This randomized controlled trial of 398 men who have sex with men and transgender women found text message reminders were superior to standard care over 48 weeks for near-perfect adherence to pre-exposure prophylaxis measured by intracellular tenofovir diphosphate >1246 fmol/punch.
Keywords: MSM, HIV, text messaging, randomized controlled trial, preexposure prophylaxis
Abstract
Background
Adherence is critical for efficacy of tenofovir disoproxil fumarate/emtricitabine (FTC) as preexposure prophylaxis (PrEP).
Methods
Between February 2013 and February 2016, 398 men who have sex with men and transgender women were randomized 1:1 to receive individualized texting for adherence building (iTAB) or standard care (SoC) for 48 weeks. The primary endpoint was dried blood spot (DBS) tenofovir diphosphate (TFV-DP) concentrations at both week 12 and the last on-drug visit of >719 fmol/punch (ie, adequate adherence). Secondary outcomes included DBS TFV-DP concentrations of >1246 fmol/punch (ie, near-perfect adherence) and plasma FTC >350 ng/mL (consistent with dosing within the past 24 hours).
Results
Concentrations >719 fmol/punch of TFV-DP were found in 88.6% of participants at week 12 and 82.5% at week 48. For the primary endpoint, the study arms did not differ (72.0% in iTAB and 69.2% in SoC; P > .05). For the secondary composite endpoint of >1246 fmol/punch the iTAB arm was superior to SoC (33.5% vs 24.8%; P = .06), reaching statistical significance when adjusting for age (odds ratio, 1.56 [95% confidence interval, 1.00–2.42]; P < .05). At week 48, iTAB was superior to SoC for near-perfect adherence (51.0% vs 37.4%; P = .02). At week 12, iTAB was superior to SoC for dosing in past 24 hours by plasma FTC (47.5% vs 33.3%; P = .007), but not at weeks 24, 36, and 48 (all P > .05).
Conclusions
Automated text messaging is a low-burden tool that improves durability of near-perfect PrEP adherence.
Clinical Trials Registration
The efficacy of tenofovir disoproxil fumarate (TDF) combined with emtricitabine (FTC) for preexposure prophylaxis (PrEP) is documented in several randomized controlled trials [1–5]. In the absence of PrEP, human immunodeficiency virus (HIV) incidence of at-risk men who have sex with men (MSM) has been found to be high in prevention studies such as Project EXPLORE (2.1 per 100 person-years) [6] and in the control groups for PrEP studies such as Intervention Préventive de l’Exposition aux Risques avec et pour les Gays (iPERGAY) (6.60 per 100 person-years) [7] and Pre-exposure Option for reducing HIV in the UK: immediate or Deferred (PROUD) (9.0 per 100 person-years) [8]. The pre-exposure prophylaxis initiative (iPrEx) study showed that TDF/FTC reduced the risk of HIV infection by 42% compared to placebo overall and by 68% among those who had 90% self-reported adherence [1]. The risk of HIV infection was reduced by >90% among study participants with high adherence as measured by tenofovir drug concentrations [9]. Real-world studies support high PrEP effectiveness for individuals who remain adherent [10, 11]. On the other hand, several large studies have also shown adverse consequences of PrEP poor adherence [2, 12–14]. Therefore as PrEP use expands, novel approaches to maximize adherence are needed.
Use of short message service (SMS), widely known as text messaging, to improve adherence to antiretroviral medication in persons living with HIV infection has been studied in multiple, mostly small, short-duration clinical studies. Several studies and a meta-analysis suggest that text messaging improves self-reported antiretroviral adherence among those living with HIV [13, 15, 16]. We developed and implemented personalized text messaging systems for adherence promotion among persons living with HIV (individualized texting for adherence building; iTAB) and shown that iTAB can be tailored to subpopulations, leading to better antiretroviral therapy dose timing [17–19]. To date, no large study has evaluated text messaging to promote adherence to PrEP, nor has any study used the objective endpoints of drug concentrations.
