Abstract
Objectives: The 2015 Workshop of the Society for Hematopathology/European Association for Haematopathology submitted small and large B-cell lymphomas (BCLs), including classical Hodgkin lymphoma (CHL), in the context of immunodeficiency.
Methods: Clinicopathologic and molecular features were studied to explore unifying concepts in malignant B-cell proliferations across immunodeficiency settings.
Results: Cases submitted to the workshop spanned small BCLs presenting as nodal or extranodal marginal zone lymphoma and lymphoplasmacytic lymphoma, Epstein-Barr virus (EBV) positive in 75% of cases. Submitted large BCLs formed a spectrum from diffuse large B-cell lymphoma (DLBCL) to CHL across immunodeficiency settings. Additional studies demonstrated overexpression of PD-L1 and molecular 9p24 alterations in the large BCL spectrum and across different immunodeficiency settings.
Conclusions: Small BCLs occur in all immunodeficiency settings, and EBV positivity is essential for their recognition as immunodeficiency related. Large BCLs include a spectrum from DLBCL to CHL across all immunodeficiency settings; immunohistochemical and molecular features are suggestive of shared pathogenetic mechanisms involving PD-L1 immune checkpoints.
Keywords: PD-L1, 9p24, Iatrogenic immunodeficiency, Posttransplant lymphoproliferative disorder, Autoimmunity, Primary immunodeficiency, Large B-cell lymphoma, Classical Hodgkin lymphoma, T-cell/histiocyte-rich B-cell lymphoma, Marginal zone lymphoma
Upon completion of this activity you will be able to:
• recognize the challenges in the classification of B-cell and classical Hodgkin lymphomas in immunodeficiency settings.
• discuss the salient histologic, immunophenotypic, and molecular features of small B-cell lymphomas in immunodeficiency settings.
• describe the overlap in histologic, immunophenotypic, and molecular features of large B-cell lymphomas and classical Hodgkin lymphomas in immunodeficiency settings.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™ per article. Physicians should claim only the credit commensurate with the extent of their participation in the activity. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Exam is located at www.ascp.org/ajcpcme.
Drs Daphne De Jong and John K. C. Chan chaired Session 2 of the workshop on B-cell and classical Hodgkin lymphomas associated with immunodeficiency and, together with Margaretha G. M. Roemer, functioned as the co-lead authors of this article. In this session, a similar approach was taken as in Session 1 (covered in Part 1 of the workshop report) to extend the spectrum of immunodeficiency-associated B-cell proliferations to those with features of various lymphoma classes according to World Health Organization (WHO) nomenclature in immunocompetent patients. These include cytologically low-grade B-cell proliferations, most of which exhibit plasmacytoid differentiation, as well as histologically more aggressive lymphomas such as diffuse large B-cell lymphoma (DLBCL) and its morphologic variants, Burkitt lymphoma and classical Hodgkin lymphoma (CHL). Similarities and important differences were noted in various immunodeficiency backgrounds that were further substantiated by additional molecular studies by Dr Roemer. Here, we discuss the spectrum and boundaries of these proliferations and explore possible unifying and differing aspects in oncogenesis.
Concept of Monomorphic vs Polymorphic B-Cell Proliferations
The spectrum of posttransplant lymphoproliferative disorders (PTLDs) has been the subject of extensive study, and among the immunodeficiency-related lymphoproliferations in the WHO classification, their terminology and definitions are the most clearly defined. Therefore, PTLD serves as the conceptual basis for B-cell lymphoproliferative disorders (B-LPDs) in other immunodeficiency states.1 The boundary between polymorphic vs monomorphic PTLD as defined in the WHO classification, however, raises a number of challenges. Polymorphic PTLD is defined as a lesion that shows a full range of B-cell maturation stages admixed with variable numbers of large transformed cells/immunoblasts, some of which may show Hodgkin/Reed-Sternberg–like morphology.2,3 The transition between polymorphic and monomorphic PTLD is subjective. Conceptually, the morphologic distinction between polymorphic PTLD and monomorphic PTLD was designed to predict response to treatment and especially to guide the choice between tapering of immunosuppression (± rituximab) only vs the need to add chemotherapy.4‐7 Polymorphic PTLD was viewed as a process in which the lymphoid proliferation was not autonomous and that if immune surveillance were restored, the process might regress spontaneously. In contrast, monomorphic PTLD has been viewed as a lymphoma in which treatment beyond reduction of immunosuppression is required. In practice, for many pathologists, the distinction has been based on the assessment of whether there is a preponderance of large transformed cells/immunoblasts. It should be realized, however, that the current pathologic definitions have proven not to be sufficient to predict this clinical behavior, and in practice, B-LPDs that entirely fulfill the criteria for monomorphic B-LPDs do regress upon reduction of immunosuppression only.6,7 Polymorphic PTLD is driven by Epstein-Barr virus (EBV) alone in most instances, whereas monomorphic PTLD may have secondary genetic aberrations leading to deregulated growth of the lymphoid cells. Thus far, only few experimental data are available to support this notion, however.8‐13 On clinical grounds, polymorphic PTLD usually occurs closer to the time of the transplant, whereas monomorphic PTLD occurs late, often many years after the transplant. Therefore, the currently used morphologic definitions do not serve their intended purpose. Ideally, the distinction between polymorphic and monomorphic PTLD, or rather the classes that require chemotherapy or not, should be determined on objective pathogenetic and biological features. Such features have yet to be identified and validated, and collaborative and multi-institutional studies of large numbers of well-defined cases are needed to move this area forward.
Once the determination is made that the lesion is a monomorphic PTLD, then the WHO recommends that further subclassification follow the WHO categories of lymphomas in immunocompetent patients. The range of morphologies considered acceptable for the designation of monomorphic PTLD is broad and includes those with sheets of transformed cells (typical of DLBCL and Burkitt lymphoma), those with scattered large cells in a T-cell and histiocyte-rich background (typical of T-cell and histiocyte-rich large B-cell lymphoma [THRLBCL]), and those that resemble CHL. This boundary therefore is a continuum with polymorphic PTLD and is well recognized as a problematic one with little agreement even among experts. While typical cases of polymorphic PTLD and monomorphic PTLD are not difficult to separate, there are B lymphoproliferations in immunodeficiency settings that clearly overlap this boundary. Furthermore, similar proliferations that straddle this boundary occurring in immunodeficiency backgrounds other than the posttransplant setting are not included in the WHO classification, and guidelines as to how they should be classified are not well defined, leading to inconsistent use of WHO terminology. Among workshop cases, a similar spectrum of lesions was noted in various immunodeficiency backgrounds; however, some differences specific to certain immunodeficiency backgrounds were also noted.
Small B-Cell Lymphomas
Although large B-cell proliferations are the more prototypical manifestation of overt lymphoma in immune deficiency states, small B-cell lymphomas are recorded and cover a specific spectrum of diseases. These are marked by plasmacytoid/plasmacytic differentiation and are morphologically similar to extranodal marginal zone lymphoma (EMZL), nodal marginal zone lymphoma (NMZL), and lymphoplasmacytic lymphoma (LPL).14‐16 Incidental reports of follicular lymphoma and B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (B-CLL/SLL) do not provide an argument for a specific association with immune deficiency states and may be considered incidental in contrast to the group mentioned above.17 Currently, small B-cell lymphomas are not formally included as PTLDs but are mentioned in the context of iatrogenic proliferations and human immunodeficiency virus (HIV)–related proliferations.
A total of 16 cases of small B-cell lymphomas were submitted to the workshop, with most (six of 16) arising in the posttransplant setting, five of 16 cases with probable immune senescence related to aging, three of 16 in the iatrogenic/autoimmune setting, one of 16 virally related (hepatitis C virus), and one of 16 presenting in the primary immune deficiency setting. No HIV-related cases were submitted.
Differential Diagnosis of Small B-Cell Lymphomas With Plasmacytic Differentiation
EMZL, mucosa-associated lymphoid tissue (MALT) type, may be the most prevalent type of cytomorphologically indolent B-cell lymphoma in immune deficiency states and has been described in the posttransplant setting.15,16,18‐21 Among solid organ transplant patients, the interval to development of EMZL is mostly extended (median, 116 months).18 Based on limited data in the literature and supported by the selection of cases submitted to the workshop, primary locations are most frequently reported in the skin and subcutaneous soft tissue and usually present with limited stage disease. Complete response is generally seen after reduction of immunosuppression with or without antiviral therapy, rituximab, or chemotherapy. Many EMZLs diagnosed in the immunodeficiency setting stand out by their prominent plasmacytic differentiation, and almost all reported cases are EBV positive. Prototypical examples are illustrated in Image 1.
