Abstract
Objectives: The 2015 Workshop of the Society for Hematopathology/European Association for Haematopathology aimed to review primary immunodeficiency and related lymphoproliferations.
Methods: Primary immunodeficiencies were divided into immune dysregulation, DNA repair defects, low immunoglobulins, and combined immunodeficiencies.
Results: Autoimmune lymphoproliferative syndrome (ALPS) is a prototypical immune dysregulation-type immunodeficiency, with defects in T-cell signaling or apoptosis, expansion of T-cell subsets, and predisposition to hemophagocytic lymphohistiocytosis. DNA repair defects directly predispose to malignancy. Low immunoglobulin immunodeficiencies such as common variable immunodeficiency (CVID) have underlying T-cell repertoire abnormalities predisposing to autoimmunity and B-cell lymphoproliferations. The full spectrum of B-cell lymphoproliferative disorders occurs in primary immunodeficiency.
Conclusions: Lymphoproliferations in primary immunodeficiency mirror those in other immunodeficiency settings, with monomorphic B- and sometimes T lymphoproliferative disorders enriched in DNA repair defects. Distinctive T-cell subset expansions in ALPS, CVID, and related entities can mimic lymphoma, and recognition of double-negative T-cell or cytotoxic T-cell expansions is key to avoid overdiagnosis.
Keywords: Primary immunodeficiency, Common variable immunodeficiency, Autoimmune lymphoproliferative syndrome, T-cell lymphoma, Double negative T cells
Upon completion of this activity you will be able to:
list examples of nonmalignant proliferations associated with primary immunodeficiencies.
describe features of primary immunodeficiency-associated proliferations that mimic malignancy.
list specific associations of childhood lymphoma and primary immunodeficiency.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™ per article. Physicians should claim only the credit commensurate with the extent of their participation in the activity. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Exam is located at www.ascp.org/ajcpcme.
Drs Dita Gratzinger and Elaine Jaffe chaired Session 5 of the workshop on primary/congenital immunodeficiency and functioned as the co-lead authors of this article. We received 49 cases of primary immunodeficiency disorders, most with associated lymphoproliferations. The primary immunodeficiencies and how they predispose to lymphoproliferative disorders are discussed in the first section; specific examples of primary immunodeficiency-related lymphoproliferative disorders reviewed at the workshop are discussed in the second section.
Types of Primary Immunodeficiency
We broadly classified the primary immunodeficiencies by clinicopathologic phenotype1 as follows: immune dysregulation, characterized by lymphoproliferation and dysfunctional immune phenomena such as autoimmunity and hemophagocytic lymphohistiocytosis; DNA repair defects, which are often syndromic and carry a risk of nonhematologic malignancy; low immunoglobulins, which typically present with repeated bacterial infections; and combined immunodeficiencies, with defects of both cellular and humoral immunodeficiency Table 1.
Table 1.
Number and Type of Primary Immunodeficiency Cases Submitted by Subtype of Primary Immunodeficiencya
| Immune Dysregulation | DNA Repair Defects | Low Immunoglobulins | Combined Immunodeficiencies |
|---|---|---|---|
| ALPS (7) | AT (4) | CVID (14) | DOCK8 deficiency (2) |
| XLP (4) | Nijmegen (1) | Immunoglobulin subset deficiency (4) | SCID (2) |
| Chédiak-Higashi (2) | Bloom syndrome (1) | PIK3CD (2) | CHARGE (1) |
| RALD (1) | CMRD (1) | XLA (1) | Δ22q11.2 (1) |
| Perforin mutation (1) | Hypomorphic RAG1 (1) | Wiskott-Aldrich (1) | |
| Down syndrome (1) |
ALPS, autoimmune lymphoproliferative syndrome; AT, ataxia telangiectasia; CHARGE, coloboma of the eye, heart defects, atresia of the choanae, retardation of growth and/or development, genital and/or urinary anomalies, and ear malformations; CMRD, congenital mismatch repair deficiency; CVID, common variable immunodeficiency; Δ22q11.2, chromosome 22q11.2 deletion syndrome, formerly known as DiGeorge or velocardiofacial syndrome; DOCK8, dedicator of cytokinesis 8 deficiency; PIK3CD, phosphatidylinositol 3-kinase C δ activating kinase mutation; RAG1, recombination-activating gene 1; RALD, RAS-associated leukoproliferative disorder; SCID, severe combined immunodeficiency; XLA, X-linked agammaglobulinemia; XLP, X-linked lymphoproliferative disorder.
Numbers in parentheses represent the number of cases in the category.
Immune Dysregulation
Immune dysregulation-associated primary immunodeficiencies are dominated by defects in T-cell and/or natural killer (NK)–cell signaling or apoptosis. These, in turn, lead to expansion of dysfunctional T-cell subsets and/or virally transformed B cells that are immune to cytotoxic killing by the dysfunctional T and/or NK cells. Impaired cytotoxicity predisposes to hemophagocytic lymphohistiocytosis, a massive expansion and activation of ineffectual cytotoxic T cells that in turn trigger massive release of proinflammatory cytokines and macrophage activation.2 Autoimmune phenomena represent failure of clearance of autoreactive cells, whether due to an intrinsic failure in apoptosis or due to failure of central or peripheral tolerance mechanisms. Many have dysgammaglobulinemias, likely due to defective T-cell help in shaping the antigen-specific immunoglobulin response. Predisposition to non-Hodgkin lymphoma, especially Epstein-Barr virus–positive (EBV+) B-cell non-Hodgkin lymphoma, is associated with impaired monitoring and killing of transformed B cells. Importantly, there is wide phenotypic variation within molecularly defined syndromes, likely due to a combination of the specific type of mutation present, the genetic background of the effected individual, and environmental exposures.
Autoimmune lymphoproliferative syndrome (ALPS) is caused by a failure of lymphocyte apoptosis,3 which leads to aberrant accumulation of T lymphocytes, including a characteristic but not entirely specific4 accumulation of CD4/CD8 double-negative T-cell receptor α/β-positive T cells (DNTs). Common manifestations include lymphadenopathy, splenomegaly, and predisposition to B-cell lymphoproliferative disorders and autoimmunity. Splenic sequestration and autoimmunity can cause chronic multilineage cytopenias; however, splenectomy should be avoided as it predisposes to life-threatening sepsis in these patients.5
Revised diagnostic criteria for ALPS developed at a National Institutes of Health workshop in 20096 require the following characteristics for a definitive diagnosis: a combination of unexplained chronic lymphadenopathy/splenomegaly, elevated DNTs, and either proof of defective lymphocyte apoptosis or a pathogenic mutation in FAS (a tumor necrosis factor receptor family member), FASLG (FAS ligand), or CASP10 (caspase 10, which transmits proapoptotic signals) for definitive diagnosis. While most patients with an initial diagnosis of ALPS are children, the diagnosis should also be considered in adult patients.7 Lymph nodes from patients with ALPS show paracortical hyperplasia with increased DNTs that are CD45RA+, CD45RO– and frequently positive for cytotoxic markers perforin, T-cell restricted intracellular antigen-1 (TIA-1), and CD57.8 These features could potentially cause confusion with mature T-cell lymphoma, particularly in adult patients.