Tenofovir diphosphate (TFV-DP) measured in dried blood spots (DBSs) provides an indicator of durable adherence due to a 17-day half-life. In iPrEx [1], MSM with TFV-DP intracellular concentrations commensurate with 4 or more TDF/FTC doses per week had 96% protection against HIV infection (≥719 fmol/punch, or the lower quartile value for DBS concentrations in pharmacokinetic [PK] studies) [9]. Similarly, consistent daily dosing (perfect adherence) can also be determined as TFV-DP concentrations ≥1246 fmol/punch [20]. In contrast, the short half-life of emtricitabine can provide an indication of recent dosing. A PK study of HIV-uninfected individuals who stopped TDF/FTC after achieving steady state showed FTC plasma trough concentrations of ≥350 ng/mL at 24 hours after the last dose [21], providing an indicator of dosing in the last 24 hours. This randomized controlled trial was designed to determine the effectiveness of a iTAB to support PrEP adherence in MSM and transgender women over 48 weeks using both intracellular TFV-DP and plasma FTC drug concentrations.
METHODS
Study Setting
The California Collaborative Treatment Group enrolled subjects from February 2013 to February 2015 at 4 Southern California medical centers (University of California, San Diego; University of Southern California; Harbor–University of California Los Angeles; and Long Beach Health Department). Subjects were followed for a minimum of 48 weeks ending in February 2016. All participants provided written informed consent to participate in the institutional review board–approved study. Sample size of 200 subjects per group was based on a 2-sided α of .05, with 94% power to detect a difference of 15% between study arms, assuming the adherence rate for the standard of care (SoC) arm is 68%.
Eligibility Criteria
Eligible participants were English- or Spanish-speaking HIV-uninfected MSM and transgender women (aged >18 years); HIV was confirmed by a negative fourth-generation antigen-antibody assay or a third-generation antibody assay plus HIV nucleic acid amplification test (NAAT). Participants had a persistent elevated risk of HIV infection determined as (1) ≥1 HIV-infected partner for ≥4 weeks; (2) condomless anal intercourse with ≥3 HIV-positive or unknown status male partners in prior 3 months; or (3) condomless anal sex with ≥1 male partner plus a sexually transmitted infection (STI) in prior 3 months. Participants were required to have acceptable laboratory values in the past 30 days, most importantly a calculated creatinine clearance ≥60 mL/minute by the Cockcroft-Gault formula [22]. Exclusion criteria included active hepatitis B; proteinuria 2+ or higher; and signs or symptoms of acute HIV infection.
Study and Intervention Design
Participants were randomized 1:1 to individualized texting for adherence building (iTAB), a personalized, 2-way, fully automated text-messaging intervention vs SoC. Randomization was stratified by study site and assigned at baseline through the electronic data capture system. Participants in both the SoC and iTAB arms received brief HIV prevention and adherence counseling with provision of study drug by study staff consistent with Centers for Disease Control and Prevention (CDC) guidelines [23]; only participants in the iTAB arm received text messages.
The iTAB intervention was developed in the context of behavioral theory. Significant formative work went into developing iTAB for PrEP including focus groups and pilot testing to generate, hone, and revise the iTAB text messaging content. Importantly, based on feedback from participants, an emphasis was placed on getting participants to engage with the text messaging system (see Supplementary Materials for additional information). On a daily basis, participants received a mix of health promotion and “factoid” messages at a personally selected time consistent with when they planned to take PrEP. Participants worked with study staff to choose messages consistent with their preferences from 440 different messages. Participants could also create their own messages. At the end of each text message, participants were asked to respond with a single letter indicating whether they did or did not take PrEP. Results of iTAB responses were not discussed with participants at study visits; however, if participants reported any difficulties with the system, staff would provide assistance.
Study Procedures and Measures
Study visits occurred at baseline and weeks 4, 12, 24, 36, and 48. If subjects missed 2 consecutive visits prior to week 48 they were discontinued from the study. Subjects were allowed to continue past week 48 on study drug through week 96 or until the last subject completed his or her week 48 visit. The primary endpoint was a priori defined at 48 weeks. At each visit, participants completed a confidential in-person interview and computer-assisted self-interviewing questionnaires. STI screening assessments at baseline and every 3–6 months included syphilis (serum rapid plasma reagin and, if positive, confirmatory treponemal test), as well as NAAT of urine and swabs of pharynx and rectum for chlamydia and gonorrhea (Hologic Aptima).
