||
| Median (interquartile range) | 80450 (4789891) | 68500 (4789093) | 100200 (3899891) | .43 |
| Primigravida | 4/52 (7.7) | 0/14 (0.0) | 4/38 (10.5) | .56 |
| Multigravida | ||||
| History of preterm births | 1/52 (1.9) | 0/14 (0.0) | 1/38 (2.6) | 1.00 |
| History of abortion | 17/52 (32.7) | 6/14 (42.9) | 11/38 (28.9) | .20 |
| Number of full-term births | 47/52 (90.4) | 13/14 (92.9) | 34/38 (89.5) | .43 |
| Have children who are currently alive | 47/52 (90.4) | 13/14 (92.9) | 34/38 (89.5) | .51 |
| HCV risk factors | ||||
| Working in health sector | 3/52 (5.8) | 0/14 (0.0)a | 3/38 (7.9) | 1.00 |
| History of jaundice | 0/52 (0.0)a | 0/14 (0.0)a | 0/38 (0.0)a | N/A |
| History of liver disease | 3/52 (5.8) | 3/14 (21.4) | 0/38 (0.0) | .02 |
| History of surgery | 34/52 (65.4) | 11/14 (78.6) | 23/38 (60.5) | .33 |
| History of blood transfusion | 4/52 (7.7) | 1/14 (7.1) | 3/38 (7.9) | 1.00 |
| History of needle-stick injury | 1/52 (1.9) | 1/14 (7.1) | 0/38 (0.0)a | .27 |
| History of tattoos | 0/52 (0.0)a | 0/14 (0.0)a | 0/38 (0.0)a | N/A |
| History of endoscopy | 1/52 (1.9) | 1/14 (7.1) | 0/38 (0.0)a | .27 |
| History of renal dialysis | 0/52 (0.0)a | 0/14 (0.0)a | 0/38 (0.0)a | N/A |
| History of dental treatment | 42/52 (80.8) | 13/14 (92.9) | 29/38 (76.3) | .25 |
| History of intravenous injection with needle sharing | 0/52 (0.0)a | 0/14 (0.0)a | 0/38 (0.0)a | N/A |
Abbreviation: HCV, hepatitis C virus.
aToo sparse for inference.
After adjusting for all host and viral factors (Table 2), the IL28B CT allele had a near statistically significantly lower odds of clearance than the CC allele (odds ratio [OR], 0.75; 95% CI, 0.57,1.00; P = .05), but not the TT allele (OR, 0.91; 95% CI, 0.61,1.38; P = .64). When adjusting only for potential predictors of clearance (baseline viral load, age, and prior births), the association remained near significant.
Table 2.
Unadjusted and Adjusted Comparison of 52 Postpartum Women Tested for the Interleukin-28B Gene Allele Who Spontaneously Cleared Their Chronic Hepatitis C Virus Infection and Those Who Remained Persistently Infecteda
| Characteristic | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.00 (.97, 1.03) | .97 | 1.01 (.97, 1.04) | .62 | ||
| Log maternal hepatitis C viral load at baseline | 1.01 (.94, 1.07) | .67 | 1.01 (.93, 1.07) | .73 | ||
| Interleukin-28B gene (rs12979860) | ||||||
| CC allele (n = 28) | Reference | – | Reference | – | Reference | – |
| CT allele (n = 17) | 0.79 (.62, 1.01) | .07 | 0.78 (.60, 1.01) | .08 | 0.75 (.57, 1.00) | .05 |
| TT allele (n = 7) | 0.93 (.64, 1.41) | .66 | 0.93 (.62, 1.42) | .69 | 0.91 (.61, 1.38) | .64 |
| Number of full-term births (n = 47) | 0.96 (.87, 1.07) | .50 | 0.94 (.84, 1.05) | .30 | ||
| History of abortion (n = 17) | 1.05 (.87, 1.26) | .54 | ||||
| History of surgery (n = 34) | 1.23 (.91, 1.68) | .15 | ||||
| History of blood transfusion (n = 4) | 0.95 (.51, 1.80) | .83 | ||||
| History of dental treatment (n = 42) | 1.21 (.87, 1.65) | .29 | ||||
Model 1 is unadjusted; model 2 adjusts for age, log baseline viral load, and number of full-term births; and model 3 adjusts for the same variables in model 2 as well as risk factors for infection. Cells with data too sparse to be calculated were excluded from the adjusted model (history of preterm births, working in the health sector, history of jaundice, history of liver disease, history of needle-stick injury, history of tattoos, history of endoscopy, history of renal dialysis, and history of intravenous injection with needle sharing). The variable “having children who are currently alive” strongly correlated with “number of full-term births” and was also excluded from the model. ORs were estimated using logistic regression. P values derived using permutation tests based on 5000 permutations. CIs were derived using 5000 bootstrap replicates.