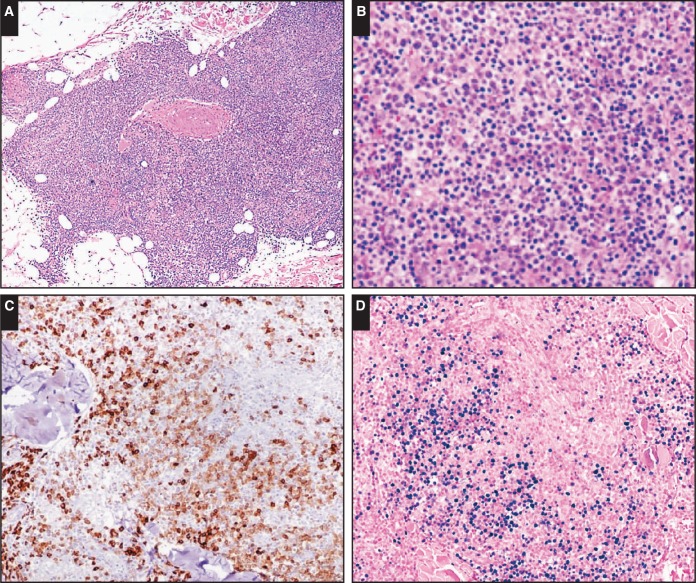
Image 1.
Primary cutaneous extranodal marginal zone lymphoma. Case SH2015-116 (Dr Gong, National Cancer Institute) of a 70-year-old male patient with multiple skin lesions exemplifies a primary cutaneous extranodal marginal zone lymphoma in the immunodeficiency setting (immune senescence related) with a spectrum of small lymphocytes to plasma cell differentiation (A-C, CD79a; A, x4; B and C, x10). EBV-encoded small RNA (EBER) is positive in up to 50% of neoplastic cells (D, EBER, x10).
Extanodal Marginal Zone Lymphomas
Of seven submitted cases of EMZL, four were cutaneous, two of which arose in a posttransplant setting in kidney and cardiac transplant patients and presented after an interval of 25 and 7 years, respectively. Case SH2015-200 (Dr Gibson, University of Pennsylvania) of a 24-year-old cardiac transplant patient showed the typical spectrum of B-cell differentiation with a prominent plasmacytic component. Class switching has been reported in all examples in the posttransplant setting, and remarkably, expression of immunoglobulin A is seen rather than immunoglobulin G, as is common in immune-competent patients.18 In all instances, EBV-encoded small RNA (EBER) was positive.
Very similar cases of primary cutaneous EBV + EMZLs were submitted from two patients with probable immune senescence related to aging: case SH2015-116 (Dr Gong, National Cancer Institute) and case SH2015-427 (Dr Soderquist, University of Pennsylvania), both from 70-year-old patients and both showing dominant plasmacytic differentiation. One additional case (case SH2015-150; Dr Belyaeva, University Pennsylvania) of a 75-year-old patient with a long history of rheumatoid arthritis and methotrexate (MTX) treatment had lesions in cutaneous and nodal locations, with the skin lesions again showing prominent plasmacytic differentiation as above.
Based on the small number of cases reported in the literature and submitted to the workshop, it appears that very similar primary cutaneous EBV+ B-LPDs with plasmacytic differentiation, diagnosed as EMZL, may occur in a broader immune-deficient background and not just in the posttransplant setting as described thus far. This underlines the need for a unifying approach in the study of immunodeficiency-related LPDs.
Posttransplant immunodeficiency has also been implicated in the development of EMZL in other MALT sites, particularly the stomach.20,21 Interestingly, however, these gastric EMZLs are generally EBV– but Helicobacter pylori positive. Therefore, convincing support of a direct association of these lesions with the immunodeficiency state is lacking, and a coincidental association is more likely. It may be that the permissive context of the decreased immune surveillance facilitates survival and proliferation of potentially neoplastic B-cell clones in H pylori–induced lymphoid tissue that might otherwise have been eradicated before progressing to overt lymphoma. Thereby, immunodeficiency may indirectly increase the risk for evolution from H pylori gastritis to EMZL. Similar arguments might hold true for EBV– salivary gland locations (case SH-2015-510; Dr Low, City of Hope Medical Center). However, it should be noted that there are no formal epidemiologic studies reported for an increased risk for EMZL, both EBV+ and EBV–, in the immunodeficiency setting, and thereby this model remains hypothetical.
Nodal B-Cell Lymphomas With Plasma Cell Differentiation
Nodal small B-cell lymphomas are only very rarely described in the immunodeficiency setting and seem to be limited, in contrast to immunocompetent patients, to lesions that exhibit plasmacytoid/plasmacytic differentiation such as LPL and NMZL.14‐16 In immunocompetent patients, the differential diagnosis between these entities can be very challenging due to lack of strict criteria. In the upcoming WHO classification, the molecular alterations with proven MYD88 mutation in the appropriate morphologic and clinical context will become more important, thereby improving the delineation of LPL based on positive diagnostic parameters.3 Thus far, no unequivocal positive diagnostic markers for NMZL have been established.22 The differential diagnosis may be even more difficult in immunodeficient patients, and the diagnostic boundaries between polymorphic B-LPD and small B-cell lymphomas with plasmacytic differentiation may be even more difficult to define.
Five submitted cases of cytomorphologically low-grade nodal disease highlight the differential diagnostic issues. Of these, three patients sought treatment 2 to 7 years after organ transplant (age range, 17-50 years), one patient had a primary immune dysregulation syndrome (Nijmegen breakage syndrome), and one patient did not have any known cause for immunodeficiency at age 62 years. All had widespread disease. The common morphologic features consisted of architectural effacement by a nodular or diffuse infiltrate of small B cells with varying components of monotypic plasma cells. Clonality testing was not consistently available. One case showed dominant plasma cell differentiation (case SH2015-17; Dr Murphy, University of Washington) but lacked further features supporting classification as LPL.
Principally because the full range of B-cell differentiation stages was not consistently present and a component of immunoblasts was not obvious, a diagnosis of NMZL was favored over polymorphic B-LPD. The characteristic architecture of marginal zone lymphoma (MZL) with perifollicular neoplastic marginal zones resulting in a target-like appearance was lacking in all examples. Therefore, the differential diagnostic arguments could be considered somewhat subjective. Moreover, although all cases were positive for EBV, the distribution of EBV+ cells was variable and ranged from uniform to only a small proportion of the lymphoma cells. This variability further supports the argument that these nodal small B-cell proliferations are a variant in the spectrum of polymorphic B-LPD that is typical for immunodeficiency settings rather than being a direct correlate of NMZL in immunocompetent patients.
This notion of a morphologic spectrum between MZL-like proliferations with plasmacytic differentiation and cases resembling polymorphic B-LPD may be further expanded based on the basis of two submitted cases of patients in whom multiple sites were biopsied. Both patients had iatrogenic immunodeficiency and both nodal and extranodal disease (gastrointestinal, skin). Case SH2015-6 (Dr Wang, Cincinnati Children’s Hospital Medical Center) is illustrative. A 17-year-old male patient with Crohn disease treated with azathioprine and 5-aminosalicylic acid had widespread nodal and extranodal, predominantly gastrointestinal, disease. Gastric and colonic biopsy specimens showed plasmacytic proliferation, which in an immunocompetent patient would suggest EMZL with plasmacytic differentiation. EBER was positive in approximately 20% of the tumor cells. An axillary lymph node, however, showed a more polymorphous EBV+ proliferation covering the full spectrum of differentiation of B cells admixed with immunoblasts and would be consistent with the designation of polymorphic B-LPD Image 2 and Figure 1.