A quarter of cases of ALPS have been reported to show concurrent features of sinus histiocytosis with massive lymphadenopathy9 (also known as Rosai-Dorfman disease [RDD]), which may be a clue to the underlying diagnosis. Lymph nodes are enlarged and contain abundant histiocytes with characteristic nuclear features of RDD; these are characteristically S100+ and contain intact lymphocytes and plasma cells (emperipolesis). A single case of malignant progression to histiocytic sarcoma has been reported.10
Ras-related leukoproliferative disorder (RALD) is a rare and complex disorder caused by an activating somatic or germline Neuroblastoma RAS viral oncogene homolog (NRAS) or Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation. RALD brings together a predisposition to ALPS-like autoimmunity with or without double-negative T-cell lymphoproliferations and a predisposition to develop myeloid malignancy, particularly juvenile myelomonocytic leukemia (JMML).11‐13 Despite identical activating retrovirus associated sequence (RAS) mutations to those seen in JMML, monocytosis, and splenomegaly, patients with RALD pursue a much more indolent clinical course than that seen in JMML.14
Chédiak-Higashi syndrome is one of a group of immunodeficiency disorders that are associated with partial oculocutaneous albinism as a result of organelle trafficking dysfunction,15 including Griscelli syndrome, Hermansky-Pudlak syndrome, and deficiency of a mitogen-activated protein kinase pathway protein involved in late endosomal-lysosomal binding, mitogen-activated protein-binding protein-interacting protein.16 The organellar trafficking dysfunction leads to dysfunctional secretion of cytotoxic granules by cytotoxic T cells and NK cells, as well as platelet dysfunction, abnormal delivery of melanosomes, and neutrophil granule abnormalities. These functional deficits explain the variable combinations of immune deficiencies, predisposition to hemophagocytic lymphohistiocytosis, albinism, and bleeding diathesis usually presenting in early childhood; neurologic dysfunction develops later in the disease course. Chédiak-Higashi syndrome in particular is due to loss of function of the lysosomal trafficking regulator lysosomal trafficking regulator (LYST) and manifests with partial albinism, severe pyogenic infections, a mild bleeding diathesis, and neurologic dysfunction. Infection-related mortality is high; those who survive are at high risk for hemophagocytic lymphohistiocytosis (so-called accelerated phase).
Perforin is crucial for NK-cell and cytotoxic T-cell–mediated cell lysis; it forms the pore through which granzyme proteases are injected into the target cell.17 Complete perforin deficiency as a result of biallelic loss-of-function mutations results in a severe familial hemophagocytic lymphohistiocytosis (HLH) phenotype with development of HLH in infancy.18 Patients with missense mutations may instead present with relatively late-onset HLH, autoimmune symptoms, and/or lymphoma.19 Heterozygous missense perforin (PRF) mutations have also been reported to be associated with pediatric anaplastic lymphoma receptor tyrosine kinase+ (ALK+) anaplastic large cell lymphoma.20
X-linked lymphoproliferative disorder (XLP) is caused by defects in Src homology-2 domain protein 1A, which interferes with signaling downstream of a subset of surface receptors in NK and T cells, particularly an inability to kill EBV-transformed B cells.21 This in turn predisposes to fulminant infectious mononucleosis, a massive lymphoproliferative reaction to primary EBV infection often associated with hemophagocytosis, progressive dysgammaglobulinemia, and lymphoproliferative disorders.22
DNA Repair Defects
Disorders of DNA repair have pleiotropic effects on development and directly predispose to various forms of malignancy, including hematolymphoid malignancy due to accumulation of DNA damage. B-cell and T-cell antigen receptors must undergo controlled recombination, rearrangement, somatic mutation, and proliferation to produce the various and tailored components of the specific immune response. Inability to repair accompanying DNA damage leads to decreased and limited antigen-specific repertoires. Ataxia telangiectasia, Nijmegen breakage syndrome, and Bloom syndrome are well-known syndromes associated with DNA repair defects; less well understood is constitutional mismatch repair deficiency, which is caused by biallelic germline mutations of mismatch repair genes. These patients have a predisposition to childhood cancers, including non-Hodgkin lymphoma.23
Low Immunoglobulins
Primary immunodeficiencies dominated by immunoglobulin deficiency, and therefore infectious manifestations are nevertheless commonly associated with autoimmune or inflammatory manifestations, which are likely due to an associated T-cell repertoire abnormality that fails to repress or deplete self-reactive immune populations. The concomitant predisposition to predominantly B-cell–derived lymphoid hyperplasias and lymphomas may be partially due to inability to suppress EBV-transformed or other abnormal clonal B-cell populations.
Combined variable immunodeficiency (CVID) is characterized by antibody underproduction, leading to low serum levels of immunoglobulin G, immunoglobulin A, and/or immunoglobulin M and a heterogeneous clinical phenotype spanning infection, autoimmunity, lung and gastrointestinal disease, and propensity to lymphadenopathy and splenomegaly, granulomatous disease, and lymphoma.24 The definition is a functional one, and patients may be diagnosed in childhood or adulthood. In most cases, the underlying molecular etiology is as yet unclear, but patients have been found to have decreased diversity of the naive B-cell pool, decreased somatic hypermutation in memory repertoires, and abnormal clonal expansion of unmutated B cells likely contributing to autoimmune phenomena.25 Patients also have a T-cell defect, manifesting as a clonal and restricted T-cell repertoire.26 Immunoglobulin subclass deficiency by contrast is primarily associated with a susceptibility to infection.27
Patients with an activating mutation of the phosphatidyl- inositol 3-kinase C δ subunit (PIK3CD) have clinical phenotypes that overlap with those of CVID, including recurrent sinopulmonary infections, lymphoid hyperplasia, and cytomegalovirus (CMV) or EBV viremia.28 Patients have an underlying T-cell defect manifesting as a deficiency of naïve T cells and an excess of senescent effector T cells. A subset of patients has elevated immunoglobulin M and develop lymphoproliferative disorders.29
Patients with X-linked agammaglobulinemia have Bruton’s tyrosine kinase mutations, resulting in B-cell maturation arrest and marked paucity of immunoglobulins; consequently, clinical presentations are dominated by onset of infection susceptibility as protection from maternally derived immunoglobulins wanes.30 There is concomitant contraction and skewing of the T-cell repertoire31 and an underrecognized but high incidence of autoimmune/inflammatory symptoms.32
Complete loss of function recombination-activating gene 1/2 (RAG1/2) mutations results in a severe combined immunodeficiency (SCID), discussed below. A subset of patients with hypomorphic RAG1/2 mutations manifests the so-called γ-δ variant of SCID, which is dominated clinically by autoimmunity and severe CMV infection.33 These patients maintain production of specific antibodies and have a marked deficiency of α-β but not γ-δ T cells, which undergo a CMV-driven expansion.34 In mouse models, γ-δ T cells promote expansion of marginal zone B cells and thus contribute to the size of the mature, antibody-producing B-cell pool.35 Other patients with hypomorphic RAG1/2 mutations present with granulomatous disease with variable immunodeficiency.36,37 The granulomatous and autoimmune manifestations are associated with broad-spectrum antibodies, which are thought to be a feature of immune dysregulation, which is then amplified by responses to recurrent and chronic viral infections.38
Combined Immunodeficiencies
While many primary immunodeficiencies dominated by immunoglobulin deficiency have been found to have a concomitant abnormal and constricted T-cell repertoire, combined immunodeficiencies have overt T- and B-cell immunodeficiencies. In some cases, autoimmune manifestations dominate the clinical picture.