Abbreviations: CI, confidence interval; OR, odds ratio.
a14 women spontaneously cleared their chronic HCV infection and 38 remained persistently infected.
Interestingly, all 14 women who had spontaneously cleared by the end of the study were among the 15 who had an undetectable level by 12 months, making undetectable viremia at 12 months a strong predictor of final clearance with a sensitivity of 100%, specificity of 97.4%, positive predictive value of 93.3%, and a negative predictive value of 100%. This indicates that any mother who did not have an undetectable viral load by 12 months after delivery was not likely to subsequently clear her viremia.
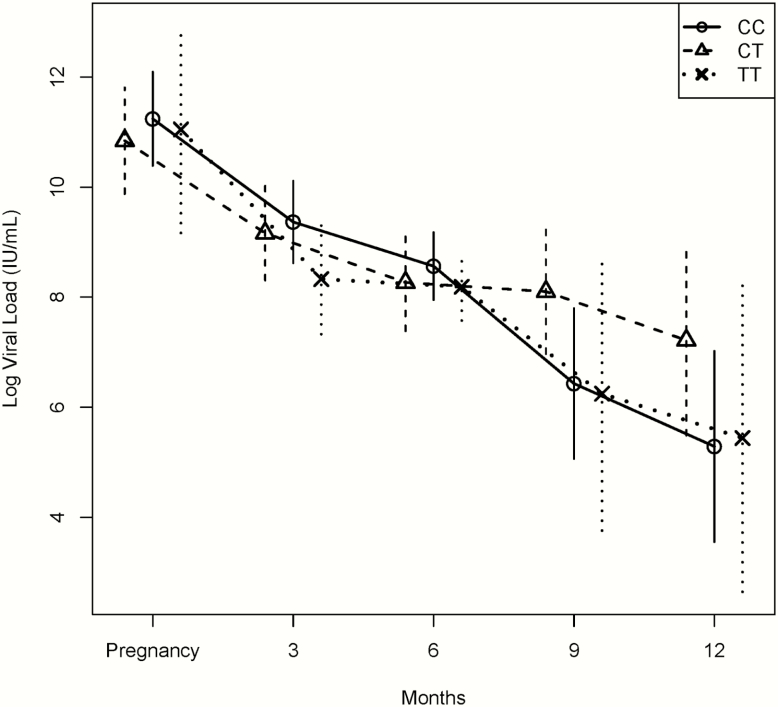
Among the 37 postpartum women with known IL28 B status who had >0.5 log drop by 3 months after giving birth, 21/37 (56.8%) had the CC allele, 11/37 (29.7%) had the CT allele, and 5/37 (13.5%) had the TT allele (P = .833). Also, among the 25 women who had a >2.0 log drop by 12 months after giving birth (compared to their pregnancy viral load), there was a statistically significant difference between the IL28 B alleles with respect to the proportion of those who cleared within each allele, where 17/28 (60.7%) of those with the CC genotype, 4/17 (23.5%) of those with the CT genotype, and 4/7 (57.1%) of those with the TT genotype cleared the virus (P = .049). After adjusting for predictors of clearance (age and number of prior births), the GEE analysis that compared the difference in change in mean log viral load over time from baseline between the 3 genotypes was statistically significant (P = .009), with the CC allele showing the greatest decline by the last visit (Figure 2). Unadjusted analysis and an analysis that adjusted for risk factors of infection yielded nearly identical results
Figure 2.
Viral load of women from pregnancy until 12 months postpartum, comparing those with interleukin-28B gene (IL28B) CC, CT, and TT alleles. The trajectories of average viral load decline (log transformed) over time, regardless of clearance, were compared among the 3 alleles (IL28B CC, CT, and TT alleles) using generalized estimating equations and found to be statistically significant (P = .009), with the CC allele showing the greatest decline by the last visit. Each point represents the mean log transformed viral load. The error bars represent 95% confidence intervals of log transformed viral load for each of the 3 alleles at each time point (baseline at last trimester of pregnancy and 3-, 6-, 9-, and 12-month follow-up visits).