Plasma FTC was assessed at weeks 12, 24, 36, and 48 by the UCSD Pediatrics Pharmacology Laboratory, using liquid chromatography-mass spectrometry in accordance with a previously published method [24]. DBS concentrations for intracellular TFV-DP and intracellular emtricitabine triphosphate (FTC-TP) were performed at week 12 (if completed) and the last study visit following week 12 (week 24, 36, or 48) where the participant was on drug and completed the visit. Both TFV-DP and FTC-TP were measured using a liquid chromatography–tandem mass spectrometry assay previously validated for the determination of TFV and FTC in human plasma [20]. Additional measures that were assessed at each visit included sexual behaviors, sexual compulsivity (Sexual Compulsivity Scale), depressive symptoms (Patient Health Questionnaire-9 [PHQ-9]), alcohol and substance use structured clinical interview for diagnostic and statistical manual of mental disorders (SCID) substance use screening questionnaire, Drug Abuse Screening Test [DAST-10], and Alcohol Use Disorders Identification Test [AUDIT]), and HIV literacy (HIV Knowledge Questionnaire–18) [9, 25–30].
Statistical Analysis
Baseline characteristics were summarized and compared between study arms using Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Primary analyses were performed on a modified intent-to-treat population, defined as randomized participants who completed the baseline visit (n = 398). The original primary outcome was based on self-reported adherence and FTC concentrations but prior to completion of the study, database lock, or any analysis of adherence, the primary endpoint was changed to DBS TFV-DP concentrations with funding provided by Gilead Sciences. The primary outcome was a composite outcome for being “adherent” defined by DBS TFV-DP concentrations that have been associated with taking ≥4 doses of TDF in past week (≥719 fmol/punch) 20 at week 12 visit and, if continued on PrEP past week 12, the last study visit through week 48 (eg, week 24, 36, or 48). Subjects missing or discontinuing prior to the week 12 visit were considered nonadherent. If week 12 was the last study visit on-drug, then adherence was based only on that one week 12 value. Secondary analyses for drug concentrations included the composite outcome for DBS TFV-DP concentration associated with taking 7 doses of TDF in past week (≥1246 fmol/punch; ie, “near-perfect adherence” [20]), as well as looking at TFV-DP concentrations at week 12 and 48 separately for those who completed those visits. Plasma FTC adherence was defined as a binary endpoint of >350 ng/mL at weeks 12, 24, 36, and 48. The different adherence outcomes were compared between the study arms using Fisher exact test and multivariable logistic regression models adjusting for baseline factors that were associated with the outcome. Early study discontinuation and adverse events were compared between study groups using Fisher exact test. Incidence of HIV seroconversion during the study was calculated by dividing the number of events by the total number of follow-up person-years, with 95% confidence intervals calculated using the exact Poisson confidence limits. A P value of <.05 was considered statistically significant. Statistical analyses were performed in R (http://cran.r-project.org), version 3.3.2.
RESULTS
Study Flow
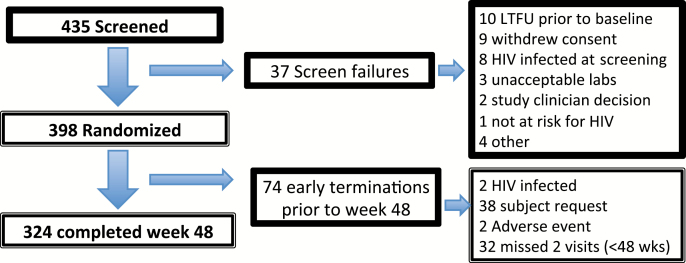
From 435 participants completing a screening visit, a total of 398 individuals (395 MSM and 3 transgender women) met the study eligibility criteria and were randomized and completed a baseline visit. Two hundred participants were randomized to the iTAB arm and 198 were randomized to the SoC arm. Of 37 screen failures (Figure 1), 8 subjects had newly diagnosed HIV (1.8% of all screened). Other reasons for screen failures included loss to follow-up prior to baseline (n = 10) and a withdrawn consent (n = 9). At week 48, 324 (81.4%) participants were still on study.
Figure 1.
Consort diagram. Abbreviations: HIV, human immunodeficiency virus; LTFU, lost to follow up.
Baseline Analysis
The mean age of participants was 35.2 years old (range, 19–64 years; Table 1). Fifty percent were non-Hispanic white, 28% were non-black Hispanic, and 15% identified as black alone or as part of multiple racial identity. Despite randomization, the iTAB arm as compared to the SoC arm (1) had lower education, HIV literacy, and likelihood of reporting income; (2) were less likely to have had an HIV-infected sexual partner for the past 4 weeks or longer; (3) had higher levels of depressive symptoms (PHQ-9); and (4) had higher mean sexual compulsivity scores.