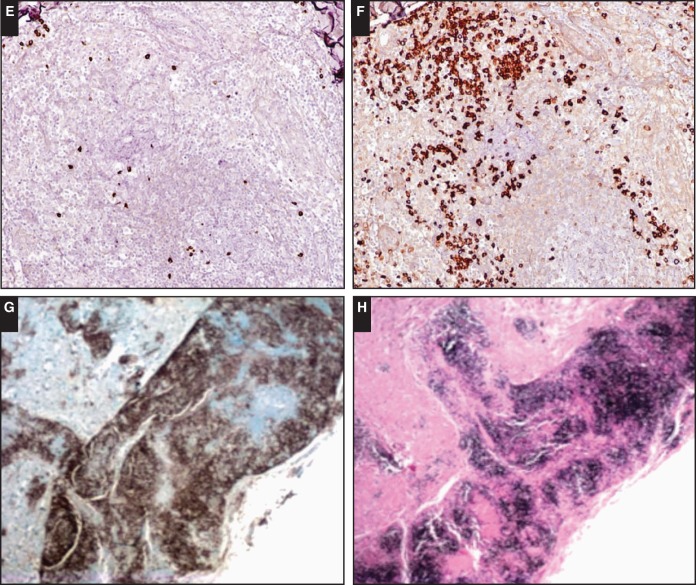
Image 2.
The morphologic spectrum of nodal B-cell lymphomas with plasma cell differentiation. Case SH2015-6 (Dr Wang, Cincinnati Children’s Hospital Medical Center) illustrates the spectrum of indolent B-cell proliferations with plasmacytic differentiation. In a 17-year-old male patient with iatrogenic immunodeficiency, a gastric lesion shows features consistent with extranodal marginal zone lymphoma (A, B, CD79a; C, EBV-encoded small RNA [EBER]).
Figure 1.
Overlapping morphologic and immunohistochemical features of nodal polymorphic B-cell lymphoproliferative disorder (B-LPD) (as discussed in Part 1 of the workshop report) and nodal marginal zone lymphoma (MZL), listing examples across all immunodeficiency settings.
Conclusions: Small B-Cell Lymphoma
Small B-cell proliferations are a rare but bona fide manifestation of immunodeficiency-related B-cell proliferations and are characterized by prominent plasmacytoid/plasmacytic differentiation. First reported in the posttransplant setting, these proliferations have now been reported in the setting of iatrogenic immunodeficiency, primary immunodeficiency, and immune senescence related to aging. In contrast to small B-cell lymphomas in immunocompetent patients, those presenting in immunodeficiency settings are virtually always EBER+. EBER reactivity in small B-cell lymphomas with plasmacytic differentiation may aid in their recognition as likely immunodeficiency related. Therefore, when encountering a rare small B-cell proliferation in immunodeficient patients suggestive of EMZL, NMZL, or LPL, it is worth performing additional studies such as EBER in situ hybridization and molecular clonality assessment to aid in the diagnosis.
Based on case reports to date and cases submitted to the workshop, immunodeficiency-related EMZL seems predominantly a primary cutaneous disease. For other locations such as gastric and salivary gland disease, especially when EBER is negative, a direct association with immunodeficiency may be more debatable. The distinction of nodal small B-cell proliferations in the immunodeficiency setting from polymorphic B-cell lymphoproliferative disorder may be subjective, and the arguments that these proliferations are better incorporated into the spectrum of immunodeficiency-related polymorphic B-LPD than considered related to NMZL in immunocompetent hosts are of merit.
Large B-Cell Lymphomas
Large B-cell lymphomas are by far the most frequently reported lymphoid proliferations in immunodeficiency states. They cover a large morphologic spectrum ranging from typical DLBCL to large cell proliferations with a dominant reactive immune infiltrate with morphologic features similar to those of THRLBCL and CHL.23‐26 In the WHO classification, it is recommended that these lymphomas be classified according to their counterparts in immunocompetent patients, but various classes are listed separately in sections on PTLD, iatrogenic-associated, and HIV-associated settings. Nomenclature is nonuniform between the different immunodeficiency backgrounds, however, and the criteria to distinguish various lesions are not consistent or specified across categories. This, for example, holds true for nomenclature currently used such as Hodgkin-type, Hodgkin-like, and TCHRBCL in the primary immunodeficiency, HIV, iatrogenic, and posttransplant settings, which refers to the difficult differential diagnosis of “gray zone-type” proliferations with ambiguous morphology and immunophenotypes with features of DLBCL and CHL.
Depending on the immunodeficiency background and the clinical condition of the patient, the first treatment approach may be the correction of the immunodeficiency, if applicable. In iatrogenic immunodeficiency, removal of immunosuppressive treatment results in complete regression of lymphoma in a varyingly reported but considerable percentage of patients. Rituximab monotherapy is also an attractive approach with relatively high success rates and is the method of choice in organ transplant patients in whom the transplant organ should not be put at risk for rejection.4‐6,27‐30 Both nonchemotherapeutic strategies are equally successful in EBV+ and EBV– proliferations.6,7,29
Once chemotherapy is chosen, treatment protocols are selected similarly to what is done in immunocompetent patients.7,31 Therefore, the diagnosis of large B-cell lymphoma vs CHL by the pathologist will likely determine therapy. A total of 94 cases of large B-cell proliferations spanning features of DLBCL, TCHRBCL, and CHL are listed in Table 1 according to their category of underlying immunodeficiency or immune dysregulation.
Table 1.
Spectrum of Large B-Cell Lymphomas and EBV Status of Cases Submitted to the Workshop
| Immunodeficiency Setting | Aging/Immune Senescence, No./Total No. (%) | Autoimmune/Iatrogenic, No./Total No. (%) | HIV, No./Total No. (%) | PID, No./Total No. (%) | PTLD, No./Total No. (%) | Total EBV+, No./Total No. (%) |
|---|---|---|---|---|---|---|
| No. of cases | 9 | 28 | 4 | 17 | 36 | 69/89 (78) |
| DLBCL | 5/5 (100) | 9/12 (75) | 1/2 (50) | 9/14 (64) | 9/23 (39) | 33/51 (65) |
| DLBCL, HL-like | 4/4 (100) | 6/7 (86) | 0 | 1/1 (100) | 5/5 (100) | 16/17 (94) |
| CHL | 0 | 9/9 (100) | 2/2 (100) | 2/2 (100) | 7/8 (88) | 20/21 (95) |
| Total cases | 9/9 (100) | 24/28 (86) | 3/4 (75) | 12/17 (71) | 21/36 (58) |
CHL, classical Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV+, Epstein-Barr virus positive; HIV, human immunodeficiency virus; HL, Hodgkin-like; PID, primary immunodeficiency; PTLD, posttransplant lymphoproliferative disorder.
Morphologic Spectrum of Large B-Cell Lymphomas, Including CHL
Examples of the complete spectrum of histologically aggressive B-cell proliferations were received by the workshop in all immune deficiency states, including the primary immunodeficiencies. The latter are separately discussed in Part 5 of the workshop report.
Forty-one cases were diagnosed as DLBCL and showed confluent sheets of large transformed cells with a vesicular chromatin structure and one or more nucleoli. All cases had expression of the complete range of B-cell defining markers such as CD20, CD79a, PAX5, BOB1, and OCT2. EBV positivity ranged from 39% in the posttransplant setting to 75% in the iatrogenic immunodeficiency setting, while immune senescence–related cases were all EBV+ by definition. While formerly an age limit was set for EBV + DLBCL, it is now recognized that this disease may occur in all age groups.32‐37
Twenty-one cases were diagnosed as CHL, based on the presence of scattered large, often multinucleated tumor cells in a mixed background of small lymphocytes, plasma cells, histiocytes, and eosinophils. The tumor cells exhibited a phenotype consistent with CHL, lacking a complete range of B-cell markers and most often expressing CD15. EBER was generally positive except in one patient who had a postrenal transplantation. A diagnosis of TCHRBCL was favored in 16 cases, based on a complete B-cell immunophenotype (CD20, CD79a, PAX5, BOB1, OCT2), (almost completely) lacking eosinophils and B cells in the background infiltrate, which was dominated by T cells and histiocytes. All cases were positive for CD30, although CD15 was seen sporadically.
It was noted that in various cases, the distinction among the three classes (DLBCL, TCHRBCL, and CHL) tended to be morphologically arbitrary; intermediate phenotypes were found and different components spanning the spectrum could be present within a single patient or single biopsy sample. Examples are illustrated in Image 3. In a case of an 83-year-old female patient without known causes of immunodeficiency and who had generalized lymphadenopathy (case SH2015-201; Dr Thibodeaux, University of Pennsylvania), morphology was fully consistent with CHL. However, the tumor cells were positive for the complete range of B-cell differentiation markers as well as for CD30, CD15, LMP1, and EBER. Therefore, a diagnosis of aggressive B-cell lymphoma with features intermediate between large B-cell lymphoma and CHL might be considered. The clinical context, however, does not imply a diagnostic dilemma between primary mediastinal B-cell lymphoma and CHL, which is the main context of the so-called mediastinal gray zone lymphoma, and this classification therefore seems to be inappropriate in the present context. In a series of 46 patients with EBV + DLBCL younger than 45 years, Nicolae and coworkers34 noted seven cases of so-called gray zone lymphoma, further attesting to the presence of a true continuum. The nomenclature in the WHO classification does not distinguish this group of cases; to put together these cases for further discussion and study by the panel, we have employed the descriptive term of DLBCL, Hodgkin lymphoma–like.