DISCUSSION
These results describe the largest reported cohort of HCV-infected women with postpartum spontaneous resolution. In this cohort, the IL28B favorable CC genotype was the single best predictor for a significant postpartum drop in viral load by 12 months, which was, in turn, a predictor for subsequent spontaneous viral clearance. We were able to leverage several key advantages to obtain these results, including high local seroprevalence, busy obstetric service with a large catchment area, and an existing study infrastructure that was conducting universal HCV screening during prenatal care. The inclusion of more than 50 viremic pregnant women who could be prospectively followed has previously only been possible in studies conducted over many years across multiple institutions [1, 4, 26]. In contrast, these data were collected over 2 years at just 1 institution [18, 27].
The postpartum period has been previously recognized as a window for spontaneous resolution. While a nonpregnant adult has an exceedingly low chance for spontaneous resolution, small studies have demonstrated a spontaneous clearance rate of up to 20% [8, 9]. More detailed studies have described a pattern of declines in HCV RNA levels in the postpartum period [10]. Immunologic analyses on a subset of women have shown that after the “immune tolerant” setting of pregnancy, there is a tremendous surge of HCV-specific T cells and increased immune activity targeted against the virus [10]. None of these prior studies had more than a few women with spontaneous resolution, so no analysis of specific risk factors could be performed.
The association of the favorable IL28B genotype in nonpregnant adults was first identified as part of a genome-wide association study in adults with sustained response to IFN therapy [11]. It was then associated with spontaneous resolution of acute HCV infection [12] and a better response to IFN/ribavirin treatment when it was started early [28, 29]. IL28B induces IFN-λ and mediates the innate immune response to RNA viruses that is distinct from IFN-α [30]. Studies that examined the intrahepatic gene expression of patients with sustained response to pegylated-IFN therapy compared to those patients who were nonresponders or relapsers showed that those with favorable IL28B genotypes had lower baseline IFN-stimulated gene (ISG) expression [31]. The approval of direct-acting antiviral (DAA) agents against HCV has diminished the role of IL28B significantly, as virtually all patients respond to therapy. However, when treating genotype 1b with sofosbuvir/ledipasvir in patients with a favorable CC allele, current American Association for the Study of Liver Diseases guidelines allow for a shortened 8-week course at the discretion of the practitioner [32–34].
The role of IL28B in HCV-infected pregnant women is related to how their immune system must become tolerant to the foreign antigens in the fetus [35]. There is a shift from a Th-1 to a Th-2 bias in the immune responses (which explains the improvement in autoimmune conditions such as rheumatoid arthritis, multiple sclerosis, and autoimmune hepatitis), along with a suppression of HCV-specific T cells and a rise in HCV RNA during pregnancy [10]. After delivery, the immune system shifts back to the Th-1 bias, which is accompanied by a surge in HCV-specific T cells. In a postpartum HCV-infected woman who has the favorable IL28B genotype, prior data would suggest that she has relatively low baseline expression of ISG in her liver [31]. These genes would then respond to cytokine signals secreted during the HCV-specific T-cell surge in the postpartum period. Future studies are necessary to prove this hypothesis.
Given these results, what role would IL28B testing play in the care of pregnant women with HCV? In ideal circumstances, every patient with chronic infection would be treated with DAAs, and virtually all would clear their infection. In reality, there is a constant triage when selecting patients who would benefit most from therapy. Given that women with the unfavorable IL28B genotype are unlikely to spontaneously resolve their infection in the postpartum period, they would be the ones most likely to benefit from therapy, thereby eliminating their viremia and the risk of vertical transmission in future pregnancies. Based on our findings, those with the CC genotype are likely to have the steepest viral load decline and subsequently clear more often. Regardless of genotype, the women who had an undetectable viral load level by 12 months were most likely to spontaneously clear, while those who had viremia were unlikely to improve after that time point.
Hence, it seems that women should be followed in the postpartum period until at least 12 months after delivery, thus saving the cost and effort of a treatment course. This triage by viral load at 12 months and by IL28B genotype may help more patients get treated in resource-poor settings where DAAs are either not available or available but, even when discounted to the local price, is far too expensive, as is the case for Egypt. If the medical community could replicate the natural sequence of tolerance and release of tolerance with the subsequent viral clearance in nonpregnant patients, this knowledge would facilitate production of a vaccine against HCV.
There are some limitations to our study, in particular, the sparsity of data in some of the HCV risk factors where there were too few women to elicit a statistically significant difference between the IL28B alleles at the 12-month time point. However, after accounting for the rate of viral load decline, the CC allele had a significantly faster rate of viral load decline, even after adjusting for potential predictors of clearance, indicating its strong association. An important limitation is that we were unable to test for the more predictive rs368234815 variant (IFNL4-∆G/TT) [36]. At the start of this study, there was no IL28B testing available in Egypt, and since the Egyptian Ministry of Health and the Cairo University IRB do not allow for shipping samples abroad, we could not perform any additional testing beyond the rs12979860 variant, which was the only commercially available test in Egypt.