Table 1.
Baseline Characteristics
| Characteristic |
iTAB Arm
(n = 200) |
Standard of Care
(n = 198) |
P Value |
|---|---|---|---|
| Gender | |||
| Male | 197 (98.5) | 198 (100) | |
| Transgender women | 3 (1.5) | 0 (0) | .25 |
| Age, mean (SD) | 35.1 (9.8) | 35.4 (8.7) | .44 |
| Race | .90 | ||
| Asian | 7 (3.6) | 5 (2.6) | |
| Black | 26 (13.2) | 26 (13.5) | |
| White | 147 (74.6) | 148 (76.7) | |
| Multiple | 14 (7.1) | 10 (5.2) | |
| Other | 3 (1.5) | 4 (2.1) | |
| Hispanic ethnicity | 61 (30.8) | 58 (29.4) | .83 |
| English primary language | 188 (94.0) | 192 (97) | .23 |
| Education | |||
| Less than high school | 4 (2.0) | 0 (0) | .05 |
| High school | 20 (10.0) | 11 (5.6) | |
| Some college | 77 (38.5) | 72 (36.4) | |
| Bachelor’ degree | 64 (32.0) | 68 (34.3) | |
| Some postgraduate | 5 (2.5) | 14 (7.1) | |
| Advanced degree | 30 (15.0) | 33 (16.7) | |
| Household income | |||
| <$2000/mo | 43 (21.5) | 42 (21.2) | .02 |
| ≥$2000/mo | 115 (57.5) | 134 (67.7) | |
| Refuse | 42 (21.0) | 22 (11.1) | |
| ≥1 HIV+ partner for >4 wk | 88 (44.0) | 109 (55.1) | .04 |
| Condomless sex with ≥3 HIV+/ unknown partners past 3 mo | 139 (70.2) | 137 (69.2) | >.99 |
| Condomless sex with ≥1 partner and had STI in past 6 mo | 31 (15.5) | 35 (17.7) | .59 |
| Any STI at baseline | 54 (27.0) | 50 (25.3) | .73 |
| Any substance use (not marijuana) past 3 mo | 152 (76.8) | 136 (69.4) | .11 |
| DAST-10 | |||
| No or low | 118 (59.6) | 131 (66.9) | .30 |
| Moderate | 67 (33.9) | 53 (27.0) | |
| Substantial | 13 (6.6) | 12 (6.1) | |
| PHQ-9, mean (SD) | 5.2 (4.9) | 4.2 (4.4) | .03 |
| Sexual compulsivity score, mean (SD) | 1.7 (0.5) | 1.6 (0.5) | .04 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: DAST-10, Drug Abuse Screening Test; HIV, human immunodeficiency virus; iTAB, individualized texting for adherence building; PHQ-9, Patient Health Questionnaire-9; SD, standard deviation; STI, sexually transmitted infection.
Study Follow-up
Seventy-four participants terminated study prior to week 48 (42 [21%] in iTAB vs 32 [16.2%] in SoC; P = .25). The most common reasons for early termination were by participant request or per protocol discontinuation for missing 2 consecutive visits prior to week 48. Two serious adverse events occurred: pancreatitis, unrelated to study drug, and Fanconi syndrome, related to study drug. Two HIV seroconversions occurred over 577 person-years of follow-up with an incidence rate of 0.35 per 100 person-years (95% confidence interval [CI], .04–1.25 per 100 person-years). Both cases were in the iTAB arm (incidence rate, 0.7 per 100 person-years [95% CI, .08–2.52]) compared to 0 (95% CI, 0–1.27) in the SoC arm (P = .25). Both of the HIV seroconverters reported discontinuing drug prior to seroconversion.