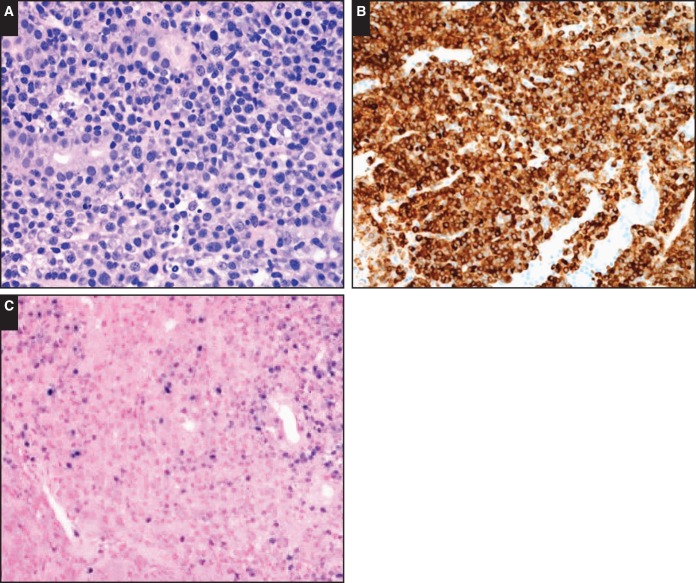
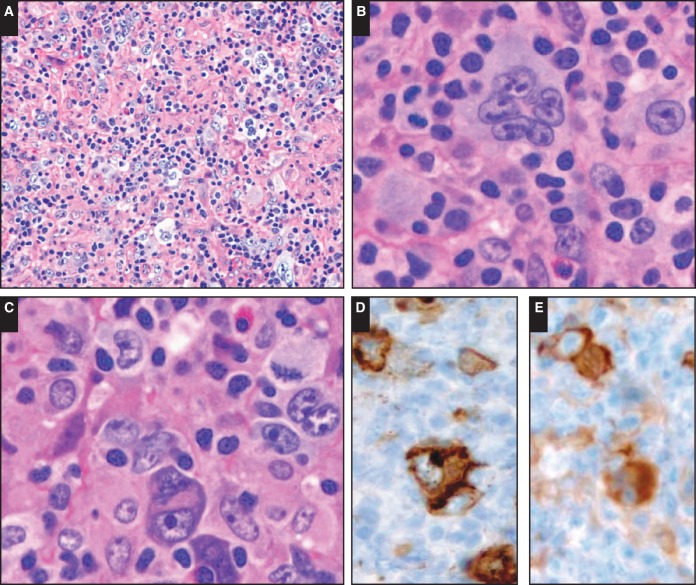
Image 3.
The morphological spectrum of large B-cell proliferations. Case SH2015-116 (Dr Thibodeaux, University of Pennsylvania) of an 83-year-old female patient shows a lesion with morphology of Hodgkin-type tumor cells in a Hodgkin-type background (A-C). The tumor cells express a complete B-cell phenotype but also CD15 (D) and fascin (E).
In four cases, overlapping morphology and immunophenotypes were observed within single biopsy samples. Case SH2015-510 (Dr Low, City of Hope Medical Center) was that of an 81-year-old patient with a long history of rheumatoid arthritis and treatment with MTX, steroids, and tumor necrosis factor–α inhibitor who developed an aggressive EBV+ lymphoma, possibly in the context of an underlying EBV– EMZL in the salivary gland. Large EBER+ multinucleated Hodgkin-like cells were present in areas with a dense reactive background pattern and merged into confluent sheets with necrosis, which was consistent with the diagnosis of DLBCL. Similar classification problems were encountered in cases of large B-cell lymphoma that arose in a patient with B-CLL who was treated with fludarabine, cyclophosphamide, and rituximab (SH2015-298); a renal transplant patient (SH2015-308); and a renal transplant patient with common variable immunodeficiency (SH2015-122). This underlines that a similar range of overlapping morphologic and immunophenotypic features is found in these lesions irrespective of the immunodeficiency background (posttransplant, iatrogenic, and immune senescence), both in young and elderly patients.
These features challenge the principle that large B-cell lymphomas represent separate entities equivalent to morphologically similar lymphoproliferations encountered in immune-competent individuals Figure 2. An alternative hypothesis might be that large B-cell lymphomas arising in immunodeficiency states share similar pathogenetic mechanisms that may culminate in a variety of different morphologies, although additional studies are required to explore this further. Nevertheless, highly unusual clinical presentations of well-defined lymphoma entities should provide food for thought in this respect, and a diagnosis of CHL with a presentation as a single soft tissue mass in the right buttock (SH2015-222; Dr Blankenship, Austin) in a patients with systemic lupus erythematosus treated with MTX should not be readily accepted as CHL despite its apparent similarity of morphology and immunophenotype to CHL in immunocompetent patients.
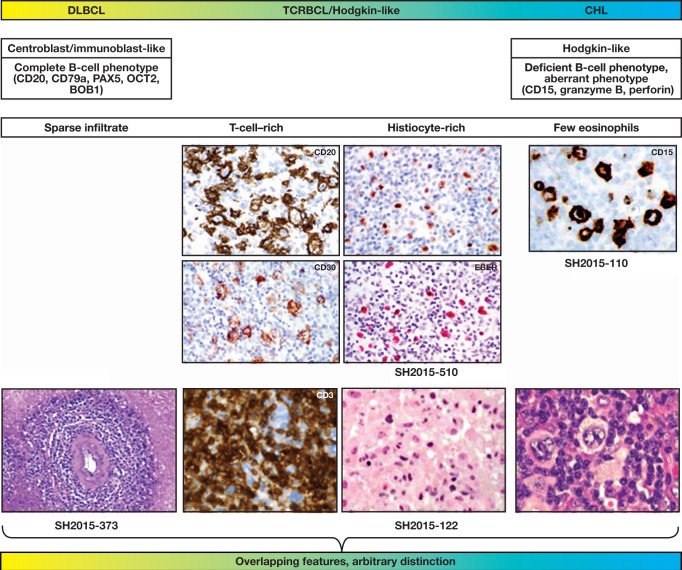
Figure 2.
Large B-cell lymphomas in immunodeficiency states form a spectrum with arbitrary boundaries. The figure illustrates the overlapping features between diffuse large B-cell lymphoma (DLBCL), T-cell–rich B-cell lymphoma (TCRBCL), and classical Hodgkin lymphoma (CHL). While at the extremes of the spectrum, the morphology and immunophenotype of the tumor cells and the composition of the reactive infiltrate may be typical and comparable to the corresponding entities in immunocompetent patients; however, frequently, discordant features are present in immunodeficient patients, precluding unequivocal classification.
Molecular Pathogenesis of Large B-Cell Lymphomas in the Immunodeficiency Setting
Thus far, the pathogenesis of immunodeficiency-related B-LPD has been studied in most detail in the posttransplant and immune senescence settings.1,38,39 Here, we would like to discuss possible common underlying pathogenetic mechanisms across the various immunodeficiency settings in light of the common morphologic spectrum.
Most immunodeficiency-related B-LPDs are reported to be ABC-type proliferations based on studies of PTLD and age-related immune senescence B-LPD. This holds true of both EBV+ and EBV– PTLD.40‐44 The mechanisms behind this common phenotype are different, however, and in EBV– cases, a spectrum of MYD88, CD79B, and CARD11 mutations results in constitutive nuclear factor–κB activation as in immunocompetent patients, while in EBV+ proliferations, EBV seems to substitute for this mechanism, suggesting a unique oncogenesis rather than a common type of ABC-DLBCL.42,43,45‐47 Indeed, the landscape of chromosomal gains and losses in EBV+ PTLD and immune senescence–related B-LPD bears important similarities and differs from EBV– cases and those in immunocompetent patients, including ABC-DLBCL. Although difficult to compare between studies in different immunodeficiency settings, amplification of the 9p24.1 region containing CD274 (PD-L1), PDCD1LG2 (PD-L2), and JAK2 seems to be most characteristic,47 and this is reflected in the high protein expression of PD-L1 that is shared by EBV+ PTLD, immune senescence–related B-LPD, and EBV+ iatrogenic immunodeficiency-related B-LPD (60%-100%, >20% tumor cells) in contrast to DLBCL in immunocompetent patients34,48‐50 but very similar to the genotypic characteristics that are shared by DLBCL in immune-privileged sites (central nervous system [CNS], testis), primary mediastinal B-cell lymphoma, and CHL.51‐53 In these diseases, immune evasion mediated by a tolerogenic microenvironment via dysregulation of the PD-1/PD-L1 immune checkpoint is essential and distinguishes these diseases from common types of DLBCL.