Wiskott-Aldrich syndrome is an X-linked combined immunodeficiency characterized by atopic dermatitis and increased susceptibility to infections; the underlying defect is deficiency of Wiskott-Aldrich syndrome protein,39 a protein involved in cytoskeletal-based stabilization of lipid rafts. Autoimmune manifestations of Wiskott-Aldrich syndrome appear to be due to resulting changes in the strength of B-cell receptor and Toll-like receptor signaling, which in turn promote enrichment of self-reactive transitional and naive B cells.40,41
Dedicator of cytokinesis 8 deficiency, the loss of a guanine nucleotide exchange factor likely involved in cytoskeletal dynamics, manifests as an autosomal recessive hyper–immunoglobulin E syndrome associated with eosinophilia, severe atopic dermatitis, and human papillomavirus and staphylococcal infections.42 Similar to Wiskott-Aldrich syndrome, the humoral component of the immunodeficiency is thought to result from disruption of the B-cell immunologic synapse43; T cells are also affected, with decreased long-term survival of CD8+ memory T cells presumably further contributing to a deficiency of long-term cellular memory.44
SCID, Omenn syndrome (combined immunodeficiency with eosinophilia, erythroderma, and lymphadenopathy), and a range of milder primary immunodeficiencies are caused by loss-of-function RAG1/2 mutations, leading to defects in productive T-cell receptor and B-cell receptor gene rearrangements ranging from complete to partial deficiency.45 Rarer defects in other components of the recombination machinery such as DCLRE1C (DNA cross-link repair 1C, which encodes the homologous end-joining protein ARTEMIS) lead to a similar spectrum of phenotypes.46RAG1/2 mutations cause variable defects in recombinase activity, which likely combine with modifying genetic and environmental factors to produce the spectrum of phenotypes, from the complete absence of B and T cells in RAG1/2 null SCID, to so-called leaky SCID with some T and/or B cells, to Omenn syndrome, to sometimes later onset presentations dominated by variable immunodeficiency, granulomatous disease, and/or autoimmunity.45,47 Some even present in adult life with a CVID-like presentation with suppurative infections, few CD4+ T cells, and defective immunoglobulin production against bacterial polysaccharide antigens.48
Chromosome 22q11.2 deletion syndrome, formerly known as DiGeorge or velocardiofacial syndrome, includes conotruncal cardiac anomalies, hypoplastic thymus, and hypoparathyroidism; variable immunodeficiency is seen according to the degree of thymic hypoplasia.49 As more patients grow into adulthood, a progressive decrease in class-switched memory B cells has also been documented, likely due to ineffective T-cell help.50 Patients with CHARGE association (coloboma of the eye, heart defects, atresia of the choanae, retardation of growth and/or development, genital and/or urinary anomalies, and ear malformations) due to CHD7 mutations or deletions51 have significant phenotypic overlap with chromosome 22q11.2 deletion syndrome, but the combined immunodeficiency in at least some patients with CHARGE is just beginning to be recognized. A subset of patients with CHARGE has low thymic output with decreased T cells and insufficient antibody response to childhood vaccines.52
Down syndrome is associated with developmental abnormalities as well as increased leukemia risk. Less well known is the associated thymic hypofunction with reduced central tolerance, possibly due to thymic stromal defects,53 which in turn is thought to lead to increased propensity to autoimmune disorders.54 B-cell numbers are decreased overall in children with Down syndrome, with dysfunctional B-cell maturation55 and a marked deficiency in switched memory B cells. Together, these immunologic abnormalities result in a polygenic mild primary combined immunodeficiency with a propensity to autoimmunity and increased risk of infection.
Primary Immunodeficiency-Related Lymphoproliferations
The World Health Organization (WHO) 2008 describes lymphoproliferative disorders associated with specific primary immunodeficiencies.56 Cases received for the workshop beautifully illustrate these associations, with the addition of more recently described entities such as mucocutaneous ulcer57 and unusual B-cell maturation in the newly described entity of activating PIK3CD mutation-related immunodeficiency.28 We also describe a range of T-cell proliferations in primary immunodeficiency that inhabit a gray zone between reactive proliferations and neoplasia and that pose a danger of overdiagnosis of T-cell malignancy. We also describe highly characteristic lymphoproliferations associated with specific immunodeficiency states; knowledge of these can prevent overdiagnosis of lymphoma and allow for recognition of clinically important primary immunodeficiency states. Finally, we discuss the relationship of primary immunodeficiency-related lymphoproliferations with those described in secondary immunodeficiency settings, for which the prototype is posttransplant lymphoproliferative disorder (PTLD).58
Range of EBV+ and EBV– B-Cell Proliferations in Primary Immunodeficiency
The whole spectrum of EBV+ and EBV– B-cell lymphoproliferations described in secondary immunodeficiency settings, ranging from hyperplasias to polymorphic and monomorphic PTLD, is also seen in primary immunodeficiency. Cases received for the 2015 workshop Table 2 encompassed the whole spectrum of B-cell lymphoproliferations described in the WHO 2008 chapter on posttransplant lymphoproliferative disorders,58 as well as the more recently described EBV+ mucocutaneous ulcer (MCU).57
Table 2.
Primary Immunodeficiency-Related Cases Submitted to the Workshop Cover the Full Spectrum of B-Cell Lymphoproliferative Diseasea
| Characteristic | Hyperplasia | MCU | Low-Grade Lymphoma | Polymorphic B-cell LPD | CHL | DLBCL | Burkitt | Plasma- blastic |
|---|---|---|---|---|---|---|---|---|
| Immune dysregulation | ALPS XLP | Chédiak-Higashib XLPb | Chédiak-Higashib | XLP (2) | Wiskottb | |||
| DNA repair | ATb Nijmegenb | Bloomb | Nijmegenb | AT (2) CMRD Nijmegen | ||||
| Low immunoglobulin | CVID (5) PIKC3D | CVID (2) | CVID (2,6b) | CVIDb | CVID (2,2b) IgSD (2b) | |||
| PIKC3Db | ||||||||
| Combined immunodeficiency | CHARGEb | CHARGEb DOCK8b | Δ22q11.2b SCID (2b) |
ALPS, autoimmune lymphoproliferative syndrome; AT, ataxia telangiectasia; CHARGE, coloboma of the eye, heart defects, atresia of the choanae, retardation of growth and/or development, genital and/or urinary anomalies, and ear malformations; CHL, classical Hodgkin lymphoma; CMRD, congenital mismatch repair deficiency; CVID, common variable immunodeficiency; Δ22q11.2, chromosome 22q11.2 deletion syndrome, formerly known as DiGeorge or velocardiofacial syndrome; DLBCL, diffuse large B-cell lymphoma; DOCK8, dedicator of cytokinesis 8 deficiency; IgSD, immunoglobulin subset deficiency; LPD, lymphoproliferative disorder; MCU, mucocutaneous ulcer; PIK3CD, phosphatidylinositol 3-kinase C δ activating kinase mutation; SCID, severe combined immunodeficiency; XLP, X-linked lymphoproliferative disorder.