TFV-DP Adherence at Weeks 12 and 48
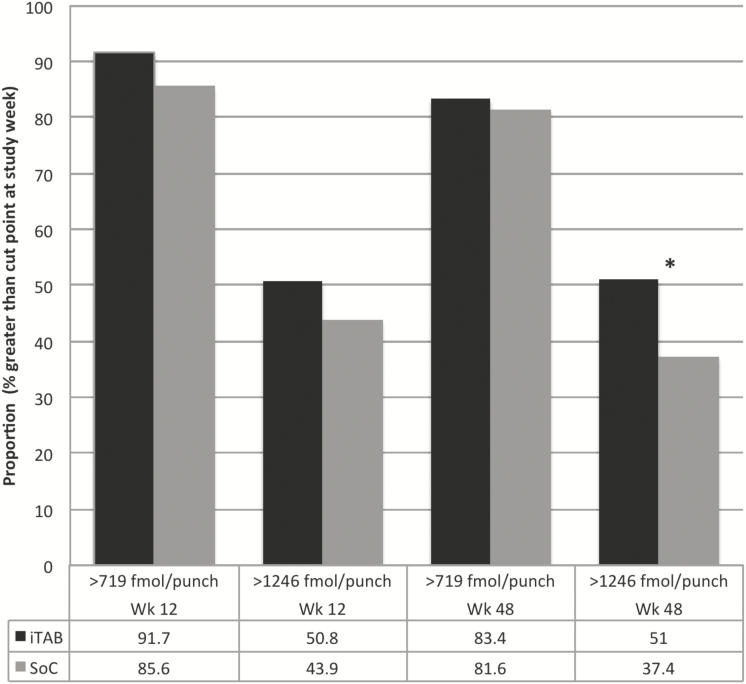
Overall adherence was high, with 320 of 361 (88.6%) subjects at week 12 and 264 of 320 (82.5%) of participants retained on study drug achieving TFV-DP ≥719 fmol/punch, whereas 171 (47.4%) at week 12 and 141 (44.1%) at week 48 had TFV-DP ≥1246 fmol/punch. Among participants with a DBS concentration at week 12, the iTAB arm had 166 (91.7%) who attained ≥719 fmol/punch compared with 154 (85.6%) in the SoC arm (P = .07; Figure 2). At week 48, 131 in the iTAB arm (83.4%) attained ≥719 fmol/punch compared with 133 (81.6%) in SoC (P = .77). For perfect adherence at week 12, the iTAB arm had 92 (50.8%) who attained a DBS level ≥1246 fmol/punch compared with 79 (43.9%) in SoC (P = .21). At week 48, participants in the iTAB arm showed better near-perfect adherence (≥1246 fmol/punch): 80 in the iTAB arm (51%) vs 61 (37.4%) in SoC arm (P = .02).
Figure 2.
Adherence by tenofovir diphosphate at week 12 and 48. Includes only subjects who complete study week. Numbers in cells represent percentage of subjects with a value greater than the stated cut point at the study week alone. *P < .05. Abbreviations: iTAB, individualized texting for adherence building; SoC, standard of care; Wk, study week.
Primary TFV-DP Adherence Endpoint
For the primary analysis, we used a composite outcome of DBS TFV-DP ≥719 fmol/punch at week 12 visit and the last study visit through week 48 (eg, week 24, 36, or 48); no statistical difference was seen between the arms (144 [72.0%] in iTAB vs 137 [69.2%] in SoC; P = .58). In logistic regression (Table 2) adjusting for education, income, white race, non-English language, and baseline depressive symptom score, the adjusted odds ratio for iTAB predicting the primary adherence outcome (≥719 fmol/punch) was 1.37 (95% CI, .87–2.17; P = .18).
Table 2.
Multivariable Logistic Regression Comparing Primary Adherence Outcome (≥719 fmol/Punch) Between the Study Arms
| Characteristic | Adjusted OR | (95% CI) | P Value |
|---|---|---|---|
| iTAB arm | 1.37 | (.87–2.17) | .18 |
| College or greater education | 2.10 | (.99–4.45) | .05 |
| Monthly income of ≥$2000 | 0.91 | (.50–1.65) | .75 |
| Income refused to answer | 0.48 | (.23–.99) | .05 |
| White race | 1.72 | (1.02–2.90) | .04 |
| Primary language other than English | 0.41 | (.15–1.12) | .08 |
| PHQ-9 score | 0.96 | (.92–1.02) | .18 |
Multivariable logistic regression includes arm of study in intent-to-treat analysis for association with having >719 fmol/punch at week 12 and (if there was one) the last study visit up until week 48. Missing was considered nonadherent. Covariates were included in the model if they were associated with the outcome (P < .15) in univariate analysis.