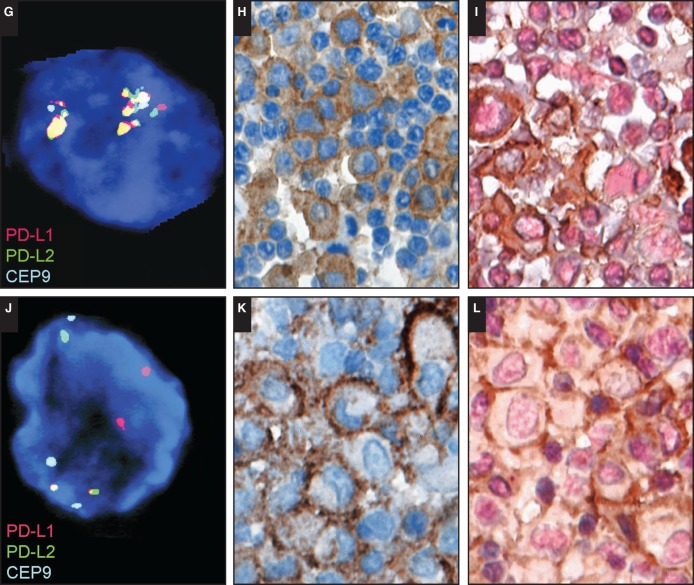
To assess for a role of immune checkpoint abnormalities in B-LPDs across immunodeficiency settings, the panel performed additional PD-L1 and PD-L2 immunohistochemistry (anti–PD-L1 clone: E1L3N, rabbit mAb from Cell Signaling Technology, Danvers, MA; PD-L2 clone: D7U8C, rabbit mAb from Cell Signaling Technology, performed in the Department of Pathology, Stanford University, Stanford, CA) on a series of 54 submitted B-LPDs. PD-L1 was expressed in most cases in tumor cells irrespective of EBV status and immunodeficiency setting in 22 of 22 DLBCLs; 13 of 13 CHLs; four of four large B-cell lymphomas, Hodgkin-like; seven of eight plasmablastic lymphomas; and nine of 12 polymophic B-LPDs Table 2. Expression of PD-L2 was only very sporadically observed. Moreover, expression of PD-L1 could be appreciated in macrophages. In a selection of these cases (n = 25), 9p24.1 alterations were studied by fluorescent in situ hybridization (FISH) according to methods previously reported52 (performed by M. G. M. Roemer in collaboration with A. H. Ligon and M. A. Shipp, Dana-Farber Cancer Institute, Boston, MA). Results are listed in Table 3 and illustrated in Image 4. Of 23 evaluable cases, seven of seven EBV– and 11 of 16 EBV+ large B-cell lymphomas showed alterations at the PD-L1/PD-L2 locus, ranging from low-level polysomy to high-level copy gain and amplification. Two cases with structural chromosomal rearrangements were noted (one EBV–, one EBV+). Most important, 9p24.1 genetic alterations were again observed across all immunodeficiency settings, including posttransplant, immune senescence, iatrogenic/autoimmune, HIV infection, and primary immunodeficiency, and occurred in both EBV+ as well as EBV– cases. This finding substantiates the proposed “unifying approach” for classification of immunodeficiency-related lymphoproliferations across immunodeficiency settings. Moreover, similar alterations were found in the morphologic classes of DLBCL, TCRBCL/Hodgkin-like, and CHL, and these preliminary data support the notion that large B-cell proliferations in the immunodeficiency setting may form a single spectrum driven by a specific pathogenetic mechanism rather than direct counterparts of lymphoproliferative disorders and lymphomas in immunocompetent patients. This small series precludes strong conclusions regarding the role of EBV in this context, and further studies are warranted to confirm and validate these observations. Based on current literature and supported by data derived from cases submitted to the workshop, a common oncogenesis across various immunodeficiency settings with a dominant role for immune evasion and a tolerogenic microenvironment is suggested.
Table 2.
PD-L1 and PD-L2 Expression and Correlation With EBV in Various Immunodeficiency Settings: Large B-Cell Lymphoma/CHL Spectrum
| Immunodeficiency Setting | Aging/Immune Senescence, No./Total No. (%) | Autoimmune/Iatrogenic, No./Total No. (%) | HIV, No./Total No. (%) | PID, No./Total No. (%) | PTLD, No./Total No. (%) | Total, No./ Total No. (%) |
|---|---|---|---|---|---|---|
| No. of cases | 5 | 12 | 7 | 16 | 14 | 54 |
| DLBCL EBV+ | 2/2 (100) | 4/4 (100) | 1/1 (100) | 4/4 (100) | 3/3 (100) | 22/22 (100) |
| DLBCL EBV– | — | — | — | 2/2 (100) | 6/6 (100) | |
| DLBCL-HL EBV+ | — | 2/2 (100) | — | 2/2 (100) | — | 4/4 (100) |
| DLBCL-HL EBV– | — | — | — | — | — | |
| CHL EBV+ | — | 4/4 (100) | — | 4/4 (100) | 4/4 (100) | 13/13 (100) |
| CHL EBV– | — | — | — | — | 1/1 (100) | |
| PBL EBV+ | — | — | 3/3 (100) | — | 1/1 (100) | 7/8 (87) |
| PBL EBV– | 1/2 (50) | — | — | 1/1 (100) | 1/1 (100) | |
| Polymorphic B-LPD EBV+ | 1/1 (100) | 0/1 | 2/3 (67) | 3/3 (100) | 1/1 (100) | 9/12 (75) |
| Polymorphic B-LPD EBV– | — | 0/1 | — | — | 2/2 (100) | |
| Total, No. (%) | 4/5 (80) | 10/12 (83) | 6/7 (86) | 16/16 (100) | 19/19 (100) |
B-LPD, B-cell lymphoproliferative disorder; CHL, classical Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV+, Epstein-Barr virus positive; EBV–, Epstein-Barr negative; HIV, human immunodeficiency virus; HL, Hodgkin-like; PBL, plasmablastic lymphoma; PID, primary immunodeficiency; PTLD, posttransplant lymphoproliferative disorder.
Table 3.