Numbers in parentheses represent the number of cases in the category. The underlying primary immunodeficiencies of workshop B-cell lymphoproliferative cases are organized by category of immunodeficiency (rows) and diagnostic category (columns).
Epstein-Barr virus positive.
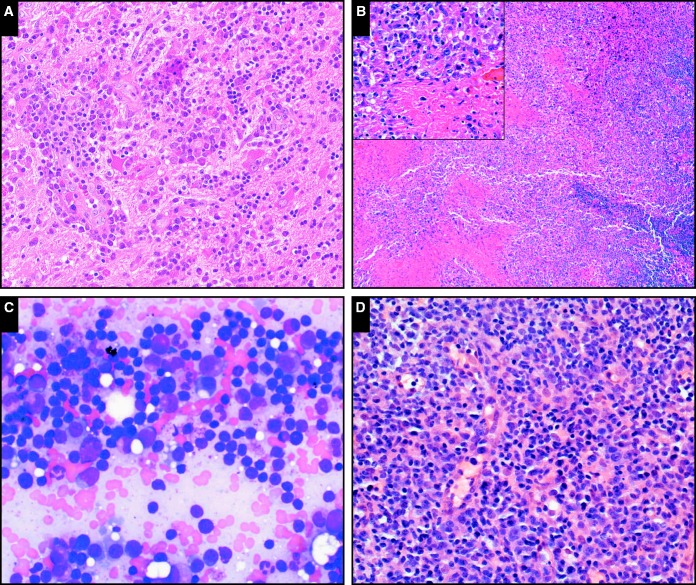
Notable examples of hyperplasias in the setting of primary immunodeficiency include plasmacytic hyperplasia involving the brain in a patient with CVID (case SH2015-467; Image 1A). EBV+ MCU is recognized in settings of advanced age or iatrogenic immunosuppression59; we received a typical case in a patient with CHARGE syndrome (case SH2015-178; Image 1B). Low-grade lymphomas were infrequent among submissions and included extranodal marginal zone lymphomas as well as a case of MYD88-mutated lymphoplasmacytic lymphoma involving the bone marrow of a patient with CVID (case SH2015-223; Image 1C); this patient later progressed to diffuse large B-cell lymphoma involving the spleen. Polymorphic B-cell lymphoproliferative disorders resembling those described in the posttransplant setting were particularly frequently represented among submitted cases, and most were EBV+; case SH2015-82 Image 1D is an excellent illustrative example. The most frequent monomorphic B lymphoproliferative disorders received were diffuse large B-cell lymphomas, over half of them EBV+; classical Hodgkin lymphoma, Burkitt lymphoma, and plasmablastic lymphoma were also represented.
Image 1.
The full range of immunodeficiency-related B-cell lymphoproliferations described in the posttransplant setting are also seen in the setting of primary immunodeficiency. A, Plasmacytic hyperplasia involving the brain in a patient with common variable immunodeficiency (CVID; case SH2015-467) (H&E, x20). B, Epstein-Barr virus–positive mucocutaneous ulcer in a patient with CHARGE (coloboma of the eye, heart defects, atresia of the choanae, retardation of growth and/or development, genital and/or urinary anomalies, and ear malformations) syndrome (case SH2015-178); note the sheets of atypical cells surrounding areas of necrosis (inset, including forms with Hodgkin-like morphology; x20); the cuff of small mature lymphocytes is visible at right (H&E, x4). C, Lymphoplasmacytic lymphoma involving the bone marrow of a patient with CVID (SH2015-223) resembles that seen in nonimmunodeficiency settings; here the lymphoplasmacytic infiltrate is visualized in a bone marrow aspirate smear (Giemsa, x40). D, Polymorphic B lymphoproliferative disorder in a patient with CVID (SH2015-82); shows scattered large atypical cells in a background of small lymphocytes and plasma cells (H&E, x20).
CVID was highly represented among submitted cases, and the entire spectrum of B-cell lymphoproliferative disorders was manifest. In contrast, DNA repair defect–associated lymphoproliferations tended to be frank lymphomas.
Several patients had more than one lymphoproliferation; these are summarized in Table 3. There was no sequential pattern in terms of EBV reactivity (ie, an EBV+ lymphoproliferation could be followed by an EBV– lymphoproliferation or vice versa). There was also no specific sequential pattern in the occurrence of hyperplasias, polymorphic lymphoproliferative disorders (LPDs), and primary immunodeficiency-related lymphomas; none of the patients with multiple diagnoses had antecedent hyperplasias, and polymorphic B-cell LPDs were diagnosed either before or after a diagnosis of lymphoma.
Table 3.
Patients With Primary Immunodeficiency and More Than One Lymphoproliferation Follow No Particular Pattern of Diagnostic Category or Epstein-Barr Virus Status
| Case No. | Immunodeficiency | Diagnosis 1 | Diagnosis 2 | Diagnosis 3 |
|---|---|---|---|---|
| SH2015-47 | Nijmegen | DLBCL | MZLa | CHLa |
| SH2015-82 | CVID | Polymorphic B-cell LPDa | DLBCLa | |
| SH2015-392 | CVID | Polymorphic B-cell LPDa | DLBCL | |
| SH2015-223 | CVID | LPL | DLBCL | |
| SH2015-226 | CVID | DLBCL | Polymorphic B-cell LPDa | CHLa |
| SH2015-178 | CHARGE | MCUa | Polymorphic B-cell LPDa |
CVID, common variable immunodeficiency; CHARGE, coloboma of the eye, heart defects, atresia of the choanae, retardation of growth and/or development, genital and/or urinary anomalies, and ear malformations; CHL, classical Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; LPD, lymphoproliferative disorder; LPL, lymphoplasmacytic lymphoma; MCU, Epstein-Barr virus–positive mucocutaneous ulcer; MZL, marginal zone lymphoma.
Epstein-Barr virus positive.
T-Cell and Histiocytic Proliferations and Borderlines With Malignancy
Unlike the B lymphoproliferative disorders, primary immunodeficiency-related T lymphoproliferative disorders differ from those seen in acquired immunodeficiency settings Table 4. Mature T-cell lymphomas are outnumbered by T-cell hyperplasias and atypical lymphoproliferations, particularly double-negative T-cell proliferations, cytotoxic T-cell infiltrates, and mixed T-cell–histiocytic infiltrates, including cutaneous granulomatous infiltrates, EBV-related hemophagocytic lymphohistiocytosis, and RDD.
Table 4.
Primary Immunodeficiency-Related Cases Submitted to the Workshop Cover a Spectrum of T Lymphoproliferative and Histiocytic Disordersa
| Characteristic | T-ALL | Nodal Hyperplasia | Extranodal LPD | Granulomatous | HLH | Rosai-Dorfman |
|---|---|---|---|---|---|---|
| Immune dysregulation | ALPS | ALPS | Perforin deficiencyb | ALPS RALD | ||
| XLPb | ||||||
| DNA repair | CMRD | AT | ||||
| Low immunoglobulin | CVID | CVID (3) | CVID | |||
| Hypomorphic RAG | ||||||
| Combined immunodeficiency | DiGeorge | Δ22q11.2b Down syndromeb |
ALPS, autoimmune lymphoproliferative syndrome; AT, ataxia telangiectasia; CMRD, congenital mismatch repair deficiency; CVID, common variable immunodeficiency; Δ22q11.2, chromosome 22q11.2 deletion syndrome, formerly known as DiGeorge or velocardiofacial syndrome; HLH, hemophagocytic lymphohistiocytosis; Hypomorphic RAG, hypomorphic recombinase-activating gene 1/2 deficiency; LPD, lymphoproliferative disorder; RALD, RAS-associated leukoproliferative disorder; T-ALL, T lymphoblastic leukemia/lymphoma; XLP, X-linked lymphoproliferative disorder.