Abbreviations: CI, confidence interval; iTAB, individualized texting for adherence building; OR, odds ratio; PHQ-9, Patient Health Questionnaire-9.
In the secondary analysis used the composite outcome of DBS TFV-DP ≥1246 fmol/punch, there was a trend favoring iTAB compared to SoC (67 [33.5%] vs 49 [24.8%], respectively; P = .06). Of baseline factors, only age predicted adherence for the composite outcome at ≥1246 fmol/punch (older individuals were more adherent). After adjusting for age, the iTAB arm was more adherent for the composite adherence outcome using ≥1246 fmol/punch (odds ratio [OR], 1.56 [95% CI, 1.00–2.42]) and age remained a significant predictor (OR, 1.03 [95% CI, 1.01–10.6]).
FTC Adherence Analysis
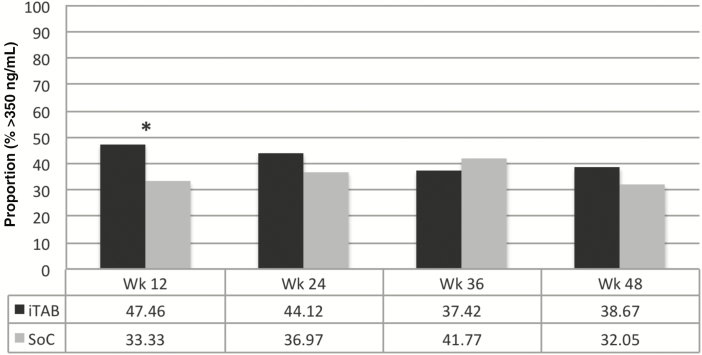
At week 12, the iTAB arm had a higher proportion of subjects with plasma FTC concentrations greater than the 24-hour trough of >350 ng/mL, ie, dosing in past 24 hours, compared to those in the SoC arm (47.5% vs 33.3%; P = .007); this difference did not persist in week 24, 36, or 48 (all P > .05; see Figure 3). Mean plasma FTC concentrations were consistently higher in the iTAB arm, but this only was statistically significant at week 12 (400.3 ng/mL vs 299.3 ng/mL; P = .009).
Figure 3.
Proportion with plasma emtricitabine concentration >24-hour trough (>350 ng/mL). *P < .05. Abbreviations: iTAB, individualized texting for adherence building; SoC, standard of care; Wk, study week.
DISCUSSION
This randomized, controlled trial enrolled MSM and transgender women compared a personalized daily text messaging (iTAB) system to SoC for adherence to TDF/FTC PrEP over 48 weeks. Using a priori composite endpoints, the text messaging intervention did not significantly improve rates of minimally acceptable adherence (≥719 fmol/punch), our primary outcome, but did improve near-perfect adherence (≥1246 fmol/punch) a secondary outcome, through week 48. Specifically, for the near-perfect composite adherence endpoint using DBS TFV-DP data and adjusting for age, the OR was 1.56 (95% CI, 1.00–2.42) for the texting arm. For participants who completed week 48, 13.5% more participants crossed the threshold for near-perfect adherence in the iTAB arm compared to SoC. Finally, we observed significantly more with dosing in past 24 hours by FTC concentrations among those assigned to the iTAB arm at week 12; however, this did not persist in later study visits.
Overall, the evidence supporting text messaging for improving PrEP adherence using biologic endpoints is somewhat mixed. The observed early (week 12) higher rates of adherence as per FTC concentrations suggest that text messaging may help to support routine dosing during PrEP initiation. Additionally, text messaging appears to help maintain near-perfect DBS levels of adherence over 48 weeks. We know that adherence tends to wane over time as was observed in this study across all study groups over 48 weeks. Despite this decrease over time, we hypothesize that the text messaging group who evidenced near-perfect adherence are likely to have formed strong daily habits that may lead to more sustainable adherence over the long term that will continue to stay above adequate thresholds even if adherence waned. Persons who are currently above an adequate threshold for adherence may subsequently fall below this threshold; therefore, maximizing adherence, even if above necessary levels seems proactively beneficial.