Another limitation is the possibility that the IL28B distribution in this cohort may not be representative of that in the general Egyptian population. However, while some regional studies describe IL28B distribution in chronic HCV-infected nonpregnant adults [22–25], they are small and not representative of the overall population. However, they do offer a very similar distribution to what we observed in our study, with little evidence of departure from Hardy-Weinberg equilibrium. Furthermore, given that our cohort included all eligible women who went to the antenatal clinic at a major clinical center over a period of 2 years, our cohort most likely constitutes a representative sample of pregnant women from the densely populated Nile Delta of northern Egypt.
Finally, we were not able to conduct viral genotyping. However, the predominant genotype in Egyptian patients is genotype 4 (93.1%), with genotypes 1, 2, and 3 accounting for 3.8%, 0%, and 0.8%, respectively [37]. While genotypes 2 and 3 potentially have a higher rate of spontaneous clearance, their proportion in Egyptian patients is so minuscule that we do not expect that it had an impact on our findings.
Our study also had several strengths, particularly the large number of HCV-infected women enrolled at a single site over a short time period, the very high acceptance rate to join the study (100%), and excellent follow-up (100%) until completion of the study.
In conclusion, we describe the largest cohort of postpartum HCV-infected women who spontaneously resolved their viremia and present evidence that this phenomenon is predictable and can allow for carefully planned studies of immunological changes in similar cohorts that can inform immune therapies and vaccines. This resolution is strongly associated with viral load at 12 months after delivery and the presence of the favorable IL28B genotype. The use of these 2 markers of clearance in the future triage of HCV-infected pregnant women may allow more targeted treatment of patients who are not likely to spontaneously resolve their HCV.
Notes
Acknowledgments. We acknowledge the volunteers who agreed to participate in this study, and the Cairo University staff who made this study possible, particularly the nurses, ancillary staff, and data entry personnel.
Financial support. This work was supported by a grant from the US–Egypt Science and Technology Joint Fund and the Egyptian Science and Technology Development Fund (grant 4586 to S. S. E-K. and H. E-G.) and a grant from the Merck Investigator Studies Program (to R. J.). This research also benefited from a 2010 Duke Global Health Institute Travel Award (to R. J.).
Potential conflicts of interest. R. J. reports HCV clinical trial support from Gilead and Abbvie, and consulting fees from Abbvie, all unrelated to this work. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ceci O, Margiotta M, Marello F et al. . Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: a 24-month prospective study. J Pediatr Gastroenterol Nutr 2001; 33:570–5. [DOI] [PubMed] [Google Scholar]
- 2. Shebl FM, El-Kamary SS, Saleh DA et al. . Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J Med Virol 2009; 81:1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeung LT, To T, King SM, Roberts EA. Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat 2007; 14:797–805. [DOI] [PubMed] [Google Scholar]
- 4. Mast EE, Hwang LY, Seto DS et al. . Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005; 192:1880–9. [DOI] [PubMed] [Google Scholar]
- 5. Irshad M, Khushboo I, Singh S, Singh S. Hepatitis C virus (HCV): a review of immunological aspects. Int Rev Immunol 2008; 27:497–517. [DOI] [PubMed] [Google Scholar]
- 6. Raghuraman S, Park H, Osburn WO, Winkelstein E, Edlin BR, Rehermann B. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis 2012; 205:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hattori Y, Orito E, Ohno T et al. . Loss of hepatitis C virus RNA after parturition in female patients with chronic HCV infection. J Med Virol 2003; 71:205–11. [DOI] [PubMed] [Google Scholar]
- 9. Lin HH, Kao JH. Hepatitis C virus load during pregnancy and puerperium. BJOG 2000; 107:1503–6. [DOI] [PubMed] [Google Scholar]
- 10. Honegger JR, Kim S, Price AA et al. . Loss of immune escape mutations during persistent HCV infection in pregnancy enhances replication of vertically transmitted viruses. Nat Med 2013; 19:1529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ge D, Fellay J, Thompson AJ et al. . Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461:399–401. [DOI] [PubMed] [Google Scholar]