The underlying primary immunodeficiencies of workshop T-cell and histiocytic cases are organized by category of immunodeficiency (rows) and diagnostic category (columns).
Epstein-Barr virus positive.
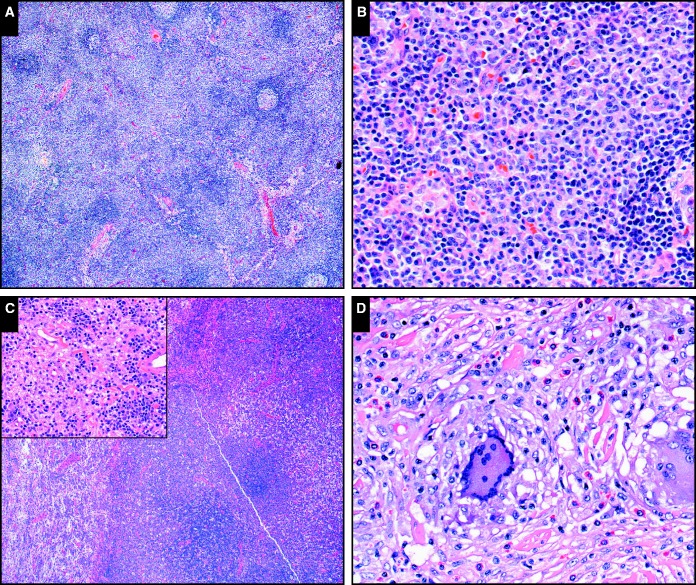
DNT proliferations were, as expected, a prominent feature of ALPS; submitted cases illustrate the extent and variability of such ALPS-related lymphoproliferations. In addition to lack of CD4, CD8, and CD45RO, ALPS-related T-cell proliferations are generally positive for cytotoxic markers such as perforin, TIA-1, and CD57.8 Their mitotic activity (and Ki-67 proliferative index) is high, likely due to tonic Akt/mTOR pathway activation.60 Classic ALPS-related lymphadenopathy is a paracortical lymphoid hyperplasia composed of small CD45RO– lymphocytes (case SH2015-33; Image 2A and Image 2B). ALPS-related DNT proliferations may form masses and involve extranodal sites. Case SH2015-140 illustrates the complicated clinical course of a patient with ALPS and extensive DNT proliferations. An initial lymph node biopsy specimen shows a classic paracortical DNT proliferation; subsequent biopsy specimens show nodal lymphoid effacement, followed by bone marrow, thymic, and renal involvement (case SH2015-140; Image 2C). Despite the extranodal involvement, partial tissue effacement, and intermediate cell size with high Ki-67, the lesions were not clonal by T-cell gene rearrangement analysis. While the predominant T-cell expansion in ALPS is that of α-β T cells, γ-δ DNT proliferation has also been described in this setting.61 We received one such case (case SH2015-415) in an infant with lymphadenopathy and hepatosplenomegaly whose lymph node biopsy specimen showed a highly proliferative γ-δ DNT infiltrate.
Image 2.
Benign or indeterminate T-cell lymphoproliferations in the setting of primary immunodeficiency. A, B, Classic autoimmune lymphoproliferative syndrome (ALPS)–related paracortical hyperplasia (SH2015-33) composed of small- to medium-sized round lymphocytes, which form pale-staining expansile paracortical collections at low power and have small visible nucleoli and slight nuclear irregularity at high power (A, H&E, x4; B, H&E, x20). C, More extensive, mass-forming oligoclonal double-negative T-cell proliferation in the setting of ALPS (SH2015-140); in addition to paracortical expansion, there is a sheet-like process obliterating underlying lymph node architecture (H&E, x4). Inset: small- to medium-sized lymphocytes have visible nucleoli and moderate nuclear membrane irregularity (H&E, x20). D, Cutaneous dermal lymphohistiocytic lesion with a clonal cytotoxic T-cell infiltrate in a patient with immunoglobulin G4 subset deficiency and CD4 T-cell lymphopenia (SH2015-410). Small mature lymphocytes with slightly irregular nuclear contour are embedded in a granulomatous infiltrate with scattered eosinophils (H&E, x20).
ALPS-related proliferations have several features that may suggest malignancy, including partial tissue effacement, high proliferative index, and an unusual immunophenotype (DNT, CD45-negative, cytotoxic, sometimes γ-δ T-cell receptor positive). Features that should favor benign lymphoproliferation include the clinical setting (a young or pediatric patient with chronic lymphadenopathy and features of immunodeficiency), an unusual immunophenotype that is nevertheless typical of a particular expanded T-cell subset, and failure to demonstrate clonality by gene rearrangement studies. Of these, knowledge of, or suspicion for, an underlying primary immunodeficiency is arguably the most crucial and highlights the necessity of clinicopathologic correlation and open communication with the clinical team.
A second cluster of T-cell hyperplasias was in patients with CVID and cytotoxic T-cell proliferations. Subsets of patients with CVID have increased circulating cytotoxic CD8+ T cells; this phenotype is associated with low-memory B cells, autoimmunity, and splenomegaly.62 Caution is therefore required to diagnose T-cell large granular lymphocyte leukemia in the setting of CVID. Patients with CVID who undergo liver biopsy often have findings of nodular regenerative hyperplasia with sinusoidal cytotoxic T-cell infiltrates,63,64 as illustrated by case SH2015-185.
We received two cases of granulomatous CD8+ cytotoxic T-cell–rich cutaneous infiltrates of uncertain malignant potential; there is overlap among these cases and the proposed entity of CD8+ granulomatous cutaneous T-cell lymphoma, which has been described in a small case series including two of four patients with primary immunodeficiency.65 These include a patient with chromosome 22q11.2 deletion (DiGeorge) syndrome and a maculopapular lymphohistiocytic rash involving the extremities, showing a cytotoxic T-cell immunophenotype and a clonal T-cell receptor gene rearrangement (SH2015-148, submitted by Dr. Morlote). A second case (SH2015-410), submitted by Drs Siddiqi and Fitzgibbons, occurred in a patient with immunoglobulin G4 subset deficiency and CD4 lymphopenia; a 15-cm indurated thigh lesion was biopsied and showed a lymphohistiocytic infiltrate with, again, a cytotoxic T-cell immunophenotype and a clonal T-cell receptor gene rearrangement Image 2D. The size of the lesion and clonality might favor the proposed diagnosis of cutaneous lymphoma; however, given the paucity of described cases, the known propensity for benign mixed T-cell/histiocytic infiltrates in the setting of immunodeficiency, and the lack of specificity of T-cell gene rearrangements in this setting, we recommend a cautious approach to diagnosis and active conversation with treating physicians to avoid potential overtreatment. This histologic picture of a cytotoxic T-cell infiltrate and a granulomatous background raises a broad differential of reactive, neoplastic, and infectious processes, including immunodeficiency.