Given that protective levels of PrEP are associated with approximately 4 doses per week, there are some that suggest less than daily dosing could a viable strategy, but when considering the possibility of less than daily dosing, it is important to note that the most sustainable behaviors are those that are done daily [31]. The HPTN 067 Adapt Study of on-demand PrEP found that, compared to daily dosing, participants who were using PrEP on demand were more likely to forget or not take a dose prior to possible exposures [32]. While iPERGAY was a successful on-demand dosing schedule, participants had a high median number of doses per month (ie, 18 doses/month), suggesting routine PrEP use >4 days per week (ie, not entirely episodic) [33]. Furthermore, no study has shown noninferiority of episodic dosing to daily dosing for efficacy. The approved usage for TDF/FTC with daily dosing remains the most reliable means of HIV prevention, and text message prompts and motivators could be utilized to maintain optimal adherence.
Our study reached individuals at risk for HIV (HIV prevalence at screening was 1.8%) who were predominantly MSM who exhibited high retention and adherence over 48 weeks, which supports TDF/FTC as a durable, well-tolerated prevention intervention. Our low HIV incidence rate was similar to that observed in another large-scale US PrEP demonstration project (ie, Demo Project, 0.4 per 100 person-years) [34]. There are several potential reasons for the high adherence and retention rates found in this demonstration project: (1) Many participants in this cohort were enthusiastic PrEP early adopters who entered the study when insurance coverage was not widely available. As a result, participants may have used the study as the avenue to access PrEP; (2) CDC PrEP practice guidelines were delivered with experienced research staff that may have a stronger PrEP knowledge base than the general community, and (3) adherence in a research study is likely better than in the community as participants know adherence is being monitored. Additional real-world challenges such as managing prescription refills and insurance payments may decrease adherence, and it is unknown how effective text messaging would be in the context of these structural barriers. Our study was not specifically examining predictors of PrEP adherence; however, as expected, demographic factors appeared to be particularly important including race, education, and income. The impact of these factors warrants continued study.
Other limitations include that, although our sample was relatively diverse, the diversity may not represent those who suffer disparities in HIV prevention or those who struggle with PrEP adherence. Our study did not include many individuals 18–24 years of age, whereas text message interventions for adherence to HIV medications appear to have the strongest effect among populations where there is greater nonadherence (eg, youth) [15, 35]. Future text messaging in the context of PrEP may be best done among those persons who are at greatest risk for nonadherence and/or among more challenged subgroups (eg, young MSM, black MSM, women, transgender women).
In summary, adherence to PrEP in this study of mostly MSM was high with correspondingly low HIV incidence. Although the primary outcome was not significant for sustained adequate adherence, iTAB daily texting improved overall durability of near-perfect adherence through 48 weeks of follow-up. Individualized daily texting for adherence is a low-burden ancillary tool that could be used to maximize long-term PrEP effectiveness by increasing near-perfect adherence. Future studies plan to examine the role of changing HIV risk over time on adherence and variable trajectories of adherence, as well as predictors of intervention engagement, including how specific messages may influence PrEP adherence.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank our participants for volunteering for this study. We also acknowledge the efforts of our study nurses Edward Seefried, Connie A. Funk, Sadia Shaik, Ruben Lopez, Sandra Diaz, and Robert Jimenez, as well as other study staff including Kelly Walsh, Marvin Hanashiro, Daisy Villafuerte, Janisse Mercado, Ramiro Correa, Michael Crump, Fang Wang, and Luis Mendez.
Financial support. This work was supported by the California HIV Research Program (CHRP: EI-11-SD-005). Additional funding includes National Institute of Allergy and Infectious Diseases grants; study drug was provided by Gilead Sciences; and dried blood spot drug concentrations were paid for through a grant by Gilead Sciences.