PD-L1/PD-L2 Expression and 9p24.1 Genetic Alterations in Large B-Cell Lymphoma/Classical Hodgkin Lymphoma Spectrum Across Immunodeficiency Settings in 23 Evaluable Cases
| SH2015 No. | Diagnosis | Setting | EBV | HHV8 | PD-L1 Expression | PD-L2 Expression | Tumor Cells, % | Disomy, % | Polysomy, % | No. of Copies | Copy Gain, % | No. of Copies | Amplification, % | No. of Copies | Rearrangement, % | Relative Loss, % | No. of Copies |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 276 | DLBCL, LyG-like | HIV | + | – | + | + | 95 | 86 | 14 | 3-4F:1-2A | |||||||
| 192 | DLBCL | Immune senescence | + | + | – | 95 | 36 | 64 | 3-4 | ||||||||
| 241 | DLBCL | Immune senescence | + | + | – | 95 | 8 | 92 | |||||||||
| 22 | DLBCL | Latrogenic (MTX) | + | + | – | 90 | 100 | ||||||||||
| 164 | DLBCL | Latrogenic (polychemotherapy) | + | + | – | 95 | 100 | ||||||||||
| 71 | DLBCL | HIV | + | – | + | – | 95 | 18 | 4 | 4-5F:2A | 78 | 6-15+F | |||||
| 46 | DLBCL | PID (ATM) | + | + | – | 70 | 76 | 24 | 3-4 | ||||||||
| 47 | DLBCL | PID (Nijmegen breakage syndrome) | – | + | – | 10 | 32 | 20 | 3-5 | 20 | 3-6F:1-3A | 28 | 1-4F:3-6A | ||||
| 190 | DLBCL | PID (hypogammaglobulinemia) | – | + | – | 70 | 66 | 22 | 3-4 | 12 | 3-4F:2A | ||||||
| 400 | DLBCL | PID (SCID/ADA deficiency) | + | + | – | 95 | 62 | 12 | 3-4 | 26 | 3-4F:1-2A | ||||||
| 78 | DLBCL | Posttransplant | – | + | – | 95 | 10 | 90 | |||||||||
| 195 | DLBCL | Posttransplant | + | + | – | 100 | 100 | ||||||||||
| 196 | DLBCL | Posttransplant | – | + | – | 90 | 90 | 10 | 3 | ||||||||
| 286 | DLBCL | Posttransplant | – | + | + | 95 | 76 | 8 | 3-4 | 16 | 3-5F:1-3A | ||||||
| 318 | DLBCL | Posttransplant | – | + | – | 95 | 96 | 4 | 3 | ||||||||
| 417 | DLBCL | Posttransplant | + | + | + | 95 | 8 | 10 | 3-7F:2-3A | 82 | 6-10+F | ||||||
| 484 | DLBCL | Posttransplant | – | + | – | 95 | 14 | 68 | 3-5 | 18 | 4-6F:1-4A | ||||||
| 504 | DLBCL | Posttransplant | + | + | – | 95 | 16 | 52 | 3-5 | 32 | 3-6F:2-4A | ||||||
| 432 | DLBCL (from LGBCL) | Immune senescence | + | + | – | 80 | 100 | ||||||||||
| 510 | DLBCL/CHL (from LGBCL) | Latrogenic (MTX) | + | + | – | 90 | 96 | 4 | 3 | ||||||||
| 70 | Composite DLBCL/CHL | HIV | + | – | + | – | 50 | 98 | 2 | 3 | |||||||
| 265 | DLBCL, TCHRBCL-like | Latrogenic (MTX) | + | + | + | 90 | 62 | 10 | 3-4 | 28 | 3-8F:1-3A | ||||||
| 10 | DLBCL, TCHRBCL-like | Posttransplant | + | + | – | 10 | 100 |
ADA, adenosine deaminase deficiency; ATM, ataxia telangiectasia mutation; CHL, classical Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; HHV8, human herpesvirus 8; HIV, human immunodeficiency virus; LGBCL, low-grade B-cell lymphoma; LyG, lymphomatoid granulomatosis; MTX, methotrexate; PID, primary immunodeficiency; Posttransplant, posttransplant lymphoproliferative disorder; SCID, severe combined immune deficiency; TCHRBCL, T-cell and histiocyte-rich large B-cell lymphoma; +, positive; –, negative.
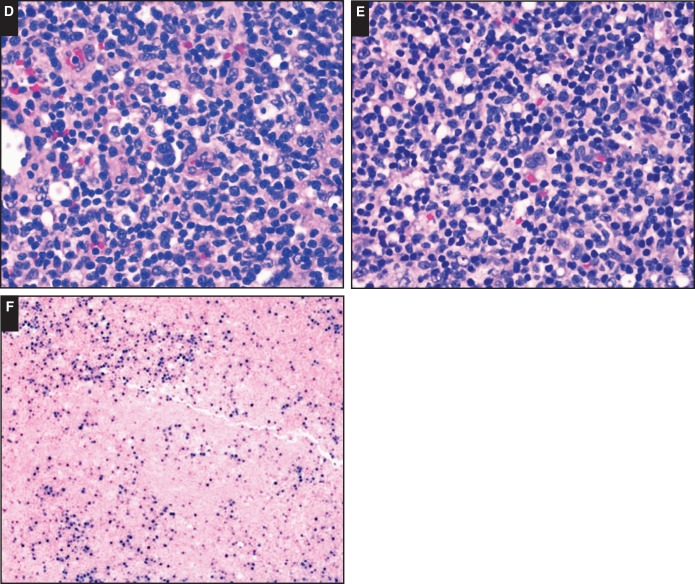
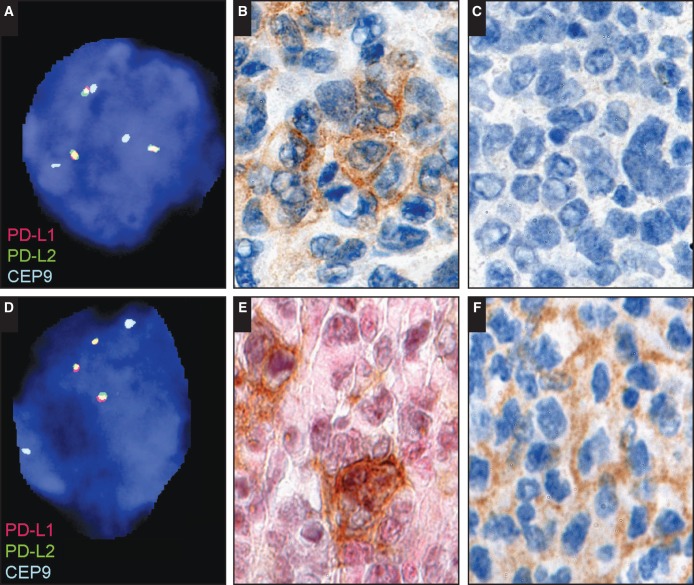
Image 4.
Examples of PD-L1 and PD-L2 protein expression and 9p24.1 genetic alterations by fluorescent in situ hybridization analysis. Low-level copy gain (polysomy; A) in a case of diffuse large B-cell lymphoma (DLBCL), immune senescence–related, Epstein-Barr virus (EBV)–positive (SH2015-192) expression of PD-L1 protein (B) and lack of PD-L2 protein (C) by immunohistochemistry. High-level copy gain (D) in a case of DLBCL, posttransplant, EBV+ (SH2015-286) expression of PD-L1 protein in a subset of PAX5+ large atypical B cells (PD-L1, brown; PAX5, pink; E) and expression of PD-L2 protein (F) by immunohistochemistry.
Clinicopathologic Correlations
A high proportion of immunodeficiency-related B-cell lymphomas may present at extranodal sites. However, only in the posttransplant setting do formal epidemiological data suggest an excess of extranodal gastrointestinal presentations compared with immunocompetent patients.14,53‐55
Specific clinicopathologic correlations, such as hepatosplenic T-cell lymphoma and thiopurine treatment, are well established in the field of immunodeficiency-related T-cell lymphomas. It has been reported that CHL may be more prevalent in the MTX and HIV settings.56‐58 This notion is not supported by the submitted cases to the workshop, however. This may be due to selection bias in the submitted cases but may also result at least in part from the use of different classification criteria in the morphologic spectrum of DLBCL, Hodgkin-like proliferations, and CHL across immunodeficiency settings as described above. One highly specific association of primary CNS lymphomas in the iatrogenic setting should be mentioned. In an epidemiologic survey, Crane and coworkers59 noted a steep increase in the incidence of primary CNS B-LPD to 36% of all PTLD and iatrogenic B-LPD cases diagnosed between 2005 and 2014 compared with preceding 5-year cohorts. The common denominator in primary CNS in both the posttransplant and the iatrogenic setting was the use of mycophenolate mofetil (MMF), particularly when patients were taking MMF in the absence of calcineurin inhibitors. Calcineurin inhibitors were associated with a strong protective effect against primary CNS lymphoma when given alone or in combination with MMF.59 Case SH2015-337 (Dr Crane, Johns Hopkins School of Medicine) is a prototypical example of a 76-year-old patient who was treated with azidothymidine and steroids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss vasculitis) and later with MMF and subsequently had multifocal cerebral lymphoma. The morphology and immunophenotype typically showed overlapping features of large B-LPD as described above with mostly EBV+Hodgkin-like tumor cells. After discontinuation of MMF and rituximab treatment, the lymphoma regressed almost completely and the patient remained stable.