The few overt T-cell lymphomas received were restricted to DNA repair defect–associated primary immunodeficiencies, again raising the question of whether there is a true predisposition to T-cell lymphoma in immunodeficiency outside of some specific contexts. A rather unusual case was that of T lymphoblastic lymphoma in the setting of congenital mismatch repair deficiency; the child had a germline biallelic deletion of post meiotic segregation increased 2 (PMS2) and also developed diffuse large B-cell lymphoma at a young age (case SH2015-177, submitted by Dr Van Krieken). In case reports of congenital mismatch-repair deficiency-related hematologic malignancies, T lymphoblastic lymphoma and Burkitt lymphoma are most frequently reported.66
We also received a case of ataxia telangiectasia–related prodromal T-cell prolymphocytic leukemia (SH2015-48, submitted by Drs Tan and Chisholm), reflecting a known association of these two rare entities.67 Outside of the context of ataxia telangiectasia, a subset of patients with T-cell prolymphocytic leukemia has been shown to have an indolent prodrome of months to years.68 It is unclear whether this indolent prodrome can be prospectively distinguished from aggressive disease and whether a relatively stable asymptomatic clonal lymphoproliferation with a T-cell prolymphocytic leukemia immunophenotype in a patient with ataxia telangiectasia belongs to this indolent category.
The diagnosis of T-cell lymphoma in the primary immunodeficiency setting is confounded by frequent nonmalignant expansions of minor T-cell subsets and should be rendered using strict criteria and with full knowledge of the clinical setting, particularly in non-DNA repair defect–associated primary immunodeficiencies. Architectural effacement, T-cell gene rearrangement, and cytogenetic abnormalities, as well as to a lesser extent immunophenotypic aberrancies and cytologic atypia, may be helpful but should be used cautiously and in combination with awareness of known associations such as both α-β and γ-δ T-cell proliferations in ALPS, cytotoxic T-cell infiltrates in CVID, and clonal cutaneous lymphohistiocytic infiltrates in multiple primary immunodeficiency settings.
Associations Among Specific Immunodeficiency States and Specific Proliferations/Lymphoma
We have discussed above the known association of ataxia telangiectasia and T-cell prolymphocytic leukemia or its prodrome, as well as ALPS and double-negative α-β and γ-δ T-cell proliferations. The association of ALPS with RDD is also well documented, and indeed we received an illustrative case of a 6-year-old boy with ALPS and both a paracortical DNT expansion and characteristic sinusoidal S100+ histiocytes with emperipolesis (SH2015-49, submitted by Dr Krishnan). RAS-associated leukoproliferative disorder, or RALD, has features of both ALPS-like double-negative T-cell lymphoproliferations and autoimmunity as well as predisposition to juvenile myelomonocytic leukemia-like monocytic proliferation. Interestingly, we received a well-documented case of RDD disease in a patient with RALD (SH2015-342, submitted by Dr. Brousse), an association not yet reported in the literature.
CVID (and X-linked agammaglobulinemia) has been previously reported to be associated with intestinal nodular hyperplasia with giardiasis69; we received an illustrative case (SH2015-229, submitted by Drs Oh and Park) that also had a paucity of plasma cells in the lamina propria, a further clue to the diagnosis.70 Two cases of Burkitt lymphoma were submitted to the workshop, both in the setting of XLP disorder. Both were EBV–, and in both cases, apparent recurrence of Burkitt lymphoma was clonally distinct (cases SH2015-65, submitted by Drs Shafernak, Leuer, and Jennings, and SH2015-285, submitted by Dr Zhou and colleagues). Burkitt lymphoma in a young boy, and particularly Burkitt lymphoma with a clonally distinct recurrence, should trigger XLP testing. A final unusual case was received from a patient with germline PIK3CD mutation who was found to have hematogone hyperplasia with a partial B-cell maturation arrest (case SH2015-431, submitted by Dr Dulau-Florea and colleagues). An abnormal hematogone maturation pattern in a patient with decreased mature B cells could be a clue to an underlying primary immunodeficiency or bone marrow failure disorder with a B-cell component.71
Parallels and Differences Among Primary and Other Immunodeficiency Settings
As in acquired immunodeficiency settings, the entire spectrum of hyperplasia, polymorphous LPDs, and B-cell lymphomas is seen in the setting of primary immunodeficiency, with a mix of EBV+ and EBV– lymphomas noted. EBV– lymphoid hyperplasias were more commonly submitted in the setting of primary immunodeficiency, but this may be an ascertainment bias of this workshop since otherwise unexplained EBV– lymphoid hyperplasias are not a defined entity in the acquired immunodeficiency setting. The greatest difference among primary and acquired immunodeficiency settings was in the spectrum of T lymphoproliferative disorders. Few overt T-cell lymphomas were received in the category of primary immunodeficiency, and these were restricted to DNA repair defect–associated primary immunodeficiencies, again raising the question of whether there is a true predisposition to T-cell lymphoma in immunodeficiency outside of some specific contexts such as thiopurine-related hepatosplenic T-cell lymphoma. By contrast, the primary immunodeficiency-related T-cell lymphoproliferative category was dominated by expansions of particular T-cell subsets, such as α-β and γ-δ T-cell proliferations in ALPS and extranodal cytotoxic T-cell infiltrates in CVID. These may simply represent very specific epiphenomena of particular underlying molecular mechanisms in a subset of primary immunodeficiencies; however, it is also possible that such benign T-cell proliferations are underrecognized at this time in other immunodeficiency settings. As iatrogenic immunosuppression begins to target highly specific pathways through small-molecule or biologic-based inhibition, we may anticipate that such proliferations will become evident outside of the primary immunodeficiency setting.
Conclusions
The number of distinct primary immunodeficiencies is large and increasing in the era of cost-efficient molecular discovery; fortunately, these can be usefully subdivided into broadly clinical and pathophysiologic groups. The range of lymphoproliferations seen in primary immunodeficiency mirrors those seen in the posttransplant and other immunodeficiency settings, with monomorphic B and sometimes T lymphoproliferative disorders enriched in those primary immunodeficiencies associated with DNA repair defects. Distinctive T-cell subset expansions seen in ALPS, CVID, and related entities can mimic lymphoma, and recognition of characteristic double-negative T-cell or cytotoxic T-cell expansions is key to avoid overdiagnosis. Themes and specific associations useful to the practicing diagnostic hematopathologist are summarized in Table 5.
Table 5.