- 12. Thomas DL, Thio CL, Martin MP et al. . Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nattermann J, Timm J, Nischalke HD et al. ; East German HCV Study Group The predictive value of IL28B gene polymorphism for spontaneous clearance in a single source outbreak cohort is limited in patients carrying the CCR5Δ32 mutation. J Hepatol 2011; 55:1201–6. [DOI] [PubMed] [Google Scholar]
- 14. Shi X, Pan Y, Wang M et al. . IL28B genetic variation is associated with spontaneous clearance of hepatitis C virus, treatment response, serum IL-28B levels in Chinese population. PLoS One 2012; 7:e37054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takaki A, Wiese M, Maertens G et al. . Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med 2000; 6:578–82. [DOI] [PubMed] [Google Scholar]
- 16. Kandeel A, Genedy M, El-Refai S, Funk AL, Fontanet A, Talaat M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int 2017 January; 37(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Kamary SS, Hashem M, Saleh DA et al. . Reliability of risk-based screening for hepatitis C virus infection among pregnant women in Egypt. J Infect 2015; 70:512–9. [DOI] [PubMed] [Google Scholar]
- 18. Jhaveri R, Hashem M, El-Kamary SS et al. . Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis 2015; 2:ofv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schröter M, Zöllner B, Schäfer P, Laufs R, Feucht HH. Quantitative detection of hepatitis C virus RNA by light cycler PCR and comparison with two different PCR assays. J Clin Microbiol 2001; 39:765–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyer S. Papers on human genetics. Englewood Cliffs, N.J: Prentice-Hall, 1963. [Google Scholar]
- 21. Hardy GH. Mendelian proportions in a mixed population. 1908. Yale J Biol Med 2003; 76:79–80. [PMC free article] [PubMed] [Google Scholar]
- 22. Abdelwahab SF, Zakaria Z, Sobhy M et al. . Differential distribution of IL28B.rs12979860 single-nucleotide polymorphism among Egyptian healthcare workers with and without a hepatitis C virus-specific cellular immune response. Arch Virol 2015; 160:1741–50. [DOI] [PubMed] [Google Scholar]
- 23. Carapito R, Poustchi H, Kwemou M et al. . Polymorphisms in EGFR and IL28B are associated with spontaneous clearance in an HCV-infected Iranian population. Genes Immun 2015; 16:514–8. [DOI] [PubMed] [Google Scholar]
- 24. Ezzikouri S, Alaoui R, Rebbani K et al. . Genetic variation in the interleukin-28B gene is associated with spontaneous clearance and progression of hepatitis C virus in Moroccan patients. PLoS One 2013; 8:e54793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurbanov F, Abdel-Hamid M, Latanich R et al. . Genetic polymorphism in IL28B is associated with spontaneous clearance of hepatitis C virus genotype 4 infection in an Egyptian cohort. J Infect Dis 2011; 204:1391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis 2005; 192:1872–9. [DOI] [PubMed] [Google Scholar]
- 27. El-Kamary SS, Hashem M, Saleh DA et al. . Reliability of risk-based screening for hepatitis C virus infection among pregnant women in Egypt. J Infect 2015; 70:512–9. [DOI] [PubMed] [Google Scholar]
- 28. Deuffic-Burban S, Castel H, Wiegand J et al. . Immediate vs. delayed treatment in patients with acute hepatitis C based on IL28B polymorphism: a model-based analysis. J Hepatol 2012; 57:260–6. [DOI] [PubMed] [Google Scholar]
- 29. Mangia A, Santoro R, Copetti M et al. . Treatment optimization and prediction of HCV clearance in patients with acute HCV infection. J Hepatol 2013; 59:221–8. [DOI] [PubMed] [Google Scholar]
- 30. Hayes CN, Imamura M, Aikata H, Chayama K. Genetics of IL28B and HCV–response to infection and treatment. Nat Rev Gastroenterol Hepatol 2012; 9:406–17. [DOI] [PubMed] [Google Scholar]
- 31. Urban TJ, Thompson AJ, Bradrick SS et al. . IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology 2010; 52:1888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Afdhal N, Reddy KR, Nelson DR et al. ; ION-2 Investigators Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–93. [DOI] [PubMed] [Google Scholar]
- 33. Feld JJ, Kowdley KV, Coakley E et al. . Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370:1594–603. [DOI] [PubMed] [Google Scholar]
- 34. American Association for the Study of Liver D. Recommendations for testing, managing, and treating hepatitis C. 2017. Available at: http://hcvguidelines.org/sites/default/files/HCV-Guidance_April_2017_a.pdf. Accessed 9 June 2017. [Google Scholar]
- 35. Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol 2007; 178:3345–51. [DOI] [PubMed] [Google Scholar]
- 36. Honegger JR, Tedesco D, Kohout JA et al. . Influence of IFNL3 and HLA-DPB1 genotype on postpartum control of hepatitis C virus replication and T-cell recovery. Proc Natl Acad Sci U S A 2016; 113:10684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61(1 Suppl):S45–57. [DOI] [PubMed] [Google Scholar]