Other novel types of chemotherapy may cause relative immune suppression in specific immunologic cellular compartments, leading to various forms of B-LPD. The dasatinib-related hyperplasias, described in Part 1 of the workshop report, are an example. However, morphologically malignant proliferations may also occur within the spectrum of DLBCL to CHL. In EBV+ proliferations, an association with the presumed immunodeficiency seems likely, but in EBV– cases, this association may not be proven. Both lenalidomide and alemtuzumab (anti-CD52 treatment) lead to severe imbalances in T-cell subsets, including suppression of Treg populations, and cases of EBV+ B-LPD are also reported in this setting.60‐63 These cases should prompt alertness for as yet undefined immunodeficiency states with the expanding clinical use of novel immune modulatory drugs, since new patterns of LPD may emerge with these new therapeutic agents. Fludarabine has been used in B-CLL treatment over a much longer period, and more data are available. Indeed, in fludarabine-treated patients, a high incidence of EBV+ B-cell proliferations has been described covering the spectrum of DLBCL to CHL, which are generally not clonally related to the underlying CLL. Therefore, these lesions do not comply with the strict definition of large cell (Richter) transformation, which is now limited to a direct, clonally related transformation of the original indolent B-CLL/SLL but rather should be regarded as iatrogenic B-LPD. Two cases of B-CLL treated with fludarabine illustrate this concept: SH2015-183 (Dr Gong, Thomas Jefferson University) and SH2015-298 (Dr Ozkaya, Istanbul University), both with EBV+ B-LPD with features of DLBCL and with CHL-like tumor cells.
Conclusions: Large B-Cell Proliferations
The spectrum of B-cell proliferations with morphologically malignant features is broad and spans the spectrum of DLBCL, THRLBCL, and CHL. A similar distribution is seen across various immunodeficiency settings; there are also some specific associations between specific iatrogenic immune modulators and manifestations of LPD. The morphologic range in individual cases, both synchronous and metachronous, together with preliminary genetic data on the role of 9p24 alterations, suggests that these various appearances may represent a single oncogenetic spectrum with varying morphologies and may not show a one-to-one correspondence to their morphologic counterparts in immunocompetent patients.
Table 4 summarizes the key features of small and large B-cell lymphomas associated with immunodeficiency that were addressed in this workshop session.
Table 4.
Summary Table: B-Cell and Classical Hodgkin Lymphomas Associated With Immunodeficiency
| Small B-cell lymphomas |
| Must meet the criteria for specific lymphoma diagnosis in an immunocompetent host |
| Cannot unequivocally be designated as immunodeficiency related if EBV– |
| EBER ISH and clonality assessments should always be included in the diagnostic workup of small B-cell lymphoma in the immunodeficiency setting |
| EMZL in the immunodeficiency setting is most appropriate for primary cutaneous lesions and (almost) invariably EBV+ in contrast to other extranodal sites |
| The morphologic overlap between NMZL and polymorphic B-LPD is extensive, and the boundary is often subjective and arbitrary |
| CHL |
| Must meet the criteria for CHL in an immunocompetent patient |
| Must be separated from mucocutaneous ulcer, polymorphic LPD, and DLBCL with Hodgkin-like cells |
| Be wary of unusual clinical presentation, extranodal localization, and aberrant immunophenotype |
| Shows overlapping features with THRLBCL and cannot always be reproducibly separated |
| More likely forms a spectrum with DLBCL and THRLBCL, and some cases may or may not represent the counterpart of CHL in immunocom petent patients |
| Large B-cell lymphoma |
| Mycophenolate mofetil specifically predisposes to the development of cerebral large B-cell lymphoma; calcineurin inhibitors exert a protective effect in this setting |
| Distribution of EBV+ and EBV– DLBCL varies in immunodeficiency settings (iatrogenic, PTLD, immune senescence, HIV related) |
| Most tumor cells should be demonstrably EBV+ for the diagnosis of EBV+ DLBCL |
| A polymorphous background is helpful to separate large B-cell lymphoma from polymorphic B-LPD with Hodgkin-like cells |
| A subset of EBV+ DLBCL exhibits a prominent T-cell/histiocyte-rich or Hodgkin-like background, and this spectrum may not be reproducibly separated from each other |
| Frequently shows deregulation of the PD-1/PD-L1/2 axis, potentially inducing a tolerogenic microenvironment and immune evasion |
| Molecular mechanisms, including 9p24.1 copy number alterations that contain the PDL1/PDL2/JAK2 locus, are likely shared in all immunodeficiency settings and offer opportunities for targeted therapy |
B-LPD, B-cell lymphoproliferative disorder; CHL, classical Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; EMZL, extranodal marginal zone lymphoma; HIV, human immunodeficiency virus; ISH, in situ hybridization; LPD, lymphoproliferative disorder; NMZL, nodal marginal zone lymphoma; PTLD, posttransplant lymphoproliferative disorder; THRLBCL, T-cell/histiocyte-rich large B-cell lymphoma.
Acknowledgments
Acknowledgments: We thank Margaret A. Shipp, MD, Scott J. Rodig, MD, PhD, Azra H. Ligon, PhD, and colleagues at the Department of Medical Oncology, Dana-Farber Cancer Institute (Boston, MA) for supporting and supervising the PDL1/PDL2/9p24.1 FISH analysis on a selection of cases submitted to the workshop. PD-L1 and PD-L2 immunohistochemistry, including PAX5 double staining, was performed by Shuchun Zhao, PhD, at the Department of Pathology, Stanford University School of Medicine, under the supervision of Dr Natkunam.
References
- 1. Morscio J, Dierickx D, Tousseyn T. Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorders: what do we know so far? Clin Dev Immunol. 2013;2013;150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed.Lyon, France: IARC; 2008. [Google Scholar]
- 3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Styczynski J, van der Velden W, Fox CP, et al. Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petrara MR, Giunco S, Serraino D, et al. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369:37-44. [DOI] [PubMed] [Google Scholar]
- 6. Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013;8:173-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nassi L, Gaidano G. Challenges in the management of post-transplant lymphoproliferative disorder. Hematol Oncol. 2015;33(suppl 1):96-99. [DOI] [PubMed] [Google Scholar]
- 8. Djokic M, Le Beau MM, Swinnen LJ. Post transplant lymphoproliferative disorder subtypes correlate with different recurring chromosomal abnormalities. Gene Chromosomes Cancer. 2006;45:313-318. [DOI] [PubMed] [Google Scholar]
- 9. Poirel HA, Bernheim A, Schneider A, et al. Characteristic pattern of chromosomal imbalances in post-transplantation lymphoproliferative disorders: correlation histopathological subcategories and EBV status. Transplantation. 2005;80:176-184. [DOI] [PubMed] [Google Scholar]
- 10. Vakiani E, Nandula SV, Subramaniyam, et al. Cytogenetic analysis of B-cell posttransplant lymphoproliferations validate the World Health Organization classification and suggests inclusion of florid follicular hyperplasia as a precursor lesion. Hum Pathol. 2007;38:315-325. [DOI] [PubMed] [Google Scholar]
- 11. Vakiani E, Basso K, Klein U, et al. Genetic and phenotypic analysis of B-cell post-transplant lymphoproliferative disorders provides insights into disease biology. Hematol Oncol. 2008;26:199-211. [DOI] [PubMed] [Google Scholar]
- 12. Cesarman E, Chadburn A, Liu YF, et al. BCL6 gene mutations in post-transplantantion lymphoproliferative disorders predict response to therapy and clinical outcome. Blood. 1998;92:2294-2302. [PubMed] [Google Scholar]
- 13. Knowles DM, Cesarman E, Chadburn A, et al. Correlative morphological and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85:552-565. [PubMed] [Google Scholar]
- 14. Caillard S, Lelong C, Pessione F, et al. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: a report of 230 cases from the French registry. Am J Transplant. 2006;6:2735-2742. [DOI] [PubMed] [Google Scholar]
- 15. Clarke CA, Morton LM, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013;109:280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knight JS, Tsodikov A, Cibrik DM, et al. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354-3362. [DOI] [PubMed] [Google Scholar]
- 17. Vajdic CM, van Leeuwen MT, Turner JJ, et al. No excess risk of follicular lymphoma in kidney transplant and HIV-related immunodeficiency. Int J Cancer. 2010;127:2732-2735. [DOI] [PubMed] [Google Scholar]