Summary Table: Primary/Congenital Immunodeficiencies
| Primary immunodeficiencies can be functionally subdivided into four clinicopathologic categories: immune dysregulation, DNA repair defect, low immunoglobulin, and combined immunodeficiency types. |
| Immune dysregulation–type primary immunodeficiencies are characterized by defects in T-cell and/or natural killer–cell signaling or apoptosis, expansion of T-cell subsets and/or virally transformed B cells, and predisposition to hemophagocytic lymphohistiocytosis. |
| Examples of immune dysregulation–type primary immunodeficiencies include autoimmune lymphoproliferative syndrome, Chédiak-Higashi syndrome, and Ras-associated leukoproliferative disorder. |
| DNA repair defects–type primary immunodeficiencies have pleiotropic effects on development and directly predispose to various forms of malignancy. |
| Examples of DNA repair defect–driven primary immunodeficiencies include ataxia telangiectasia, Nijmegen breakage syndrome, and congenital mismatch repair deficiency. |
| Low immunoglobulin–type primary immunodeficiencies are associated with a T-cell repertoire abnormality; failure to repress self-reactive clones may lead to autoimmunity, and failure to inhibit clonal outgrowth may predispose to Epstein-Barr virus–transformed or other B-cell lymphoproliferations. |
| Examples of low immunoglobulin–type primary immunodeficiencies include common variable immunodeficiency and immunoglobulin subset deficiency. |
| Combined immunodeficiencies manifest symptoms of both B- and T-cell deficiencies, with a combination of infectious and autoimmune complications and predisposition to lymphoproliferative disorders. |
| Examples of combined-type primary immunodeficiencies include severe combined immunodeficiency and Down syndrome, which is associated with a mild polygenic combined immunodeficiency. |
| Expansions of T-cell subsets in the primary immunodeficiency setting can mimic lymphoma. |
| Double-negative T-cell expansions in autoimmune lymphoproliferative syndrome have a high proliferative index, monomorphic appearance, and CD4/CD8 double-negative, cytotoxic T-cell immunophenotype; both α-β or less commonly γ-δ T-cell expansions can occur. |
| Cytotoxic T-cell expansions occur in common variable immunodeficiency and manifest as intrasinusoidal lymphocytosis in the liver and marrow and as large granular lymphocytosis in the peripheral blood; clonal T-cell gene rearrangements may be present. |
| The full spectrum of immunodeficiency-related B-cell lymphoproliferative disorders seen in other immunodeficiency settings also occurs in primary immunodeficiency, ranging from hyperplasias to mucocutaneous ulcer to polymorphic B-cell lymphoproliferative disorders and Hodgkin and non-Hodgkin lymphomas. |
References
- 1. Bousfiha A, Jeddane L, Al-Herz W, et al. The 2015 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2015;35:727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Saint BG, Sepulveda FE, Maschalidi S, et al. Cytotoxic granule secretion by lymphocytes and its link to immune homeostasis. F1000Research. 2015. http://f1000research.com/articles/4-930/v1. Accessed April 2, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher GH, Rosenberg FJ, Straus SE, et al. Dominant interfering fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935-946. [DOI] [PubMed] [Google Scholar]
- 4. Tarbox JA, Keppel MP, Topcagic N, et al. Elevated double negative T cells in pediatric autoimmunity. J Clin Immunol. 2014;34:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Price S, Shaw PA, Seitz A, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123:1989-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oliveira JB, Bleesing JJ, Dianzani U, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35-e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lambotte O, Neven B, Galicier L, et al. Diagnosis of autoimmune lymphoproliferative syndrome caused by FAS deficiency in adults. Haematologica. 2013;98:389-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim MS, Straus SE, Dale JK, et al. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol. 1998;153:1541-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maric I, Pittaluga S, Dale JK, et al. Histologic features of sinus histiocytosis with massive lymphadenopathy in patients with autoimmune lymphoproliferative syndrome. Am J Surg Pathol. 2005;29:903-911. [DOI] [PubMed] [Google Scholar]
- 10. Venkataraman G, McClain KL, Pittaluga S, et al. Development of disseminated histiocytic sarcoma in a patient with autoimmune lymphoproliferative syndrome (ALPS) and associated Rosai-Dorfman disease. Am J Surg Pathol. 2010;34:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oliveira JB, Bidère N, Niemela JE, et al. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104:8953-8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takagi M, Shinoda K, Piao J, et al. Autoimmune lymphoproliferative syndrome–like disease with somatic KRAS mutation. Blood. 2011;117:2887-2890. [DOI] [PubMed] [Google Scholar]
- 13. Lanzarotti N, Bruneau J, Trinquand A, et al. RAS-associated lymphoproliferative disease evolves into severe juvenile myelo-monocytic leukemia. Blood. 2014;123:1960-1963. [DOI] [PubMed] [Google Scholar]
- 14. Calvo KR, Price S, Braylan RC, et al. JMML and RALD (Ras-associated autoimmune leukoproliferative disorder): common genetic etiology yet clinically distinct entities. Blood. 2015;125:2753-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dotta L, Parolini S, Prandini A, et al. Clinical, laboratory and molecular signs of immunodeficiency in patients with partial oculo-cutaneous albinism. Orphanet J Rare Dis. 2013;8:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bohn G, Allroth A, Brandes G, et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med. 2007;13:38-45. [DOI] [PubMed] [Google Scholar]
- 17. Law RHP, Lukoyanova N, Voskoboinik I, et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature. 2010;468:447-451. [DOI] [PubMed] [Google Scholar]
- 18. Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957-1959. [PubMed] [Google Scholar]
- 19. Tesi B, Chiang SCC, El-Ghoneimy D, et al. Spectrum of atypical clinical presentations in patients with biallelic PRF1 missense mutations. Pediatr Blood Cancer. 2015;62:2094-2100. [DOI] [PubMed] [Google Scholar]
- 20. Ciambotti B, Mussolin L, d’Amore ESG, et al. Monoallelic mutations of the perforin gene may represent a predisposing factor to childhood anaplastic large cell lymphoma. J Pediatr Hematol Oncol. 2014;36:e359-e365. [DOI] [PubMed] [Google Scholar]
- 21. Nichols KE, Ma CS, Cannons JL, et al. Molecular and cellular pathogenesis of X-l inked lymphoproliferative disease. Immunol Rev. 2005;203:180-199. [DOI] [PubMed] [Google Scholar]
- 22. Tangye SG. XLP: clinical features and molecular etiology due to mutations in SH2D1A encoding SAP. J Clin Immunol. 2014;34:772-779. [DOI] [PubMed] [Google Scholar]
- 23. Wimmer K, Kratz CP, Vasen HFA, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium “care for CMMRD” (C4CMMRD). J Med Genet. 2014;51:355-365. [DOI] [PubMed] [Google Scholar]
- 24. Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematol Educ Program Am Soc Hematol Am Soc Hematol Educ Program. 2012;2012:301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roskin KM, Simchoni N, Liu Y, et al. IgH sequences in common variable immune deficiency reveal altered B cell development and selection. Sci Transl Med. 2015;7:302ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramesh M, Hamm D, Simchoni N, et al. Clonal and constricted T cell repertoire in common variable immune deficiency. Clin Immunol Orlando Fla. 2015;61:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jefferis R, Kumararatne DS. Selective IgG subclass deficiency: quantification and clinical relevance. Clin Exp Immunol. 1990;81:357-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lucas CL, Kuehn HS, Zhao F, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crank MC, Grossman JK, Moir S, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34:272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ochs HD, Smith CI. X-linked agammaglobulinemia: a clinical and molecular analysis. Medicine (Baltimore). 1996;75:287-299. [DOI] [PubMed] [Google Scholar]