Potential conflicts of interest. Gilead Sciences provided research funding support and study drug. R. H. is Professor Emeritus at the University of California, San Diego, and is a current employee of Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Grant RM, Lama JR, Anderson PL et al. ; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baeten JM, Donnelly D, Ndase P. et al. Antiretrovial Prophylaxis for HIV-1 Prevention among Heterosexual Men and Women, N Eng J Med, 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thigpen MC, Kebaabetswe PM, Paxton LA et al. ; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 4. Gandhi M, Glidden DV, Liu A et al. ; iPrEx Study Team. Strong correlation between concentrations of tenofovir (TFV) emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx open label extension: implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis 2015; 212:1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koss CA, Bacchetti P, Hillier SL et al. Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses 2017. doi:10.1089/aid.2016.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koblin B, Chesney M, Coates T; EXPLORE Study Team Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet 2004; 364:41–50. [DOI] [PubMed] [Google Scholar]
- 7. Molina JMC, Spire B, Pialoux G et al. ; ANRS Ipergay Study Group. On demand PrEP with oral TDF/FTC in MSM: results of the ANRS Ipergay trial. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2015. [Google Scholar]
- 8. McCormack S, Dunn DT, Desai M et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson PL, Glidden DV, Liu A et al. ; iPrEx Study Team. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marcus JL, Volk JE, Pinder J et al. Successful implementation of HIV preexposure prophylaxis: lessons learned from three clinical settings. Curr HIV/AIDS Rep 2016; 13:116–24. [DOI] [PubMed] [Google Scholar]
- 11. McCallister SM, Guzman R, Shvachko V, Rawlings R, Mera R; Gilead Sciences Inc HIV-1 seroconversion across 17 international demonstration projects with pre-exposure prophylaxis (PrEP) with oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF). American Society for Microbiology, Boston, MA, 2016. [Google Scholar]
- 12. Dai JY, Hendrix CW, Richardson BA et al. Pharmacological measures of treatment adherence and risk of HIV infection in the VOICE study. J Infect Dis 2016; 213:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Damme L, Corneli A, Ahmed K et al. ; FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marrazzo JM, Ramjee G, Richardson BA et al. ; VOICE Study Team. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012; CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One 2014; 9:e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore DJ, Montoya JL, Blackstone K et al. Preliminary evidence for feasibility, use, and acceptability of individualized texting for adherence building for antiretroviral adherence and substance use assessment among HIV-infected methamphetamine users. AIDS Res Treat 2013; 2013:585143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montoya JL, Georges S, Poquette A, Depp CA, Atkinson JH, Moore DJ; Translational Methamphetamine AIDS Research Center (TMARC) Group Refining a personalized mHealth intervention to promote medication adherence among HIV+ methamphetamine users. AIDS Care 2014; 26:1477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore DJ, Poquette A, Casaletto KB et al. ; HIV Neurobehavioral Research Program (HNRP) Group. Individualized texting for adherence building (iTAB): improving antiretroviral dose timing among HIV-infected persons with co-occurring bipolar disorder. AIDS Behav 2015; 19:459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castillo-Mancilla JR, Zheng JH, Rower JE et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blum MR, Chittick GE, Begley JA, Zong J. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol 2007; 47:751–9. [DOI] [PubMed] [Google Scholar]
- 22. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014 clinical practice guideline Available at: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Accessed 7 December 2017.
- 24. Kromdijk W, Pereira SA, Rosing H, Mulder JW, Beijnen JH, Huitema AD. Development and validation of an assay for the simultaneous determination of zidovudine, abacavir, emtricitabine, lamivudine, tenofovir and ribavirin in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2013; 919–920:43–51. [DOI] [PubMed] [Google Scholar]
- 25. Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV knowledge questionnaire. AIDS Educ Prev 2002; 14:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. First MBW, Williams JBW Spitzer RL, Gibbon M.. Structured clinical interview for DSM-IV-TR axis I disorders, clinical trials version (SCID-CT). New York: Biometrics Research, New York State Psychiatric Institute, 2007. [Google Scholar]
- 27. Skinner HA. The drug abuse screening test. Addict Behav 1982; 7:363–71. [DOI] [PubMed] [Google Scholar]
- 28. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 30. Kalichman SC, Rompa D. Sexual sensation seeking and sexual compulsivity scales: reliability, validity, and predicting HIV risk behavior. J Pers Assess 1995; 65:586–601. [DOI] [PubMed] [Google Scholar]
- 31. Neal DT, Wood W, Quinn JM. Habits—a repeat performance. Curr Direct Psychol Sci 2006; 15:198–202. [Google Scholar]
- 32. Bekker LGH, Amico R et al. HPTN 067/ADAPT Cape Town: a comparison of daily and nondaily PrEP dosing in African women. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2015. [Google Scholar]
- 33. Molina JM, Capitant C, Spire B et al. ; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 34. Liu AY, Cohen SE, Vittinghoff E et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ingersoll KS, Dillingham RA, Hettema JE et al. Pilot RCT of bidirectional text messaging for ART adherence among nonurban substance users with HIV. Health Psychol 2015; 34S:1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.