- 18. Gibson SE, Swerlow SH, Craig FE. EBV-positive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the post-transplant setting: a distinct type of posttransplant lymphoproliferative disorder? Am J Surg Pathol. 2011;35:807-815. [DOI] [PubMed] [Google Scholar]
- 19. Joshi VV, Gagnon GA, Chadwick EG, et al. The spectrum of mucosa-associated lymphoid tissue lesions in pediatric patients infected with HIV: a clinicopathologic study of six cases. Am J Clin Pathol. 1997;107:592-600. [DOI] [PubMed] [Google Scholar]
- 20. Hsi ED, Singleton TP, Swinnen L, et al. Mucosa-associated lymphoid tissue type lymphomas occurring in post-transplantation patients. Am J Surg Pathol. 2000;24:100-106. [DOI] [PubMed] [Google Scholar]
- 21. Shehab TM, Hsi ED, Poterucha JJ, et al. Helicobacter pylori–associated gastric MALT lymphoma in liver transplant recipients. Transplantation. 2001;71:1172-1175. [DOI] [PubMed] [Google Scholar]
- 22. van den Brand M, van Krieken JH. Recognizing nodal marginal zone lymphoma: recent advances and pitfalls. A systematic review. Haematologica. 2013;98:1003-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age- related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamel OW, Weiss LM, van de Rijn M, et al. Hodgkin's disease and lymphoproliferations resembling Hodgkin's disease in 33 patients receiving long-term low-dose methotrexate therapy. Am J Surg Pathol. 1996;20:1279-1287. [DOI] [PubMed] [Google Scholar]
- 25. Loo EY, Medeiros LJ, Aladily TN, et al. Classical Hodgkin lymphoma arising in the setting of iatrogenic immunodeficiency: a clinicopathologic study of 10 cases. Am J Surg Pathol. 2013;37:1290-1297. [DOI] [PubMed] [Google Scholar]
- 26. Asano N, Yamamoto k, Tamaru J, et al. Age-related Epstein-Barr virus–associated B-cell lymphoproliferative disorders: comparison with EBV-positive classic Hodgkin lymphoma in the elderly patients. Blood. 2009;113:2629-2636. [DOI] [PubMed] [Google Scholar]
- 27. Neparidze N, Lacy J. Malignancies associated with Epstein-Barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12:358-371. [PubMed] [Google Scholar]
- 28. Singavi AK, Harrington AM, Fenske TS. Post-transplant lymphoproliferative disorders. Cancer Treat Res. 2015;165:305-327. [DOI] [PubMed] [Google Scholar]
- 29. Evens AM, Roy R, Sterrenberg D, et al. Post-transplantation lymphoproliferative disorders: diagnosis, prognosis, and current approaches to therapy. Curr Oncol Rep. 2010;12:383-394. [DOI] [PubMed] [Google Scholar]
- 30. Bower M. The management of lymphoma in the immunosuppressed patient. Best Pract Res Clin Haematol. 2002;15:517-532. [DOI] [PubMed] [Google Scholar]
- 31. Choquet S, Trappe R, Leblond V, et al. CHOP-21 for the treatment of posttransplant lymphoproliferative disorders (PTLD) following solid organ transplantation. Haematologica. 2007;92:273-274. [DOI] [PubMed] [Google Scholar]
- 32. Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-lymphoproliferative disorders: a clinicopathological study of 22 patients. Am J Surg Pathol. 2003;27:16-26. [DOI] [PubMed] [Google Scholar]
- 33. Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated lymphoproliferative disorders constitute a distinct clinicopathological group: a study of 96 patients. Clin Cancer Res. 2007;13:5124-5132. [DOI] [PubMed] [Google Scholar]
- 34. Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015;126:863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ok CY, Ye Q, Li L, et al. Age cut-off for Epstein-Barr virus–positive diffuse large B-cell lymphoma—is it necessary? Oncotarget. 2015;6:13933-13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beltran BE, Morales DH, Suh C, et al. EBV-positive diffuse large B-cell lymphoma in young adults: is this a distinct disease entity? Ann Oncol. 2015;26:548-555. [DOI] [PubMed] [Google Scholar]
- 37. Hong JY, Yoon DH, Suh C. EBV-positive diffuse large B-cell lymphoma in young adults: is this a distinct disease entity? Ann Oncol. 2014;26:1-8. [DOI] [PubMed] [Google Scholar]
- 38. Sarkozy C, Salles G, Falandry C. The biology of aging and lymphoma: a complex interplay. Curr Oncol Rep. 2015;17:32. [DOI] [PubMed] [Google Scholar]
- 39. Capello D, Rossi D, Gaidano G. Post-transplant lymphoproliferative disorders: molecular basis of disease histogenesis and pathogenesis. Hematol Oncol. 2005;23:61-67. [DOI] [PubMed] [Google Scholar]
- 40. Klapper W, Kreuz M, Kohler CW, et al. Patient age at diagnosis is associated with the molecular characteristics of diffuse large B-cell lymphoma. Blood. 2012;119:1882-1887. [DOI] [PubMed] [Google Scholar]
- 41. Craig FE, Johnson LR, Harvey SA, et al. Gene expression profiling of Epstein-Barr virus–positive and –negative monomorphic B-cell posttransplant lymphoproliferative disorders. Diagn Mol Pathol. 2007;16:158-168. [DOI] [PubMed] [Google Scholar]
- 42. Gebauer N, Gebauer J, Hardel TT, et al. Prevalence of targetable oncogenic mutations and genomic alterations in Epstein-Barr virus–associated diffuse large B-cell lymphoma of the elderly. Leuk Lymph. 2015;56:1100-1106. [DOI] [PubMed] [Google Scholar]
- 43. Morscio J, Dierickx D, Ferreiro JF, et al. Gene expression profiling reveals clear differences between EBV-positive and EBV-negative post-transplant lymphoproliferative disorders. Am J Transplant. 2013;13:1305-1316. [DOI] [PubMed] [Google Scholar]
- 44. Montes-Moreno S, Odqvist L, Diaz-Perez JA, et al. EBV-positive diffuse large B-cell lymphoma of the elderly is an aggressive post-germinal center B-cell neoplasm characterized by prominent nuclear factor-kB activation. Mod Pathol. 2012;25:968-982. [DOI] [PubMed] [Google Scholar]
- 45. Kato H, Karube K, Yamamoto Y, et al. Gene expression profiling of Epstein-Barr virus–positive diffuse large B-cell lymphoma of the elderly reveals alterations of characteristic oncogenetic pathways. Cancer Sci. 2014;105:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Azevedo de Andrade T, Evangelista AF, Froes Campos AH, et al. A microRNA signature profile in EBV+ diffuse large B-cell lymphoma of the elderly. Oncotarget. 2014;5:11813-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoon H, Park S, Ju H, et al. Integrated copy number and gene expression profiling analysis of Epstein-Barr virus–positive diffuse large B-cell lymphoma. Genes Chromosomes Cancer. 2015;54:383-396. [DOI] [PubMed] [Google Scholar]
- 48. Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Loo P, Thousseyn T, Vanhentenrijk V, et al. T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolerogenic host immune response. Haematologica. 2010;95:440-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kwon D, Kim S, Kim PJ, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68:1079-1089. [DOI] [PubMed] [Google Scholar]
- 51. Chapuy B, Roemer MGM, Steward C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127:869-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roemer MGM, Advani RH, Ligon A, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caillard S, Lamy FX, Quelen C, et al. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682-693. [DOI] [PubMed] [Google Scholar]
- 54. Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222-230. [DOI] [PubMed] [Google Scholar]
- 55. Morton M, Coupes B, Roberts SA, et al. Epidemiology of posttransplantation lymphoproliferative disorder in adult renal transplant recipients. Transplantation. 2013;95:470-478. [DOI] [PubMed] [Google Scholar]
- 56. Glaser SL, Clarke CA, Gley ML, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area 1988-1998. Cancer. 2003;98:300-309. [DOI] [PubMed] [Google Scholar]
- 57. Biggar RJ, Jaffe SE, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:37-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hasserjian RP, Chen S, Perkins SL, et al. Immunomodulator agent-related lymphoproliferative disorders. Mod Pathol. 2009;22:1532-1540. [DOI] [PubMed] [Google Scholar]
- 59. Crane GM, Powell H, Kostadinov R, et al. Primary CNS lymphoproliferative disease, mycophenolate and calcineurin inhibitor usage. Oncotarget. 2015; 6:33849-33866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kluin-Nelemans HC, Coenen JL, Boers JE, et al. immunodeficiency lymphoma after alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Blood. 2008;112:1039-1041. [DOI] [PubMed] [Google Scholar]
- 61. Xiao W, Chen WW, Sorbara L, et al. Hodgkin Lymphoma variant of Richter transformation: morphology, EBV status, clonality and survival analysis—with comparison to Hodgkin-like lesion. Hum Pathol. 2016;55:108-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheah CY, Spagnolo D, Frost F, Cull G. Synchronous biphenotypic Richter syndrome with EBV positive nodal classical Hodgkin lymphoma and bone marrow diffuse large B-cell lymphoma. Histopathology 2016;69:707-710. [DOI] [PubMed] [Google Scholar]
- 63. Pálmason R, Lindén O, Richter J. Case-report: EBV driven lymphoproliferative disorder associated with ruxolitinib. BMC Hematol. 2015;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]