- 31. Ramesh M, Simchoni N, Hamm D, et al. High-throughput sequencing reveals an altered T cell repertoire in X-linked agammaglobulinemia. Clin Immunol Orlando Fla. 2015;161:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hernandez-Trujillo VP, Scalchunes C, Cunningham-Rundles C, et al. Autoimmunity and inflammation in X-linked agammaglobulinemia. J Clin Immunol. 2014;34:627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Villartay J-P, Lim A, Al-Mousa H, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005;115:3291-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ehl S, Schwarz K, Enders A, et al. A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest. 2005;115:3140-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang Y, Getahun A, Heiser RA, et al. γδ T cells shape preimmune peripheral B cell populations. J Immunol Baltim Md 1950. 2016;196:217-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuetz C, Huck K, Gudowius S, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med. 2008;358:2030-2038. [DOI] [PubMed] [Google Scholar]
- 37. De Ravin SS, Cowen EW, Zarember KA, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walter JE, Rosen LB, Csomos K, et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest. 2015;125:4135-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635-644. [DOI] [PubMed] [Google Scholar]
- 40. Kolhatkar NS, Brahmandam A, Thouvenel CD, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome. J Exp Med. 2015;212:1663-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Metzler G, Kolhatkar NS, Rawlings DJ. BCR and co-receptor crosstalk facilitate the positive selection of self-reactive transitional B cells. Curr Opin Immunol. 2015;37:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Randall KL, Lambe T, Johnson AL, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lambe T, Crawford G, Johnson AL, et al. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol. 2011;41:3423-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corneo B, Moshous D, Güngör T, et al. Identical mutations in RAG1 or RAG2 genes leading to defective V(D)J recombinase activity can cause either T-B-severe combined immune deficiency or Omenn syndrome. Blood. 2001;97:2772-2776. [DOI] [PubMed] [Google Scholar]
- 46. Volk T, Pannicke U, Reisli I, et al. DCLRE1C (ARTEMIS) mutations causing phenotypes ranging from atypical severe combined immunodeficiency to mere antibody deficiency. Hum Mol Genet. 2015;24:7361-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee YN, Frugoni F, Dobbs K, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol. 2014;133:1099-1108.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geier CB, Piller A, Linder A, et al. Leaky RAG deficiency in adult patients with impaired antibody production against bacterial polysaccharide antigens. PLoS One. 2015;10:e0133220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gul KA, Øverland T, Osnes L, et al. Neonatal levels of T-cell receptor excision circles (TREC) in patients with 22q11.2 deletion syndrome and later disease features. J Clin Immunol. 2015;35:408-415. [DOI] [PubMed] [Google Scholar]
- 50. Derfalvi B, Maurer K, McDonald McGinn DM, et al. B cell development in chromosome 22q11.2 deletion syndrome. Clin Immunol Orlando Fla. 2016;163:1-9. [DOI] [PubMed] [Google Scholar]
- 51. Vissers LELM, van Ravenswaaij CMA, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955-957. [DOI] [PubMed] [Google Scholar]
- 52. Wong MTY, Lambeck AJA, van der Burg M, et al. Immune dysfunction in children with CHARGE syndrome: a cross-sectional study. PLoS One. 2015;10:e0142350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lima FA, Moreira-Filho CA, Ramos PL, et al. Decreased AIRE expression and global thymic hypofunction in Down syndrome. J Immunol Baltim Md 1950. 2011;187:3422-3430. [DOI] [PubMed] [Google Scholar]
- 54. Giménez-Barcons M, Casteràs A, Armengol M. d P, et al. Autoimmune predisposition in Down syndrome may result from a partial central tolerance failure due to insufficient intrathymic expression of AIRE and peripheral antigens. J Immunol Baltim Md 1950. 2014;193:3872-3879. [DOI] [PubMed] [Google Scholar]
- 55. Carsetti R, Valentini D, Marcellini V, et al. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur J Immunol. 2015;45:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Krieken JH, Onciu M, Elenitoba-Johnson KS, et al. Lymphoproliferative diseases associated with primary immune disorders In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. 4th ed Lyon, France: IARC; 2008:336-339. [Google Scholar]
- 57. Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer—a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Swerdlow SH, Weber SA, Chadburn A, et al. Post-transplant lymphoproliferative disorders In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. 4th ed Lyon, France: IARC:343-349. [Google Scholar]
- 59. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Völkl S, Rensing-Ehl A, Allgäuer A, et al. Hyperactive mTOR pathway promotes lymphoproliferation and abnormal differentiation in autoimmune lymphoproliferative syndrome. Blood. 2016;128:227-238. [DOI] [PubMed] [Google Scholar]
- 61. van den Berg A, Tamminga R, de Jong D, et al. FAS gene mutation in a case of autoimmune lymphoproliferative syndrome type IA with accumulation of gammadelta+ T cells. Am J Surg Pathol. 2003;27:546-553. [DOI] [PubMed] [Google Scholar]
- 62. Viallard J-F, Blanco P, André M, et al. CD8+HLA-DR+ T lymphocytes are increased in common variable immunodeficiency patients with impaired memory B-cell differentiation. Clin Immunol Orlando Fla. 2006;119:51-58. [DOI] [PubMed] [Google Scholar]
- 63. Ziol M, Poirel H, Kountchou GN, et al. Intrasinusoidal cytotoxic CD8+ T cells in nodular regenerative hyperplasia of the liver. Hum Pathol. 2004;35:1241-1251. [DOI] [PubMed] [Google Scholar]
- 64. Szablewski V, René C, Costes V. Indolent cytotoxic T cell lymphoproliferation associated with nodular regenerative hyperplasia: a common liver lesion in the context of common variable immunodeficiency disorder. Virchows Arch Int J Pathol. 2015;467:733-740. [DOI] [PubMed] [Google Scholar]
- 65. Gammon B, Robson A, Deonizio J, et al. CD8(+) granulomatous cutaneous T-cell lymphoma: a potential association with immunodeficiency. J Am Acad Dermatol. 2014;71: 555-560. [DOI] [PubMed] [Google Scholar]
- 66. Lavoine N, Colas C, Muleris M, et al. Constitutional mismatch repair deficiency syndrome: clinical description in a French cohort. J Med Genet. 2015;52:770-778. [DOI] [PubMed] [Google Scholar]
- 67. Suarez F, Mahlaoui N, Canioni D, et al. Incidence, presentation, and prognosis of malignancies in ataxia-telangiectasia: a report from the French national registry of primary immune deficiencies. J Clin Oncol off J Am Soc Clin Oncol. 2015;33:202-208. [DOI] [PubMed] [Google Scholar]
- 68. Garand R, Goasguen J, Brizard A, et al. Indolent course as a relatively frequent presentation in T-prolymphocytic leukaemia. Groupe Français d’Hématologie Cellulaire. Br J Haematol. 1998;103:488-494. [DOI] [PubMed] [Google Scholar]
- 69. Washington K, Stenzel TT, Buckley RH, et al. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol. 1996;20:1240-1252. [DOI] [PubMed] [Google Scholar]
- 70. Malamut G, Verkarre V, Suarez F, et al. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol. 2010;105:2262-2275. [DOI] [PubMed] [Google Scholar]
- 71. Ganapathi KA, Townsley DM, Hsu AP, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125:56-70. [DOI] [PMC free article] [PubMed] [Google Scholar]